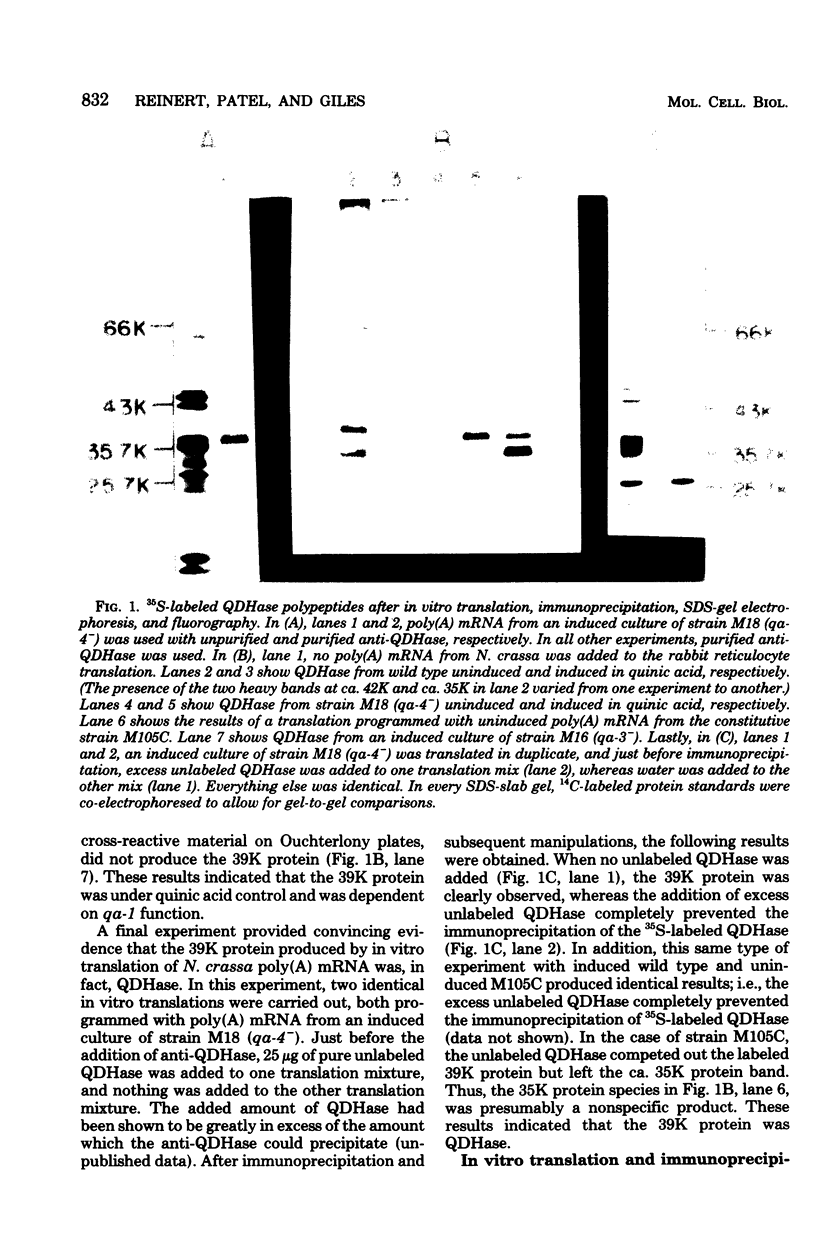
Abstract
An in vitro protein-synthesizing system (rabbit reticulocyte) was programmed with total polyadenylated messenger ribonucleic acid from wild type and various mutants in the qa gene cluster of Neurospora crassa. The products of two of the qa genes, quinate dehydrogenase (qa-3+) and dehydroshikimate dehydratase (qa-4+), were identified by specific immunoprecipitation and sodium dodecyl sulfate-slab gel electrophoresis. The results indicated that for both genes induction of a specific enzyme activity by quinic acid depends on the de novo synthesis of a specific polypeptide and on the de novo appearance of specific messenger ribonucleic acid detectable by the in vitro translation assay. Furthermore, the results indicated that the appearance of this messenger ribonucleic acid is under the control of the qa-1 gene. The simplest interpretation of these results appears to be that induction of enzyme activity in the qa system is mediated by events at the transcriptional level.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea J. L., Giles N. H. Purification and characterization of quinate (shikimate) dehydrogenase, an enzyme in the inducible quinic acid catabolic pathway of Neurospora crassa. Biochim Biophys Acta. 1978 May 11;524(1):1–14. doi: 10.1016/0005-2744(78)90097-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Genetic evidence on the organization and action of the qa-1 gene product: a protein regulating the induction of three enzymes in quinate catabolism in Neurospora crassa. Proc Natl Acad Sci U S A. 1975 Feb;72(2):553–557. doi: 10.1073/pnas.72.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E., Pueyo C., Barea J. L., Giles N. H. Genetical and biochemical characterization of QA-3 mutants and revertants in the QA gene cluster of Neurospora crassa. Genetics. 1978 Sep;90(1):69–84. doi: 10.1093/genetics/90.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleff R. S. The inducible quinate-shikimate catabolic pathway in Neurospora crassa: genetic organization. J Gen Microbiol. 1974 Apr;81(2):337–355. doi: 10.1099/00221287-81-2-337. [DOI] [PubMed] [Google Scholar]
- Chaleff R. S. The inducible quinate-shikimate catabolic pathway in Neurospora crassa: induction and regulation of enzyme synthesis. J Gen Microbiol. 1974 Apr;81(2):357–372. doi: 10.1099/00221287-81-2-357. [DOI] [PubMed] [Google Scholar]
