Abstract
We measured life span and fecundity of three reproductive modes in a clone of the monogonont rotifer Brachionus manjavacas subjected to chronic caloric restriction (CCR) over a range of food concentrations or to intermittent fasting (IF). IF increased life span 50%–70% for all three modes, whereas CCR increased life span of asexual females derived from sexually or asexually produced eggs, but not that of sexual females. The main effect of CR on both asexual modes was to delay death at young ages, rather than to prevent death at middle ages or to greatly extend maximum life span; in contrast CR in sexual females greatly increased the life span of a few long-lived individuals. Lifetime fecundity did not decrease with CCR, suggesting a lack of resource allocation trade-off between somatic maintenance and reproduction. Multiple outcomes for a clonal lineage indicate that different responses are established through epigenetic programming, whereas differences in life-span allocations suggest that multiple genetic mechanisms mediate life-span extension.
Key Words: Caloric restriction, Life span extension, Aging, Resource allocation, Rotifer
Although caloric restriction (CR) is the only mechanism known to increase life span in a wide range of taxa, there is no consensus on the origin of, or mechanisms controlling, this phenomenon (1–3). One hypothesis is that the response to CR evolved to cope with periodic food shortage in the environment, allowing an individual to survive until food again becomes available and reproduction can resume. Implicit in this premise is that there is a trade-off between reproduction and somatic growth and maintenance, and that when resources are limiting energy is not available for both. The presumption of a trade-off is supported by some but not all studies (4–8) and requires further testing. A trade-off between fecundity and life span also suggests that different modes of reproduction within the same species (sexual vs asexual, males vs females) might have different responses to CR. It is often difficult to test and distinguish differences in life span and fecundity responses to CR due to reproductive mode from those due to interspecific differences.
CR extends life span in many, but not all, monogonont rotifer species, at least under conditions tested thus far (4,5,7,9). Monogonont rotifers are basal, triploblast, microscopic, aquatic invertebrates with a number of advantages as a model system for the study of aging (10). Their small size and ease of culturing allows testing of multiple treatments with a high degree of replication. As facultatively sexual organisms, monogonont rotifers generally reproduce asexually, with a diploid female producing diploid eggs by mitosis that hatch into other asexual (amictic) females, giving rise to a clonal population. In response to a quorum sensing mechanism, sexual (mictic) females are produced that generate haploid eggs through meiosis. If unfertilized, these haploid eggs hatch into haploid males. If fertilized, a diapausing diploid egg is formed, which hatches into an amictic female. These diapausing or “resting” eggs may overwinter or be desiccated, and hatch in response to positive environmental conditions. Males do not feed, so studies of CR are conducted on amictic females arising from sexually derived diapausing eggs, amictic females hatching from mitotically derived asexual eggs, or mictic females hatching from mitotically derived asexual eggs.
Life-span extension in calorically restricted rotifers is sometimes associated with a decline in fecundity, lending support to the idea that reproduction and somatic maintenance are mutually exclusive endeavors under resource limitation. In low nutrient environments, the Brachionus plicatilis Ishikawa strain doubled its life span but decreased fecundity to less than half that under ad-libitum (AL) food conditions (9). The mean life span and relative fecundity of Cephalodella sp. were both 50% lower in CR animals than in AL animals, although Elosa worallii had a 50% increase in life-span offset by a near cessation of reproduction in CR animals (7). Additional studies have shown that starvation prior to first reproduction increased life span more than starvation after the onset of reproduction, and that starved rotifers had a smaller body size, suggesting a trade-off between somatic maintenance, longevity, and reproduction (8).
Many of the previous studies on the effect of CR on longevity and reproduction in rotifers have been in the context of the ecology of resource limitation and population dynamics, although rotifers have also been used to test multiple evolutionary theories of aging (5,11). Unfortunately, most studies that found a lack of life-span extension compared distantly related species and examined only one or two levels of CR, so it is unclear whether the optimally restricted diet for each species simply was not tested. These studies generally examined the effects of either differing periods of starvation or chronic caloric restriction (CCR), but not both, for amictic female rotifers of a single or distantly related species (4,5,7,9,12). This earlier work has provided useful insights about the maintenance of populations in the field or of life span under particular food regimens, but a more comprehensive assessment of multiple reproductive modes and a variety of levels and types of CR could provide a better understanding of the origins and mechanisms of CR-induced longevity.
In this study, we measured life span and fecundity of three different reproductive modes of the monogonont rotifer Brachionus manjavacas subject to six food concentrations ranging from AL feeding to starvation, and to alternate day AL feeding and starvation. By examining a range of CR levels and regimens for multiple reproductive types within a clonal isolate, we were able to dissect the plasticity of life span and reproductive responses and relate these to potential origins and mechanisms.
Methods
Cultures
An isolate of the monogonont rotifer B manjavacas (13), collected from the Azov Sea region in Russia and propagated continuously in the laboratory since 1983 with periodic resting egg collection and storage, is our model system for aging studies. Rotifers were fed the chlorophyte Tetraselmis suecica, which was maintained in 2-L flasks of gently bubbled, 15-ppt artificial seawater (ASW) f/2 medium (Guillard). Both rotifer and algae cultures were kept at 21°C on a 12:12 hour light:dark cycle. Cultures of T suecica used for CR studies were maintained in semicontinuous log phase growth by daily removal of approximately 20% of the culture and replacement with f/2 medium. Maternal females were maintained in AL food conditions for at least 1 week prior to experiments to prevent known life-span-extending maternal effects of CR on offspring (14).
