Abstract
Rationale
Apoptotic cell phagocytosis (efferocytosis) is mediated by specific receptors and is essential for resolution of inflammation. In chronic inflammation, apoptotic cell clearance is dysfunctional and soluble levels of several apoptotic cell receptors are elevated. Reports have identified proteolytic cleavage as a mechanism capable of releasing soluble apoptotic cell receptors, but the functional implications of their proteolysis are unclear.
Objective
To test the hypothesis that ADAM17-mediated cleavage of apoptotic cell receptors limits efferocytosis in vivo.
Methods and Results
In vivo comparison of macrophage efferocytosis in wildtype and Adam17-null hematopoietic chimeras demonstrates that ADAM17 deficiency leads to a 60% increase in efferocytosis and an enhanced anti-inflammatory phenotype in a model of peritonitis. In vitro uptake of phosphatidylserine liposomes identifies the dual-pass apoptotic cell receptor CD36 as a major contributor to enhanced efferocytosis, and CD36 surface levels are elevated on macrophages from Adam17-null mice. Further, temporal elevation of CD36 expression with inflammation may also contribute to its impact. Soluble CD36 from macrophage-conditioned media is comprised of two species based on Western blotting, and mass spectrometry identifies three N-terminal peptides, which represent probable cleavage sites. Levels of soluble CD36 are decreased in Adam17-null conditioned media, providing evidence for involvement of ADAM17 in CD36 cleavage. Importantly, enhanced efferocytosis in vivo by macrophages lacking ADAM17 is CD36 dependent and accelerates macrophage clearance from the peritoneum, thus promoting resolution of inflammation and highlighting the impact of increased apoptotic cell uptake.
Conclusions
Our studies demonstrate the importance of ADAM17-mediated proteolysis for in vivo efferocytosis regulation, and suggest a possible mechanistic link between chronic inflammation and defective efferocytosis.
Keywords: Proteolysis, metalloproteinase, inflammation, macrophage, apoptotic cells
INTRODUCTION
Efficient phagocytosis of apoptotic cells (efferocytosis) is an essential component of tissue homeostasis, wound healing, and the resolution of inflammation. Professional phagocytes, such as macrophages, employ a variety of transmembrane receptors to rapidly recognize and ingest apoptotic cells.1 After engulfing an apoptotic cell, macrophages actively dampen inflammation by releasing anti-inflammatory cytokines such as transforming growth factor-β and interleukin-10, as well as pro-resolving lipid mediators and eicosanoids which promote macrophage efflux and the resolution of inflammation.2, 3 However, if apoptotic cells are not rapidly cleared, secondary necrosis ensues, resulting in leakage of toxic intracellular antigens, tissue damage, and amplified inflammation. Defective efferocytosis is frequently observed in the context of chronic inflammation, with pathological sequelae ranging from non-resolving foot ulcers in diabetes to necrotic core expansion in atherosclerosis.4, 5 However, the underlying mechanisms responsible for deficient apoptotic cell uptake are poorly understood.
Biological fluids from patients with chronic inflammatory diseases also show elevated levels of soluble apoptotic cell receptors, including CD36, Mer tyrosine kinase (MerTK), and lectin-type oxidized LDL receptor (LOX)-1.6-8 CD36 is a two-pass transmembrane receptor,9 while MerTK10 and LOX-111 are type I and type II transmembrane proteins, respectively. MerTK and LOX-1 can be proteolytically cleaved by the transmembrane protease ADAM17.12, 13 Although the ubiquitously expressed ADAM17 was first described for its role in the shedding of cell-associated tumor necrosis factor-α,14, 15 it has since been shown to cleave a variety of cell surface proteins involved in inflammation.16, 17 Regulation of ADAM17 is poorly understood, and lack of reagents to monitor its levels and activity have hampered progress. Unlike soluble enzymes that cleave substrates at specific consensus sequences, ADAM17 cleaves its substrates at a membrane-proximal site in which stalk length and distance from the membrane appear to be important.18 ADAM17 membrane proximal cleavage of its substrates releases almost the entire extracellular domain, and the soluble ectodomain can often act as an antagonist, which has been shown for LOX-1 and MerTK.19, 20 Thus proteolysis of apoptotic cell receptors has the potential to rapidly decrease receptor surface levels and release a soluble antagonist, both of which could exacerbate deficient efferocytosis. However, the functional contribution of proteolysis to the regulation of apoptotic cell uptake in vivo has not been tested.
