Abstract
Background.
The prevalence of obesity in older adults is increasing but concerns exist about the effect of weight loss on muscle function. Demonstrating that muscle strength and power are not adversely affected during “intentional” weight loss in older adults is important given the wide-ranging negative health effects of excess adiposity.
Methods.
Participants (N = 88; age = 70.6 ± 3.6 years; body mass index = 32.8 ± 4.5kg/m2) were randomly assigned to one of four intervention groups: pioglitazone or placebo and resistance training (RT) or no RT, while undergoing intentional weight loss via a hypocaloric diet. Outcomes were leg press power and isometric knee extensor strength. Analysis of covariance, controlling for baseline values, compared follow-up means of power and strength according to randomized groups.
Results.
Participants lost an average of 6.6% of initial body mass, and significant declines were observed in fat mass, lean body mass, and appendicular lean body mass. Compared with women not randomized to RT, women randomized to RT had significant improvements in leg press power (p < .001) but not in knee extensor strength (p = 0.12). No significant differences between groups in change in power or strength from baseline were detected in men (both p > .25). A significant pioglitazone-by-RT interaction for leg press power was detected in women (p = .006) but not in men (p = .88).
Conclusions.
In older overweight and obese adults, a hypocaloric weight loss intervention led to significant declines in lean body mass and appendicular lean body mass. However, in women assigned to RT, leg power significantly improved following the intervention, and muscle strength or power was not adversely effected in the other groups. Pioglitazone potentiated the effect of RT on muscle power in women but not in men; mechanisms underlying this sex effect remain to be determined.
Key Words: Obesity, Resistance training, Muscle strength, Muscle power, Voluntary weight loss.
OVERWEIGHT and obesity are associated with a wide range of diseases (1) and constitute one of the most prevalent and disabling problems among older adults in the United States (2). Obese persons have poorer relative strength, an increased risk for mobility limitations, and increased risk of impaired physical function, all of which contribute to a higher risk for institutionalization and death (3–5).
In spite of this, whether or not overweight or obese older adults should engage in “intentional” weight loss is controversial (6–8). One concern is that while voluntary weight loss reduces fat mass, it also reduces lean body mass (LBM), which may lead to impairments in muscle strength and power, resulting in limitations in physical function (9,10). Therefore, a strong rationale exists for incorporating resistance exercise in weight loss interventions for overweight or obese older adults (11,12). Currently, there are limited data on the effect of weight loss combined with resistance training (RT) on changes in LBM and muscle strength and power in this population.
Fat infiltration in muscle has been associated with reduced strength and lower extremity function and with increased risk of mobility loss in older men and women (13,14). Pioglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist used in treating type 2 diabetes (T2D) appears to change the distribution of fat within the body, resulting in a significant decrease in the visceral-to-subcutaneous fat ratio (15). Interestingly, pioglitazone has also been shown to decrease intramyocellular lipid content in individuals with T2D (16,17). Therefore, treatment with pioglitazone may be a novel approach to preserve or improve muscle function in older adults undergoing weight loss treatment. Whether or not it may work synergistically with resistance exercise to augment increases in muscle strength and power has not been studied.
We examined the effects of RT and/or pioglitazone treatment on muscle power and strength in overweight or obese older women and men without T2D undergoing voluntary weight loss via caloric restriction. This is a secondary data analysis from a study designed to examine two differing strategies for affecting two different tissue compartments during weight loss: RT to reduce lean mass loss and pioglitazone to accelerate visceral fat loss (18).
METHODS
Study Design
Optimizing Body Composition for Functioning in Older Adults (OPTIMA) was a 16-week single-site 2 × 2 randomized single-blind study (18). Participants were randomly assigned to one of four intervention groups to receive either pioglitazone or placebo and RT or no RT. In addition, all participants received a hypocaloric diet achieved using meal replacement bars and shakes (Slim-Fast Optima, Unilever Inc.). We used concealed random allocation, and randomization was performed separately for men and women. The study was designed to analyze men and women separately. This study was reviewed and approved by the Wake Forest Medical Center Institutional Review Board and registered at http://www.clinicaltrials.gov/ (NCT00315146). All participants provided written informed consent.
Participants
The recruitment and participant characteristics are reported in Shea and colleagues (18). We targeted older adults with a clinical indication for weight loss therapy. Inclusion criteria were as follows: a score of 3–10 on the Short Physical Performance Battery (SPPB) and weight change of less than 5% over the previous 6 months. Eligibility criteria were based on National Heart, Lung, and Blood Institute recommendations: (i) a body mass index (BMI) ≥ 30kg/m2 or (ii) a BMI of 25–29.9kg/m2 or a waist circumference of >88.9cm (women) or >101.6cm (men), plus at least one of the following: clinically manifest coronary disease, other atherosclerotic diseases, sleep apnea, osteoarthritis of the knee or hip, hypertension (treatment systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg), low-density lipoprotein cholesterol ≥ 160mg/dL, high-density lipoprotein cholesterol ≤ 35mg/dL, impaired fasting glucose (110–125mg/dL), or family history of premature coronary heart disease. Medical exclusions are detailed in Shea and colleagues (18), and we excluded those currently involved in high-intensity aerobic or RT or a weight loss regimen.