Experimental Conditions
In this study, we conducted life table experiments to examine the effects of seven different food concentrations on life span and reproduction for three reproductive modes of B manjavacas. The diapausing, or “resting,” eggs of B manjavacas, the result of sexual reproduction, were collected from 200-L batch cultures, desiccated, and stored at −20°C for approximately 2 years before these experiments. Amictic eggs were removed from mature amictic females by vortexing and then isolated by micropipette. Neonates of both diapausing and amictically derived eggs < 3 hours old were individually isolated into 1mL of T suecica at a concentration of 6×105 cells mL−1 (equal to 100% of AL) in 24-well plates. The AL concentration was chosen based on previous aquaculture studies that showed maximal grazing and specific growth rates in Brachionus rotifers under comparable food conditions (15–17). All individuals were fed at AL concentrations for the first 20 hours then maintained individually in 1mL at the treatment food concentrations for the duration of life span. CR was attained by diluting algae with 15-ppt ASW to 75%, 50%, 25%, 10%, and 0% of AL concentrations. Intermittently fasted (IF) individuals were fed on alternate days at 100% and 0% of AL.
Each day of the experiment, life span, reproductive status (prereproductive, reproductive, or postreproductive), and number of offspring were scored, and the original female was moved to a new well with clean water and T suecica. Amictic females hatched from sexually derived resting eggs (RE-amictic) and amictic females hatched from mitotically derived amictic eggs (AE-amictic) both produce only amictic, diploid female offspring. Mictic females hatched from mitotically derived amictic eggs (AE-mictic) produced haploid eggs by meiosis; as the AE-mictic females did not copulate prior to egg production their eggs developed as haploid males. Kaplan–Meier survival curves and tests for significance were calculated using Prism 5.0d and Excel 2008. Daily age-specific hazard rates (µx) were determined using the simplified version of the Sacher estimate: µx = −ln(1 − q x) where q x is the age-specific probability of death in the interval Δx (18). Hazard rates were calculated for each treatment until only four individuals persisted.
Results
Effect of CR on Life Span
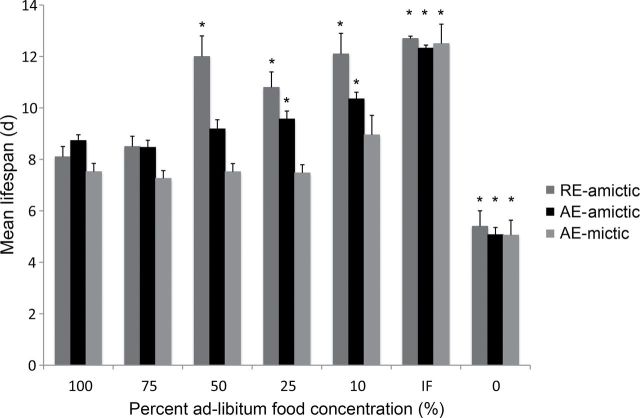
The three reproductive types of B manjavacas had different trends in survival curves across the range of food concentrations (Figure 1). Survival curves for amictic females hatched from resting eggs (RE-amictic) had gradual slopes, whereas those for amictic or mictic females hatched from amictic eggs (AE-amictic and AE-mictic) were in general steeper with a flattening of the tail caused by the extended survival of only a few individuals over the last days of the experiment. For amictic females under CCR, a significant extension of life span was not seen until food was restricted to 50% of AL or less. Survival curves, hazard rate curves, and mean and median life-span measures indicated that there was no change in life span until food concentration dropped below a threshold of 3×105 cells mL−1 (Figures 1–3; Table 1). Below 50% of AL feeding, decreasing food concentration led to increasing mean, median, and maximum life span, but the magnitude of the increase depended upon reproductive mode.
Figure 1.
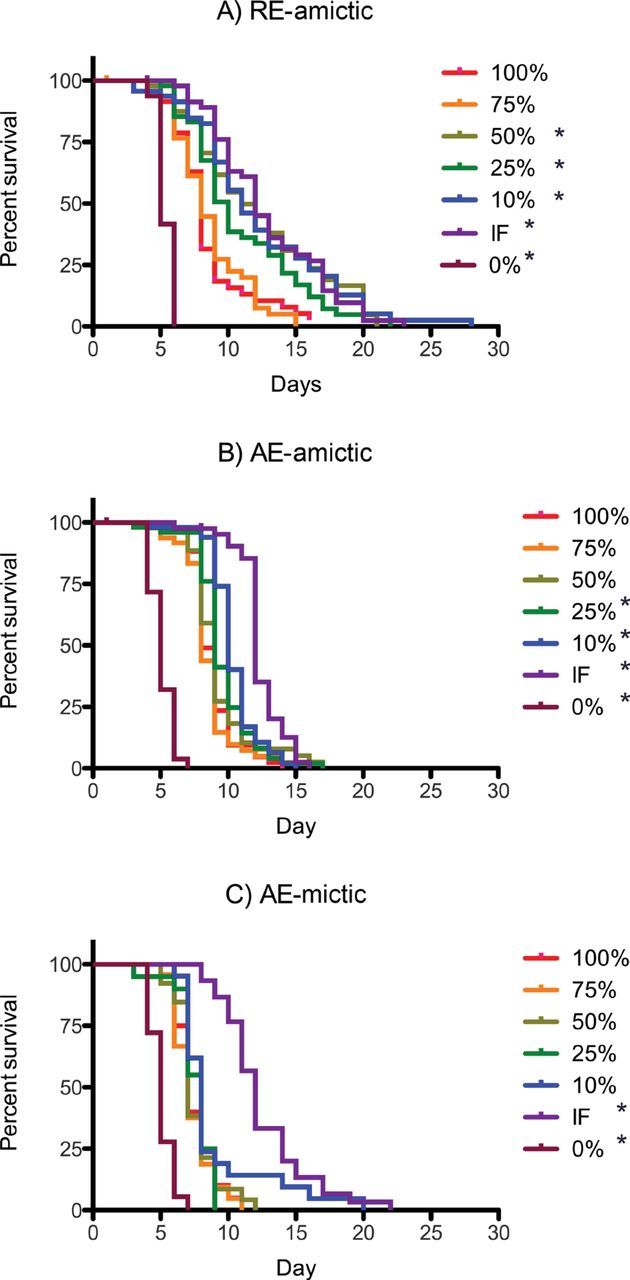
Kaplan–Meier survival curves for (A) RE-amictic, (B) AE-amictic, and (C) AE-mictic females of Brachionus manjavacas subjected to constant caloric restriction at 0–100% of ad-libitum feeding or to intermittent fasting. * indictates significant difference between treatment and 100% ad-libitum feeding (p < .05, Mantel–Cox test).