The current investigation tests the hypothesis that efferocytosis and its concomitant anti-inflammatory consequences are limited by proteolytic cleavage of apoptotic cell receptors from the macrophage surface. We show that ADAM17 deletion enhances macrophage-mediated efferocytosis in vivo, resulting in an augmented anti-inflammatory response. Additionally, we report that macrophage CD36 surface levels are elevated in the absence of ADAM17, and that there are three probable N-terminal cleavage sites in CD36. The absence of ADAM17 leads to a decrease in soluble CD36 levels. Blockade of CD36 in vivo is sufficient to abolish enhanced efferocytosis by Adam17-null macrophages, a process which also accelerates resolution of inflammation. Together these studies establish that ADAM17-mediated proteolysis of CD36 is an important post-translational mechanism controlling apoptotic cell phagocytosis, inflammation and its resolution.
METHODS
An expanded Methods section describing all procedures is available in the Online Data Supplement at http://circres.ahajournals.org
Hematopoietic chimeric mice
Adam17ΔEx5/ΔEx5 (Adam17 −/−) or WT hematopoietic chimeras were generated as previously described using C57BL/6 ES cells.21, 22 All mouse experiments were approved by the University of Washington Institutional Animal Care and Use Committee.
Sterile peritonitis model
Thioglycollate peritonitis was induced by injection of 1 ml of 4% sterile thioglycollate (BD Diagnostic, 2321398). Peritoneal cells (thioglycollate-elicited cells) were collected after 4 days by injection and removal of 5 ml PBS containing 5 mmol/L EDTA.
Flow cytometry
Staining of freshly isolated cells for flow cytometric analyses (FACScan, BD Pharmingen, 10-50,000 events) used antibodies listed in the Online Data Supplement. Flow data were analyzed using FlowJo 7.5 software (TreeStar).
Fab preparation
Protein-L purified IgAs from hybridoma media (anti-CD36, Clone CRF-D 2717, Roy Silverstein), or non-immune IgA (Sigma Aldrich, M-1421), were partially reduced to facilitate papain cleavage,23 and incubated with immobilized papain (Pierce, 20341) to generate Fab fragments.
In vivo efferocytosis
Thymuses (4-6 week C57BL/6 mice) were harvested, mechanically dissociated and filtered to yield a single-cell suspension. Thymocytes were labeled with TAMRA-SE dye (Molecular Probes, C-1171), and in vivo analysis of apoptotic cell uptake was performed.24
Liposome binding and uptake
Phosphatidylserine (PS)-rich liposomes (equal parts PS to phosphatidylcholine) were prepared with a 1% mole fraction of the fluorescent dye, 1-dioctadecyl-3,3-tetramethylindocarbocianin perchlorate (DiI, Sigma, 42364) by extrusion through a 0.1 μm polycarbonate membrane.25 Thioglycollate-elicited cells from WT or ADAM17 null hematopoietic chimeras were plated and macrophages (> 95%) adherent after 2 hours were used for binding and uptake studies.
Soluble CD36 characterization
Thioglycollate-elicited macrophages were plated 2 hours and adherent cells were cultured with 1,000,000 U/L human macrophage colony-stimulating factor (gift from Chiron), or other stimulants, in Opti-MEM (Invitrogen) for 4, 6 or 24 hours at 37° C. Conditioned media was centrifuged at 300 × g for 10 minutes to remove cell debris, followed by 28,300 × g for 140 minutes at 4°C to minimize microparticle content,26 and levels of CD36 were determined by ELISA using antibodies recognizing the extracellular domain of CD36. The resulting media were directly run on SDS-PAGE for Western analysis, or immunoprecipitated and run on SDS-PAGE for mass spectrometry (MS).
Identification of potential cleavage sites in soluble CD36
Gel bands corresponding to CD36 were detected by Coomassie staining, and were verified by CD36 immunoblot analysis of adjacent lanes. CD36 fractions were excised, subjected to standard in-gel digestion with trypsin, and digested peptides were analyzed by liquid chromatography-mass spectrometry (LCMS) analysis.
Statistical analysis
For statistical analysis, the Student’s t-test was performed using the InStat software, version 3.0b. All error bars represent standard error of the mean.
