Abstract
Background
Protease activated receptors (PARs) are expressed on structural and immune cells. Control of initiation, duration, and magnitude of PAR effects is linked to the level of receptor expression, availability of proteases, and the intracellular signal transduction machinery. We investigated nematode infection-induced changes in PAR2 expression and the impact on smooth muscle and epithelial responses to PAR2 agonists.
Methods
Smooth muscle and epithelial cell function were assessed in wild type, and IL-4, IL-13 or STAT6 gene-deficient mice following treatment with vehicle, Nippostrongylus brasiliensis or Heligmosomoides polygyrus, or IL-13. The role of enteric nerves was determined using tetrodotoxin to block nerve conduction. Expression of PAR2 was assessed by real-time PCR, western blot and immunohistochemistry.
Key Results
Nematode infection induced a STAT6- and IL-13-dependent up-regulation of PAR2 mRNA expression. The infection-induced hypercontractility to PAR2 agonists required STAT6/IL-13 and was neurally-mediated. In contrast, the infection-induced decrease in epithelial secretion to PAR2 agonists was partly dependent on STAT6 and independent of enteric nerves. The hyposecretion was correlated with decreased PAR2 immunofluorescent staining on the apical surface of epithelial cells, but enhanced lamina propria immunostaining for PAR2.
Conclusions & Inferences
This is the first study to demonstrate an immune regulation of PAR2 expression that impacts both smooth muscle and epithelial cell responses to PAR2 agonists. Differences in responses between smooth muscle and epithelial cells are related to the contribution of enteric nerves. These data provide a mechanism by which activation of PAR2 in immune-based pathologies can induce both transient and long-lasting changes in gut function.
Keywords: Nematode infection, IL-13, IL-4, epithelial cell, smooth muscle, PAR2
Introduction
Protease activated receptors (PARs) are member of the family of seven-transmembrane-, G-protein-coupled receptors (GPCR). There are four PARs identified to date: PAR1, PAR3 and PAR4, activated specifically by thrombin, and PAR2 activated by trypsin or human mast cell tryptase. PARs also have a distinctive mechanism of activation from other GPCR. The mechanism involves enzymatic cleavage at a specific consensus sequence on their extracellular domain resulting in exposure of a new N-terminus that serves as tethered ligand that binds and activates the receptor1. PARs are termed “one shot” receptors with termination of signaling involving desensitization, internalization, and lysosomal degradation2. The magnitude and duration of the effect of proteases working through PAR, therefore, is determined by the balance among the abundance and/or distribution of the serine proteases that activate these PARs and the number and availability of PARs on the cell surface2–4.
In the gut, enteric neurons, epithelial and smooth muscle cells all express PARs5–8 as do a number of immune cells, including dendritic cells, mast cells, macrophages, and T cells9–12. In addition, inflammatory cells are a rich source of proteases that activate PARs. Activation of PARs on structural cells is linked to a variety of physiological activities including epithelial secretion, mucosal barrier function, smooth muscle relaxation/contraction13–16. Endogenous serine proteases, such as trypsin, play a well-known physiological role in digestion; however, recent studies indicate that PARs are critical players in a number of gut pathologies17 including inflammatory bowel disease (IBD)14, 18, 19and functional disorders such as irritable bowel syndrome20 (IBS). Under these conditions, there is an increase in protease expression, generation and/or activation of PARs that orchestrate tissue responses including inflammation, pain, repair, and altered gut function 21.
Enteric nematode infection up-regulates IL-4 and IL-13, which bind to receptors that are linked to STAT6 signaling22. Infection is associated also with stereotypic STAT6-dependent alterations in gut function that are orchestrated by the interactions between immune and structural cells, including smooth muscle cells, epithelial cells, and nerves, that facilitate worm expulsion23–25. We showed previously23 that nematode-infection up-regulated PAR1 expression and increased small intestinal smooth muscle contractility to PAR1 agonists by a mechanism that was dependent on IL-13 and STAT6, but not on IL-4. Others suggested that PAR2 contributed to expulsion of N. brasiliensis26. These data support a role for the immune regulation of PARs, such as PAR2, in infection-induced changes in gut function that are a critical component of Th2-mediated protective immunity. The aim of the current study was to investigate the hypothesis that there is an immune regulation of PAR2 expression that alters both smooth muscle and epithelial cell responses to PAR2 agonists.
Methods
Animals and treatments
Wild-type (WT) BALB/c mice 8–12 week old (National Cancer Institute, Frederick, MD) or mice deficient in expression of STAT6 (STAT6−/−), IL-4 (IL-4−/−) or IL-13 (IL-13−/−) (NIAID/Taconic) were used for each experiment. Animal use for these studies was approved by the University of Maryland School of Medicine and USDA ARS, Beltsville, Animal Care and Use Committees. The experiments were conducted in accordance with the principles set forth in the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals (2002 revision), Office of Laboratory Animal Welfare, NIH. Mice were inoculated subcutaneously with 500 infective third-stage N. brasiliensis larvae (L3)27 and adult worms are expelled by day 9 after inoculation. Some groups of mice were infected with Heligmosomoides polygyrus (H. polygyrus, U.S. National Helminthological Collection no. 81930). Mice were inoculated orally with 200 L3 using a ball-tipped feeding tube and then cured with the antihelmintic drug, pyranthal tartrate, 2 weeks after inoculation. These same mice then were reinfected 28 days later and studied 10 days after the second inoculation. We chose to study re-infected mice because there is a greater induction of IL-4 during a secondary infection. Separate groups of WT or STAT6−/− mice received IL-13 (10µg/mouse/ iv) daily for 6 days and were studied on day 7. This dose effectively clears infection in immune deficient mice 28.
Gene expression
Total RNA was extracted with TRIzol (Invitrogen, Grand Island, NY) according to the manufacture’s instructions. RNA samples (2µg) were reverse-transcribed to cDNA using the First Strand cDNA Synthase Kit (MBI Fermentas, Hanover, MD) with random hexamer primer. Real-time quantitative PCR (RT-qPCR) was performed using the iCycler detection system (Bio-Rad, CA). Primer sequences were designed using Beacon Designer 4.0 (Premier Biosoft International, Palo Alto, CA), and synthesized by the Biopolymer Laboratory of the University of Maryland. Primer sequences for IL-4 and IL-13 were described previously29 and for PAR2 (forward sequence CACCACCTGTCACGATGTGCT; reverse sequence CTCAGTAGGAGGTTTTAACAC). PCR was performed in a 25µl volume using SYBR green Supermix (Bio-Rad, Hercules, CA). Amplification conditions were: 95°C for 3 min, 50 cycles of 95°C for 15s, 60°C for 15s, and 72°C for 20s. The fold-changes in mRNA expression were relative to the respective vehicle-treated groups of mice after normalization to 18s rRNA29.
Smooth muscle
Segments of small intestine (1 cm) were flushed to remove contents and suspended longitudinally in organ baths. One end of the tissue was attached to an isometric tension transducer (Model FT03; Grass Medical Instruments, Quincy, MA, USA) and the other end was fixed to the bottom of the bath. Tissues were stretched to a load of 9.9mN (1 g) as previous experiments showed that this load stretched tissues to their optimal length for active contraction. Tissues were allowed to equilibrate for 30–45min in Krebs’ buffer, replacing the bath solution every 10 min throughout the entire study. Tension was recorded using a Grass model 79 polygraph (Grass Medical Instruments, Quincy, MA, USA) and expressed as force per cross section in mN/cm2 16. Response curves to trypsin (1 nM-1µM) and PAR2 activating peptide, SLIGRL, (1µM-100µM) were constructed in WT mice. Concentrations that gave the maximum response in WT mice were tested subsequently in STAT6−/− and IL-4−/− mice. We showed previously that the reverse peptide, LRGILS, is inactive in this preparation16.
Ussing chambers
Four 1-cm segments of mid-jejunum were stripped of muscle and mounted in Ussing chambers exposing 0.126 cm2 to 10 ml Krebs’ buffer. Agar-salt bridges and electrodes were used to measure potential difference and the basal short circuit current (Isc) was monitored continuously. In addition, every 50 s, the clamp voltage was adjusted to 1 V (World Precision Instruments DVC 1000 voltage clamp, Sarasota, FL),for 10 s to allow calculation of tissue resistance using Ohm’s law. After the 15-min equilibrium period, tissue resistance, a measure of tissue permeability, was determined. After a second 15-min period, concentration-dependent changes in Isc were measured after the cumulative addition of trypsin (1 nM-1µM) or SLIGRL (10 µM-100µM) to the serosal side. Some tissues were challenged with the reverse peptide LRGILS (100µM) as a control. To determine if trypsin responses could be desensitized, the mucosa was exposed to trypsin, washed and re-exposed again to the same concentration of trypsin. To determine if responses to trypsin were mediated by prostaglandins, responses in separate tissues were compared in the presence or absence of indomethacin (10µM), a cyclooxygenase (COX) inhibitor.
