Abstract
We recently demonstrated the anti-tumor efficacy of orally administered alpha-tocopheryloxyacetic acid (α-TEA), a redox silent and non-hydrolysable derivative of naturally occurring vitamin E. In order to move α-TEA closer to the clinic to benefit breast cancer patients, the present study had two goals. First to determine the minimal effective treatment dose and second to test the efficacy of dietary administration of α-TEA in the clinically relevant MMTV-PyMT mouse model of spontaneous breast cancer that more closely resembles human disease.
The minimal effective dose of α-TEA was evaluated in the transplantable 4T1 tumor model and we demonstrate a dose-dependent decrease of primary tumor growth and reduction of metastatic spread to the lung. MMTV-PyMT mice were treated with oral α-TEA starting at six weeks of age for nine weeks with no apparent signs of drug toxicity. The α-TEA treatment delayed tumor development and significantly slowed tumor progression, resulting in a 6-fold reduction of the average cumulative tumor size. In addition, oral α-TEA caused an 80% reduction in spontaneous metastases. In situ analysis of tumor tissue identified apoptosis as an important mechanism of α-TEA-mediated tumor suppression in addition to inhibition of tumor cell proliferation.
This study demonstrates, for the first time, the ability of orally administered α-TEA to delay tumor onset and to inhibit the progression and metastatic spread of a clinically relevant model of spontaneous breast cancer. Our finding of the high efficacy in this tumor model highlights the translational potential of oral α-TEA therapy.
Keywords: vitamin E analog, alpha-tocopheryloxyacetic acid, dietary administration, breast cancer
INTRODUCTION
Recently, we and others have demonstrated the anti-tumor efficacy of a novel class of chemotherapeutic drugs, the redox silent, semi-synthetic derivatives of naturally occurring vitamin E (1-3) that have been shown to exhibit selective toxicity toward tumor cells (1, 4-7). This novel drug class is epitomized by vitamin E succinate (α-tocopheryl succinate, α-TOS) and α-tocopheryloxyacetic acid (α-TEA). Both derivatives structurally share the phytyl-tail and the chroman head with vitamin E. However, the hydroxyl group at the number 6 carbon of the phenolic ring of the chroman head is replaced by an acid residue (8) that confers the anti-tumor activities (1, 2, 5, 7, 9-11). Alpha-TEA is of particular interest to us because, unlike α-TOS, α-TEA is non-hydrolysable and therefore can be administered through the more clinically relevant oral route. In order to move α-TEA closer to the clinic to be used as a new treatment option benefiting breast cancer patients, the present study had two main goals. The first goal was to determine the minimal effective dose of α-TEA. For this purpose α-TEA was incorporated into mouse chow at different concentrations and was fed to mice bearing established transplantable 4T1 tumors. We demonstrate a dose-dependent decrease of primary tumor growth and reduction of metastatic spread to the lung.
The second goal was to test the efficacy of orally administered α-TEA in a more clinically relevant murine model of spontaneously-arising breast. Although, transplantable tumor models are valuable tools in cancer research, they do not fully recapitulate human cancer etiology as they lack the physiologically relevant host and tumor microenvironments that influence spontaneous tumor progression. For this reason, we employed the transgenic MMTV-PyMT mouse model of spontaneous breast cancer to test the efficacy of α-TEA in the endogenously appropriate setting. The MMTV-PyMT mice carry the polyoma middle T oncogene driven by the mouse mammary tumor virus (MMTV) promoter (12). These mice rapidly develop multifocal mammary adenocarcinoma involving the entire mammary pad with secondary metastasis to the lung (12). MMTV-PyMT mice received a low dose of 2 mg α-TEA per day starting at 6 weeks of age until 15 weeks of age when the mice on the control diet were sacrificed due to large tumor burden. Our results show, that α-TEA treatment delayed the development of palpable tumors by more than two weeks and significantly (p<0.0001) slowed tumor progression, resulting in 85% reduction of the average cumulative tumor area, and inhibiting lung metastases by 80%. In addition, the orally administered α-TEA resulted in a 50% reduction of the average number of tumors per mouse. We also determined that apoptosis is an important mechanism of α-TEA-mediated tumor suppression in vivo.
Together these results demonstrate, for the first time, the efficacy and ease of delivery of orally administered α-TEA against spontaneous murine breast cancer that more closely resembles human breast cancer. The dramatic anti-tumor impact of the lower α-TEA dose in this model may lay the foundation for clinical testing of α-TEA in humans.
MATERIALS AND METHODS
Preparation of α-tocopheryloxyacetic acid
Alpha-TEA [(2,5,7,8-tetramethyl-(2R-(4R,8R,12-trimethyltridecyl) chroman-6-yloxy) acetic acid)] was synthesized in-house using a combination of previously described methods (1, 13), and purity was confirmed by high-performance liquid chromatography and nuclear magnetic resonance analysis.
Tumor cells and cell culture
The 4T1 tumor cell line is a variant of 410.4, a tumor subline that was isolated from a spontaneous mammary tumor in a BALB/cfC3H mouse. The 4T1 tumor is poorly immunogenic and highly metastatic and spontaneously metastasizes to the liver, lungs, bone marrow and brain (14-16), a characteristic that is shared with human breast cancers. The tumor cells were maintained in IMDM (JRH Biosciences, Lenexa, KS), containing 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA), 0.75 μg/mL fungizone and 10% fetal bovine serum (both Gemini Bio-Products, Woodland, CA).