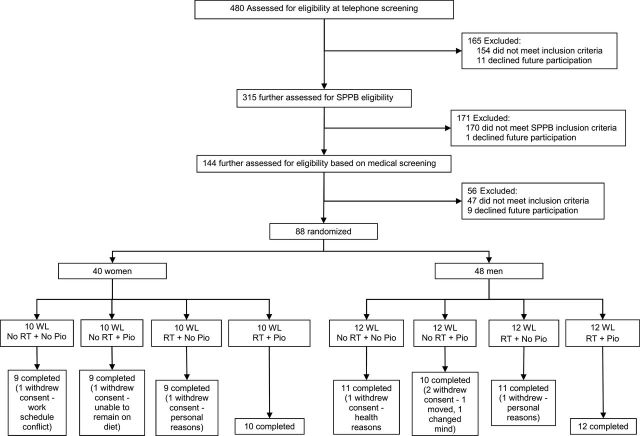
A total of 88 participants (48 men) aged 65–79 years were randomized, and 81 (44 men) completed the intervention. Of the seven who withdrew, six did so for reasons not related to the study, and one could not comply with the diet and withdrew, as previously described (18).
Measures
Baseline and follow-up assessments were conducted by blinded assessment staff. Baseline assessments were obtained over 1–2 weeks prior to randomization. Follow-up assessments were obtained within a week of the completion of the intervention.
Body composition.
Body composition was assessed using Dual-energy X-ray Absorptiometry (Hologic Delphi QDR; Bedford, MA, software Version 11.2) by a trained technician. Total body mass (TBM), fat mass (FM), LBM, and appendicular LBM (aLBM) were analyzed. The aLBM was calculated as the sum of nonbone lean mass in the arms and legs.
Leg press power.
Maximal leg press (LP) power was assessed using the Nottingham power rig (19). Five trials were collected on each leg, alternating between legs. The peak power (W) during maximal effort was recorded. The mean of five trials of both the left and right leg were reported for LP power.
Isometric knee extensor strength.
The strength of the quadriceps was assessed as the torque produced during a maximal voluntary isometric contraction with the knee at a 90° angle. Both legs were tested unless contraindicated by knee replacement. Two practice trials were given. During the actual test, participants were asked to push as hard as they could against the shin pad for 4 seconds. Two trials were performed on each leg, and the maximum torque values recorded in Newton-meters (Nm). The mean of two trials of both the left and right leg were reported for isometric knee extensor (KE) strength.
Interventions
Weight loss.
The details of the weight loss intervention have been reported previously (18). Participants attended a 45-minute group behavioral and nutrition session led by a registered dietitian 1 day a week for 16 weeks. The weight loss goal of each participant was 7% of their initial body mass. Two Slim-Fast Optima meal replacement bars or shakes were provided per day to achieve a 500-cal energy deficit. For the third meal, participants used a weekly menu plan with recipes designed to be low in fat and high in vegetables, which provided 500–750 kcal. Viactiv 500 mg calcium chews were also provided and were taken twice a day. Body mass (kg, Tanita BWB-800 scale) was recorded at the weekly sessions. If an individual was not meeting weight loss goals, energy intake was modified to produce the desired rate of weight loss. All participants were also encouraged to incorporate more physical activity into their day.
Drug.
Participants were randomly assigned to receive either pioglitazone (Actos, donated by Takeda Pharmaceuticals, Deerfield, IL) or a placebo. A blinded staff person passed out medications at the first group session, and the drug/placebo was distributed monthly thereafter. Participants were instructed to take the drug once per day at a dosage of 15mg for the first 3 weeks. Side effects were assessed at this time, but none were identified. The dosage was then increased to 30mg once per day for the next 13 weeks.
Resistance training.
The details of the RT intervention have been reported previously (18). The RT intervention was designed to increase strength and muscle mass in the major muscle groups of the body, while also improving muscle power in the lower extremity. All sessions were supervised by trained exercise interventionists. Participants attended 1 hour RT sessions 3 days a week for 16 weeks in small groups of six to eight participants. In the first 2 weeks, participants progressed incrementally toward the study goal of three sets of 8–10 repetitions for each exercise at 70% of the maximal load that they could lift one time (1RM) for each exercise. The 1RM testing was repeated every 2 weeks.