Immunofluorescent staining
Tissue sections (4 µm) were cut from frozen blocks and slides were stored at −80°C. For immunofluorescent staining, tissue slides were fixed in cold acetone for 30 min and blocked with 10% normal goat serum in PBS for 1 hr at room temperature. The slides were incubated with anti-PAR2 (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) overnight then incubated with Avidin-Alexafluor488 (1:200, Molecular Probes, Inc., Eugene, OR). The slides were cover-slipped and photographed with a Nikon E800 microscope (Melville, NY) using Nikon DXM 1200 software. The intensity of staining was assessed by first establishing settings for the samples from the individual vehicle-treated groups and then using these same conditions to evaluate the samples from the nematode-infected group, only comparing slides prepared on the same day.
Mucosal scraping and tissue fractionation
The mucosal epithelial cell layer of upper small intestine from uninfected and N. brasiliensis infected BALB/c mice (n=5/group) was gently scraped using a cover slip. The collected tissue was homogenized in cold HEPES buffer (20 mM HEPES, 100 mM KCl, pH 7) containing protease inhibitors (Thermo Scientific, Rockford, IL) and fractionated by differential centrifugation. Briefly, the total homogenate was subjected to low speed centrifugation (1,200 × g) to separate debris and non-homogenized particles and the clear homogenate was centrifuged at 80,000 × g. The cytosolic fraction (supernatant) was collected and the pellet resuspended in cold phosphate buffer (20 mM sodium phosphate, 10% glycerol, pH 6.5) containing 0.5% CHAPS. Detergent-soluble proteins (membrane integral proteins) were separated from insoluble particulate by centrifugation at 150,000 × g. The protein concentration in the total homogenates, cytosolic, and detergent-soluble membrane fractions was determined using BCA Protein Assay kit (Thermo Scientific, Rockford, IL).
Western blotting
Proteins in mucosal samples were separated by SDS-PAGE in 12% Tris-glycine Novex© gels (Invitrogen) and transferred to nitrocellulose membrane for immunostaining. Immunoreactions used rabbit polyclonal anti-PAR2 (C-17, Santa Cruz Biotechnology, CA) (Chemicon, Temecula, CA, USA) diluted 1:500, rabbit polyclonal anti-Na+/K+-ATPase diluted 1:5,000, or goat polyclonal antibody anti-GAPDH diluted 1:500 in 5% dry non-fat milk in TBS-Tween. Secondary antibodies were peroxidase-labeled goat anti-rabbit IgG or donkey anti-goat IgG (Santa Cruz Biotechnology, CA) diluted 1:1000. Immunoreactive bands were detected by chemiluminescence (Super Signal® West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and signal was acquired using a LAS 4000 UV imager (FujiFilm, Stamford, CT).
Solutions and drugs
Krebs’ buffer contained (in mM) 4.74 KCl, 2.54 CaCl2, 18.5 NaCl, 1.19 NaH2PO4, 1.19 MgSO4, and 25.0 NaHCO3, and was added on each side of the tissue mounted on Ussing chambers. The tissues were equilibrated for 15 min in Krebs’ buffer containing 12 mM glucose on the serosal side and 10 mM mannitol on the mucosal side. All drugs were obtained from Sigma-Aldrich (St. Louis, MO), unless stated otherwise. Stock solutions of trypsin (100µM in water) SLIGRL and LRGILS (1mM in water), or tetrodotoxin (TTX, 1mM in citrate buffer) were prepared and kept frozen until the day of the study.
Data analysis
Statistical analysis was performed using unpaired t-tests or one-way ANOVA, while dose responses were compared using multiple ANOVA. Analyses were followed with post hoc analysis for multiple comparisons. A value of p<0.05 was considered significant.
Results
Immune regulation of PAR2 expression during nematode infection