Animal studies
Six-to eight-week-old female BALB/c mice were purchased from the NCI Frederick facility (Frederick, MD) and the MMT-PyMT transgenic mice (FVB/N-Tg(MMTV-PyVT)634Mul/J) (17) were purchased from the University of Arizona Cancer Center Experimental Mouse Shared Services. All mice were housed at the University of Arizona Animal Facilities in accordance with the Principles of Animal Care (NIH publication No. 85-23) and all studies were approved by the University of Arizona Institutional Animal Use Committee. For the transplantable tumor model studies, 5 × 104 viable 4T1 breast cancer cells were injected sub-cutaneously (s.c.) into the right mammary fat pad of BALB/c mice. The mice received control diet until tumor establishment (day 10 post tumor-implantation, ~17 mm2) and were then switched to mouse chow containing different amounts of α-TEA. For the spontaneous tumor model, MMT-PyMT transgenic mice received control diet until 6 weeks of age (day 42) and were then fed α-TEA chow. Tumor growth was monitored by measuring the tumor length (L) and width (W) using calipers and calculating the tumor area as: A = (L × W). Visible metastatic lung nodules were enumerated by staining with India ink and Fekete’s solution (14). Alpha-TEA was incorporated into the AIN93G diet by Harlan Teklad (Madison, WI) at a concentration of 3 g α-TEA per kg chow (0.3%), 1 g/kg chow (0.1%), 0.5 g/kg chow (0.05%) and 0.25 g/kg chow (0.025%).
Histopathology of mammary glands from MMTV-PyMT mice
MMTV-PyMT mice received 0.1% α-TEA diet (~2 mg/day) starting at 6 weeks of age for 4 weeks (until 10 weeks of age) when mice were sacrificed. Mammary glands were dissected and whole mounts prepared. Briefly, the tissues were dried flat on a microscope slide, fixed and stained with carmine red (18) and examined under a microscope (Nikon TE-2000S) at 20x magnification. At the same time tissues were fixed in 10% buffered formalin and paraffin-embedded, and 5 μm sections were stained with hematoxylin and eosin (H&E) and evaluated by a veterinary pathologist based upon the system described by Lin et al. (19) and guidelines for mouse models of mammary cancer described by Cardiff et al. (20). Apoptosis and cell proliferation were determined on deparaffinized 5 μm sections by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and using an antibody specific for nuclear antigen Ki-67, respectively. The TUNEL assay was performed using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA) according to the manufacturer’s protocol. Detection of the anti-Ki-67 primary antibody was performed on a Discovery XT Automated Immunostainer (Ventana Medical Systems, Inc, Tucson, AZ). Antibodies were diluted in Discovery XT diluent and all staining was performed using VMSI validated reagents, including streptavidin-HRP and diaminobenzoate. Because the malignant tissues in the α-TEA treated-mice, particularly at the early time point (day 70), were much smaller and less contiguous in comparison to the tissues from untreated mice, microscopic fields were only scored from solid cell sheets within the sections to remove bias toward lower numbers in the α-TEA-treated groups. Fluorescein TUNEL-stained nuclei were scored as positive for apoptosis in 15 microscopic fields (200x magnification, Nikon TE-2000S) per section. Brown-stained Ki-67 positive cells were counted in 15 microscopic fields (400x magnification, Nikon TE-2000S) per section.
Determination of α-TEA levels
Sera and tissues were analyzed for α-TEA content by high performance liquid chromatography with mass spectrometric detection (HPLC/MSD). Blood was collected by terminal heart puncture and serum was isolated using microtainer serum separator tubes (BD, Franklin Lakes, NJ) and stored at −80°C. Tissue samples were snap frozen and stored in liquid nitrogen until analysis. Tissues (10 mg) were minced and digested in 15 mg/mL collagenase (Worthington Biochemical Corporation, Lakewood, NJ) and 6 mg/mL pronase E (Sigma-Aldrich, St. Louis, MO) at 37°C for 1h in 300 μL PBS. Subsequently, 300 μL 1% SDS was added and the tissue samples were homogenized by repeated pulling through a 21-g needle.
For HPLC/MSD detection of α-TEA, a 50 μL aliquot of serum or tissue digest was added to a capped 15-mL polypropylene centrifuge tube containing 200 μL of ice cold ethanol. The tubes were vortex-mixed briefly and allowed to stand for 5 min. The volume of each sample was brought up to 1.0 mL with saline and extracted with hexane:dichloromethane, 95/5 (v/v) by vortex-mixing. The samples were then centrifuged to facilitate separation of the two phases. The lower aqueous layer was frozen in an acetone–dry ice bath, and the upper organic layer was carefully decanted into a clean capped polypropylene 15-mL centrifuge tube. The organic extract was dried at room temperature under a gentle stream of nitrogen and then reconstituted with 100 μL of the mobile phase used to analyze the samples. Spiked drug-free serum samples were prepared in the same manner and were used to prepare a calibration curve. An injection of 2.0 to 5.0 μL was used for each analysis. The chromatographic system was an Agilent LC/MSD 1100 using atmospheric pressure electrospray ionization (AP-ESI) in negative ion mode. A C18 column with a mobile phase of acetonitrile:methanol:glacial acetic acid, 70/30/0.25 (v/v/v) at a flow rate of 0.5 mL/min was used for the chromatographic separation. Calibration curves, from 1.0 to 50 μg/mL, were generated by least squares quadratic curvilinear regression and had correlation coefficients (r2) ≥ 0.997.
