Abstract
Background.
Studies that have investigated the association between markers of inflammation and risk of dementia are conflicting. Therefore, the researchers conducted a systematic review and meta-analysis of observational studies with the hypothesis that an increased level of peripheral proinflammatory markers would be associated with risk of all-cause dementia or Alzheimer’s disease (AD).
Methods.
The researchers conducted a literature search of observational studies indexed in the PubMed and PsycInfo databases. Selected studies included those with at least one peripheral inflammatory biomarker and its association with risk of all-cause dementia or AD. Random effects models were used to generate pooled hazard ratios (HRs) comparing the top versus bottom quantile of inflammatory marker level. Heterogeneity was assessed using the I 2 statistic.
Results.
Seven studies were identified, combining for a total 5,717 participants, 746 cases of all-cause dementia and 565 cases of AD. An increased level of C-reactive protein was associated with a 45% increased risk of all-cause dementia (HR: 1.45; 95% CI: 1.10, 1.91). Similarly, a higher level of interleukin-6 was associated with a 32% increased risk (HR: 1.32; 95% CI: 1.06, 1.64) of all-cause dementia. For AD alone, the association with C-reactive protein was less pronounced (HR: 1.21; 95% CI: 1.03, 1.42) and interleukin-6 was not associated with risk of AD (HR: 1.06; 95% CI: 0.83, 1.35). No significant heterogeneity was found in any of the meta-analyses (I 2 = 0%–40%, p ≥ .16).
Conclusions.
An increased peripheral level of inflammatory markers is associated with a modest increase in risk of all-cause dementia. Evidence for an association with risk of AD alone is limited.
Key Words: Dementia, Alzheimer, Cognitive decline, Inflammation, c-reactive protein, Interleukin-6
Dementia affects at least one in eight individuals aged 65 and older in the United States alone (1). Several forms of dementia exist, with Alzheimer’s disease (AD) being the most prevalent, representing up to 70% of cases of dementia, alone or in combination with vascular disease. Inflammation has been linked to the etiology of dementia, either as a primary or secondary process. Supporting this theory, several lines of evidence suggest a role of inflammation in the pathogenesis of dementia. First, some observational studies suggest a neuroprotective effect of nonsteroidal anti-inflammatory drugs (NSAID), although this may be limited to NSAID use in midlife or early in the disease process, and among those with one or more apolipoprotein ε4 alleles (2,3). Second, neuropathological and neuroimaging studies have shown that even in the early stages of AD, one can observe amyloid-beta deposition, microglial activation, and upregulation of genes encoding for inflammatory proteins (4). Lastly, peripheral inflammatory markers may be indicative of more global inflammatory processes that relate directly or indirectly to dementia risk via their role in the development and progression of other conditions such as cardiovascular disease (CVD) (5) and type 2 diabetes (6).
Several observational studies show no association between peripheral inflammatory markers and risk of dementia whereas others report a positive association. This inconsistency may be attributed to differences in study design, participant-level characteristics, or lack of power. Therefore, the researchers conducted a systematic review and meta-analysis of observational studies with the hypothesis that increased levels of peripheral proinflammatory markers would be associated with greater risk of all-cause dementia or AD among nondemented adult populations.
Methods
Search Strategy
This systematic review and meta-analysis is described in accordance with the guidelines specified by the Meta-analysis of Observational Studies in Epidemiology (7). Two investigators (A.K. and J.O.) independently performed the literature search. Using the MEDLINE and PsycInfo databases, Boolean searches were conducted using keywords for the exposure (“inflammation” or “inflammatory”) and the outcome (“dementia,” “Alzheimer,” “cognition,” or “cognitive”). There were no restrictions on language or date of publication. Reference lists of relevant review articles and all articles included in the meta-analysis were hand searched. The AlzRisk epidemiology database was also consulted for additional sources (8).
Selection Criteria
The researchers selected studies included in the meta- analysis after two stages. First, the researchers reviewed the title and abstract, followed by the full text. Studies were required to report a measure of association (hazard ratio [HR], odds ratio [OR], and risk ratio) and 95% CIs or standard error for the association between a peripheral (serum and plasma) inflammatory marker and incident all-cause dementia or AD. A study was also required to have a prospective cohort, case cohort, or nested case–control design, at a minimum, adjust for age as a potential confounder, and be published in a peer-reviewed journal (abstracts or conference proceedings were excluded).