RESULTS
In vivo efferocytosis is enhanced in macrophages lacking ADAM17
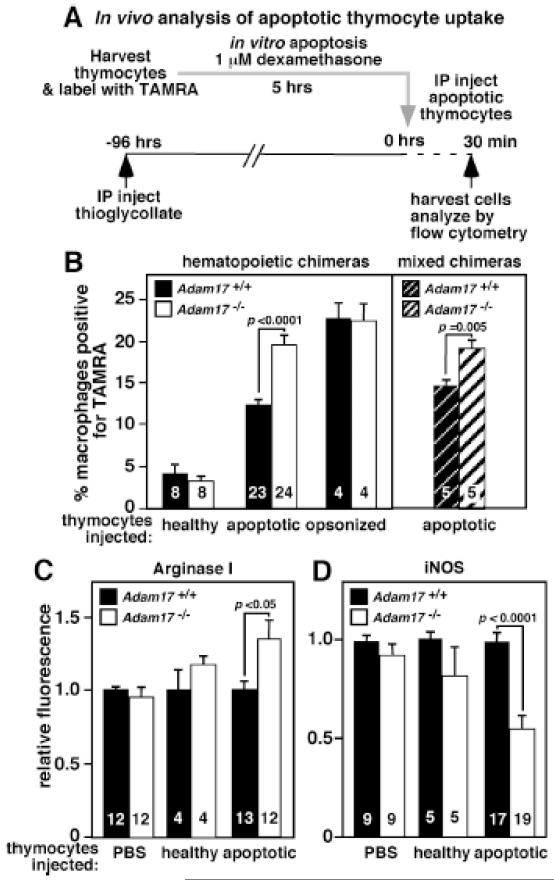
To test the hypothesis that efferocytosis and its downstream anti-inflammatory response are limited by proteolytic cleavage of macrophage apoptotic cell receptors, we evaluated the effect of leukocyte-specific deletion of the transmembrane protease ADAM17 on apoptotic cell phagocytosis in vivo. Adam17-null and WT hematopoietic chimeras were injected intraperitoneally with the sterile irritant thioglycollate to promote inflammatory monocyte influx. At 96 hours after thioglycollate, levels of cytokines released early in the response (2-4 hours) have abated and the influx of neutrophils has cleared (48 hours) leaving F4/80+ macrophages as the major leukocyte (>85%) in the peritoneal cavity.21 Fluorescently-labeled control or apoptotic thymocytes were injected into the peritoneum to evaluate efferocytosis (Figure 1A). No difference in thymocyte uptake between Adam17-null and WT macrophages is observed following injection of either healthy control or opsonized thymocytes (Figure 1B). In contrast, administration of apoptotic thymocytes demonstrates a 1.6-fold increase in Adam17-null macrophage binding and uptake of apoptotic cells relative to WT (Figure 1B). Uptake of fluorescently labeled apoptotic thymocytes was confirmed by fluorescent microscopy (Supplemental Figure 1). This significant increase in macrophage-mediated efferocytosis suggests that ADAM17 may directly or indirectly change levels of one or more apoptotic cell receptors.
Figure 1. Increased in vivo efferocytosis of apoptotic thymocytes by Adam17-null macrophages is cell autonomous, and shifts them to a less inflammatory phenotype.
A. The scheme for in vivo peritoneal macrophage uptake of TAMRA-labeled apoptotic thymocytes is shown. Healthy thymocytes and opsonized thymocytes were evaluated as controls. B. WT, Adam17-null, or mixed hematopoietic chimera mice that have ~50% WT and ~50% Adam17-null leukocytes were injected with the indicated thymocytes. The percent of TAMRA-positive macrophages was assessed by flow cytometry. C. Following in vivo exposure to PBS, healthy or apoptotic thymocytes, Adam17-null or WT macrophages were analyzed immediately following harvest for intracellular levels of arginase I, or D. cultured ex-vivo for 20 hours with lipopolysaccharide and IFNγ and analyzed for intracellular iNOS by flow cytometry. Numbers in the bars indicate the number of mice evaluated, and p values for significant differences are shown.
Mixed hematopoietic chimeras containing a 50% mixture of Adam17-null and WT bone marrow were evaluated to determine whether efferocytosis in the absence of ADAM17 is indirectly increased as a result of alteration of the extracellular inflammatory environment, for example by soluble mediators. Although both Adam17-null and WT cells in the mixed chimeras are exposed to an identical extracellular milieu, Adam17-null macrophages still display enhanced efferocytosis (Figure 1B). These results indicate that the increase in apoptotic cell phagocytosis by Adam17-null macrophages is cell intrinsic and directly impacts apoptotic cell receptor function.
Efferocytosis has been shown to actively inhibit the macrophage inflammatory response.2 To evaluate whether elevated apoptotic cell uptake alters the inflammatory phenotype of Adam17-null macrophages, intracellular protein levels of arginase I and inducible nitric oxide synthase (iNOS) were compared by flow cytometry following injection of apoptotic thymocytes (representative histograms in Supplemental Figure 2), healthy thymocytes or PBS. Following in vivo uptake of apoptotic cells, Adam17-null macrophages show a 34% elevation in arginase I levels relative to WT (Figure 1C). In addition, subsequent inflammatory stimulation ex vivo leads to a 45% reduction in iNOS induction in the null macrophages (Figure 1D). No differences in arginase I or iNOS levels were observed in Adam17-null or WT macrophages injected with control thymocytes (Figure 1C and D). Although we can’t eliminate the possibility that prior uptake of apoptotic neutrophils during the thioglycollate response may alter macrophage phenotype and/or efferocytosis, Adam17-null macrophages did not demonstrate altered arginase signaling detectable at 96 hours. Overall, ADAM17 deletion significantly augments the macrophage efferocytosis-induced anti-inflammatory phenotype.