Figure 3.
Mean life span for RE-amictic, AE-amictic, and AE-mictic females of Brachionus manjavacas subjected to constant caloric restriction at 0–100% of ad-libitum feeding or to intermittent fasting. * indicates significant difference between treatment and 100% ad-libitum feeding (p < .05, two-tailed t test with Bonferroni’s correction).
Table 1.
Life span and mean total offspring under different food concentrations
| Food Concentration (% ad libitum) | n | Minimum Life span (d) | Mean Life span (d; SEM) | Median Life span (d) | Maximum Life span (d) | Mean Total Offspring (d; SEM) | |
|---|---|---|---|---|---|---|---|
| RE-amictic | 100 | 41 | 4 | 8.1 (0.4) | 8 | 16 | 24.8 (1.2) |
| 75 | 43 | 5 | 8.5 (0.4) | 8 | 15 | 23.2 (1.5) | |
| 50 | 44 | 4 | 12.0 (0.8)** | 11 | 21 | 26.7 (1.7) | |
| 25 | 43 | 5 | 10.8 (0.6)** | 9 | 22 | 25.5 (1.8) | |
| 10 | 43 | 3 | 12.1 (0.8)** | 11 | 28 | 29.2 (2.0) | |
| IF | 44 | 6 | 12.7 (0.6)** | 12 | 23 | 31.1 (1.7)* | |
| 0 | 48 | 4 | 5.4 (0.09)** | 5 | 6 | 8.0 (0.2)** | |
| AE-amictic | 100 | 49 | 5 | 8.7 (0.2) | 8 | 14 | 31.2 (0.4) |
| 75 | 47 | 4 | 8.5 (0.3) | 8 | 15 | 30.9 (0.5) | |
| 50 | 43 | 4 | 9.2 (0.4) | 9 | 17 | 31.1 (0.6) | |
| 25 | 51 | 3 | 9.6 (0.3)* | 9 | 17 | 30.4 (1.0) | |
| 10 | 48 | 4 | 10.3 (0.3)** | 10 | 15 | 31.1 (0.6) | |
| IF | 40 | 6 | 12.3 (0.3)** | 12 | 16 | 22.2 (0.6)** | |
| 0 | 53 | 4 | 5.1 (0.1)** | 5 | 7 | 3.8 (0.1)** | |
| AE-mictic | 100 | 20 | 5 | 7.5 (0.3) | 7 | 11 | 29.2 (0.5) |
| 75 | 23 | 5 | 7.3 (0.3) | 7 | 11 | 28.8 (0.5) | |
| 50 | 25 | 5 | 7.5 (0.3) | 7 | 12 | 30.4 (0.3)* | |
| 25 | 19 | 3 | 7.5 (0.3) | 8 | 9 | 29.9 (0.9) | |
| 10 | 21 | 6 | 9.0 (0.8) | 8 | 20 | 31.0 (0.6)* | |
| IF | 30 | 8 | 12.5 (0.6)** | 12 | 22 | 31.6 (0.5)** | |
| 0 | 18 | 4 | 5.1 (0.2)** | 5 | 7 | 8.5 (0.5)** |
Notes. d = days; IF = intermittent fasting; n = number of individuals; SEM = standard error of the mean.
The difference between 100% feeding and caloric restriction regime for mean life span and mean total offspring were tested using two-tailed t test with Welch’s correction for unequal variance (*p < .05, **p < .005).
For RE-amictic females, 50% and 10% of AL produced nearly identical survival curves and a 50% increase in mean and median life span of over those for AL feeding. A maximum life span of 28 days was reached under 10% of AL. AE-amictic females had gradually increasing median life span from 8 days at 100% of AL to 10 days at 10% of AL. AE-mictic females, on the other hand, exhibited significantly extended life span only under IF. Uniformly across reproductive types, individuals in the starved treatment (0% AL) began dying on Day 4, and all were dead by Day 7.
The greatest extension in mean and median life span occurred for all reproductive types in IF individuals, with an increase in mean and median life span of 150%–171% that of individuals fed AL (Figures 1 and 3; Table 1). Maximum life span increased under IF, but was greater than 10% CCR only in AE-mictic females. Restriction of food to 10% of AL levels produced the next highest increase in mean and median life span in all reproductive types.
Hazard rate curves (Figure 2) indicated that the main effect of both types of CR on amictic females was to delay death at young ages, rather than to prevent death at middle ages or to greatly extend maximum life span, a phenomenon also reflected in the survival curves. In AE-mictic females, CR did not decrease the hazard rate at early or middle ages, but 10% CCR and IF greatly increased the life span of a few long-lived individuals.
Figure 2.
