Abstract
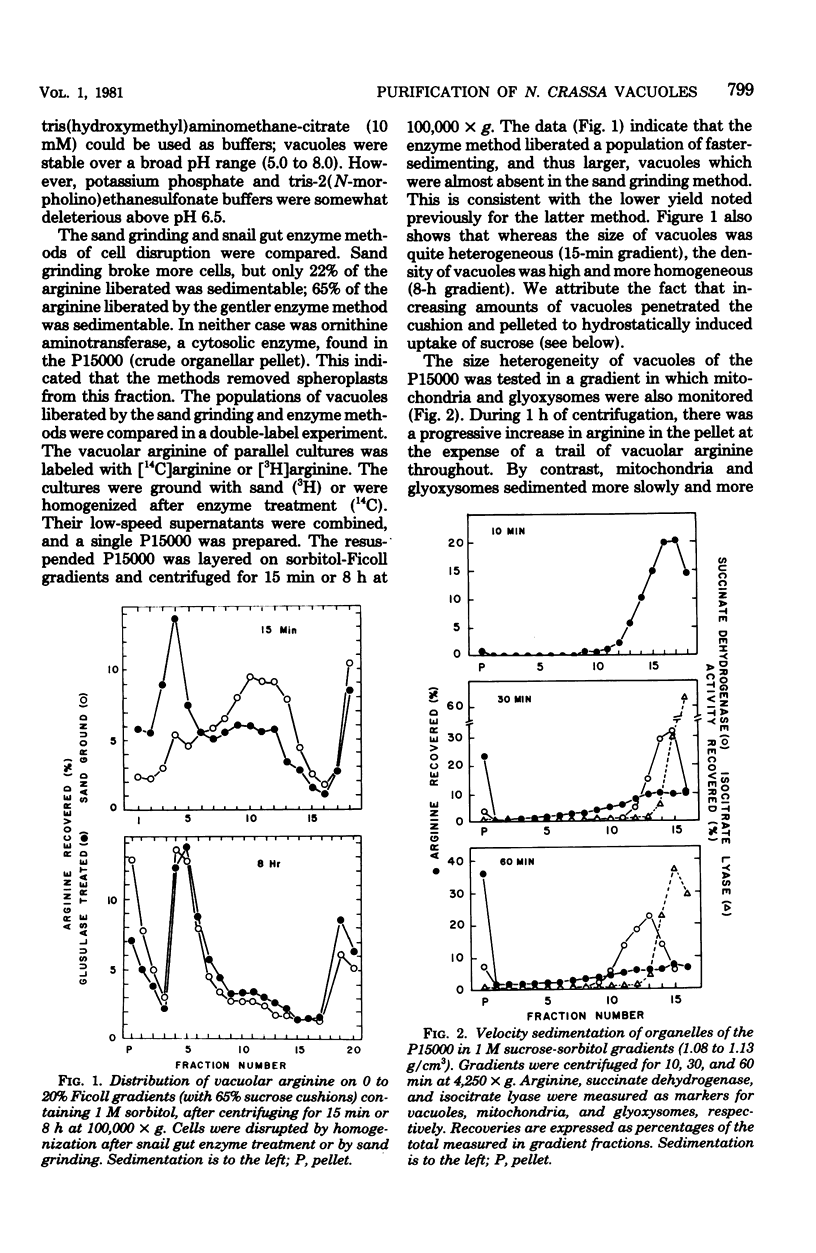
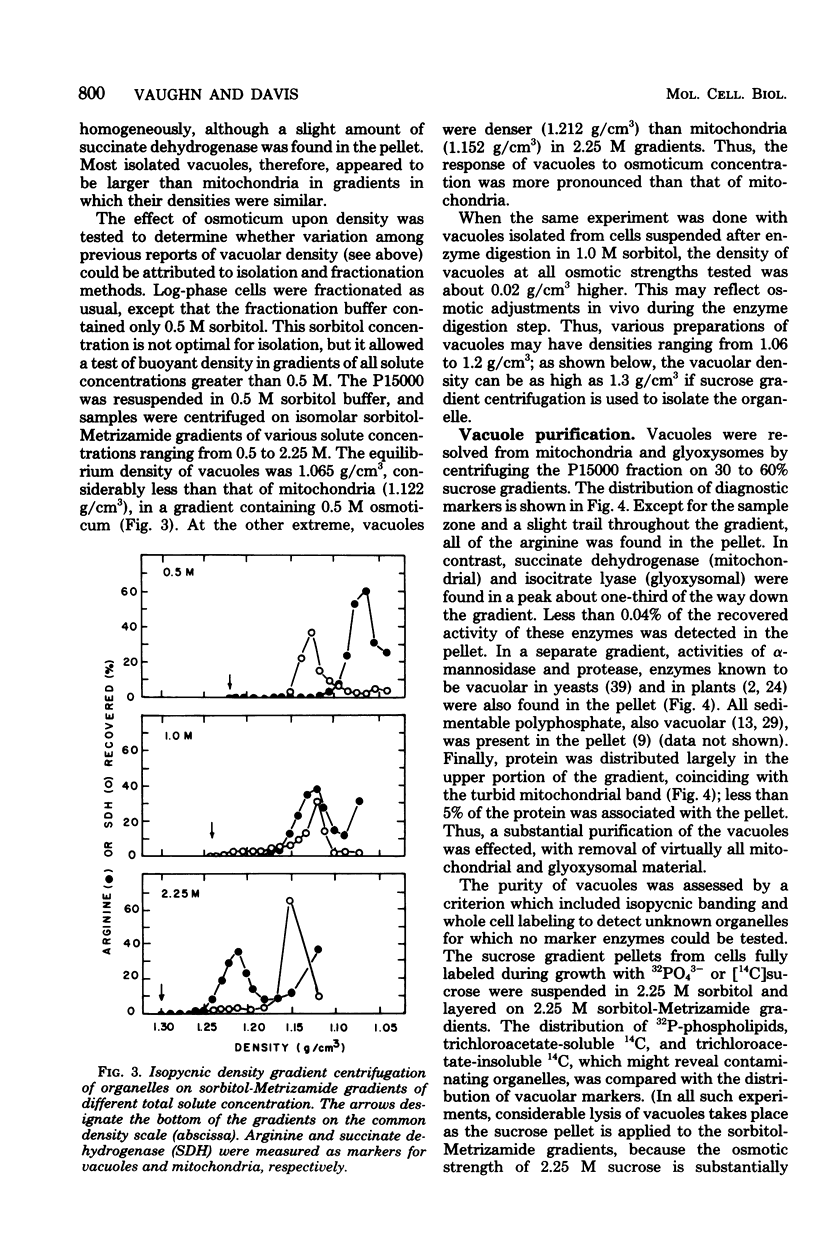
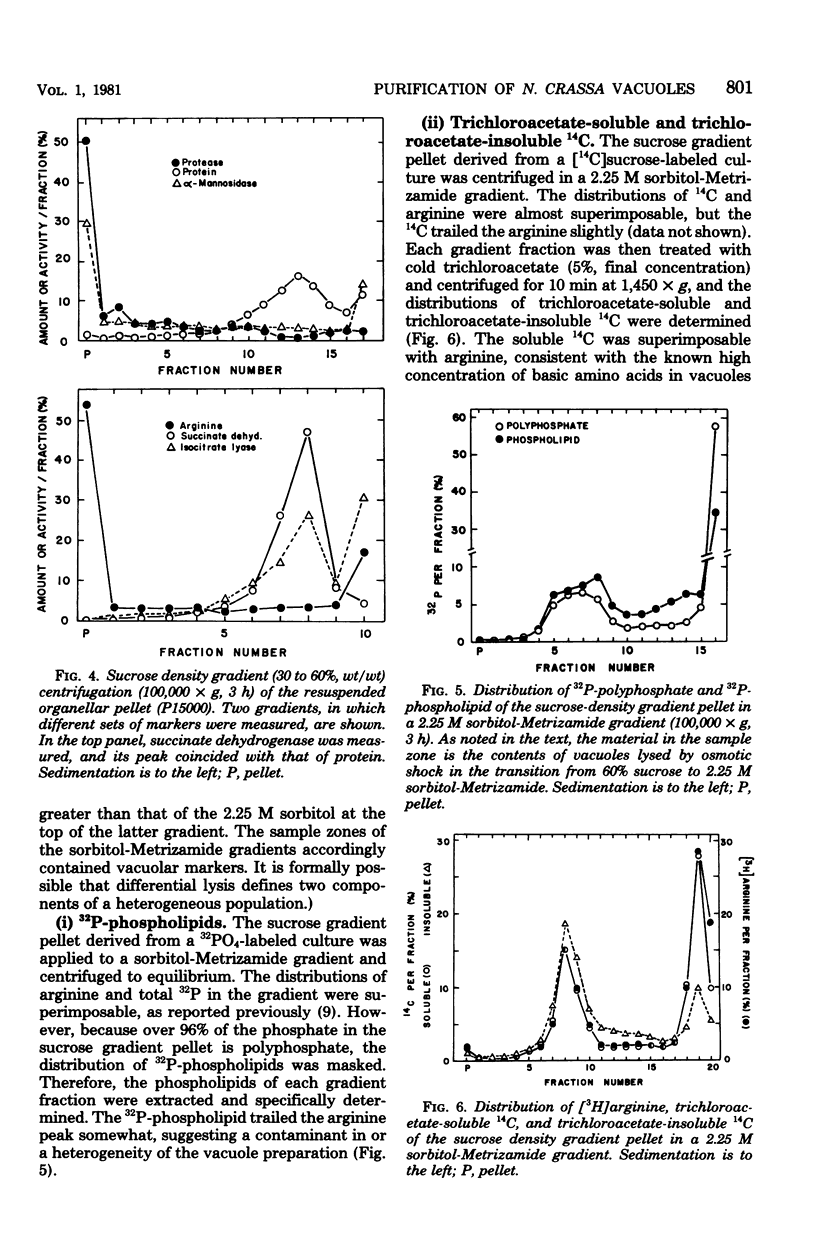
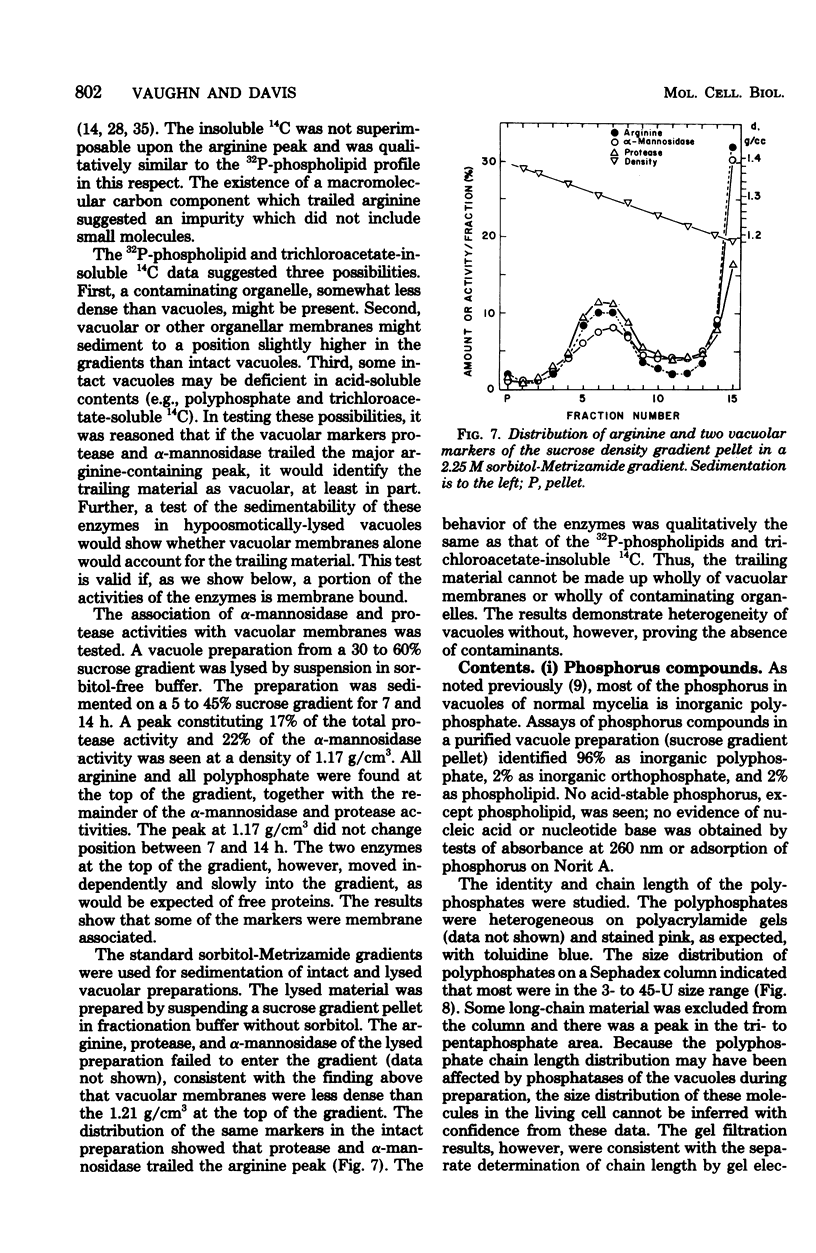
The Neurospora crassa vacuole, defined by its content of basic amino acids, polyphosphate, protease, phosphatases, and alpha-mannosidase, was purified to near homogeneity. The procedure depends upon homogenization of snail gut enzyme-digested cells in a buffer osmotically stabilized with 1 M sorbitol, differential centrifugation of the extract, and sucrose density gradient centrifugation of the organellar pellet. Isopycnic centrifugation of vacuoles in 2.25 M sorbitol-Metrizamide density gradients yielded a peak (density, 1.31 g/cm3) of vacuolar markers coincident with 32P-phospholipids, trichloroacetate-insoluble 14C, and trichloroacetate-soluble 14C. A trail of macromolecular markers in the lighter portions of the gradient reflected, at least in part, heterogeneity of the vacuoles. Almost no contamination by mitochondria or glyoxysomes was detected. Vacuoles were very heterogeneous in size as estimated by velocity sedimentation, but most were larger than mitochondria. Variations of the osmotic strength of the medium were found to alter the equilibrium density