Statistical analysis
Statistical significance of differences among data sets of treatment groups was assessed either by Student’s t-test, where applicable, or by one-way analysis of variances (ANOVA), including Tukey-Kramer post tests for multiple comparisons. To compare tumor growth rates, growth curves were transformed to linearity, and linear regression analysis was used to determine slopes that were then compared by t-test. To compare average cumulative tumor growth in the MMTV-PyMT model, growth curves were determined by non-linear regression analysis and statistically evaluated for difference by f-test. Differences of the mean number of lung metastases were evaluated by Mann-Whitney test. All analyses were performed using Prism software (GraphPad, San Diego, CA). Probability values (p) of ≤ 0.05 were considered indicative of significant differences between data sets
RESULTS
Orally administered α-TEA inhibits growth and spread of established transplantable 4T1 mammary tumors in a dose-dependent manner
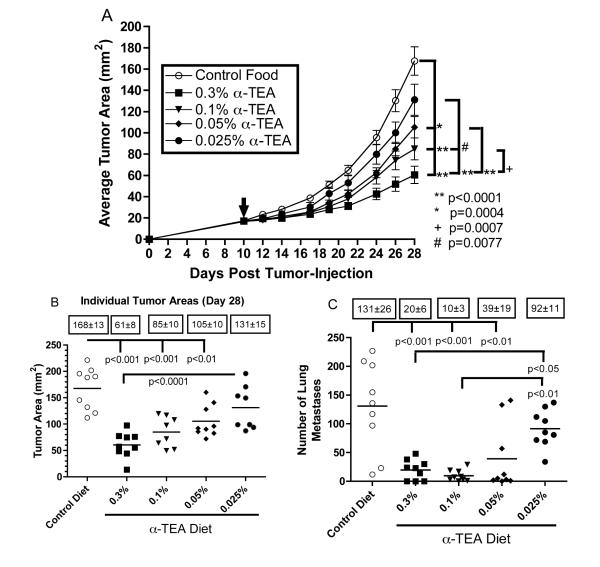
Prior to testing the efficacy of α-TEA in the more clinically relevant MMTV-PyMT mouse model of spontaneous breast cancer, we wanted to assess the minimal effective dose of α-TEA against transplantable, poorly immunogenic and highly metastatic 4T1 murine mammary cancer. Because we had already demonstrated that the relatively high dose of 6 mg α-TEA per day reduced primary tumor burden and metastatic spread in this tumor model (2), 6 mg α-TEA per day was chosen as the highest dose (0.3% α-TEA diet). Mice were injected with 4T1 tumor cells and received control diet until day 10 when tumors became palpable (~17 mm2). The mice were then transferred to mouse chow containing different doses of α-TEA (0.3%, 0.1%, 0.05%, 0.025%) for 18 days. Figure 1A shows that all doses of dietary α-TEA, except for the lowest dose (0.025%), resulted in significant and dose-dependent reductions in primary tumor growth rate and tumor size. Our results also show that while the highest α-TEA dose tested (0.3%) achieved a 64% reduction in final tumor size, the 0.1% diet still caused a 50% reduction in tumor size (Figure 1B). Even the 0.05% diet caused a moderate, but statistically significant, 38% reduction in tumor size. Not only did the oral α-TEA treatment slow down tumor growth, the 0.3%, 0.1% and 0.05% α-TEA diet also significantly reduced metastatic spread of the primary tumor to the lung by 85%, 92% and 71%, respectively (Figure 1C). Similar to the impact on primary tumors, the lowest dose tested (0.025% α-TEA diet) failed to significantly decrease lung metastases. Interestingly, in comparison to the highest dose tested (0.3%), the 0.1% dose was as efficacious in suppressing lung metastases.
Figure 1. Effect of dietary delivery of α-TEA on primary tumor growth.
BALB/c mice were injected with 4T1 mammary tumor cells in the right mammary fat pad (day 0). When tumors became palpable ( ,~17 mm2, day 10 post-tumor cell injection), mice received α-TEA in the diet for 18 days. Untreated mice received control diet throughout the study. (A) The values represent the mean tumor areas ± SEM of 9 mice per group. To compare tumor growth rates, growth curves were transformed to linearity and linear regression analysis was used to determine slopes that were then compared by t-test. (B) The values represent tumor areas of individual mice on day 28 post-tumor injection. Boxed numbers are mean tumor areas ± SEM. Differences of the mean tumor areas were determined by ANOVA including Tukey-Kramer post tests for multiple comparisons. (C) Effect of dietary delivery of
α-TEA on tumor spread. BALB/c mice with implanted 4T1 mammary tumors from the above study were sacrificed on day 28 post-tumor cell injection. To determine the number of pulmonary metastases, lungs were inflated with India ink and removed, and the surface lung metastases were counted. Boxed numbers are mean number of lung metastases ± SEM. Differences of the mean number of lung metastases were determined by ANOVA including Tukey-Kramer post tests for multiple comparisons.
,~17 mm2, day 10 post-tumor cell injection), mice received α-TEA in the diet for 18 days. Untreated mice received control diet throughout the study. (A) The values represent the mean tumor areas ± SEM of 9 mice per group. To compare tumor growth rates, growth curves were transformed to linearity and linear regression analysis was used to determine slopes that were then compared by t-test. (B) The values represent tumor areas of individual mice on day 28 post-tumor injection. Boxed numbers are mean tumor areas ± SEM. Differences of the mean tumor areas were determined by ANOVA including Tukey-Kramer post tests for multiple comparisons. (C) Effect of dietary delivery of
α-TEA on tumor spread. BALB/c mice with implanted 4T1 mammary tumors from the above study were sacrificed on day 28 post-tumor cell injection. To determine the number of pulmonary metastases, lungs were inflated with India ink and removed, and the surface lung metastases were counted. Boxed numbers are mean number of lung metastases ± SEM. Differences of the mean number of lung metastases were determined by ANOVA including Tukey-Kramer post tests for multiple comparisons.
Determination of α-TEA levels in vivo
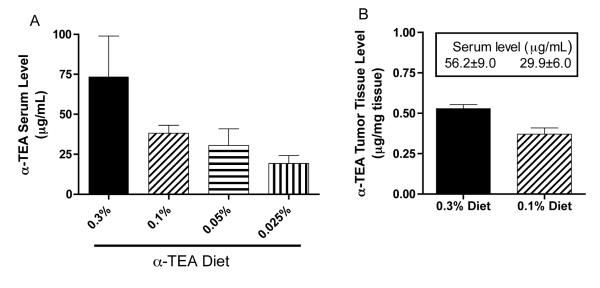
In order to correlate the clinical outcome with the actual α-TEA dose the mice received, food intake was monitored every two to three days throughout the treatment period and α-TEA serum levels were determined at the end of the study. The average food consumption among the different groups was comparable and ranged from 2.0 g chow per mouse per day (0.3% diet group) to 2.5 g chow per mouse per day (0.05% diet group), with the mice on the control diet eating an average of 2.2 g chow per mouse per day, suggesting that there is no aversion to α-TEA in the food. According to this food consumption profile, the mice on the 0.3%, 0.1%, 0.05% and 0.025% diets received an average daily α-TEA dose of 5.9 mg, 2.4 mg, 1.2 mg and 0.6 mg, respectively, that is equivalent to 295 mg, 122 mg, 62 mg, 28 mg α-TEA per kg bodyweight. To determine the actual level of circulating α-TEA, serum was collected at the end of the study (day 28 post tumor-injection) and analyzed by HPLC/MSD. Our results show a dose-dependent decrease in serum α-TEA levels ranging from 73.5 ± 25.4 μg/mL in the mice at the highest dose (0.3% diet) to 19.3 ± 4.9 μg/mL at the lowest dose (0.025% diet) (Figure 2A). Meanwhile, mice on the control diet had no detectable α-TEA levels (data not shown).