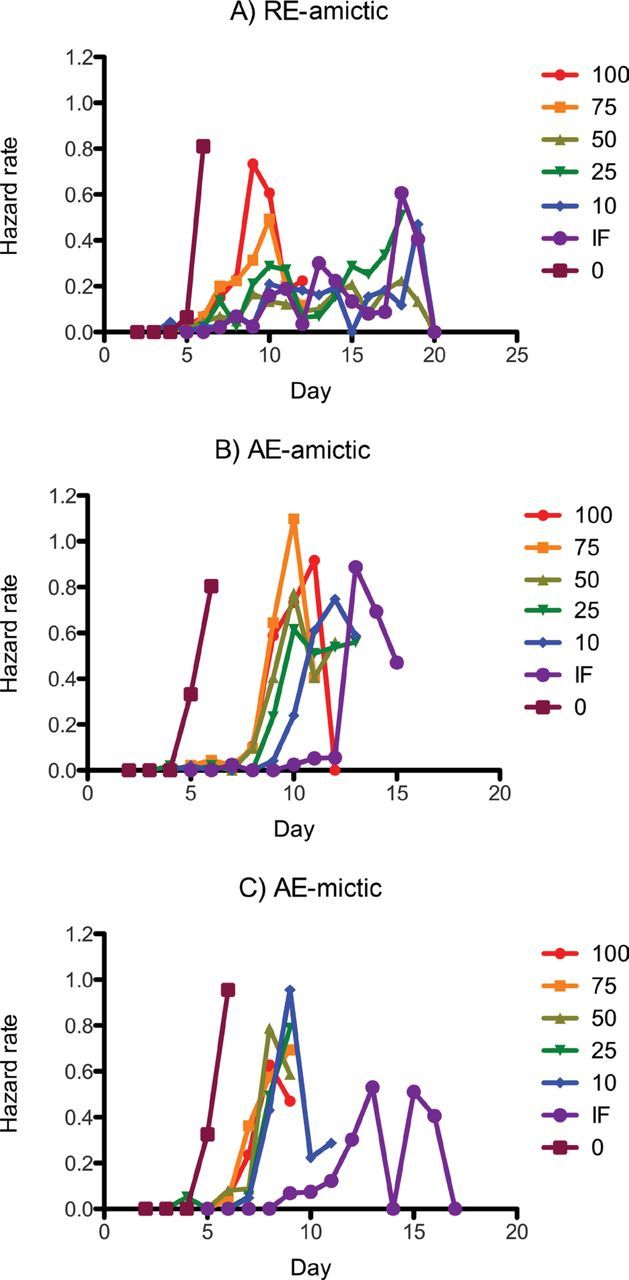
Age-specific hazard rates for (A) RE-amictic, (B) AE-amictic, and (C) AE-mictic females of Brachionus manjavacas subjected to constant caloric restriction at 0–100% of ad-libitum feeding or to intermittent fasting. Hazard rates were computed when four or more individuals were present, as sample sizes late in life were too small to adequately assess the hazard rate (18).
Effect of CR on Life-Span Allocation and Fitness
CR altered the allocation of life span to prereproductive, reproductive, and postreproductive periods differently in amictic and mictic females (Figure 4). Both types of amictic females significantly increased the reproductive portion of life span under IF (to 73% and 84%), whereas mictic females maintained a relatively constant proportion of life span in reproduction across varying levels of CCR and IF (from 51% to 58%). Conversely, mictic females increased the reproductive portion of life span to nearly 72% while starved, whereas amictic females maintained or slightly decreased their reproductive period under starvation conditions. For both AE-mictic and AE-amictic females, a significantly shorter portion of life span was spent in the prereproductive stage at very low food concentrations.
Figure 4.
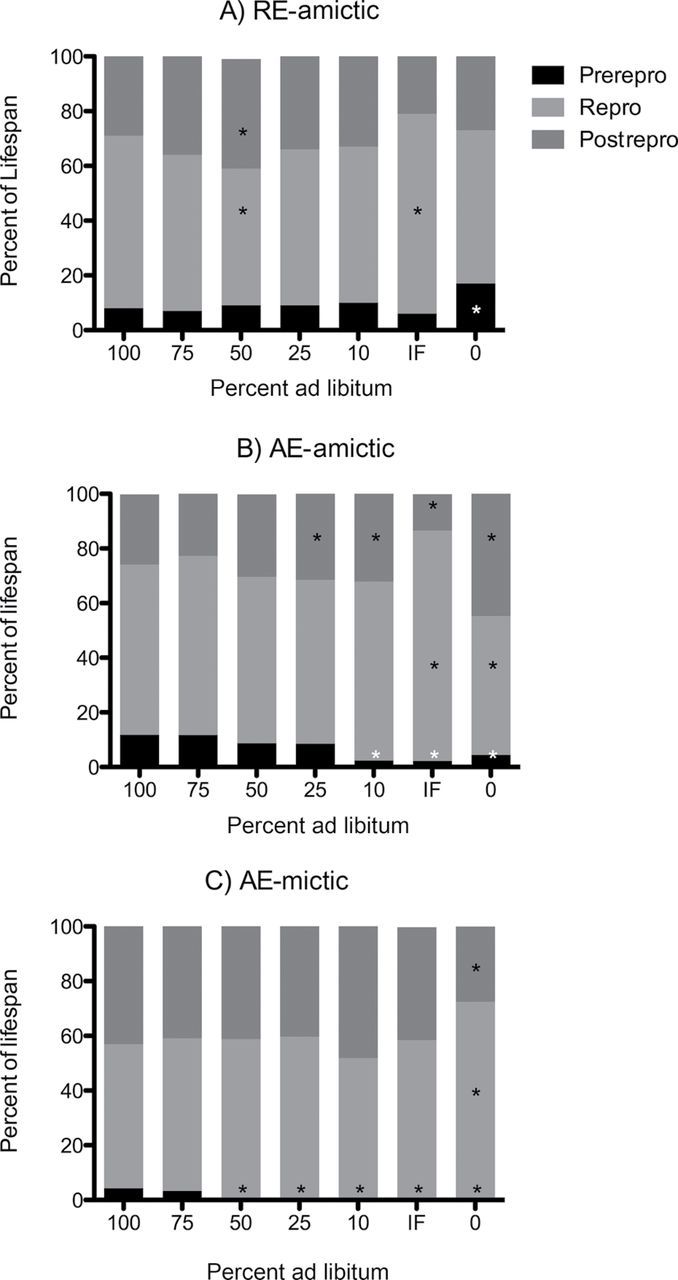
Proportion of life span allocated to prereproductive, reproductive, and postreproductive stages in (A) RE-amictic, (B) AE-amictic, and (C) AE-mictic females of Brachionus manjavacas subjected to constant caloric restriction at 0–100% of ad-libitum feeding or to intermittent fasting (IF). * indicates difference in that stage from comparable stage in 100% ad-libitum feeding treatment (p < .05, two-tailed t test with Bonferroni’s correction).