Increased phosphatidylserine liposome binding/uptake by Adam17-null macrophages is CD36 dependent and is associated with elevated CD36 surface levels
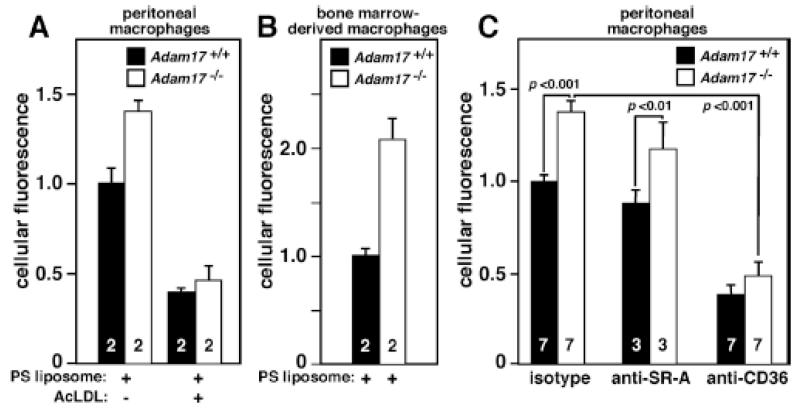
Macrophages express a variety of apoptotic cell receptors that may be modulated by ADAM17. Since macrophage efferocytosis is dependent upon apoptotic cell receptor recognition of phosphatidylserine (PS) exposed by cells undergoing apoptosis,2 PS liposomes were employed as a surrogate for apoptotic cells for an in vitro screen of potential receptors responsible for the enhanced efferocytosis by Adam17-null macrophages. Adam17-null peritoneal macrophages bind more PS liposomes in vitro (Figure 2A and 2C), but not phosphatidylcholine liposomes (data not shown), suggesting that ADAM17 deletion results in elevated levels of receptors that recognize PS exposed on apoptotic cells. Similar results were obtained with Adam17-null versus WT bone-marrow-derived macrophages (Figure 2B). Although many apoptotic cell receptors can bind PS, a unique subset of receptors shares an affinity for acetylated LDL (acLDL) as a ligand. When acLDL is added with PS liposomes, the ADAM17-dependent difference in PS liposome binding is abolished (Figure 2A). This finding suggests that the receptors responsible for elevated liposome binding by Adam17-null macrophages have the ability to directly bind both acLDL and PS liposomes - a trait shared by CD36 and SR-B1.27 However, SR-B1 surface levels were not detectable above isotype control staining of WT or Adam17 null macrophages (data not shown), thus focusing our attention on CD36.
Figure 2. Adam17-null peritoneal and bone marrow-derived macrophages show increased phosphatidylserine liposome binding/uptake, and peritoneal macrophage uptake is CD36-dependent.
A. Peritoneal macrophages from WT or Adam17-null hematopoietic chimera mice were harvested 96 hours after thioglycollate injection, and either plated and treated with or without acetylated-LDL at 4°C for 15 minutes followed by incubation with 40 μmol/L fluorescently labeled phosphatidylserine (PS) liposomes for 4 hours at 4°C. B. Bone-marrow-derived macrophages were isolated from whole bone marrow of WT or Adam17-null hematopoietic chimera mice and cultured for 7 days with M-CSF. Adherent cells were replated, and PS liposome binding was monitored as described in A. C. Peritoneal macrophages were plated and incubated with 160 μmol/L fluorescently labeled PS liposomes and 5 mg/L of either receptor-blocking or isotype control antibody for one hour at 37°C. Fluorescence was assessed by flow cytometry, numbers within the bars indicate the number of mice evaluated, and p values for significant differences are shown.
To first directly test the role of CD36 in the enhanced in vitro liposome binding and uptake by Adam17-null macrophages, the extent of inhibition by anti-CD36 or isotype control antibody was evaluated (Figure 2C). CD36 blockade abolishes the differential liposome uptake by Adam17-null peritoneal macrophages, and significantly reduces uptake by both Adam17-null and WT macrophages. In contrast, blocking antibody to SR-A, a prominent scavenger receptor, has no significant effect. Together these data identify CD36 as the primary apoptotic cell receptor leading to enhanced PS liposome binding and uptake in vitro by Adam17-null macrophages.