Data Extraction
Two investigators (A.K. and J.O.) performed data extr action using a standardized extraction form. Variables included those necessary for meta-analysis and sensitivity analysis of pooled results, participant-level characteristics, and study-level characteristics. This included the following variables from each study: manuscript title, authors, year of publication, study design, country of cohort, length of follow-up, screening method at baseline, cognitive outcomes and the diagnostic criteria used, sample size, mean or median baseline age, percentage of female participants, ethnicity, inflammatory markers measured, measure of association and 95% CIs or standard error, p value, number of cases of all-cause dementia and AD, and covariates used in published regression models. The researchers performed quality assessment for each study using a 9-point modified Newcastle–Ottawa scale for cohort studies (9).
Data Synthesis
The majority of studies reported HRs as the measure of association. Two studies reported ORs, which were considered as equivalent (10). C-reactive protein (CRP) and interleukin-6 (IL-6) were considered for meta-analysis because other markers were investigated in two or less studies. Although studies reported both categorical and continuous measures of association, categorical measures were chosen, using the lowest quantile as the reference. This decision was made a priori because the seven studies measuring CRP utilized six different assays and reported highly varying distributions of CRP levels. Therefore, the researchers did not consider it valid to compute a pooled HR per unit increase. Although comparing the highest to lowest quantile may not detect a nonlinear association, results from these studies suggested little to no indication of a nonlinear association. For three studies that did not report a categorical HR, the researchers contacted the authors to obtain additional data. The authors of two studies did not respond to the researchers’ data request. For one of these studies, the researchers estimated HRs from published figures (11). The researchers excluded the other study from the meta-analysis but reviewed it qualitatively (12).
Both fixed effects and DerSimonian and Laird random effects models were used (13). As results were similar for both, only random effects analyses are reported. Because some studies reported HRs from multiple models, the researchers decided a priori to first pool results from multivariate-adjusted models and then use additionally adjusted models for sensitivity analysis. Heterogeneity across studies was assessed using the I 2 statistic. Publication bias was assessed using an Egger’s test and visual inspection of funnel plots. All analyses were performed using STATA version 10.1 (StataCorp, College Station, TX).
Results
Study Selection
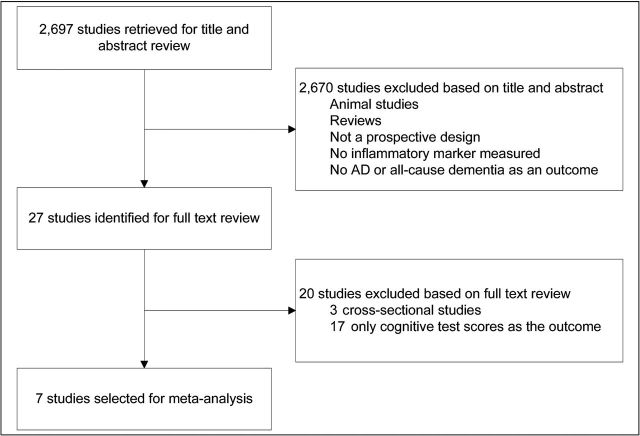
The study selection process is illustrated in Figure 1. A total of 2,697 studies were retrieved from Pubmed and PsycInfo. After reviewing the title and abstract, 2,670 studies were not eligible for meta-analysis based on the researchers’ inclusion criteria. Of the remaining 27 studies identified for full text review, 20 were excluded because they were either cross-sectional studies or did not evaluate all-cause dementia or AD as an outcome. A hand search of the reference lists for these 27 studies and relevant reviews yielded no additional studies, resulting in seven total studies for the researchers’ meta-analysis.
Figure 1.
Study selection.
Description of Studies
Table 1 depicts the seven studies included in the meta-analysis, combining for a total of 5,717 participants, 746 cases of all-cause dementia and 565 cases of AD (11,14–18). One publication was included as two distinct studies in the meta-analysis because results were reported for two separate subcohorts (17). The majority of studies measured inflammatory proteins in serum samples, whereas one study used plasma. Most studies had a majority of female participants, although two studies were comprised all male cohorts (16,17). Follow-up times ranged from 4 to 25 years. All studies used unique cohorts from either the United States or Europe. At a minimum, each study adjusted for age and gender, either through regression modeling or restriction. Although most studies utilized a prospective cohort design, there were two nested case–control studies and one case cohort study. Study quality on the 9-point modified Newcastle–Ottawa scale ranged from 6 to 9. All studies were published in 2002 or later. No evidence of publication bias was found.
Table 1.