- Hautala J. A., Jacobson J. W., Case M. E., Giles N. H. Purification and characterization of catabolic dehydroquinase, an enzyme in the inducible quinic acid catabolic pathway of Neurospora crassa. J Biol Chem. 1975 Aug 10;250(15):6008–6014. [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Lucas M. C., Jacobson J. W., Giles N. H. Characterization and in vitro translation of polyadenylated messenger ribonucleic acid from Neurospora crassa. J Bacteriol. 1977 Jun;130(3):1192–1198. doi: 10.1128/jb.130.3.1192-1198.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: preparation of efficient protein synthesis lysates and the purification and characterization of the heme-regulated translational inhibitory protein kinase. Methods Enzymol. 1979;60:459–484. doi: 10.1016/s0076-6879(79)60045-9. [DOI] [PubMed] [Google Scholar]
- Reinert W. R., Giles N. H. Proof of de novo synthesis of the qa enzymes of Neurospora crassa during induction. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4256–4260. doi: 10.1073/pnas.74.10.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Schindler D., Davies J. Inhibitors of macromolecular synthesis in yeast. Methods Cell Biol. 1975;12:17–38. doi: 10.1016/s0091-679x(08)60949-8. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Searle P. F., Williams J. G. Characterisation of bacterial clones containing DNA sequences derived from Xenopus laevis vitellogenin mRNA. Nucleic Acids Res. 1979 Feb;6(2):487–506. doi: 10.1093/nar/6.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarsky L., Kemper B. Characterization and partial purification of parathyroid hormone messenger RNA. J Biol Chem. 1978 Oct 25;253(20):7194–7201. [PubMed] [Google Scholar]
- Strøman P., Reinert W. R., Giles N. H. Purification and characterization of 3-dehydroshikimate dehydratase, an enzyme in the inducible quinic acid catabolic pathway of Neurospora crassa. J Biol Chem. 1978 Jul 10;253(13):4593–4598. [PubMed] [Google Scholar]
- Vapnek D., Hautala J. A., Jacobson J. W., Giles N. H., Kushner S. R. Expression in Escherichia coli K-12 of the structural gene for catabolic dehydroquinase of Neurospora crassa. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3508–3512. doi: 10.1073/pnas.74.8.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]