of vacuole preparations from 1.06 g/cm3 to over 1.3 g/cm3. This explains the great variation in density reported previously for the "vacuole," the "vesicle," and the "protease particle" of N. crassa, all of which appear to be the same entity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H., Morris S. J., Whittaker V. P. Adenosine triphosphatase activity associated with purified cholinergic synaptic vesicles of Torpedo marmorata. Eur J Biochem. 1977 Oct 17;80(1):313–318. doi: 10.1111/j.1432-1033.1977.tb11884.x. [DOI] [PubMed] [Google Scholar]
- Bronfman M., Beaufay H. Alteration of subcellular organelles induced by compression. FEBS Lett. 1973 Oct 15;36(2):163–168. doi: 10.1016/0014-5793(73)80360-6. [DOI] [PubMed] [Google Scholar]
- Burton E. G., Metzenberg R. L. Properties of repressible alkaline phosphates from wild type and a wall-less mutant of Neurospora crassa. J Biol Chem. 1974 Aug 10;249(15):4679–4688. [PubMed] [Google Scholar]
- Carlson S. S., Wagner J. A., Kelly R. B. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochemistry. 1978 Apr 4;17(7):1188–1199. doi: 10.1021/bi00600a009. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Vaughn L. E., Davis R. H. Basic amino acids and inorganic polyphosphates in Neurospora crassa: independent regulation of vacuolar pools. J Bacteriol. 