Study Characteristics
| Source | Study Design | Baseline Age (mean ± SD) | Female Participants (%) | Years of Follow-up (mean) | Sample Size | Number of Cases | Covariates |
|---|---|---|---|---|---|---|---|
| Schmidt et al. (16) (United States) | Nested case–control | 55.0±4.6 | 0 | 25.3 | 1,050 | Dem: 214; AD: 95 | Age, education, smoking, average cholesterol, blood pressure, years of follow-up, APOE ε4, BMI |
| Engelhart et al. (14) (Netherlands) | Case cohort | 71.7±9.0 | 52.9 | 6.5 | 727 | Dem: 188; AD: 140 | Age, gender, education |
| Ravaglia et al. (11) (Italy) | Cohort | 73.6±6.3 | 43.1 | 3.7 | 804 | Dem: 109; AD: 68 | Age, gender, education, APOE ε4, stroke, CVD, physical activity, BMI, creatinine homocysteine, serum folate, serum vitamin B12 |
| Tan et al. (18) (United States) | Cohort | 78.6±4.6 | 62.2 | 7.0 | 691 | Dem: —; AD: 44 | Age, gender |
| Sundelof et al. (17) (Sweden) | Cohort | 71.0±0.7 | 0 | 10.1 | 1,062 | Dem: 165; AD: 81 | Age, APOE ε4, diabetes, NSAIDs, aspirin, smoking, BMI, hypertension, cholesterol, stroke, education |
| Sundelof et al. (17) (Sweden) | Cohort | 77.5±0.8 | 0 | 4.8 | 749 | Dem: 70; AD: 46 | Age, APOE ε4, diabetes, NSAIDs, aspirin, smoking, BMI, hypertension, cholesterol, stroke, education |
| Eriksson et al. (15) (Sweden) | Nested case–control | 78.5 | 61.7 | 4.3 | 634 | Dem: —; AD: 91 | Age, gender |
Notes: Sundelof et al. is included twice as results were reported for two separate subcohorts. AD = Alzheimer’s disease; APOE ε4 = apolipoprotein ε4; BMI = body mass index; CVD = cardiovascular disease; Dem = dementia; NSAID = nonsteroidal anti-inflammatory drug.
The majority of studies measured both all-cause dementia and AD as cognitive outcomes. Two studies measured AD alone. For diagnosis of these outcomes, all studies used DSM-III-R (19) or DSM-IV criteria (20) for all-cause dementia and NINCDS-ADRDA criteria for AD (21).
CRP
Figure 2 shows the five studies that investigated the association between CRP and all-cause dementia (11,14,16,17). All studies reported HRs more than 1.0, although most were modest in magnitude and only one reached statistical significance. Collectively, the studies suggested a significant increased risk for higher levels of CRP (HR: 1.45; 95% CI: 1.10, 1.91). One study with a substantially longer follow-up period and younger baseline age reported a particularly strong association (HR: 2.80; 95% CI: 1.57, 5.00), potentially leading to heterogeneity. However, the I 2 test for heterogeneity was not significant (I 2 = 39%; p = .16).
Figure 2.
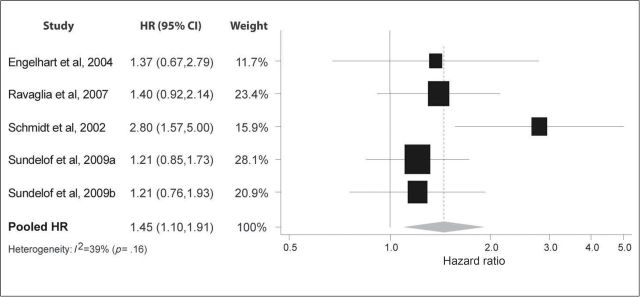
Pooled hazard ratios for C-reactive protein and incident dementia.
For AD alone, seven studies reported HRs that varied from 0.77 to 2.20 (Figure 3). Six reported a trend for an increased risk (11,14–18), and one a trend for a decreased risk (17). The pooled results indicated a modest increased risk (HR: 1.21; 95% CI: 1.03, 1.42). No heterogeneity was found (I 2 = 0%; p = .65).
Figure 3.
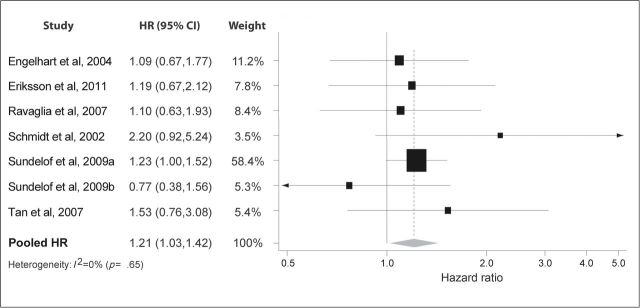
Pooled hazard ratios for C-reactive protein and incident Alzheimer’s disease.