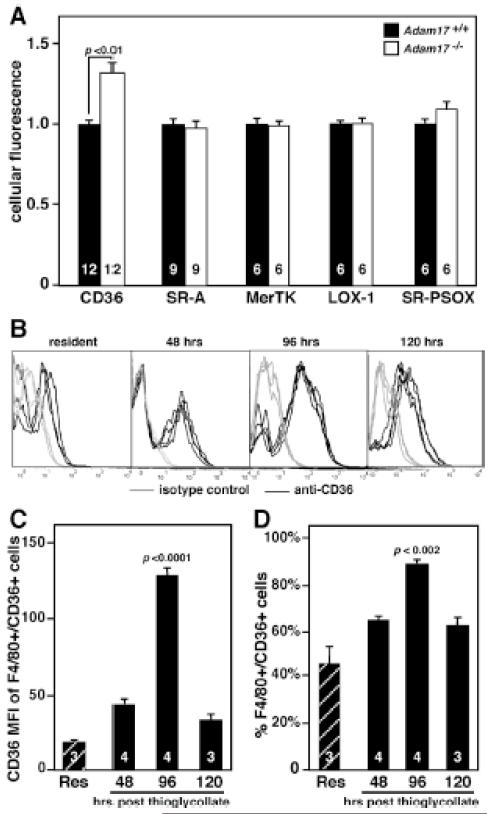
To more broadly screen for possible changes in apoptotic cell receptor surface levels, freshly isolated thioglycollate-elicited peritoneal macrophages from WT and Adam17-null chimeras were analyzed by flow cytometry. ADAM17 deletion results in a 30% increase in macrophage surface CD36 but no difference in other apoptotic cell receptors (Figure 3A), although MerTK, LOX-1 and SR-PSOX are reported substrates of ADAM17.12, 13, 28 CD36 surface levels are elevated to a similar extent on Adam17-null macrophages from mixed hematopoietic chimeras (data not shown), indicating a cell autonomous trait. Interestingly, CD36 surface levels are low in resident peritoneal macrophages but increase after thioglycollate injection, reaching a maximum at 96 hours (Figure 3B-D). These results suggest that CD36 may be important during the resolution phase of inflammation.2, 29 Also, no difference in CD36 mRNA levels is observed by qPCR analysis of Adam17-null and WT macrophages (data not shown), suggesting that ADAM17-dependent mechanisms regulate CD36 surface levels post-translationally. The combined data showing an acLDL-sensitive increase in PS liposome binding and uptake by Adam17-null macrophages, as well as elevated CD36 surface levels, implicate CD36 as a major target of ADAM17.
Figure 3. Cell surface levels of CD36 are elevated on peritoneal macrophages lacking ADAM17, and maximal expression of CD36 on WT macrophages is during the resolution phase of peritonitis.
WT resident peritoneal macrophages (Res) or peritoneal macrophages from WT or Adam17-null hematopoietic chimera mice were harvested at different times after thioglycollate injection and immediately stained with antibodies to the indicated apoptotic cell receptors and evaluated by flow cytometry. A. Levels of different apoptotic cell receptors were evaluated 96 hours after thioglycollate. B. Histograms following staining of resident peritoneal macrophages or peritoneal macrophages 48, 96 and 120 hours after thioglycollate with anti-CD36 or isotype control (n=3-4 mice/time point). C. The mean fluorescent index (MFI) of CD36 is indicated for F4/80+ macrophages that are CD36+. D. The % of F4/80+ cells expressing CD36 is shown. Numbers within bars indicate the number of mice evaluated, and p values for significant differences are shown.
ADAM17 deletion reduces levels of soluble CD36
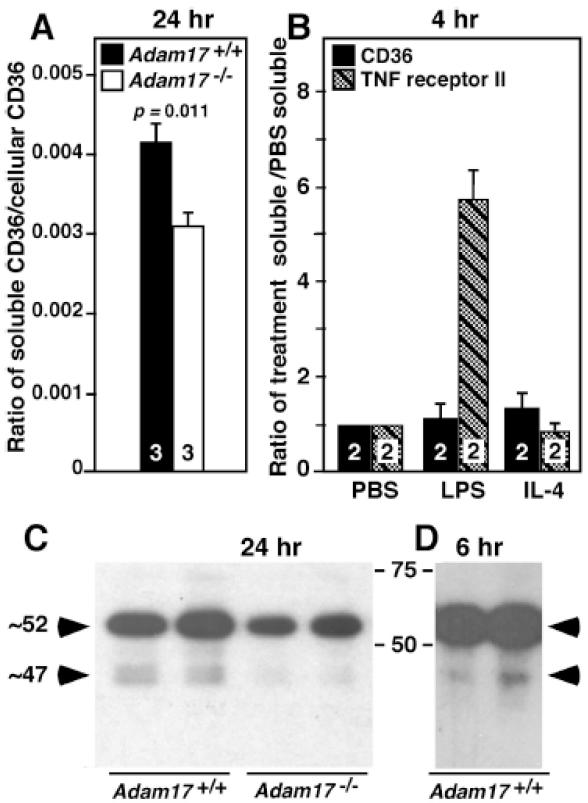
Elevated macrophage CD36 surface levels would be expected to coincide with decreased release of soluble CD36. To evaluate whether levels of soluble CD36 are altered by ADAM17 deficiency, 24-hour conditioned media were collected from adherent Adam17-null and WT peritoneal macrophages. To reduce potential microvesicle content, the media were centrifuged at high speed, which depletes microvesicles by ~75%.26 Quantification of media CD36 levels by ELISA using antibodies to the CD36 extracellular domain shows that ADAM17 deletion reduces the ratio of soluble/cellular CD36 by 25.8% (Figure 4A). Soluble forms of CD36 are not due to alternative splicing since this has been shown to occur in the 5′ noncoding region in all identified variants.30 Release of soluble CD36 from WT peritoneal macrophages was also investigated following 4-hour stimulation with PBS, LPS and interleukin (IL)-4 (Figure 4B). Although shedding of tumor necrosis factor (TNF) receptor II, another substrate of ADAM17, was increased more than 5-fold following LPS stimulation (Figure 4B), neither LPS nor IL-4 increased levels of soluble CD36 relative to PBS, suggesting its release is primarily constitutive.