In WT mice, N. brasiliensis infection increased the expression of the Th2 cytokines, IL-4 (1.0 ± 0.3 vs 95 ± 32 fold, p<0.05) and IL-13 (1.0 ± 0.2 vs 1277 ± 232 fold, p<0.01) as expected. H. polygyrus infection produced a similar up-regulation of these Th2 cytokines as described previously28 (data not shown). PAR2 mRNA expression was increased in N. brasiliensis-infected mice (Figure 1) as well as after H. polygyrus infection (2.1 ± 0.3 fold vs WT, p<0.05). The N. brasiliensis-induced increase in PAR2 expression observed in WT mice was absent in STAT6−/− or IL-13−/− mice, but was retained in IL-4−/− mice (Figure 1). A modest, but significant up-regulation of PAR2 expression was observed also in mice treated for 7 days with exogenous IL-13 (1.8 ± 0.1 fold, p<0.05).
Figure 1.
Nippostrongylus brasiliensis infection induced an IL-13 and STAT6-dependent up-regulation of PAR2 in the small intestine. This increase was independent of IL-4. The fold increases are relative to the individual vehicle groups after normalization to 18s rRNA. * p<0.05 vs respective control (n≥5 for each group).
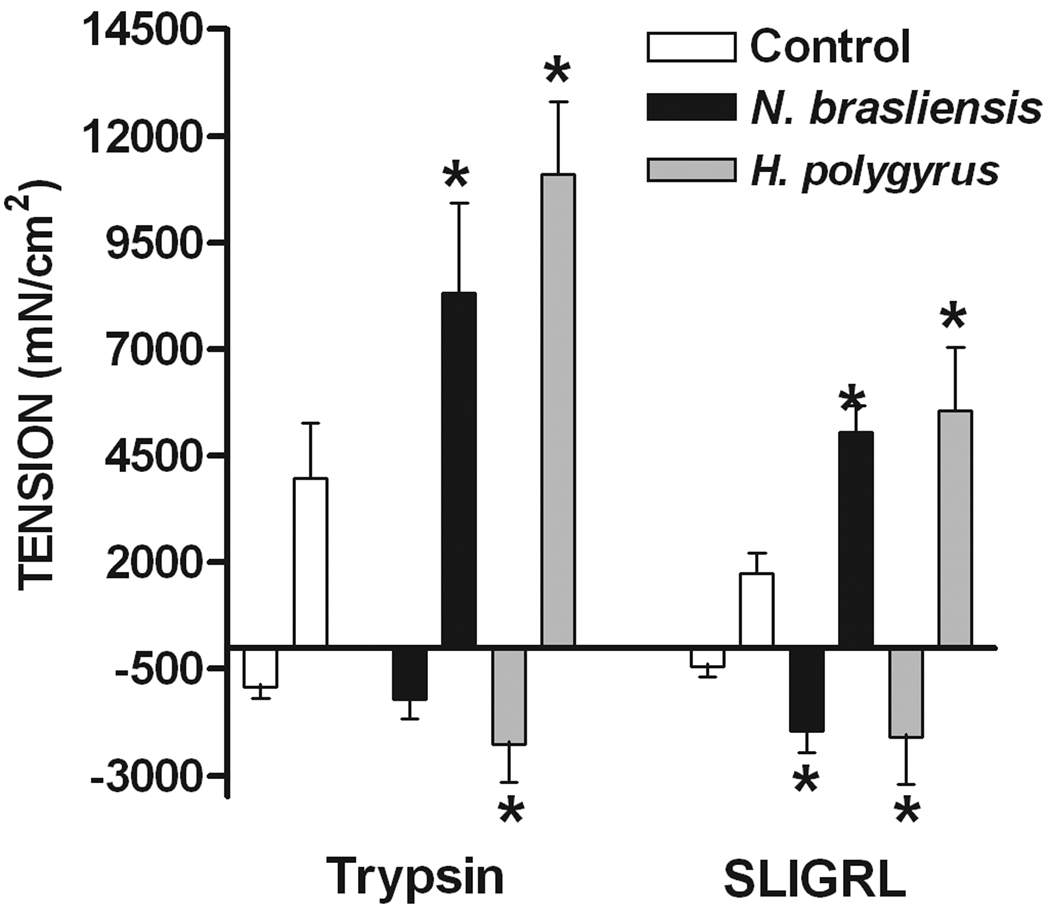
N. brasiliensis infection enhanced smooth muscle responses to PAR2 agonists
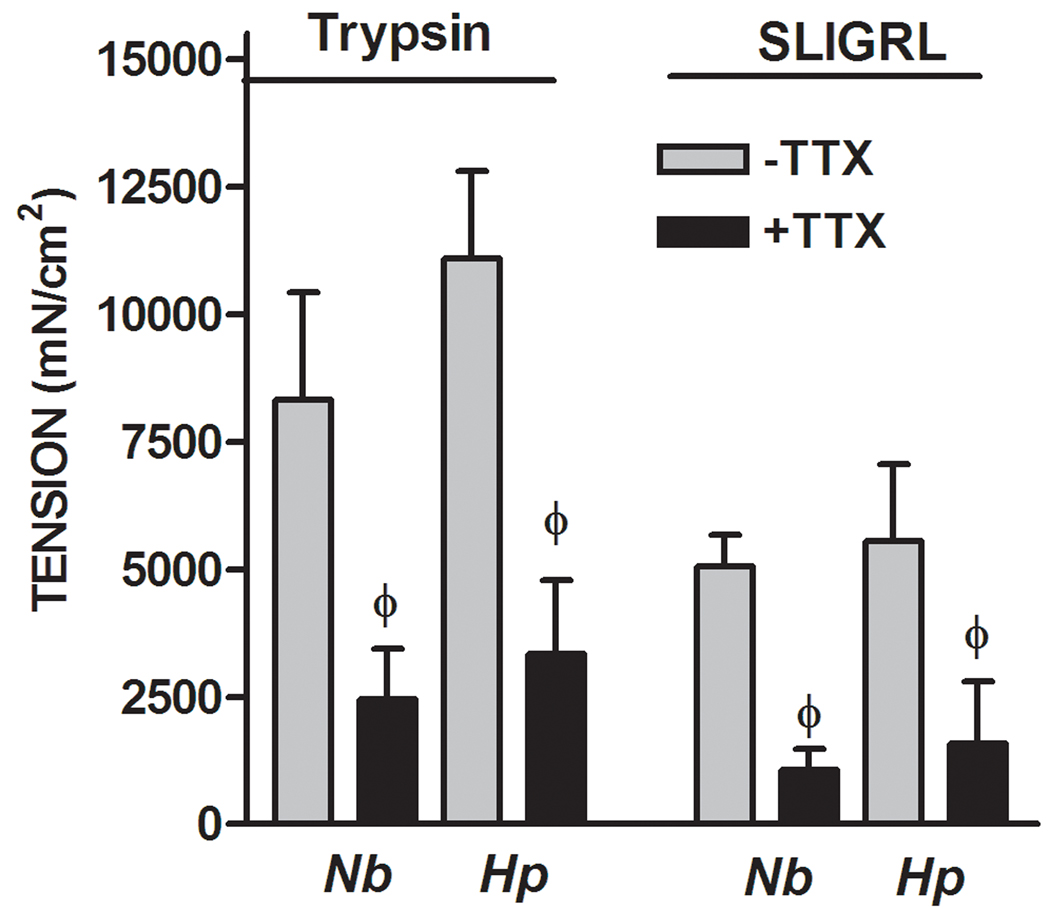
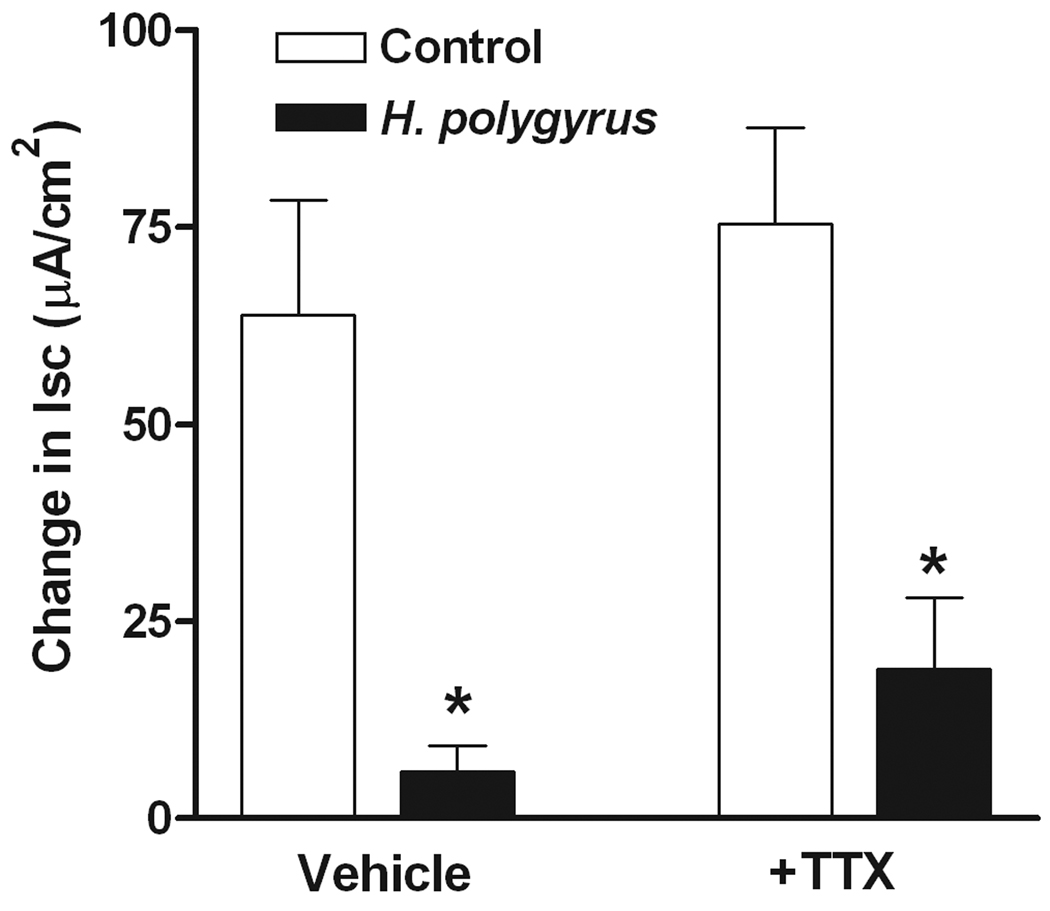
In WT mice, both trypsin and the selective PAR2 agonist, SLIGRL, induced a biphasic response in smooth muscle consisting of a small transient relaxation followed by a larger contraction (Figure 2A). Previous studies showed that these responses were not observed following exposure to the reverse peptide, LRGILS. The initial relaxation and the subsequent contraction to trypsin and SLIGRL were enhanced by both N. brasiliensis and H. polygyrus infection. Exogenous administration of IL-13 also enhanced both phases of the response to PAR2 agonists (data not shown). We and others showed previously that the contraction in response to PAR2 agonists is dependent, in part, on enteric nerves16, 30, 31. To investigate the contribution of nerves to the infection-induced increased smooth muscle contractility induced by trypsin and SLGIRL, responses were compared in the presence and absence of the neurotoxin, TTX, which blocks sodium channels and, therefore, inhibits nerve conduction. The hypercontractile responses to both trypsin and SLIGRL were reduced to controls levels in the presence of TTX in smooth muscle taken from N. brasliensis and H. polygyrus-infected mice (Figure 2B). The dependence of the infection-induced increase in the response to PAR2 agonists on enteric nerves was observed also in IL-13 treated mice (data not shown). The transient relaxation was insensitive to TTX as shown previously15.
Figure 2.
Nematode infection-induced an intestinal smooth muscle hyper-responsiveness to PAR2 agonists that was dependent on enteric nerves. (A) Intestinal smooth muscle exhibited biphasic responses to the PAR2 agonist trypsin (1µM) or PAR2 activating peptide SLIGRL (100µM) featuring an initial relaxation followed by a contraction. (B) The nematode infection-induced increase in the contractile responses to PAR2 was not observed in the presence of TTX. *p<0.05 vs respective control; Φp<0.05 vs respective response in the absence of TTX (n≥5 for each group).
PAR2 mRNA expression was increased by infection, therefore, we determined if the hypercontractility was also immune-regulated. The increased smooth muscle contractility to SLIGRL were negated in STAT6−/−, but not in IL-4−/− mice (Figure 3). In addition, administration of exogenous IL-13 to STAT6−/− mice did not change the amplitude of the contraction to SLIGRL when compared to vehicle-treated STAT6−/− mice (2542 ± 927 vs 1880 ± 851 mN/cm2).
Figure 3.
The hypercontractile smooth muscle response to PAR2 agonists in small intestine is immune-mediated. Segments of jejuna were taken from the mice and suspended longitudinally in organ baths for in vitro contractility studies in response to PAR2 activating peptide SLIGRL (100µM). The N.braslinesis infection-induced increase in smooth muscle responses to SLIGRL was not observed in STAT6−/− mice, but was retained in IL-4−/− mice. *p<0.05 vs respective control; (n≥5 for each group).