Data Analysis
All statistical analyses were performed using SAS version 9.1 (Cary, NC). Descriptive statistics of baseline data included means and standard deviations of age, height, body mass, BMI, waist circumference, comorbidities, SPPB score, LP power, and isometric KE strength for both men and women. Following the prespecified design, analyses were performed separately in men and women. Changes in body composition measures of TBM, FM, LBM, and aLBM were calculated as follow-up minus baseline values. Analysis of covariance was used to compare changes in body composition across randomized groups, controlling for baseline values. The main effect of each intervention on LP power and KE strength was determined using two-way analysis of covariance models, which contained a term for each main effect and the baseline measure of the outcome. Estimated least squares means were calculated and used to determine changes from baseline. In each model, we tested for an interaction between each main effect with respect to power and strength by entering a product term (RT × pioglitazone) into the main effect model. If the interaction term was significant, then pairwise comparisons for mean changes in the outcome among the four randomized groups were then performed using the Tukey–Kramer extension of Tukey’s Honest Significant Difference procedure. We inspected the residuals from all of our models using the SAS 9.2 diagnostics residuals option. In all cases, the residuals approximated a normal distribution. P-values < .05 were considered statistically significant.
RESULTS
Participants
Our sample was predominantly white, ranged in age from 65 to 79 years, with an average BMI of 32.8±4.5kg/m2 and a waist circumference of 116.1±26.4cm in men and 107.7±13.2cm in women (Table 1). Overall, 54% of men and 73% of women reported some difficulty with daily tasks. Participants had moderate functional ability at baseline with an average SPPB score of 9.0. At baseline, the men randomized to RT had a greater body mass than those not randomized to RT (p = 0.03, based on independent samples t test). Attendance to weekly nutrition sessions was 90%, and the overall attendance to the RT sessions was 84%. Attendance was >70% in 17 of 23 men and 17 of 19 women. Attendance was >80% in 14 of 23 men and 15 of 19 women. One female participant attended less than 50% of the sessions as a result of extended travel engagements. Overall compliance to pioglitazone (based on pill count) was 96%. For those participants assigned to RT, we examined muscle strength and power data at baseline, 8 weeks, and 16 weeks for data collected on the Keiser RT equipment (knee extension and LP). For men and women, there was a significant time effect for knee extension strength and power and LP strength and power (data available on request).
Table 1.
Demographic Information at Baseline by Randomization Group and by Main Intervention Arm
| According to randomized group | According to main intervention arm | |||||||
|---|---|---|---|---|---|---|---|---|
| No RT + No Pioglitazone | No RT + Pioglitazone | RT + No Pioglitazone | RT + Pioglitazone | RT | No RT | Pioglitazone | No Pioglitazone | |
| Women | ||||||||
| n | 10 | 10 | 10 | 10 | 20 | 20 | 20 | 20 |
| Age (y) | 70.0±2.6 | 69.8±4.1 | 71.3±4.9 | 69.0±1.7 | 70.2±3.7 | 69.9±3.3 | 69.4±3.1 | 70.7±3.9 |
| Height (cm) | 162.8±8.8 | 161.0±5.6 | 161.9±8.0 | 164.1±4.7 | 163.0±6.5 | 161.9±7.2 | 162.5±5.3 | 162.3±8.2 |
| BMI (kg/m2) | 33.8±5.4 | 34.5±5.3 | 31.1±3.1 | 33.9±5.9 | 32.5±4.8 | 34.1±5.2 | 34.2±5.4 | 32.5±4.5 |
| Waist circumference (cm)* | 110.0±15.7 | 103.9±10.5 | 103.3±9.2 | 114.0±15.4 | 108.7±13.5 | 106.8±13.2 | 109.0 ± 13.8 | 106.5±12.8 |
| Number of comorbidities | 1.2±0.6 | 1.7±1.1 | 1.6±1.0 | 1.1±1.1 | 1.4±1.0 | 1.5±0.9 | 1.4±1.1 | 1.4±0.8 |
| SPPB score (0–12)* | 8.2±1.2 | 8.6±1.1 | 9.1±1.0 | 8.7±1.3 | 8.9±1.1 | 8.4±1.1 | 8.7±1.1 | 8.7±1.2 |
| Men | ||||||||
| n | 12 | 12 | 12 | 12 | 24 | 24 | 24 | 24 |
| Age (y) | 70.5±4.7 | 69.7±2.8 | 69.5±3.9 | 69.5±3.5 | 69.5±3.6 | 70.1±3.8 | 69.6±3.1 | 70.0±4.2 |
| Height (cm) | 175.4±6.8 | 178.0±4.6 | 179.0±5.6 | 177.8±5.8 | 178.4±5.6 | 176.7±5.8 | 177.9±5.1 | 177.2 ± 6.4 |
| BMI (kg/m2) | 31.5±2.8 | 31.0±2.8 | 33.2±5.1 | 33.6±4.6 | 33.4 ± 4.7 | 31.2±2.8 | 32.3±4.0 | 32.4±4.10 |
| Waist circumference (cm)* | 126.0±47.5 | 110.4±7.9 | 116.3±11.4 | 114.4±12.8 | 115.3±11.9 | 117.0 ± 36.1 | 112.5±10.7 | 119.6±35.4 |
| Number of comorbidities | 1.8±0.8 | 1.7±1.2 | 1.5±1.2 | 1.2 ± 1.1 | 1.3±1.2 | 1.8±1.0 | 1.4±1.1 | 1.7±1.0 |
| SPPB score (0–12)* | 9.5±0.7 | 9.3±0.5 | 9.0±0.9 | 9.3±0.8 | 9.2±0.8 | 9.4±0.6 | 9.3±0.6 | 9.3±0.8 |
Notes. Data are mean ± SD. RT, resistance training; BMI, body mass index; SPPB, short physical performance battery.