Figure 4. Adam17 deletion results in a 25% decrease in the ratio of soluble to cell- associated CD36.
CD36 content and biochemical characteristics of microparticle-depleted conditioned medium and cell lysates from Adam17-null and WT peritoneal macrophages after the indicated incubation times were analyzed. A. CD36 levels in lysate and media were quantified by ELISA and expressed as the ratio of soluble to cellular CD36, and numbers within the bars indicate the number of mice of different genotypes whose macrophages were evaluated. These data are representative of 2 experiments. B. Soluble levels of CD36 and TNF receptor II were evaluated by ELISA following stimulation of WT peritoneal macrophages for 4 hours. Data is expressed relative to levels in PBS. Values are from two different experiments using macrophages from different mice determined in duplicate. C. and D. Western blot analysis of conditioned media from Adam17-null or WT peritoneal macrophages following deglycosylation with PNGase F. Each lane represents media collected from macrophages of different mice after the indicated time, and the collections for C are from a different experiment than shown in A.
Since CD36 is a highly glycosylated protein, Western analysis was performed following PNGase F treatment of media samples to assess molecular species (Figure 4C and 4D). Conditioned media (24-hour) from WT macrophages show primarily two regions of staining with apparent molecular weights of ~52 and ~47 kDa (Figure 4C). The ~47 kDa region appears as a doublet, although detection of two distinct species is variable. However, CD36 levels in both the ~52 and ~47 kDa regions are reduced in conditioned media from Adam17-null macrophages relative to WT. The ~47 kDa region is also detected in 6-hour conditioned media (Figure 4D), consistent with it not being a degradation product of the longer 24-hour incubation. Together, these observations provide the first evidence for a role of ADAM17 in the proteolytic release of soluble CD36.
Mass spectrometry identifies three probable N-terminal cleavage sites in soluble CD36
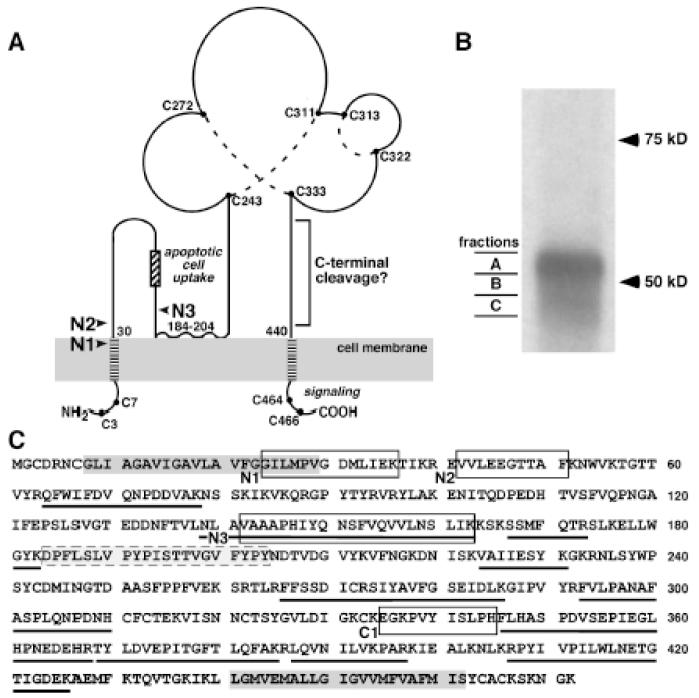
CD36 is predicted to be a dual-pass transmembrane protein with considerable extracellular topology (Figure 5A).9 To further characterize the species in soluble CD36 detected by Western analysis, conditioned media from WT macrophages were immunoprecipitated with anti-CD36 and three fractions were cut (Figure 5B) from the Coomassie-stained gel to include the ~52kDa region (fraction A), the zone between the two regions (fraction B) and the ~47kDa region (fraction C). In-gel tryptic digest followed by mass spectrometry was performed on each of the excised gel fractions. Mass spectrometry unequivocally identified CD36 in all fractions with high sequence coverage (A, 31%; B, 34%; and C, 32%), and all peptides included segments of the extracellular domain (Figure 5C). The prediction would be that peptides from both the N- and C-terminal regions of the extracellular domain with non-tryptic cleavage sites should be detected for soluble CD36.