N. brasiliensis infection altered epithelial responses to PAR2 agonists
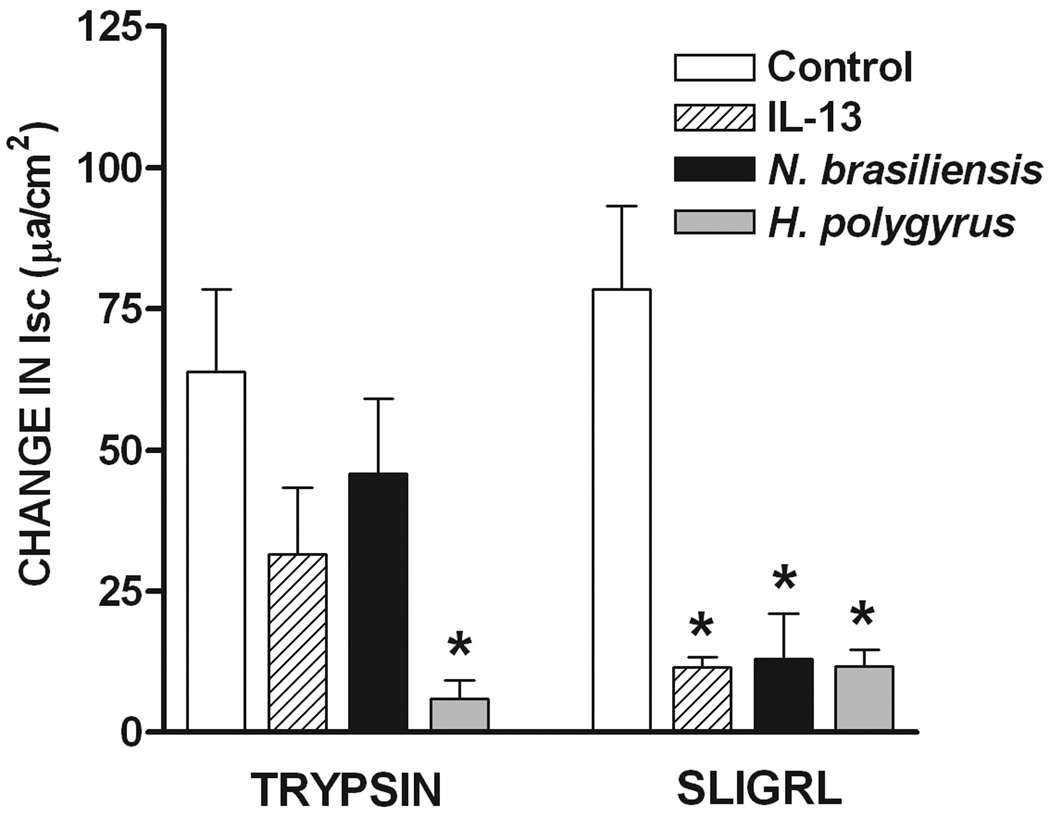
The cumulative addition of trypsin or SLIGRL, but not the reverse peptide, to the serosal side of the mucosa, induced a concentration-dependent increase in Isc (Figure 4A). In addition, the exposure to trypsin desensitized the response to a subsequent administration of trypsin (Figure 4A), similar to previous observations with PAR2 agonists in rat small intestine 15. Finally, the increase in chloride secretion in response to trypsin was unaltered by TTX (Figure 4A), but was inhibited completely by prior incubation of the tissue with the COX inhibitor, indomethacin (Figure 4B). The tissue exposed to trypsin and indomethacin was able to respond to acetylcholine. Similar control of responses was observed after exposure to SLIGRL (data not shown).
Figure 4.
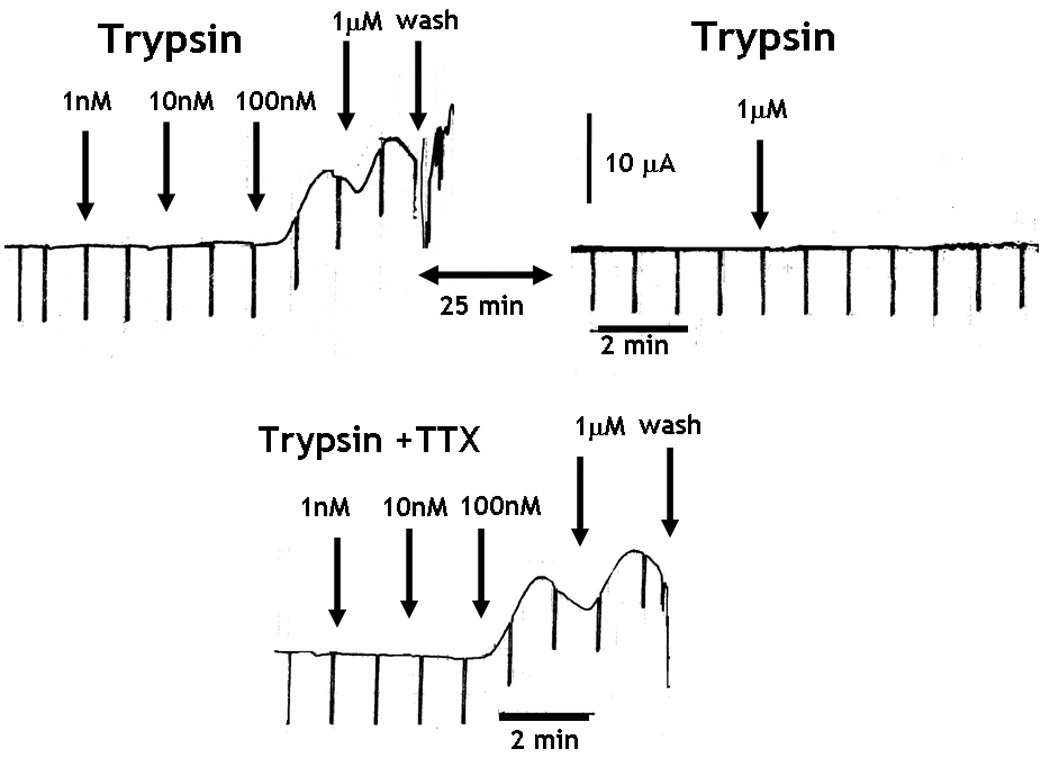
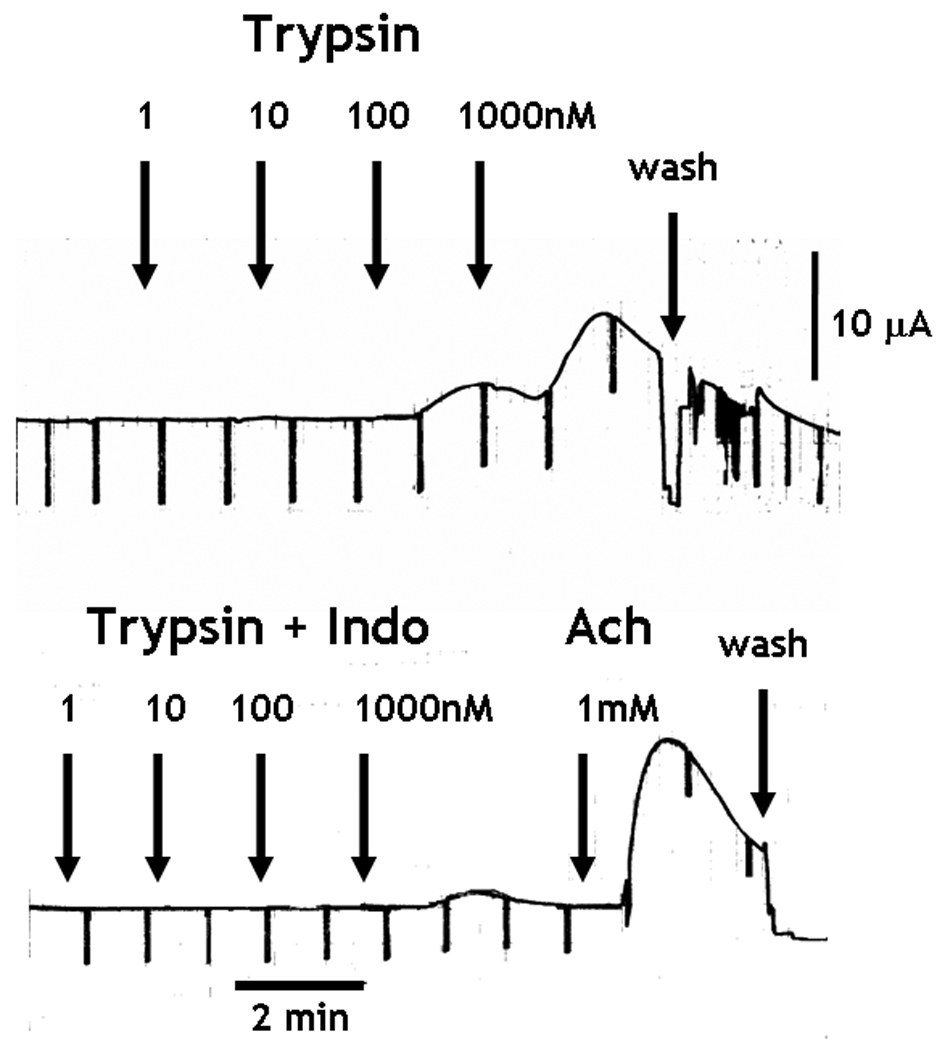
PAR2 activation-induced intestinal epithelial chloride secretion was dependent on COX generation of PGE2, but was independent of enteric nerves. (A and B) The cumulative addition of trypsin to the serosal side of mucosae mounted in Ussing chambers induced a concentration-dependent increase in Isc. (A) Prior exposure to trypsin abolished the response to a second challenge with trypsin 25 minutes later. The increase in Isc was unaltered by 1µM TTX, (B) but was abolished in the presence of 10µM indomethacin. The tissue responded to acetylcholine confirming the viability of the preparation. (C) H. polygyrus (day 14 post infection), N. brasiliensis (day 9 post infection), and in vivo IL-13 treatment (7 days) all significantly inhibited epithelial cell response to PAR2 agonist, SLIGRL. Responses to trypsin were reduced only by H. polygyrus infection *p<0.05 vs respective control.