Comorbidities: high blood pressure, heart attack, stroke, diabetes congestive heart failure, falls, fractures, cancer, and lung disease.
*Missing data.
Body Composition
Based on least squares means, female participants lost an average of 5.8kg of TBM after the 16-week intervention, a 6.6% decrease from baseline (Table 2). Male participants lost an average of 6.9kg, a 6.6% decrease from baseline. Both LBM and aLBM decreased in all groups. Women lost 2.0kg of LBM (4%) and 1.0kg of aLBM (5%), whereas men lost 2.2kg of LBM (3%) and 1.2kg of aLBM (4%). Ninety-five percent confidence intervals calculated using analysis of covariance for the mean change within each of the four treatment groups did not include zero for FM, LBM, and aLBM for men and women. Therefore, all groups had significant decreases in these variables from baseline. There were no significant main effects or interactions for TBM, FM, LBM, or aLBM.
Table 2.
Body Composition Data at Baseline and 4-Mo Follow-up by Randomization Group Note that all groups were assigned to 4-months of weight loss treatment
| No RT + No Pioglitazone | No RT + Pioglitazone | RT + No Pioglitazone | RT + Pioglitazone | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | FU | Absolute Change | BL | FU | Absolute Change | BL | FU | Absolute Change | BL | FU | Absolute Change | |
| Women | ||||||||||||
| n | 10 | 9 | 10 | 9 | 10 | 9 | 10 | 10 | ||||
| Body mass (kg) | 91.02±13.49 | 85.65±15.43 | −5.77±2.48 | 90.07±13.70 | 85.33±13.20 | −5.10±3.32 | 82.97±12.99 | 76.06±12.42 | −6.98±4.24 | 93.14±17.65 | 87.90±18.10 | −5.24±2.42 |
| Fat mass (kg) | 39.06±7.16 | 35.38±8.44 | −3.71±1.95 | 39.72±8.09 | 37.28±8.33 | −2.96±2.80 | 34.99±8.38 | 30.40±8.75 | −5.04±2.53 | 41.45±10.15 | 38.07±10.96 | −3.38±1.89 |
| LBM (kg) | 49.75±7.51 | 48.03 ± 7.93 | −2.08±1.29 | 48.22 ± 6.43 | 45.95 ± 5.88 | −2.14±1.89 | 45.93 ± 6.38 | 43.69 ± 5.22 | −1.94±2.05 | 49.45 ± 7.79 | 47.61 ± 7.25 | −1.84±1.55 |
| aLBM (kg) | 20.84±3.92 | 19.89±4.15 | −1.12±1.00 | 20.58±3.30 | 19.40±2.98 | −1.09±1.19 | 19.09±3.42 | 18.23±2.72 | −0.86±1.13 | 21.03±3.05 | 20.00±2.98 | −1.04±0.87 |
| Men | ||||||||||||
| N | 12 | 11 | 12 | 10 | 12 | 11 | 12 | 12 | ||||
| Body mass (kg) | 98.80 ± 13.71 | 91.80 ± 13.51 | −6.90±4.50 | 99.22 ± 9.69 | 90.37 ± 13.54 | −8.11±5.12 | 106.48 ± 14.87 | 100.28 ± 13.06 | −5.41±4.09 | 106.81 ± 13.84 | 100.30 ± 12.45 | −6.51±4.78 |
| Fat mass (kg) | 29.57±5.91 | 25.37 ± 6.49 | −3.90±2.56 | 29.35 ± 6.45 | 24.05 ± 8.35 | −5.48±3.18 | 36.43 ± 11.46 | 31.97 ± 10.56 | −3.60±2.40 | 34.78 ± 10.08 | 29.70 ± 9.93 | −5.08±3.72 |
| LBM (kg) | 66.48±8.87 | 63.68±8.15 | −3.01±2.23 | 67.01±5.35 | 63.46±5.90 | −2.62±2.31 | 67.21±5.80 | 65.42±5.20 | −1.84±2.10 | 68.91±7.15 | 67.46±5.63 | −1.45±2.49 |
| aLBM (kg) | 29.05 ± 4.29 | 27.55 ± 4.21 | −1.59±0.98 | 29.42 ± 2.35 | 27.75 ± 2.63 | −1.27±1.12 | 29.71 ± 3.42 | 28.74 ± 3.06 | −0.88±1.15 | 30.41 ± 3.74 | 29.52 ± 3.14 | −0.89±1.13 |
Notes. All groups were assigned to 4 months of weight loss treatment. Data are mean ± SD. RT, resistance training; BL, baseline; FU, 4-month follow-up; LBM, lean body mass; aLBM, appendicular lean body mass.