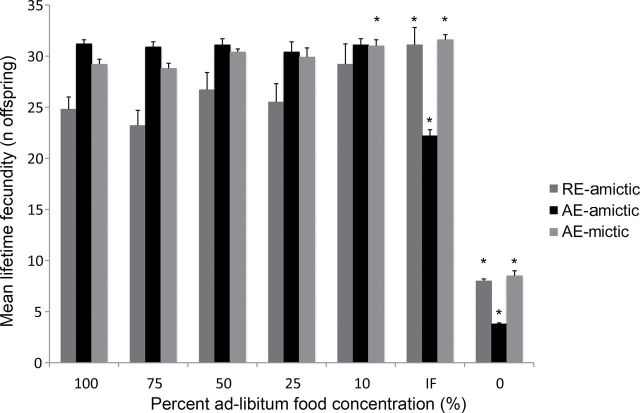
No trade-off between lifetime fecundity and life span was apparent with declining food levels, except in IF AE-amictic females (Figure 5). In fact, for RE-amictic and AE-mictic females, average lifetime fecundity generally increased with increasing CR. Lifetime fecundity was relatively constant under CCR from 10% to 100% of AL for mictic females. CR influenced average daily reproduction over the course of the experiment, however (Figure 6). For amictic females, average daily reproduction was greater at higher food concentrations for the first 5–6 days of the experiment, then shifted as females aged to become higher for IF and the lowest levels of CCR. Mictic females had similar daily reproduction across CCR food concentrations, except for IF and starved treatments, where daily reproduction was greatly reduced. Complete starvation led to declines in both daily and lifetime average reproduction in all female types.
Figure 5.
Mean lifetime individual fecundity of RE-amictic, AE-amictic, and AE-mictic females of Brachionus manjavacas subjected to constant caloric restriction at 0–100% of ad-libitum feeding or to intermittent fasting. * indicates significant difference between treatment and 100% ad-libitum feeding (p < .05, two-tailed t test with Bonferroni’s correction).
Figure 6.
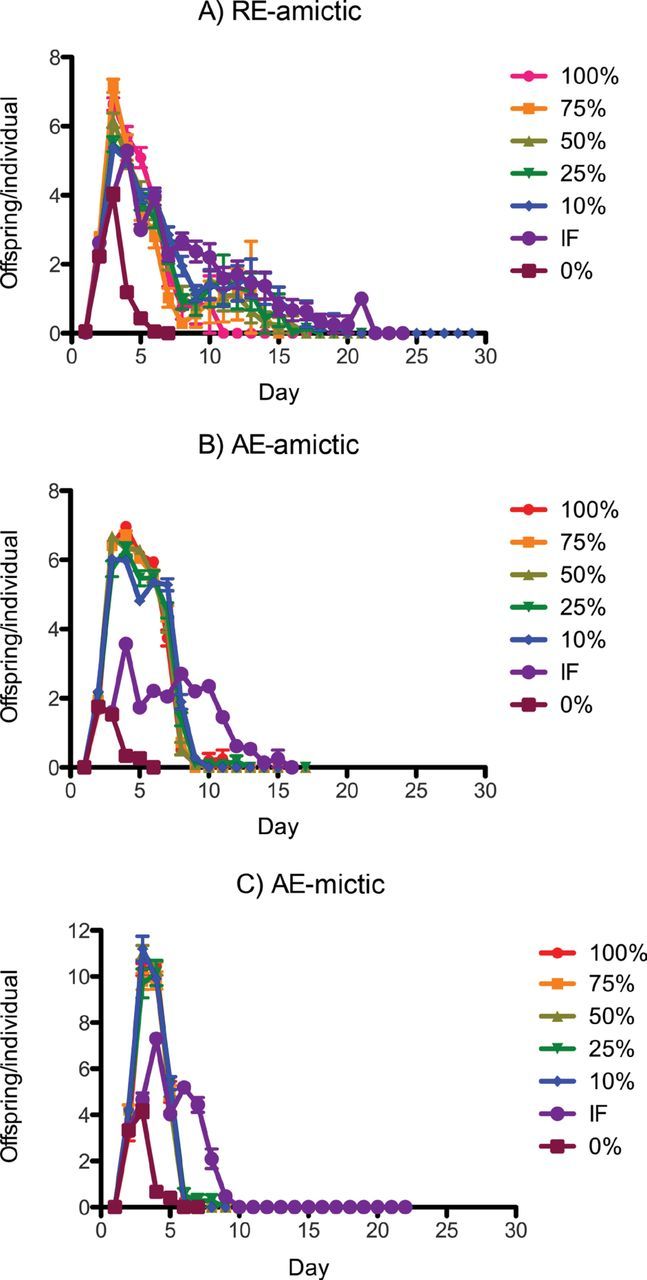
Mean daily individual fecundity of (A) RE-amictic, (B) AE-amictic, and (C) AE-mictic females of Brachionus manjavacas subjected to constant caloric restriction at 0–100% of ad-libitum feeding or to intermittent fasting.
Daily reproduction was negatively correlated with life span early in life, but positively correlated with life span late in life (Figure 7). Negative correlations for amictic females on Day 3 of the experiment were driven by the higher reproductive output of less-restricted females (with shorter life span) relative to females subject to extreme CCR or IF. In midlife, around Days 5 and 6, the association between daily fecundity and life span shifted. In late life, the reproduction of less-restricted females (100% and 75% AL) dropped to near zero, whereas the daily reproduction of longer-lived 10% AL and IF amictic females was maintained. The correlation between daily fecundity and life span for mictic females was driven almost entirely by the single point for IF treatment at all ages, because daily reproduction was unchanged by CCR for mictic females.
Figure 7.
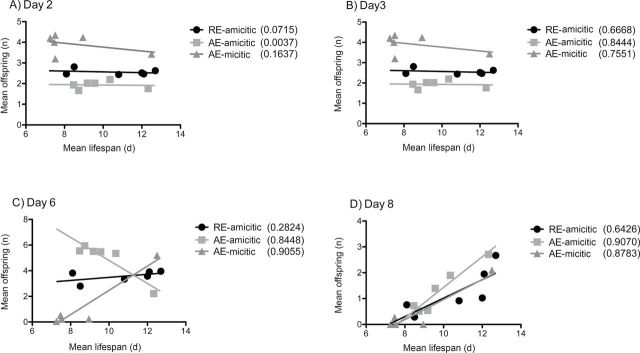
Correlation between average daily reproduction and life span for RE-amictic, AE-amictic, and AE-mictic females of Brachionus manjavacas on (A) Day 2, (B) Day 3, (C) Day 6, and (D) Day 8 of the experiment. r 2 value given for linear correlation; starved rotifers (0% ad libitum) were not included.
