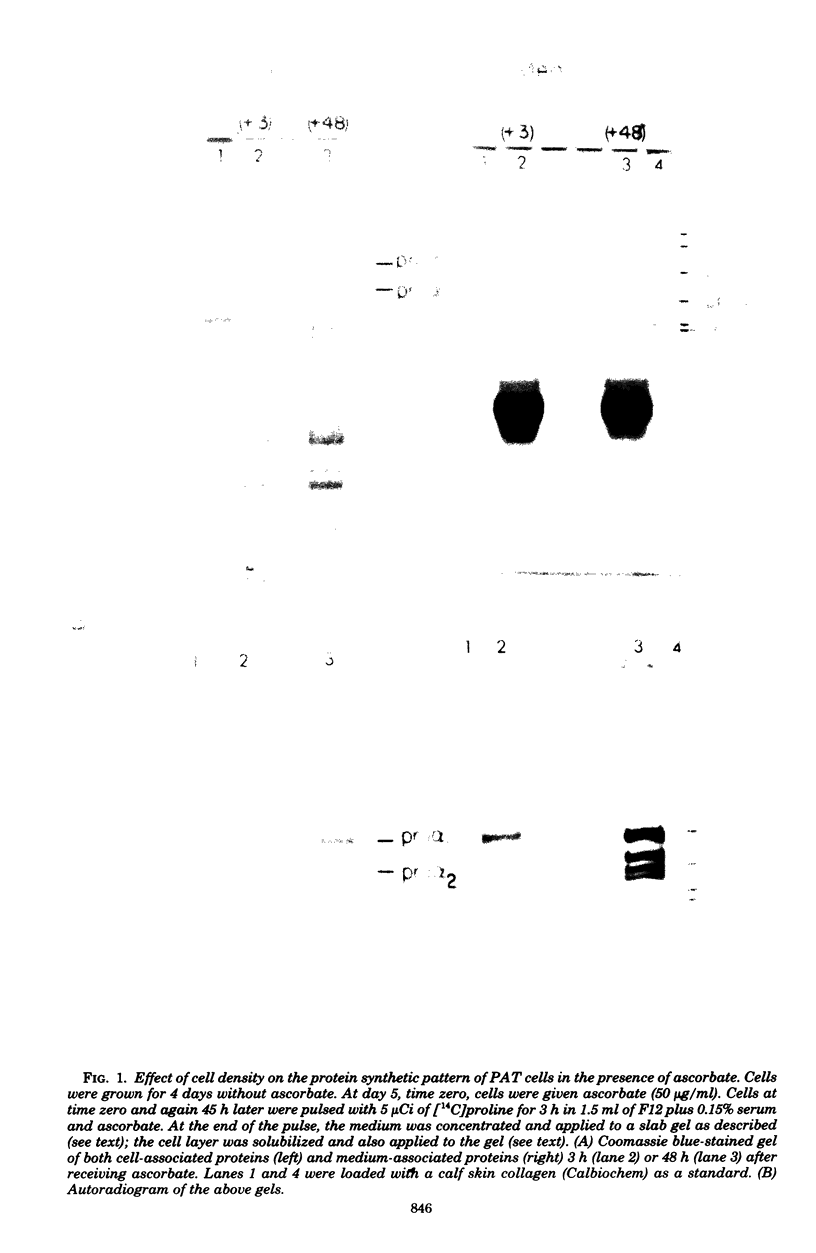
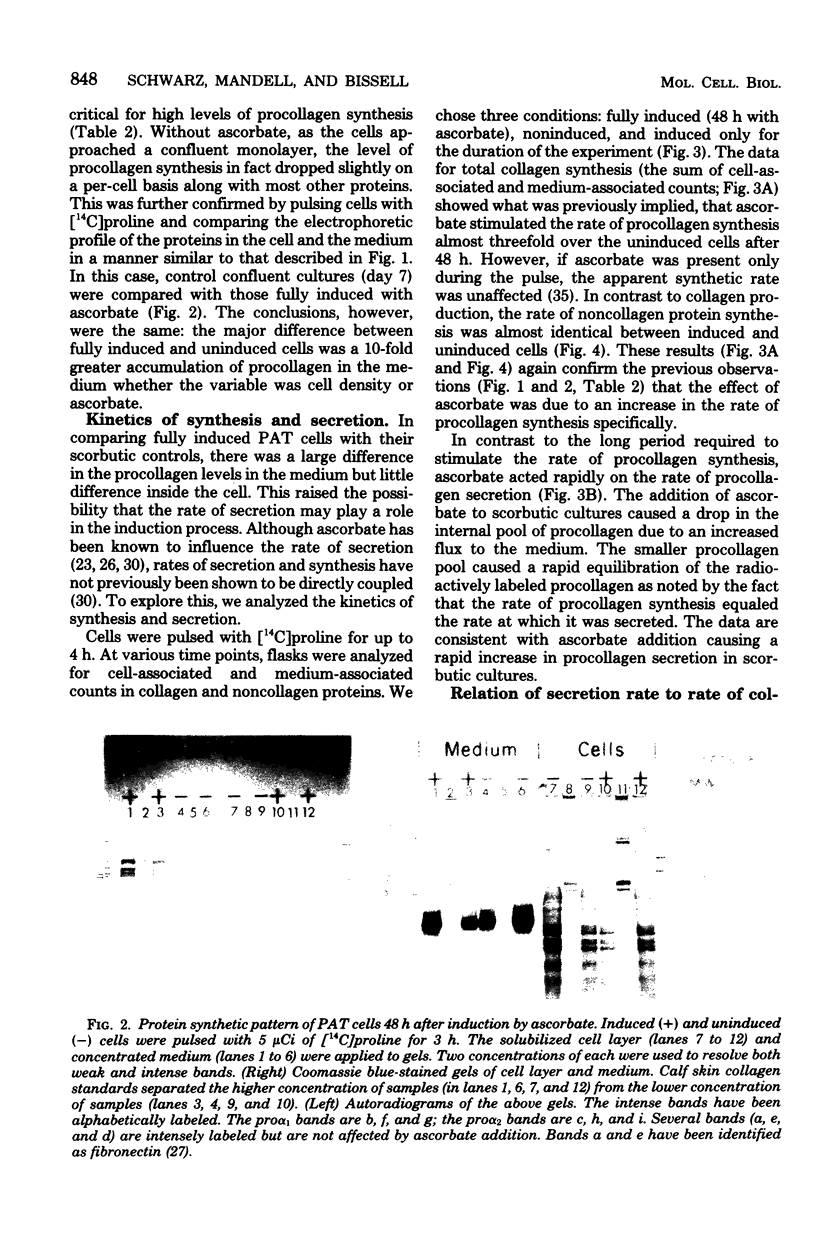
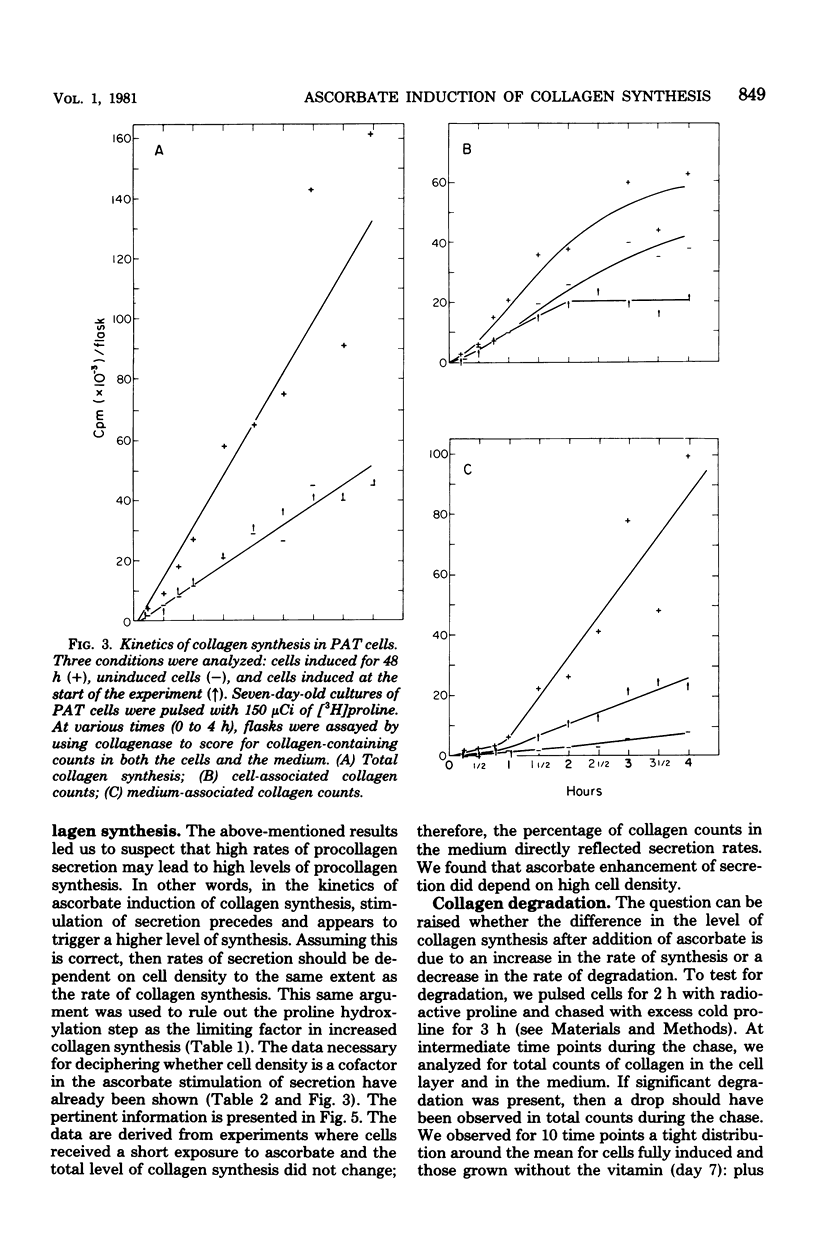
Abstract
Ascorbic acid displays the characteristics of an ideal inducer of tissue-specific function in primary avian tendon cells in culture. It is a highly specific, potent stimulator of collagen synthesis, it demonstrates slow reversible kinetics, and it has no effect on growth rate of the cultured cells. Kinetic analysis of ascorbate induction of collagen synthesis was used to determine the critical steps in this complex biosynthetic pathway. Full hydroxylation of the proline residues in collagen, although probably a necessary step for collagen induction, was in itself not sufficient for achieving either increased secretion or increased synthesis. On the other hand, an increase in secretion rate, which required both the presence of ascorbate and a high cell density, did correlate with the later stimulation in procollagen production. The process of procollagen secretion, therefore, meets the minimal requirements for the rate-limiting step. The fact that the cells maintained a large pool of intracellular procollagen despite changes in the rates of translation or secretion led us to postulate a possible feedback between the level of the internal procollagen pool and the rate of procollagen synthesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Kelley J., Brayden J. E., Low R. B., Stirewalt W. S. An ultramicro method of amino acid analysis: application to studies of protein metabolism in cultured cells. Anal Biochem. 1979 Jul 1;96(1):45–55. doi: 10.1016/0003-2697(79)90552-9. [DOI] [PubMed] [Google Scholar]
- Barnes M. J. Function of ascorbic acid in collagen metabolism. Ann N Y Acad Sci. 1975 Sep 30;258:264–277. doi: 10.1111/j.1749-6632.1975.tb29287.x. [DOI] [PubMed] [Google Scholar]