Responses to trypsin were not altered significantly following either N. brasiliensis or exogenous administration of IL-13, but were reduced after H. polygyrus infection (Figure 4C). In contrast, secretory responses to SLIGRL were inhibited following infection as well as exogenous IL-13. This reduction was unexpected given the increased PAR2 mRNA expression; therefore, we performed immunofluorescence studies to determine if infection altered PAR2 availability on the surface of epithelial cells. In control mice there is evidence of PAR2 on epithelial cells as well as on cells within the lamina propria that are likely mucosal mast cells (Figure 5A). In contrast, in N. brasiliensis-infected mice, PAR2 is visibly diminished in epithelial cells, but there is prominent staining of cells in the lamina propria (Figure 5B), consistent with the mastocytosis that is characteristic of nematode infection.
Figure 5.
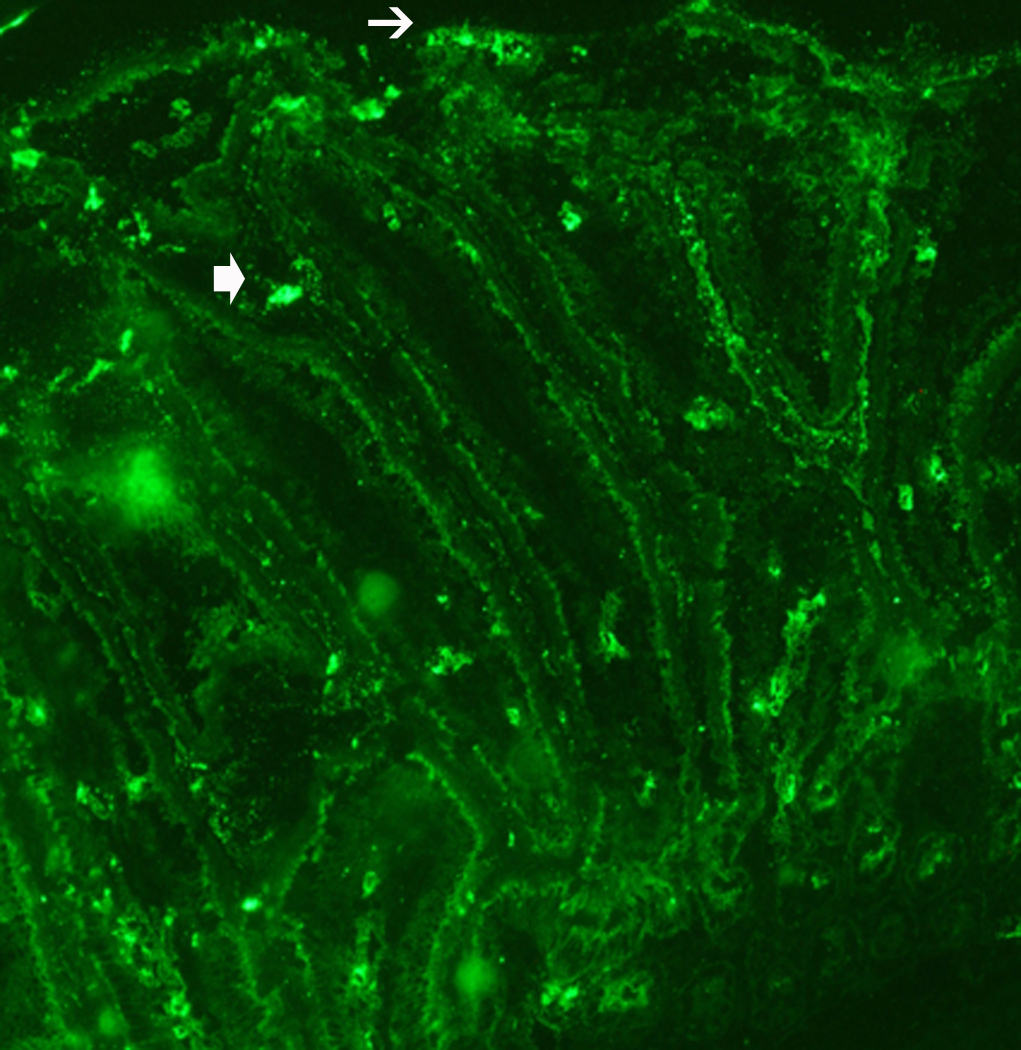
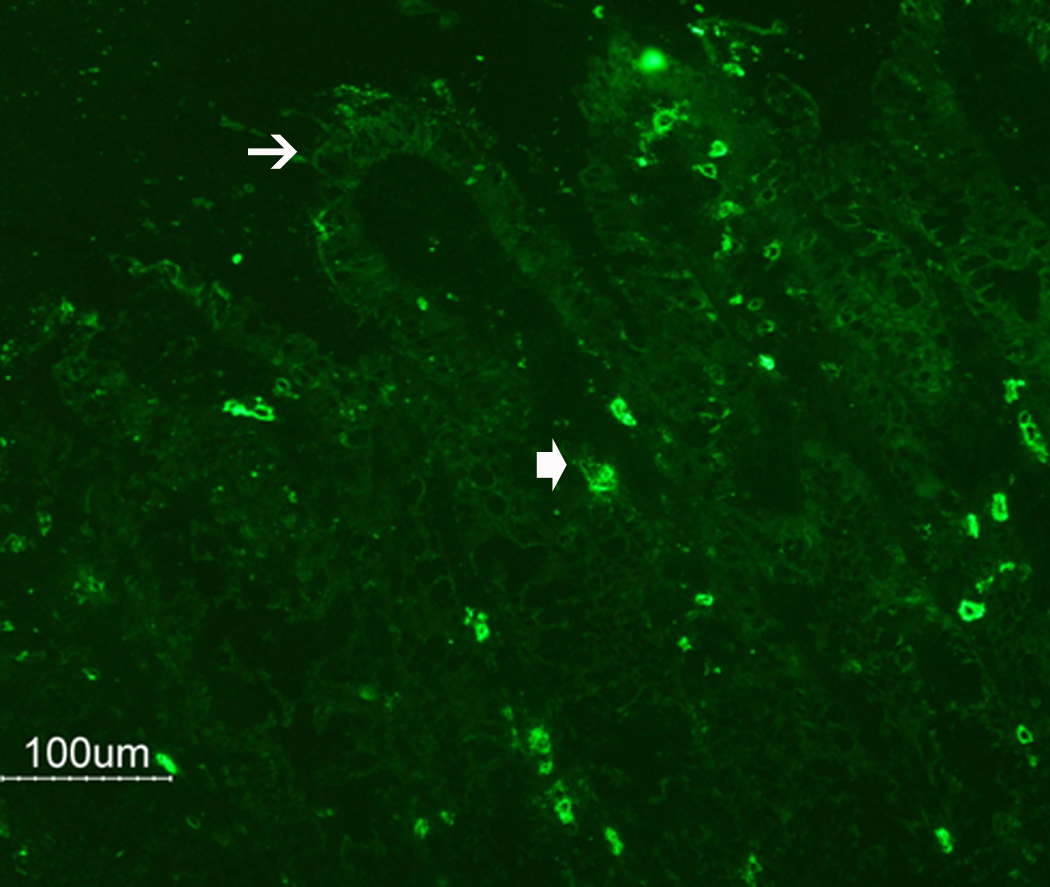
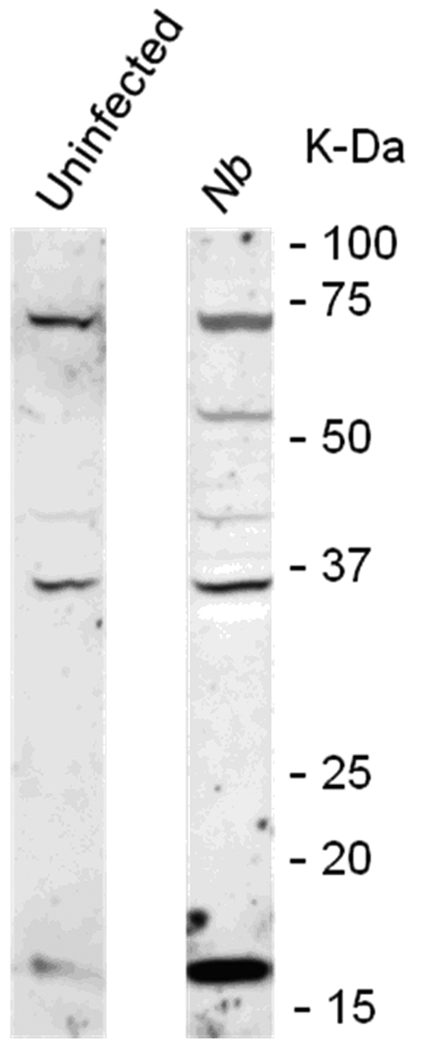
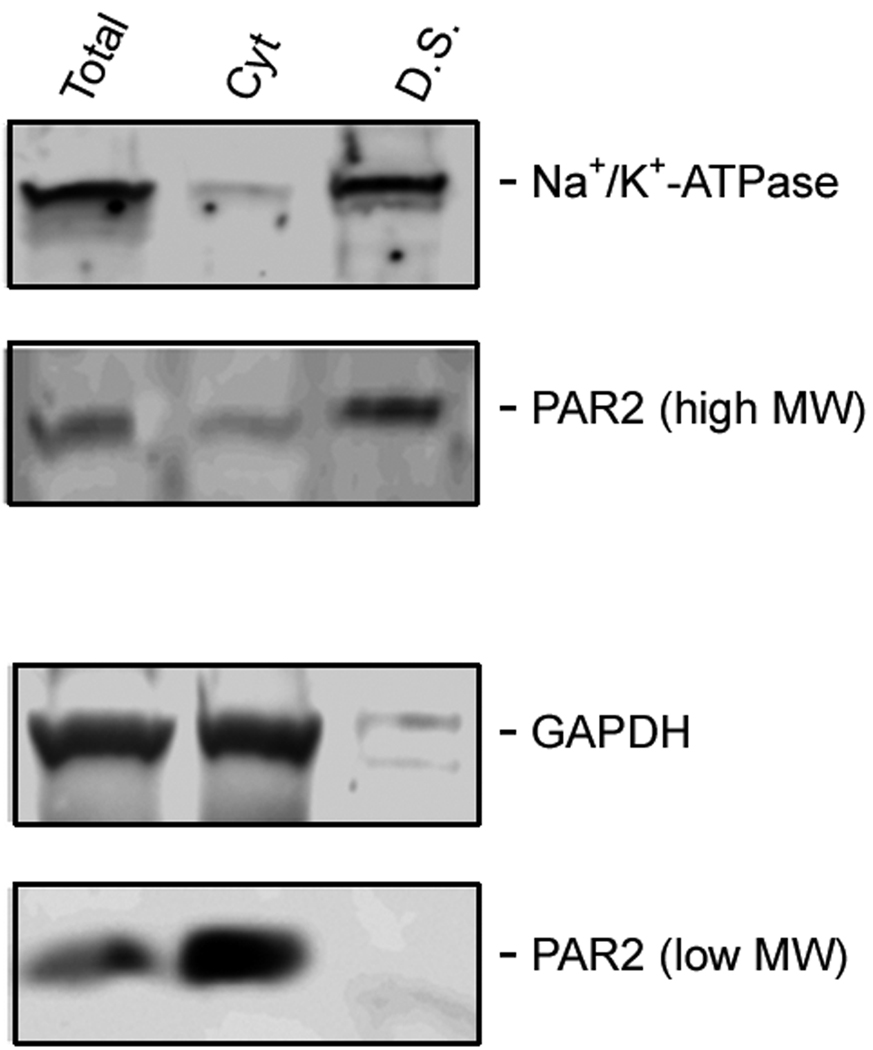
N. brasiliensis infection induced a decrease in PAR2 expression on the epithelial cell surface. (A) Frozen tissue blocks of mid-jejuna were prepared and the sections were cut for immunofluorescence staining for PAR2. The pictures are representative of each group of at least 3 mice. Original magnification, 200x. (A) Tissue from uninfected mice shows staining on epithelial cells (arrows) and on cells in the lamina propria that may be resident mast cells (arrow heads). (B) Tissue from infected animals shows diminished staining in epithelial cells (arrows) and increased staining in lamina propria cells (arrow heads). (C) A representative western blot for total PAR2 staining in scraped mucosa is shown. Total immunoreactivity was 3-fold higher (p<0.05) in infected mice, with ~80% directed to a 17 kDa form of PAR2. (D) Western blot analysis for PAR2 was performed on fractionated mucosa along with membrane or cytosolic markers (as indicated). The full size PAR2 proteins co-partitioned with plasma membrane markers (Detergent Soluble, D.S.), while the 17 kDa form was co-fractionated with cytosolic markers (Cyt), consistent with an internalization following PAR2 activation.