Muscle Power and Strength
For women, LP power and KE strength increased, except for KE strength in the no RT group (Table 3, mean change = −1.7 Nm). In men, KE decreased in all four groups, whereas for LP power increased. Among women, those assigned to RT had a 29.3-W greater improvement in mean LP power compared with those not assigned to RT (p < .001), whereas mean KE strength was not different between RT and non-RT groups (p = .12). Among men, there was no main effect of RT on lower extremity power (p = .11) or strength (p = .61). There was no main effect of pioglitazone on LP power or KE strength in either men or women (all p ≥ 0.22). There was a significant RT by pioglitazone interaction in the women with respect to LP power (p = 0.006, Figure 1). In women, pairwise comparisons suggested that a combination of RT and pioglitazone led to a potentiated effect on power compared with RT alone. No other significant interactions were detected.
Table 3.
Mean (SE) Data for Knee Extensor Strength and Leg Press Power in Men and Women Before and After Weight Loss, According to Main Intervention Arm
| Pioglitazone | No Pioglitazone | RT | No RT | |
|---|---|---|---|---|
| Women | ||||
| Mean KE strength (Nm) | ||||
| Baseline | 56.8 (3.4) | 53.3 (5.2) | 51.2 (4.8) | 59.0 (3.8) |
| Follow-up* | 59.5 (3.3) | 55.9 (3.3) | 61.5 (3.3) | 53.8 (3.5) |
| LS change (95% CI) from baseline* | 3.9 (−1.9, 10.7) | 0.3 (−6.5, 7.1) | 5.9 (−0.8, 12.5) | −1.7 (−8.7, 5.3) |
| Between-group difference*,† (95% CI) | 3.6 (−6.1, 13.2); p = .45 | 7.6 (−2.2, 17.4); p = .12 | ||
| Mean LP power (W) | ||||
| Baseline | 41.8 (4.2) | 47.8 (5.5) | 38.0 (4.2) | 51.6 (5.1) |
| Follow-up* | 71.1 (4.7) | 62.8 (4.8) | 81.6 (4.7) | 52.3 (4.9) |
| LS change (95% CI) from baseline* | 26.0 (16.5, 35.5) | 17.7 (7.9, 27.4) | 36.5 (26.8, 46.1) | 7.2 (−2.7, 17.1) |
| Between-group difference*,† (95% CI) | 8.3 (−5.3, 22.0); p = .22 | 29.3 (15.2, 43.4); p < .001 | ||
| Men | ||||
| Mean KE strength (Nm) | ||||
| Baseline | 109.3 (6.1) | 106.9 (5.5) | 112.9 (7.4) | 103.4 (3.6) |
| Follow-up* | 102.9 (6.4) | 108.0 (6.2) | 103.1 (6.3) | 107.7 (6.4) |
| LS change (95% CI) from baseline* | −8.0 (−20.9, 5.0) | −2.9 (−15.5, 9.7) | −7.8 (−20.5, 5.0) | −3.1 (−16.1, 9.9) |
| Between-group difference*,† (95% CI) | −5.1 (−23.1, 13.0); p = .58 | −4.7 (−23.0, 13.7); p = .61 | ||
| Mean LP power (W) | ||||
| Baseline | 104.3 (7.2) | 92.9 (8.2) | 96.0 (7.3) | 101.2 (8.3) |
| Follow-up* | 107.2 (8.7) | 121.8 (8.7) | 124.5 (8.5) | 104.5 (8.9) |
| LS change (95% CI) from baseline* | 7.4 (−10.2, 25.0) | 22.0 (4.4, 39.5) | 24.7 (7.5, 41.8) | 4.6 (−13.3, 22.6) |
| Between-group difference*,† (95% CI) | −14.6 (−39.6, 10.4); p = .25 | 20.0 (−4.9, 45.0); p = .11 | ||
Note. LP, leg press; KE, knee extensor; RT, resistance training; CI, confidence interval; LS, least squares.
*Follow-up means, measures of change, and between-group differences are adjusted for main effects and baseline measure.
†Differences are change in treatment group minus change in control group.
Figure 1.
Recruitment and randomization flow diagram.
Finally, given that there was a difference in body mass for men between those randomized to RT and no RT, we reran our analyses in Table 3, controlling for baseline body mass. For both men and women, there were no substantive differences in the results for KE strength or LP power.
DISCUSSION
The purpose of this analysis was to determine the effects of RT and/or pioglitazone on muscle power and strength during hypocaloric weight loss in overweight or obese older adults. All four groups lost FM and LBM following the 16-week weight loss intervention. We have previously reported that men randomized to RT lost less aLBM, and that men and women randomized to RT lost less thigh muscle volume (18). In examining the changes from baseline in muscle power, women randomized to RT improved LP power significantly more than women not randomized to RT. There were no differences in KE strength in women or men and LP power in men between participants randomized to RT versus no RT. Based on the 95% CIs, we cannot definitively state that KE strength was preserved in men or women. However, women assigned to RT showed substantial improvements in LP power (115% improvement), following the intervention in spite of a decrease in LBM and aLBM. The men assigned to RT showed improvements in LP power (30% improvement), although we did not find a significant difference between the groups (p = .11).