- Bates C. J., Bailey A. J., Prynne C. J., Levene C. I. The effect of ascorbic acid on the synthesis of collagen precursor secreted by 3T6 mouse fibroblasts in culture. Biochim Biophys Acta. 1972 Sep 29;278(2):372–390. doi: 10.1016/0005-2795(72)90241-3. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S., Baum B. J., Crystal R. G. Fibroblasts degrade newly synthesised collagen within the cell before secretion. Nature. 1978 Nov 23;276(5686):413–416. doi: 10.1038/276413a0. [DOI] [PubMed] [Google Scholar]
- Bissell M. J. The differentiated state of normal and malignant cells or how to define a "normal" cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- Blanck T. J., Peterkofsky B. The stimulation of collagen secretion by ascorbate as a result of increased proline hydroxylation in chick embryo fibroblasts. Arch Biochem Biophys. 1975 Nov;171(1):259–267. doi: 10.1016/0003-9861(75)90031-4. [DOI] [PubMed] [Google Scholar]
- Boone C. W. Malignant hemangioendotheliomas produced by subcutaneous inoculation of Balb/3T3 cells attached to glass beads. Science. 1975 Apr 4;188(4183):68–70. doi: 10.1126/science.1114343. [DOI] [PubMed] [Google Scholar]
- Breul S. D., Bradley K. H., Hance A. J., Schafer M. P., Berg R. A., Crystal R. G. Control of collagen production by human diploid lung fibroblasts. J Biol Chem. 1980 Jun 10;255(11):5250–5260. [PubMed] [Google Scholar]
- DAVIDSON E. H. DIFFERENTIATION IN MONOLAYER TISSUE CULTURE CELLS. Adv Genet. 1964;12:143–280. doi: 10.1016/s0065-2660(08)60416-2. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Collagen biosynthesis during connective tissue development in chick embryo. Dev Biol. 1972 Jul;28(3):443–453. doi: 10.1016/0012-1606(72)90028-0. [DOI] [PubMed] [Google Scholar]
- Evans C. A., Peterkofsky B. Ascorbate-independent proline hydroxylation resulting from viral transformation of Balb 3T3 cells and unaffected by dibutyryl cAMP treatment. J Cell Physiol. 1976 Nov;89(3):355–367. doi: 10.1002/jcp.1040890302. [DOI] [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Green H., Todaro G. J., Goldberg B. Collagen synthesis in fibroblasts transformed by oncogenic viruses. Nature. 