To confirm that the decreased immunofluorescent staining was due to an internalization of PAR2 as a consequence of worm infection, mucosal tissue was scraped from small intestines of non-infected and N. brasiliensis-infected WT mice (n=5), fractionated by differential centrifugation in the presence or absence of detergents to isolate membrane-bound or cytosolic proteins, and prepared for western analysis for PAR2. Total staining for PAR2 was three-fold higher in infected mice (p<0.05), with most of the immunoreactivity (~80%) directed to a low molecular weight (17 kDa) form of PAR2 (Figure 5C). Furthermore, while the full size PAR2 co-fractionated with membrane markers (which include plasma membrane, endoplasmic reticulum, and early endosome), the 17 kDa form was internalized in the cytosolic compartment, and co-fractionated with cytosolic non-membrane-bound markers (Figure 5D).
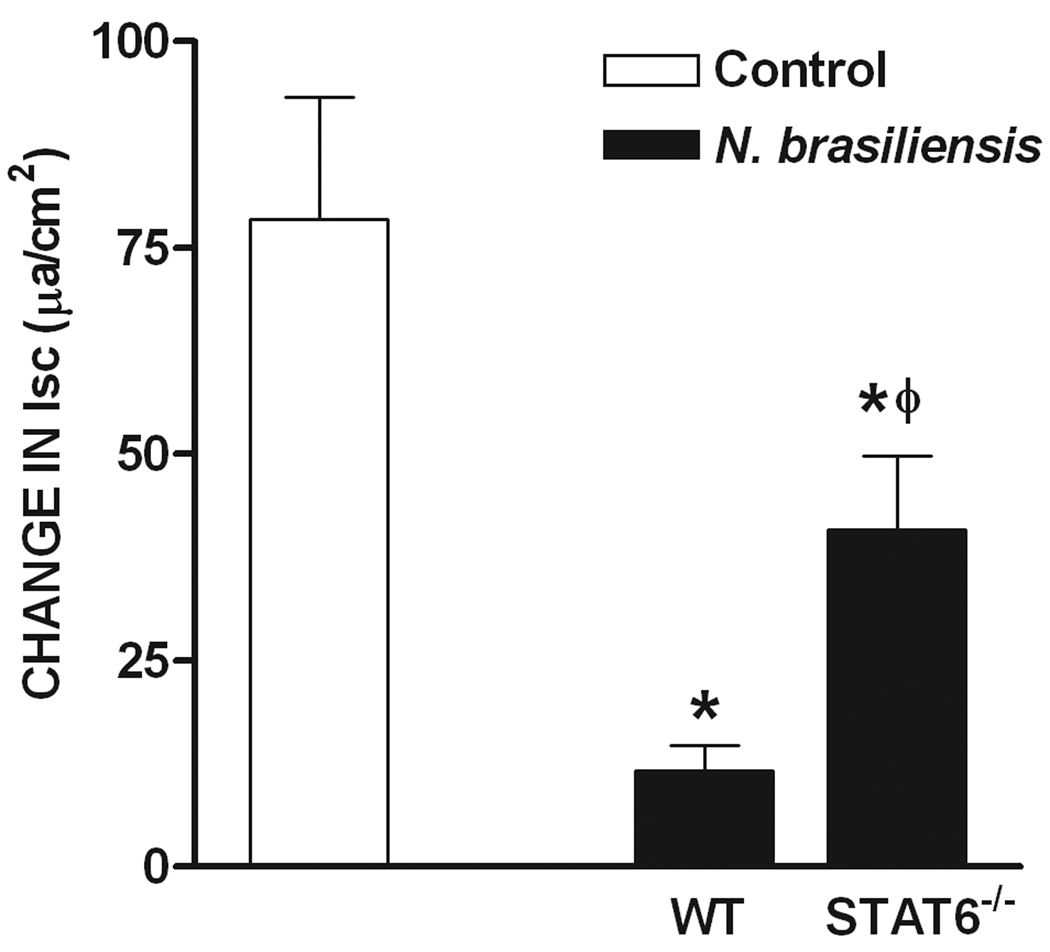
In control mice, epithelial responses to PAR2 agonists were independent of enteric nerves and the infection-induced hyposecretion to SLIGRL during infection was also unchanged by TTX treatment (Figure 6A). To determine if the infection-induced changes in epithelial responses to SLIGRL were dependent on Th2 cytokines, we compared responses in N. braseliensis-infected WT, STAT6−/−, and IL-4−/− mice. The infection-induced reduction in epithelial cell secretion in response to SLIGRL in WT mice was attenuated significantly, but not completely, in N. brasiliensis-infected STAT6−/− mice, but remained inhibited in IL-4−/− mice.
Figure 6.
Regulation of nematode infection-induced inhibition of Isc responses to serosal application of SLIGRL. (A) H. polygyrus (day 14 post infection)-induced inhibition of SLIGRL responses was similar in presence and absence of TTX. (B) N. brasiliensis (day 10 post infection)-induced inhibition of secretion in response to SLIGRL was attenuated in STAT6−/−, but was still observed in IL-4−/− mice *p<0.05 vs respective control, Φ p<0.05 vs WT infected (n≥5 for each group).