The pooled HR changed from a 45% increased risk for all-cause dementia to 21% for AD, a difference likely attributable to the cases of vascular dementia (VaD) among those with all-cause dementia. Indeed, the three studies that published HRs for VaD showed a more robust effect than for either all-cause dementia or AD alone (Schmidt et al.; HR: 5.1; 95% CI: 1.8–14.8 and Ravaglia et al.; HR: 2.93; 95% CI: 1.39–6.18). Engelhart and colleagues reported a HR per standard deviation increase of 1.31 (1.06–1.61), also higher than the equivalent measures for all-cause dementia (HR: 1.12; 95% CI: 0.99–1.25) or AD (HR: 1.10; 95% CI: 0.96–1.26).
When repeating meta-analysis using additionally adjusted models, the association between CRP and AD decreased slightly in magnitude, and was no longer significant (HR: 1.17; 95% CI: 0.97, 1.41). For all-cause dementia, there was little change in the magnitude or significance of the pooled result (HR: 1.38; 95% CI: 1.12, 1.71). One study consistently had the largest effects as well as a considerably longer follow-up time of 25 years (16). Although removing this study attenuated the pooled HR for both outcomes, it remained significant.
IL-6
In Figure 4, four studies report fairly consistent results on the association between IL-6 and all-cause dementia (11,14,17). Individually, each study reported a HR more than 1.0, although only one was statistically significant. Together, the results suggested a positive association between IL-6 level and dementia risk (HR: 1.32; 95% CI: 1.06, 1.64), with little evidence of heterogeneity (I 2 = 0%; p = .85).
Figure 4.
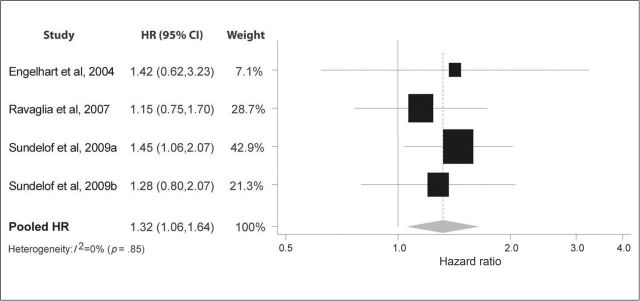
Pooled hazard ratios for interleukin-6 and incident dementia.
Figure 5 depicts six studies less consistent on the association between IL-6 and AD (11,14,15,17,18). Three studies show a trend towards an increased risk of AD, two a trend towards a decreased risk, and one study had null findings. Combined, the studies did not show a significant effect (HR: 1.06; 95% CI: 0.83, 1.35). Heterogeneity was also nonsignificant (I 2 = 0%: p = .78).
Figure 5.
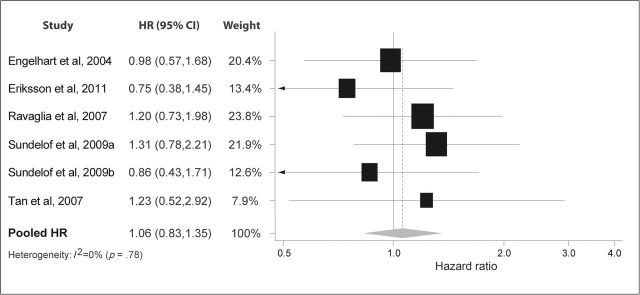
Pooled hazard ratios for interleukin-6 and incident Alzheimer’s disease.
For IL-6, only two studies reported HRs for VaD that could be compared with the other outcomes. Ravaglia and colleagues reported a HR for VaD slightly higher than for all-cause dementia or AD (HR: 1.40; 95% CI: 0.66, 2.90). Engelhart and colleagues reported a HR per standard deviation increase for VaD (HR: 1.23; 95% CI; 0.97–1.65) similar to the HR for all-cause dementia of (HR: 1.28; 95% CI: 1.06–1.55).
When conducting the meta-analysis on additionally adjusted models, there was little change in the association between IL-6 and AD. Similar sensitivity analysis was not done for all-cause dementia as all the included studies reported results from only one model.