Figure 2. Determination of α-TEA serum and tissue levels.
(A) Tumor-bearing mice received diets containing indicated amounts of α-TEA for 18 days (day 10 to day 28 post-tumor injection). Subsequently, serum was isolated and α-TEA levels of 4 to 5 individual mice per group were analyzed by HPLC/MSD. (B) Tumor-bearing mice received the 0.3% or the 0.1% α-TEA diet for 8 days (day 18 post-tumor injection). Tumor tissues and sera were isolated, and α-TEA levels of three individual mice per group were analyzed by HPLC/MSD. Boxed numbers are corresponding average serum levels ± SEM.
It is self-evident that an anti-cancer therapeutic needs to reach its target in order to be successful in eradicating a tumor. Therefore, we were interested in how much α-TEA was present in tumor tissues during α-TEA therapy. Because we had determined that the 0.3% and 0.1% diets resulted in the most primary tumor growth inhibition, we measured the α-TEA levels that were achieved after mice received the 0.3% or the 0.1% diet for 8 days. Similar to the serum levels, α-TEA levels in the tumors were dose dependent and ranged from 0.53 ± 0.01 μg/mg tumor tissue (0.3% diet) to 0.37 ± 0.02 μg/mg tumor tissue (0.1% diet). Mice on the control diet had undetectable α-TEA levels (data not shown).
Low dose oral α-TEA treatment does not cause gross toxicity
Although vitamin E analogs including α-TEA have been shown to be preferentially toxic to malignant cells (1, 4-7) and we have shown in an earlier report (2) that mice on oral α-TEA therapy lacked signs of toxicity, we wanted to determine if the prolonged α-TEA treatment resulted in toxic effects. For this purpose, body weights of the mice were monitored every five to seven days throughout the study. On average, all groups, including the control group, had no significant weight change during the study except for the 0.3% diet group that had an overall weight loss of 12.2 ± 1.9%, which is just slightly higher than the 10% weight loss that is commonly used to estimate maximum tolerated drug doses in mice (21).
Oral administration of α-TEA inhibits growth and spread of spontaneous mammary tumors
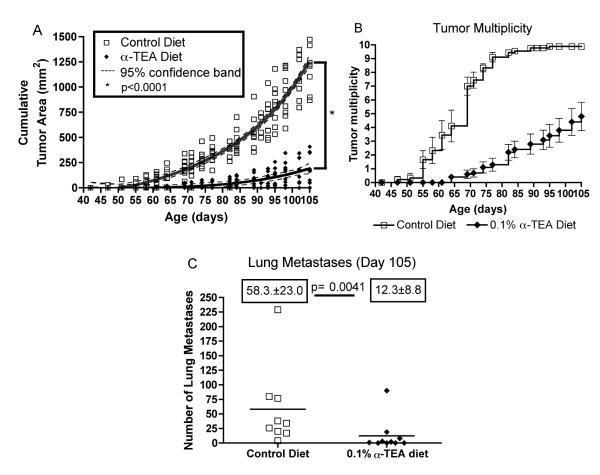
After we established that the lower 0.1% α-TEA dose was efficacious in treating established primary tumors in the transplantable 4T1 mammary cancer model, we wanted to investigate the efficacy of this dose in treating breast disease in the more clinically relevant transgenic MMTV-PyMT model of spontaneous breast cancer. For this purpose, MMTV-PyMT mice were treated with the 0.1% α-TEA diet starting at 6 weeks of age (42 days). At this age, MMTV-PyMT mice display alveolar hyperplasia and moderate mammary intraepithelial neoplasia in comparison to wild-type mice. The mice received the α-TEA diet ad libitum for 9 weeks until day 105 of age, when the mice on the control diet were sacrificed due to large tumor burden. Both groups of mice consumed similar amounts of food, with the control mice eating 2.7 g/day and the mice in the 0.1% diet group eating 2.4 g/day. Therefore, the mice on the 0.1% diet received an average daily α-TEA dose of 2.4 mg/day. Serum analysis showed that the mice had an average circulating α-TEA level of 27.2 ± 4.8 μg/mL, which was somewhat lower than the α-TEA serum level of BALB/c mice on the 0.1% diet (38.2 ± 4.9 μg/mL). The α-TEA treatment delayed the development of palpable tumors by more than two weeks (17 days) and significantly (p<0.0001) slowed tumor progression over the duration of the study, resulting in an 83% reduction of the average cumulative tumor area (Figure 3A). In addition, the α-TEA treatment reduced tumor multiplicity by 50% (Figure 3B). The average number of tumors in the α-TEA-treated group was 4.8 ± 1.0 in comparison to an average number of tumors of 9.9 ± 0.1 in the control diet group (Figure 3B). At the study endpoint (day 105), we also determined the metastatic spread of the primary tumors to the lung. Over the course of the study, the α-TEA diet significantly reduced (p=0.0041) the average number of lung metastases almost 5-fold from 58.3 ± 23.0 to 12.3 ± 8.8 (Figure 3C) with 40% (4/10) of the mice lacking visible metastatic lung nodules.
Figure 3. Effect of dietary delivery of α-TEA on the growth of spontaneously developing mammary tumors.