Discussion
Proteases are part of the profile of inflammatory mediators produced in a number of pathologies including inflammatory bowel disease (IBD) and the irritable bowel syndrome (IBS) as well as in animal models of these diseases20. PAR2 is implicated in the substance P-mediated neurogenic inflammation and enhanced responsiveness to mechanical stimuli that are thought to underlie the symptoms of visceral hypersensitivity32. Indeed, IBS patients have an elevated generation of serine proteases in the gut lumen that activate PAR233, consistent with the observation that exposure of the lumen to PAR2 agonists induced inflammation34. In this study, we investigated the effects of nematode infection-induced up-regulation of Th2 cytokines on PAR2 expression and the impact of these changes on small intestinal function. We showed an immune regulation of PAR2 expression that was associated with altered smooth muscle and epithelial cell responses to PAR2 agonists.
The nematode infection-induced up-regulation of PAR2 mRNA expression was STAT6-dependent suggesting the involvement of IL-4/IL-13. The fact that the increased PAR2 expression was observed in nematode-infected IL-4−/− mice, but not in IL-13−/− mice, indicates that PAR2 may be among a number of other genes, including PAR-1 and 5HT2A, which are specifically regulated by IL-13 and STAT629, 35. Infection-induced up-regulation of PAR2 mRNA expression was reproduced by exogenous administration of IL-13 to uninfected WT mice, and this supports the conclusion that during infection, IL-13 maintains the abundance of receptors thereby prolonging the functional effects of PAR2 agonists.
The well-documented mastocytosis associated with nematode infection36–38 provides increased local levels of mast cell protease-1 (mMCP-1), the major mast cell protease in the mouse that is required for expulsion of some nematodes38–40. Nematode infection, therefore, resulted in an increase not only in the number of PAR2, but also in the availability of agonists. The importance of this interaction is illustrated by the reports that mast cells play a key role in neurally-mediated changes in gut function associated with post-infectious IBS41.
Activation of PAR2 has variable effects on circular versus longitudinal smooth muscle and may differ further depending on the region of the GI tract 5, 6, 16, 42. In the current study, the hypercontractile response to PAR2 was dependent on STAT6, but not on IL-4. Although we did not perform studies in IL-13−/− mice, our data support a role for IL-13 in that the IL-13- induced hypercontractility to SLIGRL was not observed in STAT6−/− mice. Of interest is that both N. brasliensis and H. polygyrus infection enhanced both the relaxation and contraction phases of the smooth muscle responses to PAR2 agonists indicating that this is a general response to nematode infection, rather than a response to a specific nematode. The hypercontractility to PAR2 activation, therefore, is part of the stereotypic changes in smooth muscle contractility to acetylcholine, nerve stimulation, 5-HT and PAR1 reported previously25, 35, 43.
We also confirmed our previous data showing that PAR2 activation resulted in a small transient TTX-insensitive relaxation of longitudinal smooth muscle followed by a larger amplitude TTX-sensitive contraction29. The hypercontractile effect was almost entirely dependent on enteric nerves as TTX reduced the response to levels similar to those in uninfected controls. These data indicate that the infection-induced increase in contractility is due to the immune-mediated up-regulation of PAR2 on enteric nerves rather than smooth muscle. PAR2 and substance P play key roles in neurogenic inflammation44and while we did not explore the contribution of substance P in infection-induced hypercontractility to PAR2 agonists, we showed previously that the PAR2 actions on smooth muscle involved both NK1 and NK2 receptors16. The immune control of the nematode infection-induced hypercontractility to PAR2 agonists was consistent with immune-mediated changes in PAR2 expression that result in enhanced neurally-mediated effects on smooth muscle responses to PAR2 agonists. This effect may contribute to altered smooth muscle responses to food allergy, as well as those in IBS patients whose symptoms are linked to increased numbers of mast cells41. It is of interest to note that nematode infection-induced hypercontractility to PAR1 agonists29was not dependent on enteric nerves (unpublished data), implicating a separate outcome for the immune-mediated effects of PAR1 and PAR2 on smooth muscle function.
Epithelial responses to PAR2 agonists in murine small intestine were similar to those described in rats, including the lack of neural control and the dependence on cyclooxygenase generation of PGE215, 45, 46. We showed previously that nematode infection induced a stereotypic hyposecretion to a number of secretagogues, including acetylcholine and 5-HT47, 48 , which was mimicked by exogenous administration of IL-1349. Indeed, the increased intraluminal fluid in nematode infection is attributed to decreased absorption, rather than increased secretion47, 49. In the present study, responses to SLIGRL also were inhibited significantly in infected mice, an effect reproduced by administration of IL-13. These reduced epithelial cell responses were in contrast to the observed STAT6/IL-13-mediated up-regulation of PAR2 expression and the corresponding increases in smooth muscle responses to PAR2 agonists. These data are also opposite to that observed during Clostridium difficile (C. diff) toxicity, where increased PAR2 mRNA expression and enhanced immunoreactivity on enterocytes was associated with increased inflammation, neutrophil infiltration, and enhanced epithelial secretion 50. Since the functional response to PAR2 agonists is linked to the availability of PAR2 on the membrane, the nematode infection-induced hyposecretion suggested a loss of receptors on the epithelial cell. Immunofluorescent staining revealed an absence of PAR2 on epithelial cells in nematode-infected mice when compared to controls, but increased staining in the lamina propria consistent with mastocytosis. The reason for this pattern may be due to the ability of nematodes to generate proteases, including trypsin-like proteases that may activate PAR251–54. Activation of PAR2 resulted in receptor internalization and therefore, continuous exposure of epithelial cells to nematode proteases during the course of infection reduced the number of PAR2 on the epithelial surface due to receptor desensitization. This mechanism of control of PAR expression is consistent with the detection of high levels of cytosolic low molecular weight forms of PAR2 in infected mice, and has important implications in that PAR-mediated effects on epithelial cells and smooth muscle are dependent, in part, on expression of newly synthesized PARs and their targeting to the plasma membrane. This finding has important ramifications in that the number of PAR2 may limit the duration of the effects of luminal proteases on epithelial cell function during chronic infections. It also illustrated the differences in PAR2 effects on nerves (e.g. sensory afferents) in which agonist-triggered hyperexcitability is linked to second messenger-mediated changes in the sensitivity of channels such as transient receptor potential vanilloid 4 (TRPV4)32.
In conclusion, these studies are the first to show an immune regulation of PAR2 expression that affected function on both smooth muscle and epithelial cells. The hyposecretion to PAR2 agonists in epithelial cells and smooth muscle responses can be attributed to the reduced availability of PAR2 on the epithelial cell surface despite increased mRNA expression. The hypercontractility to PAR2 agonists in smooth muscle can be attributed to the up-regulation of PAR2 on enteric nerves and the resulting changes in the neural sensitivity that impact gut function. These downstream actions underlie the longer lasting effects of PAR2 activation that may contribute to IBS symptoms.
Aknowledgements
This work was supported by NIH grants RO1-AI/DK49316 (T.S-D), USDA CRIS project #1235-51000-055 (JFU).
Footnotes
The opinions and assertions in this article are those of the authors and do not necessarily represent those of the U. S. Department of Agriculture or the Department of Defense.
Contributions:
TSD, LN and AZ designed the studies, performed experiments, analyzed data and wrote the paper, JFU performed experiments and wrote the paper, JS, RS and KBM performed experiments and analyzed data.