Discussion
Although many of the individual studies did not report significant findings, the pooled results showed a significant increased risk of dementia for individuals with higher level of CRP and IL-6. For AD alone, there was no significant risk associated with higher IL-6 level, and the increased risk for higher CRP level was not robust to fully adjusted models used for the researchers’ sensitivity analysis.
Although the researchers’ results suggest an association between peripheral inflammatory markers and dementia, it is possible that the findings are due to the complex and overlapping relationship between inflammatory markers and CVD. For example, consistent evidence indicates that peripheral CRP levels can predict CVD (22), which can in turn act as an intermediary and contribute to the cerebrovascular pathology seen in dementia (23). CVD may also be contributing to unmeasured or residual confounding. Indeed, the studies that reported multiple models typically excluded CVD in multivariate-adjusted models but not in additionally adjusted models, when the pooled effect was attenuated. However, it should be noted that, when taking the broader perspective of cerebrovascular pathology, the additionally adjusted models may be overadjusting for factors that lie within the causal pathway.
Although few studies investigated VaD alone, the association between inflammatory markers, particularly CRP, was generally more robust with VaD than with all-cause dementia or AD alone. Individuals with VaD likely had a high prevalence of symptomatic CVD, contributing to the association between inflammatory markers and incident VaD. However, VaD alone cannot explain the researchers’ findings, as AD comprises the majority of dementia cases. Inflammation likely contributes to cognitive impairment in non-VaD dementias through similar cerebrovascular pathology. Functional imaging studies have shown that cerebrovascular dysfunction in individuals with mild cognitive impairment can precede progression to AD (24,25), emphasizing the vascular role in the pathology of non-VaD dementias. Vascular pathology is also frequently seen in postmortem examination of individuals with different forms of dementia (26).
Randomized clinical trials (RCTs) of NSAIDs can provide additional insight into the relationship between inflammation and dementia, while avoiding some of the limitations of observational studies. Several RCTs have tested the use of NSAIDs for the treatment of prevalent AD, with one early trial showing promising results (27), but subsequent RCTs unable to replicate these findings (28–32). To date, only one RCT has investigated NSAID use for the primary prevention of AD (2). The results indicated that NSAIDs had a detrimental effect on participants who developed AD early in the trial, and a protective effect on those who developed AD later in the trial. Thus, the collective findings from these RCTs suggest that NSAIDs may provide a protective effect on cognitive function early in the disease process, although are not protective or can even be harmful in the later stages of incipient disease. In the researchers’ review of observational studies, cognitively normal individuals with increased systemic inflammation, who may have been in the early stages of dementia, were found to have a higher risk of dementia.
This systematic review and meta-analysis have several strengths and limitations. Strengths include the comprehensive search methodology and exclusive use of high quality studies. In addition, few studies have investigated the effect of inflammation on cognition in the oldest old, and it is possible that there is a different association between inflammation and dementia in the young–old and the oldest old. There may also be a gender effect similar to that seen in other diseases such as CVD, where a stronger association with inflammatory markers is reported in women compared with men (33). In the meta-analysis of CRP on risk of incident AD, one study had a particularly large weight (17), potentially affecting the generalizability of results if study quality was poor. However, the researchers’ search criteria restricted the meta-analysis to only high quality studies (this particular study was rated an 8 on the 9-point modified Newcastle–Ottawa scale). Lastly, as the I 2 statistic can be underpowered with a small sample, it is possible that heterogeneity is present but undetected (34).
Important limitations inherent in observational studies can also affect the researchers’ ability to infer any causal relationship between inflammatory markers and dementia. Unmeasured or residual confounding can result if the researchers do not adequately adjust for the many factors associated with inflammation and dementia. Misclassification of the exposure is also another limitation as inflammatory marker levels can be influenced by other diseases not related to dementia, an acute inflammatory response, or even because of the normal aging process (35). Additionally, the studies measured peripheral markers in the blood rather than within the brain. Therefore, results from these studies may not reflect intracerebral inflammatory processes if peripheral levels of inflammatory markers do not correlate with levels seen in the brain. Misclassification of the outcome is another possibility. In each study, participants classified as normal passed initial screening tests, whereas those classified as demented underwent additional assessment using formal diagnostic criteria. However, this method of classification would make it more likely to classify normal participants as demented rather than the reverse, biasing the results toward the null. Reverse causation is another potential limitation as increased inflammatory levels could be both a cause and a consequence of dementia. The majority of studies measured the exposure later in life, when inflammatory marker levels could be influenced by preclinical or undiagnosed dementia. Only one study measured inflammatory markers at midlife and reported the most robust results, suggesting that midlife measurements may be a better indicator of future dementia risk (16).