MMTV-PyMT mice (10 mice) received α-TEA in the diet (0.1%) starting at 6 weeks of age (day 42) until 15 weeks of age (day 105) when mice were sacrificed. Untreated mice (9 mice) received control diet throughout the study. (A) The values represent the cumulative tumor areas per mouse. Tumor growth curves were determined by non-linear regression analysis and statistically evaluated for differences by f-test. (B) The values represent tumor multiplicity calculated as: Tumor multiplicity = [# tumors per group] / [# of mice per group]. (C) Effect of dietary α -TEA on tumor spread. Tumor-bearing MMTV-PyMT mice from the above study were sacrificed on day 105. To determine the number of pulmonary metastases, lungs were inflated with India ink and the surface lung metastases were counted. Boxed numbers are the mean numbers of lung metastases per group ± SEM. Differences were evaluated by Mann-Whitney test.
We also tested the efficacy of the 0.3% and 0.05% α-TEA diet to treat tumors in the MMTV-PyMT mice. Although we had shown in our previous studies that the 0.3% diet did not cause gross toxicity in BALB/c mice (2), several of the MMTV-PyMT mice on the 0.3% α-TEA diet died after about 10-19 days of α-TEA treatment, suggesting that these transgenic mice may be more sensitive to this higher α-TEA dose. In addition, the 0.05% α-TEA dose inhibited tumor development in the MMTV-PyMT mice in a dose dependent manner similar to the 4T1 tumor model (data not shown).
Effect of oral α-TEA on tumorigenesis in MMTV-PyMT mice
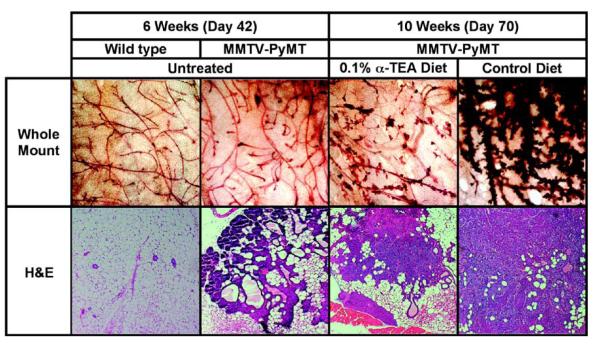
In order to determine the effect of the α-TEA on the early development of spontaneous tumors, whole mounts of mammary glands from control and α-TEA diet-fed mice were prepared after 4 weeks of α-TEA treatment. In comparison to younger mice (6 weeks of age), untreated mice at 10 weeks of age displayed a marked increase in irregular formed ductal side branches, enlarged terminal buds and multilobular tumor masses (Figure 4, compare whole mount panels 2 and 4). This is in contrast to mammary glands from mice that received short-term (4 weeks) α-TEA treatment (Figure 4, whole mount panel 3), which displayed only moderately irregular side branches and few enlarged terminal buds. Examination of H&E stained tissue sections revealed that 10-week-old untreated mice had early mammary carcinoma signified by coalescing acini with solid sheets of cells filling their lumen, nuclear atypia and loss of intact basement membranes with neoplastic epithelial cells invading the stroma. In addition, inflammation was observed (Figure 4, H&E panel 4). The mice that received 4 weeks of α-TEA treatment displayed only adenomas indicated by expansile masses composed of relatively uniform cells within acini, the basement membranes were intact and only mild inflammation was observed (Figure 4, H&E panel 3).
Figure 4. Effect of α-TEA treatment on tumorigenesis in MMTV-PyMT mice.
MMTV-PyMT mice received α-TEA in the diet (0.1% diet) starting at 6 weeks of age for 4 weeks when mice were sacrificed. Mammary glands were dissected and either whole mounts or tissue sections were prepared. To prepare whole mounts, the tissues were dried flat on a microscope slide, fixed and stained with carmine red (20x magnification). Tissue sections were prepared from formalin fixed, paraffin embedded tissues and stained with H&E (100x magnification).
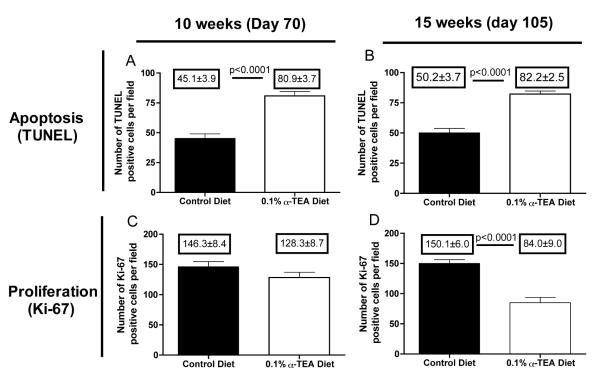
Orally active α-TEA increases apoptosis and inhibits cell proliferation in spontaneously arising breast tumors
After we demonstrated the ability of orally administered α-TEA to reduce primary tumor burden and metastatic spread, we wanted to determine if the delay of tumor development and tumor progression was due to the pro-apoptotic or anti-proliferative properties of α-TEA. For this purpose, we evaluated apoptosis and cell proliferation in tumor sections after 4 weeks (day 70 of age) and 9 weeks (day 105 of age) of α-TEA treatment by TUNEL and Ki-67 staining, respectively. Our results show (Figure 5A and B) that at both time points the average number of TUNEL positive cells/field in the α-TEA-treated group was significantly higher than in the control group (p<0.0001). There was no difference in the number of Ki-67 positive cells after short-term α-TEA treatment (4 weeks, day 70) compared to mice on the control diet (Figure 5C). However, the number of Ki-67 positive cells was significantly lower after 9 weeks of α-TEA treatment (day 105) compared to control treatment (Figure 5D). Together, these results suggest that tumor cell killing by apoptosis, at least in the earlier stages of the disease, seems to play a bigger role in the α-TEA-mediated inhibition of tumor progression.
Figure 5. α-TEA increases apoptosis and inhibits cell proliferation in MMTV-PyMT tumors.