1966 Feb 26;209(5026):916–917. doi: 10.1038/209916a0. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörlein D., Fietzek P. P., Wachter E., Lapière C. M., Kühn K. Amino acid sequence of the aminoterminal segment of dermatosparactic calf-skin procollagen type I. Eur J Biochem. 1979 Aug 15;99(1):31–38. doi: 10.1111/j.1432-1033.1979.tb13227.x. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Harsch M., Murphy L., Rosenbloom J. Effects of temperature on conformation, hydroxylation, and secretion of chick tendon procollagen. J Biol Chem. 1974 Jul 25;249(14):4480–4486. [PubMed] [Google Scholar]
- Jimenez S., Harsch M., Rosenbloom J. Hydroxyproline stabilizes the triple helix of chick tendon collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):106–114. doi: 10.1016/0006-291x(73)90960-1. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Ascorbate increases the synthesis of procollagen hydroxyproline by cultured fibroblasts from chick embryo tendons without activation of prolyl hydroxyla. Biochim Biophys Acta. 1975 Dec 5;411(2):202–215. doi: 10.1016/0304-4165(75)90300-1. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J Biol Chem. 1977 Dec 10;252(23):8391–8397. [PubMed] [Google Scholar]
- Kao W. W., Prockop D. J., Berg R. A. Kinetics for the secretion of nonhelical procollagen by freshly isolated tendon cells. J Biol Chem. 1979 Apr 10;254(7):2234–2243. [PubMed] [Google Scholar]
- Leung M. K., Fessler L. I., Greenberg D. B., Fessler J. H. Separate amino and carboxyl procollagen peptidases in chick embryo tendon. J Biol Chem. 1979 Jan 10;254(1):224–232. [PubMed] [Google Scholar]
- Levene C. I., Bates C. J. Ascorbic acid and collagen synthesis in cultured fibroblasts. Ann N Y Acad Sci. 1975 Sep 30;258:288–306. doi: 10.1111/j.1749-6632.1975.tb29289.x. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Lukens L. N. The role of hydroxylation in the secretion of collagen by mouse fibroblasts in culture. Arch Biochem Biophys. 1971 Dec;147(2):612–618. doi: 10.1016/0003-9861(71)90419-x. [DOI] [PubMed] [Google Scholar]