In conclusion, this meta-analysis provides suggestive evidence of an association between increased peripheral levels of inflammatory markers and increased risk of incident dementia. To confirm the findings of this meta-analysis, future observational studies should emphasize earlier baseline assessment in midlife. Similarly, additional RCTs of NSAIDs should focus on the prevention rather than the treatment of dementia.
Funding
This work was supported by the National Institute of Aging (K24 AG 031155, R01 AG 026720) and the Alzheimer’s Association (IIRG-08-88872).
References
- 1. Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011; 7: 208–244. 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2. Breitner JC, Baker LD, Montine TJ, et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011; 7: 402–411. 10.1016/j.jalz.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorelick PB. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci. 2010; 1207: 155–162. 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- 4. Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, van Gool WA. Neuroinflammation - an early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener Dis. 2010; 7: 38–41. 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 5. Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004; 363: 1139–1146. 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 6. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001; 286: 327–334 [DOI] [PubMed] [Google Scholar]
- 7. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283: 2008–2012 [DOI] [PubMed] [Google Scholar]
- 8. Weuve J, McQueen MB, Blacker D. The AlzRisk Database Alzheimer Research Forum; http://www.alzrisk.org/Updated August 12, 2011. Accessed September 9, 2011. [Google Scholar]
- 9. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Proceedings of the Third Symposium on Systematic Reviews Beyond the Basics: Improving Quality and Impact; 2000; Oxford, UK [Google Scholar]
- 10. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992; 135: 1301–1309 [DOI] [PubMed] [Google Scholar]
- 11. Ravaglia G, Forti P, Maioli F, et al. Blood inflammatory markers and risk of dementia: the Conselice Study of Brain Aging. Neurobiol Aging. 2007; 28: 1810–1820. 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 12. Haan MN, Aiello AE, West NA, Jagust WJ. C-reactive protein and rate of dementia in carriers and non carriers of apolipoprotein APOE4 genotype. Neurobiol Aging. 2008; 29: 1774–1782. 10.1016/j.neurobiolaging.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7: 177–188 [DOI] [PubMed] [Google Scholar]
- 14. Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004; 61: 668–672. 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 15. Eriksson UK, Pedersen NL, Reynolds CA, et al. Associations of gene sequence variation and serum levels of C-reactive protein and interleukin-6 with Alzheimer’s disease and dementia. J Alzheimers Dis. 2011; 23: 361–369. 10.3233/JAD-2010-101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002; 52: 168–174. 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 17. Sundelof J, Kilander L, Helmersson J, et al. Systemic inflammation and the risk of Alzheimer’s disease and dementia: a prospective population-based study. J Alzheimers Dis. 2009; 18: 79–87. 10.3233/JAD-2009-1126. [DOI] [PubMed] [Google Scholar]
- 18. Tan ZS, Beiser AS, Vasan RS, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007; 68: 1902–1908. 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 19. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. 3rd ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 20. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984; 34: 939–944 [DOI] [PubMed] [Google Scholar]
- 22. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003; 107: 363–369 [DOI] [PubMed] [Google Scholar]
- 23. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42: 2672–2713. 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirao K, Ohnishi T, Hirata Y, et al. The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage. 2005; 28: 1014–1021. 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 25. Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003; 30: 1104–1113. 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 26. Kovacs GG, Alafuzoff I, Al-Sarraj S, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord. 2008; 26: 343–350. 10.1159/000161560. [DOI] [PubMed] [Google Scholar]
- 27. Rogers J, Kirby LC, Hempelman SR, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993; 43: 1609–1611 [DOI] [PubMed] [Google Scholar]
- 28. Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003; 289: 2819–2826. 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 29. Pasqualetti P, Bonomini C, Dal Forno G, et al. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin Exp Res. 2009; 21: 102–110 [DOI] [PubMed] [Google Scholar]
- 30. Reines SA, Block GA, Morris JC, et al. Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004; 62: 66–71 [DOI] [PubMed] [Google Scholar]
- 31. Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology. 2002; 58: 1050–1054 [DOI] [PubMed] [Google Scholar]
- 32. Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology. 1999; 53: 197–201 [DOI] [PubMed] [Google Scholar]
- 33. Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997; 17: 1121–1127 [DOI] [PubMed] [Google Scholar]
- 34. Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index?. Psychol Methods. 2006; 11: 193–206. 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 35. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004; 39: 687–699. 10.1016/j.exger.2004.01.009 [DOI] [PubMed] [Google Scholar]