1980 Jun;142(3):945–952. doi: 10.1128/jb.142.3.945-952.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr M., Boller T., Wiemken A. Polybase induced lysis of yeast spheroplasts. A new gentle method for preparation of vacuoles. Arch Microbiol. 1975 Nov 7;105(3):319–327. doi: 10.1007/BF00447152. [DOI] [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- Indge K. J. Polyphosphates of the yeast cell vacuole. J Gen Microbiol. 1968 May;51(3):447–455. doi: 10.1099/00221287-51-3-447. [DOI] [PubMed] [Google Scholar]
- Karlin J. N., Bowman B. J., Davis R. H. Compartmental behavior of ornithine in Neurospora crassa. J Biol Chem. 1976 Jul 10;251(13):3948–3955. [PubMed] [Google Scholar]
- Lampkin S. L., 4th, Cole K. W., Vitto A., Gaertner F. H. The protease problem in Neurospora. Variable stability of enzymes in aromatic amino acid metabolism. Arch Biochem Biophys. 1976 Dec;177(2):561–565. doi: 10.1016/0003-9861(76)90467-7. [DOI] [PubMed] [Google Scholar]
- Martinoia E., Heck U., Boller T., Wiemken A., Matile P. Some properties of vacuoles isolated from Neurospora crassa slime variant. Arch Microbiol. 1979 Jan 16;120(1):31–34. doi: 10.1007/BF00413268. [DOI] [PubMed] [Google Scholar]