An important issue is whether or not the changes we observed in muscle strength and power that were not statistically significant might have clinical significance. With our sample sizes of 20 participants per group, the study had ~80% power to detect differences that were 95% of a standard deviation for each outcome: Women KE Strength SD = 14.1; Women LP Power SD = 20.1; Men KE Strength SD = 29.3; and Men LP Power SD = 40.6. Many of our between-group differences were not of this magnitude (Table 3). However, the changes we observed in muscle power for men are similar to previous studies that have reported a significant increase, and we believe they are clinically relevant and important with respect to physical function (20,21).
Our data show that a decline in muscle strength and power with a reduction in body mass and LBM is not obligatory. In fact, even if we examine the LBM data from the No RT + No Pioglitazone (−3.01kg) versus RT + Pioglitazone (−1.45kg), there is relatively little difference in changes in muscle power and strength between the groups. Previously, Sartorio and colleagues (22) showed that a 3-week hypocaloric diet with low-intensity aerobic exercise in 18- to 77-year-old obese persons resulting in ~4% weight loss improved stair-climbing performance (a functional assessment of lower extremity muscle power) by ~21% (p < .05). Villareal and colleagues (23) also used caloric restriction (~750 kcal/day deficit) plus multimodal exercise sessions three times per week for 6 months to induce an 8% loss in body mass. They observed improvements in strength and lower extremity function but no change in lean mass in either the treatment or control group. Wang and colleagues (24) observed significant declines in TBM, LBM, FM, and percent body fat in a group of older (~70 years) obese adults undergoing 6 months of weekly educational meetings, a meal replacement diet, and a three-session-per-week structured exercise program leading to ~8% loss in TBM, ~3% decline in LBM, and ~14% decline in FM. However, concentric KE strength assessed on a dynamometer increased ~25% (NS) while muscle quality increased ~37% (p < .05). There was an 11% decline in strength and a 1% decline in muscle quality in a weight-stable control group (both NS). Frimel and colleagues (11) also reported that a 6-month exercise program blunted the loss in LBM and increased 1RM muscle strength during voluntary weight loss in frail obese older adults compared with weight loss alone. Recently, Santanasto and colleagues (25) reported a 12.4% decline in peak isokinetic KE strength following 6 months of a multicomponent physical activity program combined with weight loss treatment that resulted in a 5.4% reduction in body mass, whereas strength declined only ~1% in a physical activity only group. In spite of the decline in strength, lower extremity physical function improved in the physical activity plus weight loss group. Our study adds to these data because all participants in our study received weight loss treatment, and we observed a significant increase in LP power, an important determinant of physical function (21), and no appreciable decline in muscle strength even in the group that did not do RT. Collectively, these data suggest that a 6%–7% voluntary weight loss (3%–5% loss in LBM), when combined with resistance exercise, does not have a negative impact on muscle power, or physical performance in older overweight or obese adults.
Although decline in muscle mass has been associated with declines in muscle strength (26), it is also observed that body fat has a significant inverse association with strength. Moreover, with aging strength decline has been shown to be much more rapid than the concomitant loss of muscle mass, while maintaining or gaining muscle mass does not prevent aging-associated declines in muscle strength (27). In addition, although higher FM is related to greater absolute muscle strength, it is also associated with lower muscle quality (strength per unit of lean mass), and it predicts accelerated loss of lean mass (28). Data from the Health ABC observational study show that higher muscle lipid content is associated with lower KE strength independent of subcutaneous and midthigh adiposity (13). However, weight loss reduces intramyocellular lipid content (29). One might speculate that the reduction of FM obtained in our intervention produced a synergy with the RT and/or pioglitazone treatment to offset reductions in strength/power that might have otherwise occurred due to the loss of LBM. However, it is also the case that RT leads to muscle hypertrophy and muscle architecture changes without changes in LBM (30,31) and RT increases muscle activation and recruitment (32,33), factors that might explain increases in strength and power in spite of decreases in LBM.
In older adults, it is critical to distinguish between intentional and unintentional weight loss. Our data demonstrate that older overweight and obese adults undergoing intentional weight loss during an appropriately designed intervention that reduces TBM, FM, and LBM, even with no RT do not show dramatic decline in muscle strength or power. Moreover, in women, when the weight loss intervention was combined with resistance exercise, muscle power significantly increased in spite of the loss of LBM. It is plausible that the relative stability of or increase in LP power, together with the loss of body mass we observed, confers a shift in power- or strength-to-weight ratio, which will have a favorable impact on daily activities, particularly those that involve the vertical movement of the center of mass such as stair climbing and rising from a chair (25,34).