After 4 weeks (day 70) or 9 weeks (day 105) of α-TEA treatment (0.1% diet), tumor sections were examined for cell proliferation by Ki-67 stain and apoptosis by TUNEL assay. Data are depicted as average number of Ki-67 or TUNEL-positive cells in fifteen microscopic fields per tumor sample (Ki-67: 400x magnification; apoptosis: 200x magnification). Differences were evaluated by t-test.
DISCUSSION
The anti-tumor activities of vitamin E compounds, exemplified by α-TOS and the closely related α-TEA, have been extensively characterized using in vitro systems and using transplantable xenograft and syngeneic tumor models of various origin. In recent years it has been established that α-TOS and α-TEA have the ability to inhibit tumor progression in vivo (1, 5, 7, 9, 10, 22-28). In addition, we have recently shown, using the transplantable, highly aggressive and poorly immunogenic syngeneic model of murine breast cancer (4T1), that the esterase-stable α-TEA can be administered orally with high anti-tumor efficacy (2).
In order to move α-TEA closer to the clinic to be used as a new treatment option for breast cancer, the present study had two main goals, first to determine the minimal effective dose needed to treat breast cancer and second to test the efficacy of orally administered α-TEA in a more clinically relevant murine model of spontaneously arising breast cancer.
To examine the minimally effective α-TEA dose, BALB/c mice with established 4T1 mammary tumors (day 10 post tumor-implantation) were fed mouse chow containing decreasing amounts of α-TEA. The relatively high α-TEA dose (0.3% diet) of approximately 6 mg α-TEA per day (equivalent to 300 mg/kg body weight) caused a 64% reduction of primary tumor size and 85% decrease in lung metastases (Figure 1), confirming our earlier studies in this tumor model (2). The next dose tested was a 3-fold lower formulation of the α-TEA diet designed to deliver 2 mg α-TEA per day (equivalent to 100 mg/kg bodyweight) that resulted in an anti-tumor effect similar to the 0.3% diet, achieving a 50% reduction in tumor size (Figure 1). Our findings indicate that increasing the α-TEA dose does not translate into a directly proportional anti-tumor benefit. This seems to be particularly true for the ability of α-TEA to inhibit metastasis formation, as the 0.1% diet inhibited metastatic tumor spread (92% reduction) with the same efficacy as the 0.3% α-TEA diet (85% reduction) (Figure 1C). This is also underscored by the result that the even lower 0.05% α-TEA diet dose (~1 mg/day), although it had only a moderate impact on the primary tumor (38% reduction in tumor size), was almost as effective in preventing metastasis formation as the 0.1% or the 0.3% diet. This finding suggests that the low ~1 mg/day α-TEA intake (equivalent to 50 mg/kg body weight) is below a necessary α-TEA threshold level needed to significantly suppress the primary tumor; however, it is still high enough to inhibit metastatic tumor spread. Studies using a model of residual disease, where primary tumors are surgically removed after metastasis formation (29), are currently under way to examine this possibility. If these results are confirmed, it may open the possibility to use a low α-TEA dose as a safe long-term therapy option to prevent or treat recurrent metastatic disease after debulking of the primary tumor. Another possible explanation of the findings that the 0.1% and 0.05% α-TEA diets significantly reduced lung metastasis, although there was only moderate or no significant effect on the primary tumor, may be that α-TEA is able to suppress tumor spread via different mechanisms at lower doses. In a recent report we showed that low α-TEA and α-TOS doses (ranging from ~2.5 to ~12.5 μg/mL) significantly decreased wound healing and tube formation of endothelial-like EAhy926 cells in vitro and that α-TOS also inhibited in vivo angiogenesis by induction of apoptosis of proliferating endothelial cells (30). A similar mechanism may be responsible for the anti-metastatic activity of α-TEA.
Because our results suggested that the dose-dependent suppression of tumor growth and metastatic spread may not be a linear function of the apparent α-TEA intake, we determined the circulating α-TEA serum levels. Although the 0.3% diet contained three times as much α-TEA as the 0.1% diet, the α-TEA serum level in the mice on the 0.3% diet was approximately twice as high as the α-TEA serum level in the mice on the 0.1% α-TEA diet (Figure 2). However, according to the average food intake, the daily dose of the mice on the 0.3% diet (5.9 mg/day) was indeed close to twice the average calculated dose of the mice on the 0.1% diet (2.4 mg/day). This may explain the observed similarities in anti-tumor efficacy of the 0.3% and 0.1% diet. Surprisingly, the serum level of the mice on the 0.05% diet was close to the α-TEA serum level of the mice that received the 2-fold higher α-TEA dose (0.1% diet), which may help to explain the interesting result that the low 0.05% α-TEA diet dose still had a significant suppressive impact on lung metastasis.
The transplantable 4T1 tumor model enabled us to quickly determine the minimal effective α-TEA dose, establishing that the 0.1% diet is an effective α-TEA dose against murine mammary tumors. However, the second goal of this study was to evaluate the efficacy of α-TEA therapy in a more clinically relevant murine mammary tumor model. Although transplantable tumor models are valuable tools in pre-clinical cancer research, and many mimic human disease reasonably well, they do not fully resemble human cancer etiology as they lack the physiologically intact tumor bed and spontaneously arising tumor microenvironment (31). For this reason, we employed the transgenic MMTV-PyMT mouse model of spontaneous breast cancer to test the efficacy of α-TEA therapy in a setting more closely resembling human disease. The MMTV-PyMT mice (12) develop multifocal mammary adenocarcinoma involving the entire mammary pad with secondary metastasis to the lung, making it a valuable model of human breast cancer (19). The MMTV-PyMT mice were started on the 0.1% TEA diet at 6 weeks of age, when these mice displayed alveolar hyperplasia and moderate mammary intraepithelial neoplasia and therefore represent an early disease model of breast cancer (Figure 3). This dose of ~2 mg α-TEA per day delayed the development of palpable tumors by 17 days and reduced tumor size by 85% in comparison to untreated mice (Figure 3A and B,). In addition, a promising result was that α-TEA was able to prevent lung metastases in 40% of the treated animals and reduced the average number of spontaneous lung metastases by 80% (Figure 3C). These results corroborate our earlier findings using the transplantable 4T1 tumor model that α-TEA is effective in inhibiting tumor progression and spread and suggests that α-TEA therapy may be particularly valuable to treat metastases formation.