Discussion
In this study, we surveyed the effects of seven different CR regimens on the life span and fecundity of three reproductive modes of the monogonont rotifer B manjavacas. We found that the magnitude of life-span extension and the concomitant effect on reproduction depended both on the type of CR and on the female’s reproductive type, suggesting a diversity of underlying mechanisms for the response to CR.
Threshold Response to CCR
Brachionus manjavacas appears to have a threshold below which the food concentration must drop before eliciting a life-span-extending response. We did not observe a significant increase in life span of RE-amictic females until food limitation reached 50% of AL or less, and further limiting food between 50% and 10% of AL resulted in longer mean and maximum life span. Restriction to 25% of AL was required before life span increased in AE-amictic females. Previous grazing and population growth experiments in similar species of Brachionus show that maximal specific growth rates are achieved at food levels comparable with our AL concentration and decline with decreasing food concentrations (15–17). We did not measure assimilation rates at the varying food concentrations, however, and at higher concentrations a larger proportion of food may have passed through the rotifer gut without being fully digested or incorporated.
These results suggest induction of the CR response was controlled by nutrient sensing or metabolic status, which was not triggered until perceived or consumed resources reached a critical level. This is consistent with the wide range of rapidly changing resource conditions in the natural habitats of B manjavacas, and implies that different Brachionus isolates may have different response thresholds depending on their native environment. On a practical note, these results demonstrate that one must use caution in interpreting studies in which a single level of CR has been tested; as in this study what may appear as a lack of response to CR at 75% of AL, for example, may simply be the incorrect level to induce life-span-extending mechanisms. Without examination of a range of food levels, it is impossible to determine the level of restriction at which there is maximum longevity with the fewest detrimental side effects.
Varied Response to CR of Different Reproductive Modes
Differences in the response to CR depended not only on the method of food restriction but also on the reproductive mode of the female, likely due to the differences in evolutionary pressures on different types of females. Mictic females were unable to respond to any level of CCR, but increased mean life span by 70% under IF. A similar lack of response to CCR was found previously for mictic females of the freshwater species Brachionus calyciflorus (19). The production of mictic females by B manjavacas is induced by environmental conditions primarily driven by crowding, and requires relatively low ammonia conditions (ie, good water quality) and high food concentrations (20,21). Mictic females are programmed for maximum reproductive output as quickly as possible, to allow sexual reproduction and the production of diapausing eggs that can overwinter and withstand desiccation and thereby permit the population to persevere through adverse environmental circumstances. A mechanism to delay reproduction and extend life span in low food conditions thus would not benefit mictic females or ensure passage of their genetic material, because a declining population caused by delayed reproduction would not allow the frequent random encounter between males and mictic females that is required for mating. In addition, there is a waning ability of males to recognize and mate with mictic females more than 24 hours old, further decreasing the evolutionary incentive of mictic females to live to old age (22).
Amictic females, on the other hand, had increased life span for both CCR and IF, but with different reproductive strategies under each. Under IF, average daily reproduction was lowered almost immediately and the reproductive period was extended in proportion with the extended life span, to result in the same lifetime fecundity. Amictic females would benefit from an ability to survive through low resource conditions, as their “best bet” for evolutionary success is to increase the chances for survival of their offspring by producing eggs in a hospitable environment.
Previous studies have shown that RE-amictic females are unable to respond to mixis cues, and only a portion of the AE-amictic female population can produce mictic females, although all are exposed to the same cue and all are genetically identical (20). This strong evidence of “bet-hedging” by maintaining multiple reproductive modes within the same population is also likely to influence life-span responses to CR.
Differences Between CCR and Intermittent Fasting
Differences between daily fecundity and allocation of life span to reproduction under CCR versus IF suggest that different mechanisms are employed to promote longevity under the different types of food limitation. Mean life span was greatest under IF for all reproductive types, but amictic females responded to both CCR and IF with extended life span, whereas mictic females had increased life span only under IF. Additionally, amictic females greatly increased the reproductive portion of life span under IF, but not under CCR, whereas mictic females maintained a constant reproductive portion of life span under all types of CR except starvation.
Studies in many other animal systems have reported differences in the effects of CCR and intermittent caloric restriction (ICR) or IF. Reports in multiple species, from Caenorhabditis elegans to mice, have verified that IF or ICR frequently increase longevity more than does CCR (23). Additionally, IF/ICR and CCR have diverse effects on other age-related phenotypes. Mice subjected to ICR have lower levels of mammary tumor formation, with corresponding lower IGF-1 and mTOR levels, than do mice under CCR or AL, for example (24).
Despite noticeable differences in IF/ICR and CCR phenotypes in many animal systems, the possible differences in mechanism are unclear. Few studies have addressed the genetics of multiple CR regimens simultaneously, even though cross-study comparisons are problematic, and direct comparisons could help tease apart mechanisms (25). In C elegans, diverse CCR regimens and CR mimetics evoked a variety of independent genetic pathways involved in nutrient sensing and stress response, though IF was not tested (26). Studies of IF in which animals had same overall caloric intake as AL animals, but still exhibited increased life span and stress-resistance, argued for an indirect effect of energy sensing on antiaging response in IF regimens (25).
Brachionus rotifers in their natural habitats are unlikely to see food conditions like those of the IF treatment imposed in this study. In the wild, B manjavacas would consume a variety of microbial species, and phytoplankton and bacterial dynamics in the rotifers’ aquatic habitats are much more likely to demonstrate long periods of very low or high concentrations, gradually increasing or decreasing food concentrations, or complete depletion for a period of time, rather than a simple every other day AL and starvation conditions. Thus, it seems probable that although a mechanism for adjusting life span and reproduction would have evolved in response to CCR, IF may simply be invoking a stress response in B manjavacas.