- Matile P., Jost M., Moor H. Intrazelluläre Lokalisation proteolytischer Enzyme von Neurospora crassa. Z Zellforsch Mikrosk Anat. 1965 Oct 12;68(2):205–216. [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Isolation and partial characterization of vacuoles from tobacco protoplasts. Plant Physiol. 1979 Dec;64(6):1114–1120. doi: 10.1104/pp.64.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel R., Liebl A., Hartmann A., Neupert W. Action of intracellular proteinases on mitochondrial translation products of Neurospora crassa Schizosaccharomyces pombe. Hoppe Seylers Z Physiol Chem. 1976 Mar;357(3):415–426. doi: 10.1515/bchm2.1976.357.1.415. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Schovanka I. Some physical properties of adrenal medulla chromaffin granules isolated by a new continuous iso-osmotic density gradient method. Biochim Biophys Acta. 1977 Jan 4;464(1):53–64. doi: 10.1016/0005-2736(77)90370-4. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. Regulation of polyamine synthesis in relation to putrescine and spermidine pools in Neurospora crassa. J Bacteriol. 1981 Jan;145(1):14–20. doi: 10.1128/jb.145.1.14-20.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Munkres K. D., Metzenberg R. L. A particulate fraction from Neurospora crassa exhibiting aryl sulfatase activity. Arch Biochem Biophys. 1971 Feb;142(2):623–632. doi: 10.1016/0003-9861(71)90527-3. [DOI] [PubMed] [Google Scholar]
- Urech K., Dürr M., Boller T., Wiemken A., Schwencke J. Localization of polyphosphate in vacuoles of Saccharomyces cerevisiae. Arch Microbiol. 1978 Mar;116(3):275–278. doi: 10.1007/BF00417851. [DOI] [PubMed] [Google Scholar]
- Wattiaux-de Coninck S., Dubois F., Mertens-Strijthagen J., de Schrijver C., Wattiaux R. Permeability of mitochondria to sucrose induced by hydrostatic pressure. Biochim Biophys Acta. 1980 Jul 16;600(1):173–184. doi: 10.1016/0005-2736(80)90422-8. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Anterasian G. P. Control of arginine metabolism in Neurospora. Induction of ornithine aminotransferase. J Biol Chem. 1977 Oct 25;252(20):6974–6980. [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Wiemken A. Isolation of vacuoles from yeasts. Methods Cell Biol. 1975;12:99–109. doi: 10.1016/s0091-679x(08)60954-1. [DOI] [PubMed] [Google Scholar]
- ZALOKAR M. Cytochemistry of centrifuged hyphae of Neurospora. Exp Cell Res. 1960 Feb;19:114–132. doi: 10.1016/0014-4827(60)90042-2. [DOI] [PubMed] [Google Scholar]