Figure 5. Identification of novel proteolytic cleavage sites in soluble CD36.
A. Topology and domains of CD36 and possible ADAM17 cleavage sites are indicated with arrows. Possible N-terminal ADAM17 cleavage sites are indicated with arrows. A hydrophobic region between amino acids 184-204 may interact with the plasma membrane,9 creating additional regions adjacent to the plasma membrane. All of the cysteine residues in CD36 are shown as a solid circle, and dotted lines indicate disulfide bonds determined from MS analysis.40 CD36 domain data particularly implicate the C-terminal cytoplasmic domain in assembly of signaling complexes that mediate its multiple functions.38, 39 This diagram was adapted from Silverstein and Febbraio.9 B. Soluble CD36 immunoprecipitated from microparticle-depleted 24-hour-conditioned media was separated on SDS-PAGE following PNGase F treatment. Western showing three gel fractions (A, B, C) from the major regions of CD36 seen in Figure 4 and the area between were excised as indicated for mass spectrometric analysis. C. CD36 protein sequence is shown and peptides identified in the 3 gel slices are underlined, two putative transmembrane domains are in dark gray boxes, and a hydrophobic region that may interact with the plasma membrane is the gray box with dashed outline. The unique N-terminal peptides (N1, N2 and N3) and a C-terminal non-tryptic peptide (C1) are indicated by open black boxes. All of the indicated peptides were identified in at least 2 of 3 separate samples.
Mass spectrometric analysis identified three N-terminal semi-tryptic peptides (Figure 5A, 5C and Supplemental Figure 3). Peptide N1 results from a non-tryptic proteolytic cleavage between Gly23-Gly24. This unique cleavage site is estimated to be six amino-acids within the extracellular side of the putative N-terminal transmembrane domain (http://uniprot.org), which is not a typical cleavage site for ADAM17.18 Cleavage 12 amino acids from the transmembrane domain between Glu41-Val42, a more typical pattern for ADAM17, gives rise to peptide N2. Peptide N3 consists of amino acids 139-163 and contains the ADAM17 preferred amino acids alanine at the P1 position and valine at the P1′ position of the cleavage site as observed for several ADAM17 substrates including tumor necrosis factor-α.18 Although the N3 peptide is a greater distance from the transmembrane domain than other reported ADAM17 cleavage sites, it is adjacent to a hydrophobic region between amino acids 184 and 204 (Figure 5A and 5C) that may interact with the plasma membrane.9
Only one semi-tryptic peptide (C1) was identified in the C-terminal portion of CD36 (Figure 5A, 5C and Supplemental Figure 3D). Because its cleavage site is 95 amino acids from the C-terminal transmembrane domain, ADAM17 cleavage at this site is unlikely. However, because ADAM17 may share cleavage sites with trypsin (http://merops.sanger.ac.uk), it is possible that some tryptic peptides actually result from ADAM17 cleavage such as tryptic peptides with a C-terminal cleavage site at K426 and/or K430 (Figure 4C). Use of alternative enzymes for proteomics analysis would help address this issue. Nevertheless, our data provide strong evidence for proteolytic cleavage of CD36.
Increased in vivo apoptotic cell phagocytosis by Adam17-null macrophages is CD36 dependent and promotes accelerated resolution of inflammation
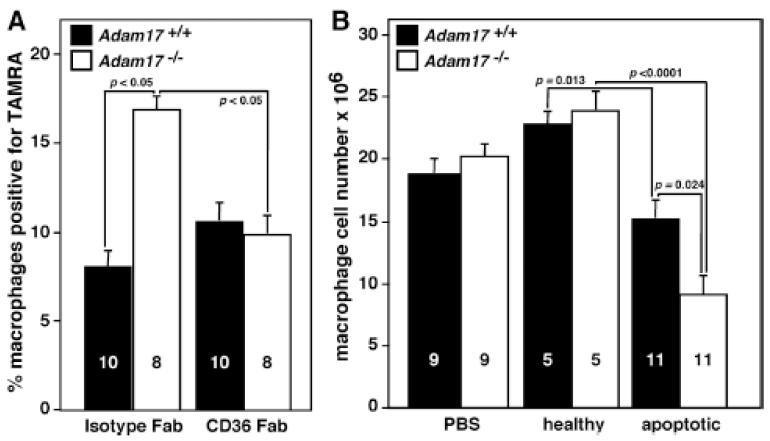
We next sought to directly test the contribution of CD36 to enhanced efferocytosis by Adam17-null macrophages in vivo. CD36-blocking or isotype control Fab were injected at the same time as the fluorescently-labeled apoptotic thymocytes into the peritoneum of chimeras. Fab was used to avoid CD36 dimerization and activation that occurs with whole IgA.31 CD36 blockade significantly decreases efferocytosis by Adam17-null, but not WT, macrophages (Figure 6A). The absence of an effect on apoptotic cell uptake by WT macrophages in vivo as compared with in vitro liposome uptake (Figure 2C) may be due to a disparate response to whole IgA used in the in vitro screen or differences in the effective dosage required in vivo. However, these in vivo data establish that CD36-mediated apoptotic cell phagocytosis is selectively enhanced in the absence of ADAM17.