Although the lower 0.1% diet dose was very effective in delaying tumor development and suppressing primary tumor growth and metastasis, we also evaluated the 0.3% α-TEA diet, but treated MMTV-PyMT mice died after approximately three weeks of drug exposure. This is in contrast to our finding that this same dose had no adverse effects in BALB/c mice as reported here and previously (2). Although we have no compelling explanation for this different outcome, it seems to suggest that the transgenic MMTV-PyMT mice are more sensitive to higher doses of α-TEA. However, more detailed toxicological studies are needed to determine the differential effect of α-TEA on mice of different genetic backgrounds.
To elucidate the mechanism of α-TEA-mediated tumor suppression, we evaluated what roles the pro-apoptotic and anti-proliferative properties of α-TEA play in vivo. Using in vitro experiments, we (2, 29) and others (3, 7, 32) have shown that Vitamin E analogs such as α-TOS and α-TEA induce apoptosis and inhibit proliferation of breast cancer and other tumor cell lines. However, this is the first report examining the mechanism of how α-TEA inhibits tumor progression in vivo in a model of spontaneous breast cancer. After 4 weeks of α-TEA treatment and at the end of the study (after 9 weeks of treatment), we examined tumor tissues for apoptosis and cell proliferation in situ. Our results show that after short-term treatment (4 weeks), there was no difference in cell proliferation in the tumor tissue (Figure 5C). Meanwhile, at the study endpoint, tumor cell proliferation was impacted by α-TEA therapy. However, at both time points there were significantly more apoptotic cells in the tumor tissue from α-TEA-treated animals in comparison to control animals. This suggests that the well-documented pro-apoptotic activties of α-TEA play a significant role in the α-TEA-mediated inhibition of tumor progression in the MMTV-PyMT tumor model.
This study demonstrates for the first time the ability of orally administered α-TEA to delay tumor onset and to inhibit the progression and metastatic spread of a clinically relevant model of spontaneous breast cancer. Our finding of the high efficacy in this tumor model highlights the translational potential of oral α-TEA therapy and may lay the foundation for clinical testing of α-TEA in metastatic breast cancer patients.
ACKNOWLEDGMENTS
We thank Lajos Szabo for synthesis of α-TEA. We also thank Deborah Bradely-Dunlop, Matthew Rausch, Michael Rak and Felicia Goodrum for technical assistance. Transgenic mice were generated by the Experimental Mouse Shared Service at the Arizona Cancer Center. The Shared Service is supported by the Cancer Center Support Grant (P30CA23074). Immunohistochemical data was generated by the TACMASS Core (Tissue Acquisition and Cellular/Molecular Analysis Shared Service) at the Arizona Cancer Center, supported by NIH grant CA23074.
Supported by Grants: NIH 5R01CA120552 and American Institute for Cancer Research 07A126.
Abbreviations
- α-TEA
alpha-tocopheryloxyacetic acid
- α-TOS
α-tocopheryl succinate
- AP-ESI
atmospheric pressure electrospray ionization
- H&E
hematoxylin and eosin
- HPLC/MSD
high performance liquid chromatography with mass spectrometric detection
- MMTV
mouse mammary tumor virus
- ANOVA
one-way analysis of variances
- PyMT
polyoma middle T oncogene
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
Footnotes
Disclosure: Laurence H. Hurley is a patent holder for the “Preparation of tocopherols, tocotrienols, other chroman and side chain derivatives for use as antitumor agents and for inducing cell apoptosis”. No commercial applications have been realized or are under development.
REFERENCES
- 1.Lawson KA, Anderson K, Menchaca M, et al. Novel vitamin E analogue decreases syngeneic mouse mammary tumor burden and reduces lung metastasis. Moleculaar Cancer Therapy. 2003;2:437–44. [PubMed] [Google Scholar]
- 2.Hahn T, Szabo L, Gold M, Ramanathapuram L, Hurley LH, Akporiaye ET. Dietary Administration of the Proapoptotic Vitamin E Analogue {alpha}-Tocopheryloxyacetic Acid Inhibits Metastatic Murine Breast Cancer. Cancer Research. 2006;66:9374–8. doi: 10.1158/0008-5472.CAN-06-2403. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil J, Tomasetti M, Mellick AS, et al. Vitamin E analogues: a new class of inducers of apoptosis with selective anti-cancer effects. Current Cancer Drug Targets. 2004;4:355–72. doi: 10.2174/1568009043332943. [DOI] [PubMed] [Google Scholar]
- 4.Neuzil J. Vitamin E succinate and cancer treatment: a vitamin E prototype for selective antitumour activity. British Journal of Cancer. 2003;89:1822–6. doi: 10.1038/sj.bjc.6601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuzil J, Weber T, Gellert N, Weber C. Selective cancer cell killing by alpha-tocopheryl succinate. British Journal of Cancer. 2001;84:87–9. doi: 10.1054/bjoc.2000.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawson KA, Anderson K, Snyder RM, et al. Novel vitamin E analogue and 9-nitro-camptothecin administered as liposome aerosols decrease syngeneic mouse mammary tumor burden and inhibit metastasis. Cancer Chemother Pharmacol. 2004 doi: 10.1007/s00280-004-0817-y. [DOI] [PubMed] [Google Scholar]
- 7.Anderson K, Simmons-Menchaca M, Lawson KA, Atkinson J, Sanders BG, Kline K. Differential response of human ovarian cancer cells to induction of apoptosis by vitamin E Succinate and vitamin E analogue, alpha-TEA. Cancer Research. 2004;64:4263–9. doi: 10.1158/0008-5472.CAN-03-2327. [DOI] [PubMed] [Google Scholar]