The results of this study highlight the value of examining an array of phenotypes when studying the effects of CR. Many reports of life-span-extending interventions in invertebrates employ fluorodeoxyuridine (FUdR), an inhibitor of DNA synthesis, to prevent hatching of offspring, making tracking of individual animals much easier. In this study, however, because maximum and medium life span were similar for 10% and IF, we would not have seen that life-span extension was occurring by different processes in the two types of CR without also measuring reproduction. Further, the life span altering properties of FUdR itself are poorly understood even for eutelic organisms, and are rarely tested against a non-FUdR control in invertebrate aging studies. Additional parameters beyond life span should be measured in studies of CR, to shed light on potential differences in mechanisms with varied modes of food limitation.
Resource Allocation Under CR
Analysis of a potential trade-off between fecundity and life span under limiting resources revealed a complex system in which initial average daily reproduction was lowered, but late life daily reproduction and lifetime fecundity were increased, relative to AL. If there were a straightforward trade-off between life span and reproduction, one might expect continually decreasing daily reproduction, and a resulting lower lifetime reproductive output, to be associated with increasing life span in more restricted individuals. Instead, in both mictic and amictic females hatched from amictic eggs, the prereproductive period shortens with both CCR and IF. This suggests a strategy to initially produce offspring as quickly as possible under limiting conditions, an observation that has been made previously in the monogonont Synchaeta pectinata (12). Over the course of this experiment, however, we observed a shift in daily reproduction between AL and CR rotifers: daily reproductive output was initially higher in AL-fed rotifers, but then switched in late life so that higher daily reproductive rates were maintained in CR individuals but declined in AL fed rotifers. In fact, there was a flat or negative correlation between life span and mean daily fecundity during the period of maximal reproduction on Days 2–5, but in later life life span and mean daily fecundity were positively correlated in all reproductive types. Resource allocation trade-offs thus may be confined to the early reproductive period, as was also reflected in the decreased hazard rate for CCR and IF rotifers in early but not in late life. Unseen trade-offs may have manifested as decreased body size, offspring viability, or long-term specific growth rate, parameters not measured in this experiment.
The evidence for a trade-off between reproduction and life span in the face of food shortage is mixed. One of the primary arguments in favor of a trade-off is that when rotifers are starved later in life, after beginning reproduction, they have lower tolerance to starvation than rotifers starved early in life (4). Additionally, many rotifer species immediately cease reproduction upon starvation, and resume reproduction upon reintroduction of feeding (7). In some studies in which rotifers are starved, either for different lengths of time or at different times in their life span, lifetime fecundity is indeed decreased, much as we observed for rotifers starved after the first day of the experiment (7). However, there are many reports where reproduction is decreased but life span is not extended, particularly when starvation occurs late in life or for extended periods (5,7,8,12). Moreover, it has been demonstrated that even without food restriction, reproduction near the end of life is more closely associated with a short life span than is early reproduction (6). In addition to our observations of increasing lifetime reproduction with decreasing food concentrations, others found that S pectinata increased the proportion of energy allocated to reproduction as food became more limiting, producing a constant egg size but reproducing earlier under limiting conditions, and that there was no significant difference in life span between restricted and unrestricted rotifers (12).
Unfortunately, evidence on both sides is predominantly correlative; it is experimentally difficult to establish a causative relationship between energy intake, reproduction, and life span. Our findings support the hypothesis that amictic monogonont rotifers have a temporally dynamic reproductive strategy, as has been found for organisms ranging from plants to rodents to birds, which allows them to deal with food limitation and to resume reproduction when conditional are favorable, and which may not or may not be directly linked to life-span extension (8,27). Such a strategy may become less flexible with age, for reasons unrelated to food concentrations. The age-specific physiological constraints due to changes in molecular pathways and the accumulation of DNA and cellular damage with increasing age provide as plausible a causative force in decreased starvation tolerance with age as does the allocation of limited resources to reproduction (28).
Conclusions
Examination of life span and fecundity in B manjavacas subjected to food limitation leads us to hypothesize that different mechanisms are employed under different CR regimens. Additionally, studying multiple reproductive modes within a clonal isolate of a single species enabled us to dissect the plasticity of life span and reproductive responses in the face of potentially conflicting evolutionary programs, without the confusion of multiple genotypes. We hypothesize that in amictic females a single mechanism invoked by a threshold response promotes increased longevity under CCR conditions, whereas different pathways, possibly related to stress response, are evoked by IF. The main effect of both types of CR was to delay death at young ages, rather than to prevent death at middle ages or to greatly extend maximum life span. In mictic females there was no increase in longevity from any level of CCR; the positive response to IF may be due to yet a third mechanism. This contrast between genetically identical organisms suggests different genetic responses are established through epigenetic programming early in development, probably due to the different reproductive strategies of asexual and sexual females. Based on studies in other organisms, CCR-induced longevity increases maybe due directly to changes in energy intake, whereas IF-induced life-span extension maybe an indirect effect of differences in resource intake, leading to upregulation of stress response mechanisms (25). Further dissection of genetic mechanisms will determine if IF and CCR work via a single universal process across reproductive types, or by overlapping or distinct nutrient sensing, metabolic, and/or hormetic pathways in rotifers and other animals.
Funding
This work was supported by the National Institute on Aging Division of Aging Biology (R01 AG037960-01).
Acknowledgments
We thank Bette Hecox-Lea and Tucker Hopkins for laboratory assistance, Terry Snell for providing B manjavacas and T suecica cultures, and two anonymous reviewers for helpful comments.