We detected a significant interaction between RT and pioglitazone treatment in women, such that pioglitazone potentiated the effect of RT on muscle power. We did not detect a similar response in men, and it is possible the interaction is a reflection of type I statistical error or a lack of statistical power for the male sample. Maher and colleagues (35) reported greater skeletal muscle PPAR-γ expression in younger women compared with men. Gender differences in PPAR-γ expression by adipocytes and inflammatory cells have also been reported (36,37). Gender differences in response to PPAR-γ agonist treatment merit further investigation.
A potential mechanism whereby pioglitazone treatment might improve muscle strength and power could be via a decrease in intramyocellular lipid content. Previously, we reported that, in women, thigh muscle attenuation decreased overall following weight loss, but there was not a significant difference in the decrease according to main intervention group. In men, an overall increase in thigh muscle attenuation was observed postweight loss, but this was also not significant according to main intervention group (from Table 2 and Supplementary Table S1 in Ref. [18]). Whether reducing intramyocellular lipid leads to improved function requires further study, but we believe that the improvements we observed in muscle function are more likely due to the effect of the weight-loss treatment as opposed to pioglitazone treatment.
This study has several limitations. First, our work represents a secondary analysis of a randomized controlled trial powered for the primary analyses that were focused on changes in body composition (18). Our participants were community dwelling and generally in good health and therefore our findings may not be generalizable to frailer overweight or obese older adults. Although strength training can improve skeletal health in older adults (38), our study was too short to detect changes in bone mineral density. We did not measure intramyocellular lipid directly but relied on muscle attenuation data from CT. Finally, the duration of our intervention was relatively short. Two recent trials suggest that weight loss interventions alone or when combined with exercise can have positive effects on physical function over the longer term (39,40). The strengths of our study include its clinical trial protocol, minimal loss to follow-up, excellent adherence to the treatments, and the use of interventions that can be implemented in a community setting.
Health care providers may be reluctant to advise older overweight and obese adults to lose weight because of uncertainty of whether the benefits of weight loss outweigh the perceived risks including a belief that lean tissue loss leads to obligatory declines in muscle strength and power which may compromise physical function. In this study of older overweight and obese men and women undergoing voluntary weight loss, muscle power and strength either improved or did not significantly change even in the participants randomized to weight loss only. Women randomized to RT improved LP power, in spite of a loss of aLBM. An intriguing observation was that pioglitazone potentiated the effect of RT on muscle power in women but not in men. Future work might be directed at understanding the potential mechanisms underlying this sex effect and to more completely explore the potential of pioglitazone to act synergistically with RT. These data add to a growing body of literature, suggesting that well-designed voluntary weight loss interventions can be implemented in older overweight and obese adults without adverse effects on muscle function.
Figure 2.
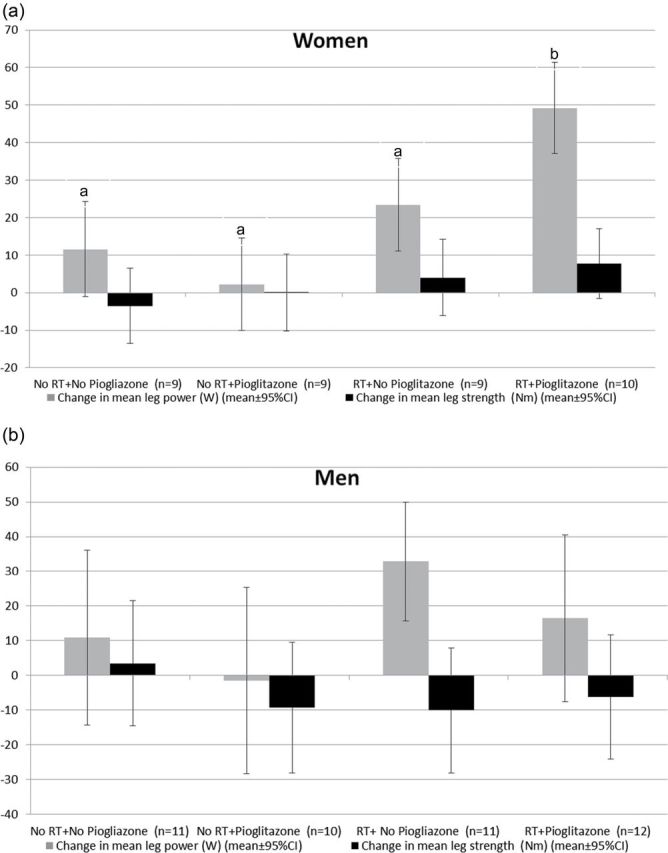
Adjusted mean (95% CI) change (adjusted for baseline measure) in LP power and KE strength according to randomized group for women (A) and men (B).
a,bGroups without common letters are significantly different (p < .05), following Tukey–Kramer procedure to adjust for multiple comparisons.
No tests were performed for KE power in women or KE power and LP power in men because the overall interaction term was not significant.