- 8.Lawson KA, Anderson K, Snyder RM, et al. Novel vitamin E analogue and 9-nitro-camptothecin administered as liposome aerosols decrease syngeneic mouse mammary tumor burden and inhibit metastasis. Cancer Chemotherapy and Pharmacology. 2004;54:421–31. doi: 10.1007/s00280-004-0817-y. [DOI] [PubMed] [Google Scholar]
- 9.Lawson KA, Anderson K, Simmons-Menchaca M, et al. Comparison of vitamin E derivatives alpha-TEA and VES in reduction of mouse mammary tumor burden and metastasis. Experimental Biolology and Medicine (Maywood) 2004;229:954–63. doi: 10.1177/153537020422900913. [DOI] [PubMed] [Google Scholar]
- 10.Ramanathapuram LV, Kobie JJ, Bearss D, Payne CM, Trevor KT, Akporiaye ET. Alpha-Tocopheryl succinate sensitizes established tumors to vaccination with nonmatured dendritic cells. Cancer Immunology and Immunotherapy. 2004;53:580–8. doi: 10.1007/s00262-004-0499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia L, Yu W, Wang P, Sanders BG, Kline K. In vivo and in vitro studies of anticancer actions of alpha-TEA for human prostate cancer cells. Prostate. 2008;68:849–60. doi: 10.1002/pros.20750. [DOI] [PubMed] [Google Scholar]
- 12.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proceedings of the National Academy of Sciences USA. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kline K, Sanders BG, Hurley L, et al. Preparation of tocopherols, tocotrienols, other chroman and side chain derivatives for use as antitumor agents and for inducing cell apoptosis. PCT International Application. 2000 [Google Scholar]
- 14.McEarchern JA, Kobie JJ, Mack V, et al. Invasion and metastasis of a mammary tumor involves TGF-beta signaling. International Journal of Cancer. 2001;91:76–82. doi: 10.1002/1097-0215(20010101)91:1<76::aid-ijc1012>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Miller FR, Heppner GH. Immunologic heterogeneity of tumor cell subpopulations from a single mouse mammary tumor. Journal of the National Cancer Institute. 1979;63:1457–63. [PubMed] [Google Scholar]
- 16.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im2002s39. Chapter 20:Unit 20 2. [DOI] [PubMed] [Google Scholar]
- 17.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Molecular and Cellular Biology. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Nguyen A, Pollard JW. Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol. 2002;247:11–25. doi: 10.1006/dbio.2002.0669. [DOI] [PubMed] [Google Scholar]
- 19.Lin EY, Jones JG, Li P, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. American Journal of Pathology. 2003;163:2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardiff RD, Anver MR, Gusterson BA, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–88. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 21.Clarke R. Issues in experimental design and endpoint analysis in the study of experimental cytotoxic agents in vivo in breast cancer and other models. Breast Cancer Research and Treatment. 1997;46:255–78. doi: 10.1023/a:1005938428456. [DOI] [PubMed] [Google Scholar]
- 22.Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z. The selective antiproliferative effects of alpha-tocopheryl hemisuccinate and cholesteryl hemisuccinate on murine leukemia cells result from the action of the intact compounds. Cancer Research. 1994;54:3346–51. [PubMed] [Google Scholar]
- 23.Malafa MP, Fokum FD, Mowlavi A, Abusief M, King M. Vitamin E inhibits melanoma growth in mice. Surgery. 2002;131:85–91. doi: 10.1067/msy.2002.119191. [DOI] [PubMed] [Google Scholar]
- 24.Barnett KT, Fokum FD, Malafa MP. Vitamin E succinate inhibits colon cancer liver metastases. Journal of Surgical Research. 2002;106:292–8. doi: 10.1006/jsre.2002.6466. [DOI] [PubMed] [Google Scholar]
- 25.Weber T, Lu M, Andera L, et al. Vitamin E succinate is a potent novel antineoplastic agent with high selectivity and cooperativity with tumor necrosis factor-related apoptosis-inducing ligand (Apo2 ligand) in vivo. Clinical Cancer Research. 2002;8:863–9. [PubMed] [Google Scholar]
- 26.Kogure K, Hama S, Manabe S, Tokumura A, Fukuzawa K. High cytotoxicity of alpha-tocopheryl hemisuccinate to cancer cells is due to failure of their antioxidative defense systems. Cancer Letters. 2002;186:151–6. doi: 10.1016/s0304-3835(02)00344-0. [DOI] [PubMed] [Google Scholar]
- 27.Prasad KN, Edwards-Prasad J. Vitamin E and cancer prevention: recent advances and future potentials. Journal of the American College of Nutrition. 1992;11:487–500. doi: 10.1080/07315724.1992.10718253. [DOI] [PubMed] [Google Scholar]
- 28.Prasad KN, Kumar B, Yan XD, Hanson AJ, Cole WC. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. Journal of the American College of Nutrition. 2003;22:108–17. doi: 10.1080/07315724.2003.10719283. [DOI] [PubMed] [Google Scholar]
- 29.Ramanathapuram LV, Hahn T, Dial SM, Akporiaye ET. Chemo-Immunotherapy of Breast Cancer Using Vesiculated alpha-Tocopheryl Succinate in Combination With Dendritic Cell Vaccination. Nutrition and Cancer. 2005;53:177–93. doi: 10.1207/s15327914nc5302_7. [DOI] [PubMed] [Google Scholar]
- 30.Dong LF, Swettenham E, Eliasson J, et al. Vitamin E analogues inhibit angiogenesis by selective induction of apoptosis in proliferating endothelial cells: the role of oxidative stress. Cancer Research. 2007;67:11906–13. doi: 10.1158/0008-5472.CAN-07-3034. [DOI] [PubMed] [Google Scholar]
- 31.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. American Journal of Pathology. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kline K, Yu W, Sanders BG. Vitamin E: mechanisms of action as tumor cell growth inhibitors. Journal of Nutrition. 2001;131:161S–3S. doi: 10.1093/jn/131.1.161S. [DOI] [PubMed] [Google Scholar]