- Parry G., Soo W. J., Bissell M. J. The uncoupled regulation of fibronectin and collagen synthesis in Rous sarcoma virus transformed avian tendon cells. J Biol Chem. 1979 Dec 10;254(23):11763–11766. [PubMed] [Google Scholar]
- Paz M. A., Gallop P. M. Collagen synthesized and modified by aging fibroblasts in culture. In Vitro. 1975 Sep-Oct;11(5):302–312. doi: 10.1007/BF02615641. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. B. Increased collagen synthesis in Kirsten sarcoma virus-transformed BALB 3T3 cells grown in the presence of dibutyryl cyclic AMP. Cell. 1974 Nov;3(3):291–299. doi: 10.1016/0092-8674(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. Regulation of collagen secretion by ascorbic acid in 3T3 and chick embryo fibroblasts. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1343–1350. doi: 10.1016/0006-291x(72)90614-6. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Schwarz R. I., Bissell M. J. Dependence of the differentiated state on the cellular environment: modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. I., Farson D. A., Bissell M. J. Requirements for maintaining the embryonic state of avian tendon cells in culture. In Vitro. 1979 Dec;15(12):941–948. doi: 10.1007/BF02619153. [DOI] [PubMed] [Google Scholar]
- Schwarz R. I., Farson D. A., Soo W. J., Bissell M. J. Primary avian tendon cells in culture. An improved system for understanding malignant transformation. J Cell Biol. 1978 Dec;79(3):672–679. doi: 10.1083/jcb.79.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R., Colarusso L., Doty P. Maintenance of differentiation in primary cultures of avian tendon cells. Exp Cell Res. 1976 Oct 1;102(1):63–71. doi: 10.1016/0014-4827(76)90299-8. [DOI] [PubMed] [Google Scholar]
- Scott P. G., Telser A. G., Veis A. Semiquantitative determination of cyanogen bromide peptides of collagen in SDS-polyacrylamide gels. Anal Biochem. 1976 Jan;70(1):251–257. doi: 10.1016/s0003-2697(76)80065-6. [DOI] [PubMed] [Google Scholar]