Note. LP, leg press; KE, knee extensor.
FUNDING
This work was supported by Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332) and Takeda Pharmaceuticals North America.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of the intervention staff, Melanie Bopp, Samantha Rogers, and Shannon Newton, and our participants.
REFERENCES
- 1. Patterson RE, Frank LL, Kristal AR, White E. A comprehensive examination of health conditions associated with obesity in older adults. Am J Prev Med. 2004;27:385–390 [DOI] [PubMed] [Google Scholar]
- 2. Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863 [DOI] [PubMed] [Google Scholar]
- 3. Jette AM, Branch LG, Sleeper LA, Feldman H, Sullivan LM. High-risk profiles for nursing home admission. Gerontologist. 1992;32:634–640 [DOI] [PubMed] [Google Scholar]
- 4. Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010;11:671–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wee CC, Huskey KW, Ngo LH, et al. Obesity, race, and risk for death or functional decline among Medicare beneficiaries: a cohort study. Ann Intern Med. 2011;154:645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sørensen TI. Weight loss causes increased mortality: pros. Obes Rev. 2003;4:3–7 [DOI] [PubMed] [Google Scholar]
- 7. Yang D, Fontaine KR, Wang C, Allison DB. Weight loss causes increased mortality: cons. Obes Rev. 2003;4:9–16 [DOI] [PubMed] [Google Scholar]
- 8. Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc. 2008;9:302–312 [DOI] [PubMed] [Google Scholar]
- 9. Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017 [DOI] [PubMed] [Google Scholar]
- 10. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896 [DOI] [PubMed] [Google Scholar]
- 11. Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryan AS. Exercise in aging: its important role in mortality, obesity and insulin resistance. Aging health. 2010;6:551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165 [DOI] [PubMed] [Google Scholar]
- 14. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 15. Basu A, Jensen MD, McCann F, Mukhopadhyay D, Joyner MJ, Rizza RA. Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes. Diabetes Care. 2006;29:510–514 [DOI] [PubMed] [Google Scholar]
- 16. Rasouli N, Raue U, Miles LM, et al. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E930–E934 [DOI] [PubMed] [Google Scholar]
- 17. Teranishi T, Ohara T, Maeda K, et al. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metab Clin Exp. 2007;56:1418–1424 [DOI] [PubMed] [Google Scholar]
- 18. Shea MK, Nicklas BJ, Marsh AP, et al. The effect of pioglitazone and resistance training on body composition in older men and women undergoing hypocaloric weight loss. Obesity (Silver Spring). 2011;19:1636–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–390 [DOI] [PubMed] [Google Scholar]
- 20. Marsh AP, Miller ME, Rejeski WJ, Hutton SL, Kritchevsky SB. Lower extremity muscle function after strength or power training in older adults. J Aging Phys Act. 2009;17:416–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sartorio A, Lafortuna CL, Conte G, Faglia G, Narici MV. Changes in motor control and muscle performance after a short-term body mass reduction program in obese subjects. J Endocrinol Invest. 2001;24:393–398 [DOI] [PubMed] [Google Scholar]
- 23. Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866 [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Miller GD, Messier SP, Nicklas BJ. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:866–871 [DOI] [PubMed] [Google Scholar]
- 25. Santanasto AJ, Glynn NW, Newman MA, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011;2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330 [DOI] [PubMed] [Google Scholar]
- 27. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 28. Koster A, Ding J, Stenholm S, et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66:888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houmard JA, Tanner CJ, Yu C, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes. 2002;51:2959–2963 [DOI] [PubMed] [Google Scholar]
- 30. Häkkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand. 2001;171:51–62 [DOI] [PubMed] [Google Scholar]
- 31. Haub MD, Wells AM, Tarnopolsky MA, Campbell WW. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am J Clin Nutr. 2002;76:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moritani T, deVries HA. Potential for gross muscle hypertrophy in older men. J Gerontol. 1980;35:672–682 [DOI] [PubMed] [Google Scholar]
- 33. Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20(5 suppl):S135–S145 [DOI] [PubMed] [Google Scholar]
- 34. Sartorio A, Lafortuna CL, Agosti F, Proietti M, Maffiuletti NA. Elderly obese women display the greatest improvement in stair climbing performance after a 3-week body mass reduction program. Int J Obes Relat Metab Disord. 2004;28:1097–1104 [DOI] [PubMed] [Google Scholar]
- 35. Maher AC, Fu MH, Isfort RJ, Varbanov AR, Qu XA, Tarnopolsky MA. Sex differences in global mRNA content of human skeletal muscle. PLoS ONE. 2009;4:e6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunn SE, Ousman SS, Sobel RA, et al. Peroxisome proliferator-activated receptor (PPAR)alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tchoukalova YD, Koutsari C, Votruba SB, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring). 2010;18:1875–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7):CD000333 [DOI] [PubMed] [Google Scholar]
- 39. Rejeski WJ, Brubaker PH, Goff DC, Jr, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171:880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]