References
- 1. Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. 10.1038/nrm1616 [DOI] [PubMed] [Google Scholar]
- 2. Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. 10.1038/35041700 [DOI] [PubMed] [Google Scholar]
- 3. Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002 [DOI] [PubMed] [Google Scholar]
- 4. Kirk KL. Life-history responses to variable environments: starvation and reproduction in planktonic rotifers Ecology. 1997;78(2):434–441. 10.1890/0012-9658(1997)078[0434LHRTVE]2.0.CO;2 [Google Scholar]
- 5. Kirk KL. Dietary restriction and aging: comparative tests of evolutionary hypotheses. J Gerontol A Biol Sci Med Sci. 2001;56A(3):B123–B129. 10.1093/gerona/56.3.B123 [DOI] [PubMed] [Google Scholar]
- 6. Snell TW, King CE. Life span and fecundity patterns in rotifers: the cost of reproduction. Evolution. 1977;31(4):882–890 [DOI] [PubMed] [Google Scholar]
- 7. Weithoff G. Dietary restriction in two rotifer species: the effect of the length of food deprivation on life span and reproduction. Oecologia. 2007;153:303–308. 10.1007/s00442-007-0739-6 [DOI] [PubMed] [Google Scholar]
- 8. Yoshinaga T, Hagiwara A, Tsukamoto K. Life history response and age-specific tolerance to starvation in Bachionus plicatilis O.F. Müller (Rotifera). J Exp Mar Biol Ecol. 2003;287:261–271. 10.1016/S0022-0981(02)00574-9 [DOI] [PubMed] [Google Scholar]
- 9. Yoshinaga T, Hagiwara A, Tsukamoto K. Effect of periodical starvation on the life history of Brachionus plicatilis O.F. Müller (Rotifera): a possible strategy for population stability. J Exp Mar Biol Ecol. 2000;253:253–260. 10.1016/S0022-0981(00)00268-9 [DOI] [PubMed] [Google Scholar]
- 10. Austad SN. Is there a role for new invertebrate models for aging research? J Gerontol A Biol Sci Med Sci. 2009;64A(2):192–194. 10.1093/gerona/gln059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Enesco HE. Rotifers in aging research; use of rotifers to test various theories of aging. Hydrobiologia. 1993;255/256:59–70. 10.1007/BF00025821 [Google Scholar]
- 12. Stelzer C-P. Resource limitation and reproductive effort in a planktonic rotifer. Ecology. 2001;82(9):2521–2533. 10.1890/0012-9658 (2001)082[2521RLAREI]2.0.CO;2 [Google Scholar]
- 13. Fontaneto D, Giordani I, Melone G, Serra M. Disentangling the morphological stasis in two rotifer species of the Brachionus plicatilis species complex. Hydrobiologia. 2007;583:297–307. 10.1007/s10750-007-0573-1 [Google Scholar]
- 14. Kaneko G, Yoshinaga T, Yanagawa Y, Ozaki Y, Tsukamoto K, Watabe S. Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Funct Ecol. 2010; 10.1111/j.1365-2435.2010.01773.x [Google Scholar]
- 15.Hansen, BW, Wernberg-Møller, T, Wittrup, L. Particle grazing efficiency and specific growth efficiency of the rotifer Brachionus plicatilis (Muller). J Exp Mar Biol Ecol. 1997;215: 217–233 . [Google Scholar]
- 16. Awaïss A, Kestemont P, Micha JC. An investigation into the mass production of the freshwater rotifer Brachionus calyciflorus Pallas. 1. An eco-physiological approach to nutrition. Aquaculture. 1992;105:325–336 DOI:10.1016/0044-8486(92)90096-4 [Google Scholar]
- 17. Montagnes D, Kimmance S, Tsounis G, Gumbs J. Combined effect of temperature and food concentration on the grazing rate of the rotifer Brachionus plicatilis . Mar Biol. 2001;139:975–979. 10.1007/s002270100632 [Google Scholar]
- 18. Curtsinger JW, Gavrilova N, Gavrilov L. Biodemography of aging and age-specific mortality in Drosophila melanogaster . In: Masoro EJ, Austad SN, eds. Handbook of the Biology of Aging. 6th ed. San Diego, CA: Elsevier Academic Press; 2005:265–292 [Google Scholar]
- 19. Galindo MD, Guisande C. The reproductive biology of mictic females in Brachionus calyciflorus Pallas. J Plankton Res. 1993;15(7):803–808. 10.1093/plankt/15.7.803 [Google Scholar]
- 20. Gilbert JJ. Environmental and endogenous control of sexuality in a rotifer life cycle: developmental and population biology. Evol Dev. 2003;5(1):19–24. 10.1046/j.1525-142X.2003.03004.x [DOI] [PubMed] [Google Scholar]
- 21. Snell TW, Boyer EM. Thresholds for mictic female production in the rotifer Brachionus plicatilis (Muller) J Exp Mar Biol Ecol. 1988;124:73–85. 10.1016/0022-0981(88)90112-8 [Google Scholar]
- 22. Snell TW, Kim J, Zelaya E, Resop R. Mate choice and sexual conflict in Brachionus plicatilis (Rotifera). Hydrobiologia. 2007;593: 151–157. 10.1007/s10750-007-9065-6 [Google Scholar]
- 23. Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. 10.1038/nature08980 [DOI] [PubMed] [Google Scholar]
- 24. Dogan S, Johannsen AC, Grande JP, Cleary MP. Effects of intermittent and chronic calorie restriction on mammalian target of rapamycin (mTOR) and IGF-I signaling pathways in mammary fat pad tissues and mammary tumors. Nutr Cancer. 2011;63(3):389–401. 10.1080/01635581.2011.535968 [DOI] [PubMed] [Google Scholar]
- 25. Anson RM, Jones B, de Cabo R. The diet restriction paradigm: a brief review of the effects of every-other-day feeding. Age. 2005;27:17–25. 10.1007/s11357-005-3286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greer EL, Brunet A. Different dietary restriction regimens extend life span by both independent and overlapping genetic pathways in C. elegans . Aging Cell. 2009;8:113–127. 10.1111/j.1474-9726.2009.00459.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nichols JD, Conley W, Batt B, Tipton AR. Temporally dynamic reproductive strategies and the concept of r- and K-selection. Am Nat. 1976;110(976):995–1005 [Google Scholar]
- 28. Rodríguez-Graña L, Calliari D, Tiselius P, Winding Hansen B, Nilsson Sköld H. Gender-specific ageing and non-Mendelian inheritance of oxidative damage in marine copepods. Mar Ecol Prog Ser. 2010;401:1–13. 10.3354/meps08459 [Google Scholar]