References
- 1.Hollenberg MD, Saifeddine M, al Ani B, Kawabata A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- 2.Amadesi S, Bunnett N. Protease-activated receptors: protease signaling in the gastrointestinal tract. Current Opinion in Pharmacology. 2004;4:551–556. doi: 10.1016/j.coph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Vergnolle N. Review article: proteinase-activated receptors - novel signals for gastrointestinal pathophysiology. Aliment Pharmacol Ther. 2000;14:257–266. doi: 10.1046/j.1365-2036.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-Activated Receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 5.Cocks TM, Sozzi V, Moffatt JD, Selemidis S. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology. 1999;116:586–592. doi: 10.1016/s0016-5085(99)70180-0. [DOI] [PubMed] [Google Scholar]
- 6.Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, Payan DG, Bunnett NW. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao C, Liu S, Hu HZ, Gao N, Kim GY, Xia Y, Wood JD. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- 8.Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bohm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colognato R, Slupsky JR, Jendrach M, Burysek L, Syrovets T, Simmet T. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–2652. doi: 10.1182/blood-2002-08-2497. [DOI] [PubMed] [Google Scholar]
- 10.Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, Coelho AM, Singh P, Grady EF, Perdue M, Bunnett NW. Mast Cell Tryptase Controls Paracellular Permeability of the Intestine: ROLE OF PROTEASE-ACTIVATED RECEPTOR 2 AND {beta}-ARRESTINS. J Biol Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Syrovets T, Paskas S, Laumonnier Y, Simmet T. Mature Dendritic Cells Express Functional Thrombin Receptors Triggering Chemotaxis and CCL18/Pulmonary and Activation-Regulated Chemokine Induction. J Immunol. 2008;181:1215–1223. doi: 10.4049/jimmunol.181.2.1215. [DOI] [PubMed] [Google Scholar]
- 12.Mari B, Guerin S, Far DF, Breitmayer JP, Belhacene N, Peyron JF, Rossi B, Auberger P. Thrombin and trypsin-induced Ca(2+) mobilization in human T cell lines through interaction with different protease-activated receptors. FASEB J. 1996;10:309–316. doi: 10.1096/fasebj.10.2.8641564. [DOI] [PubMed] [Google Scholar]
- 13.Bueno L, Fioramonti J. Protease-activated receptor 2 and gut permeability: a review. Neurogastroenterol Motil. 2008;20:580–587. doi: 10.1111/j.1365-2982.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 14.Cenac N, Chin AC, Garcia-Villar R, Salvador-Cartier C, Ferrier L, Vergnolle N, Buret AG, Fioramonti J, Bueno L. PAR2 activation alters colonic paracellular permeability in mice via IFN-{gamma}-dependent and -independent pathways. J Physiol (Lond) 2004;558:913–925. doi: 10.1113/jphysiol.2004.061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vergnolle N, MacNaughton WK, Al-Ani B, Saifeddine M, Wallace JL, Hollenberg MD. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc Natl Acad Sci U S A. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao A, Shea-Donohue T. PAR-2 agonists induce contraction of murine small intestine through neurokinin receptors. Am J Physiol Gastrointest Liver Physiol. 2003;285:G696–G703. doi: 10.1152/ajpgi.00064.2003. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin SR, Camerer E. PARticipation in inflammation. J Clin Invest. 2003;111:25–27. doi: 10.1172/JCI17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci U S A. 2001;98:13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen KK, Sherman PM, Cellars L, ndrade-Gordon P, Pan Z, Baruch A, Wallace JL, Hollenberg MD, Vergnolle N. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci U S A. 2005;102:8363–8368. doi: 10.1073/pnas.0409535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergnolle N, Cellars L, Mencarelli A, Rizzo G, Swaminathan S, Beck P, Steinhoff M, ndrade-Gordon P, Bunnett NW, Hollenberg MD, Wallace JL, Cirino G, Fiorucci S. A role for proteinase-activated receptor-1 in inflammatory bowel diseases. J Clin Invest. 2006;116:2056. doi: 10.1172/JCI21689R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vergnolle N. Protease-activated receptors as drug targets in inflammation and pain. Pharmacology & Therapeutics. 2009;123:292–309. doi: 10.1016/j.pharmthera.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Urban JF, Jr, Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ, Gamble HR, Byrd C, Donaldson D, Else K, Finkelman FD. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 23.Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells3. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226–G232. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- 24.Khan WI, Vallance BA, Blennerhassett PA, Deng Y, Verdu EF, Matthaei KI, Collins SM. Critical role for signal transducer and activator of transcription factor 6 in mediating intestinal muscle hypercontractility and worm expulsion in Trichinella spiralis-infected mice. Infect Immun. 2001;69:838–844. doi: 10.1128/IAI.69.2.838-844.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao A, McDermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 26.Devlin M, Gasser R, Cocks T. Initial support for the hypothesis that PAR2 is involved in the immune response to Nippostrongylus brasiliensis in mice. Parasitology Research. 2007;101:105–109. doi: 10.1007/s00436-007-0467-1. [DOI] [PubMed] [Google Scholar]
- 27.Urban JF, Jr, Madden KB, Cheever AW, Trotta PP, Katona IM, Finkelman FD. IFN inhibits inflammatory responses and protective immunity in mice infected with the nematode parasite, Nippostrongylus brasiliensis. J Immunol. 1993;151:7086–7094. [PubMed] [Google Scholar]
- 28.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF, Jr, Shea-Donohue T. Immune Regulation of Protease-Activated Receptor-1 Expression in Murine Small Intestine during Nippostrongylus brasiliensis Infection. J Immunol. 2005;175:2563–2569. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linden DR, Manning BP, Bunnett NW, Mawe GM. Agonists of proteinase-activated receptor 2 excite guinea pig ileal myenteric neurons. Eur J Pharmacol. 2001;431:311–314. doi: 10.1016/s0014-2999(01)01447-9. [DOI] [PubMed] [Google Scholar]
- 31.Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, ndrade-Gordon P, Hollenberg MD, Wallace JL. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- 32.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. The Journal of Physiology. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, ndrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cenac N, Coelho AM, Nguyen C, Compton S, ndrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao A, Urban JF, Jr, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology. 2006;131:568–578. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Madden KB, Urban JF, Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis25. J Immunol. 1991;147:1387–1391. [PubMed] [Google Scholar]
- 37.Donaldson LE, Schmitt E, Huntley JF, Newlands GFJ, Grencis RK. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol. 1996;8:559–567. doi: 10.1093/intimm/8.4.559. [DOI] [PubMed] [Google Scholar]
- 38.Pennock JL, Grencis RK. The mast cell and gut nematodes: damage and defence. Chem Immunol Allergy. 2006;90:128–140. doi: 10.1159/000088885. [DOI] [PubMed] [Google Scholar]
- 39.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosbottom A, Knight PA, McLachlan G, Thornton EM, Wright SW, Miller HR, Scudamore CL. Chemokine and cytokine expression in murine intestinal epithelium following Nippostrongylus brasiliensis infection. Parasite Immunol. 2002;24:67–75. doi: 10.1046/j.0141-9838.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 41.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast Cell-Dependent Excitation of Visceral-Nociceptive Sensory Neurons in Irritable Bowel Syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 42.Kawabata A, Kuroda R, Nagata N, Kawao N, Masuko T, Nishikawa H, Kawai K. In vivo evidence that protease-activated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br J Pharmacol. 2001;133:1213–1218. doi: 10.1038/sj.bjp.0704211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF, Jr, Shea-Donohue T. Immune Regulation of Protease-Activated Receptor-1 Expression in Murine Small Intestine during Nippostrongylus brasiliensis Infection. J Immunol. 2005;175:2563–2569. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunnett NW. Protease-activated receptors: how proteases signal to cells to cause inflammation and pain. Semin Thromb Hemost. 2006;32(Suppl 1):39–48. doi: 10.1055/s-2006-939553. [DOI] [PubMed] [Google Scholar]
- 45.Cuffe JE, Bertog M, Velazquez-Rocha S, Dery O, Bunnett N, Korbmacher C. Basolateral PAR-2 receptors mediate KCl secretion and inhibition of Na+ absorption in the mouse distal colon. J Physiol. 2002;539:209–222. doi: 10.1113/jphysiol.2001.013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Merwe JQ, Ohland CL, Hirota CL, MacNaughton WK. Prostaglandin E2 Derived from Cyclooxygenases 1 and 2 Mediates Intestinal Epithelial Ion Transport Stimulated by the Activation of Protease-Activated Receptor 2. J Pharmacol Exp Ther. 2009;329:747–752. doi: 10.1124/jpet.108.145466. [DOI] [PubMed] [Google Scholar]
- 47.Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF, Jr, Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 48.Shea-Donohue T, Sullivan C, Finkelman FD, Madden KB, Morris SC, Goldhill J, Pineiro-Carrero V, Urban JF., Jr The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J Immunol. 2001;167:2234–2239. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 49.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 50.Cottrell GS, Amadesi S, Pikios S, Camerer E, Willardsen JA, Murphy BR, Caughey GH, Wolters PJ, Coughlin SR, Peterson A, Knecht W, Pothoulakis C, Bunnett NW, Grady EF. Protease-Activated Receptor 2, Dipeptidyl Peptidase I, and Proteases Mediate Clostridium difficile Toxin A Enteritis. Gastroenterology. 2007;132:2422–2437. doi: 10.1053/j.gastro.2007.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhoads ML, Fetterer RH, Hill DE, Urban J. Trichuris suis: A Secretory Chymotrypsin/Elastase Inhibitor with Potential as an Immunomodulator. Experimental Parasitology. 2000;95:36–44. doi: 10.1006/expr.2000.4502. [DOI] [PubMed] [Google Scholar]
- 52.Toubarro D, Lucena-Robles M, Nascimento G, Costa G, Montiel R, Coelho AV, Sim⌡es N. An apoptosis-inducing serine protease secreted by the entomopathogenic nematode Steinernema carpocapsae. International Journal for Parasitology. 2009;39:1319–1330. doi: 10.1016/j.ijpara.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Trap C, Boireau P. [Proteases in helminthic parasites.] Vet Res. 2000;31:461–471. doi: 10.1051/vetres:2000132. [DOI] [PubMed] [Google Scholar]
- 54.Trap C, Fu B, Le GF, Liu M, Le RD, Romand T, Perret C, Blaga R, Boireau P. Cloning and analysis of a cDNA encoding a putative serine protease comprising two trypsin-like domains of Trichinella spiralis. Parasitol Res. 2006;98:288–294. doi: 10.1007/s00436-005-0075-x. [DOI] [PubMed] [Google Scholar]