Figure 6. Enhanced efferocytosis by Adam17-null macrophages is abolished by CD36 blocking Fab and resolution of inflammation is accelerated in mice lacking leukocyte ADAM17.
The in vivo apoptotic cell assay was performed as shown in Figure 1A. A. Fluorescently labeled apoptotic thymocytes, along with 80 μg of either isotype, or CD36-blocking Fab, were injected into the peritoneal cavity of Adam17-null and WT chimeric mice and TAMRA-positive macrophages assessed by flow cytometry. B. Total macrophage number in the peritoneal cavity was evaluated 30 min after injection of PBS, healthy thymocytes or apoptotic thymocytes to monitor the resolution of inflammation. The number of mice analyzed (within bars) and p values of significant differences are shown.
To investigate in vivo relevance of enhanced efferocytosis by Adam17-null macrophages, we asked whether resolution of the inflammatory response was enhanced following injection of apoptotic thymocytes. Administration of apoptotic thymocytes led to a 33% reduction in the number of WT macrophages within 30 minutes (Figure 6B), indicating that apoptotic cell uptake enhances macrophage clearance and inflammation resolution. Injection of healthy thymocytes or PBS had no effect on macrophage numbers, suggesting no cell-intrinsic acceleration of macrophage exiting at 96 hours. In contrast to WT macrophages, Adam17-null macrophage numbers were reduced 56% in response to efferocytosis (Figure 6B), demonstrating accelerated exiting and highlighting the in vivo impact of increased apoptotic cell uptake in the absence of ADAM17.
DISCUSSION
This study demonstrates for the first time that in vivo efferocytosis and its associated anti-inflammatory effects are enhanced by ADAM17 deletion, suggesting that ADAM17 normally functions to limit apoptotic cell phagocytosis. Unexpectedly, the major target of ADAM17 appears to be the dual-pass scavenger receptor CD36; its surface levels are elevated in the absence of ADAM17 and the ratio of soluble to cell-associated CD36 is decreased by 25.8%. Further, in the sterile peritonitis model, we detect no Adam17-dependent alterations in surface levels of any other major apoptotic cell receptors, including LOX-1 and MerTK, which were previously implicated as substrates of ADAM17.12, 13 Using mass spectrometry, three novel N-terminal peptides and one C-terminal peptide were detected in soluble CD36 that represent probable cleavage sites. In addition, we establish that enhanced in vivo efferocytosis in the absence of ADAM17 is CD36 dependent, and leads to accelerated clearance of macrophages. These data provide evidence for ADAM17 involvement in the shedding of a dual-pass transmembrane protein, and demonstrate the importance of proteolysis in controlling apoptotic cell uptake and resolution of inflammation associated with apoptotic cell uptake.
Recent reports have shown that ADAM17 can cleave MerTK and LOX-1 in vitro, and thus they were particularly likely targets.12, 13 However, we demonstrate no change in surface levels of either of these receptors in vivo, while CD36 levels are elevated in the absence of ADAM17. A likely explanation for the lack of effect on either MerTK or LOX-1 is that in vitro analyses have shown that their shedding requires stimulation with LPS or tumor necrosis factor-α, respectively.12, 13 Thus, ADAM17 cleavage of MerTK and LOX-1 may play a more significant role in responses to pathogens and/or on classically activated macrophages.
The ADAM17-dependent correlation between macrophage CD36 surface levels and in vivo enhanced efferocytosis further highlights the importance of CD36 as an apoptotic cell receptor. Previous experiments in CD36-deficient mice established that apoptotic cell burden in wound tissue is 2 to 3-fold greater than in WT mice, and in vitro efferocytosis was reduced by ~40% in CD36-deficient macrophages.32, 33 In our studies, elevated surface levels of CD36 in the absence of ADAM17 led to increased apoptotic cell uptake by inflammatory macrophages in vivo, and we demonstrate that the majority of ADAM17-dependent enhanced efferocytosis is abolished by CD36 blockade. Low levels of expression of CD36 on resident tissue and peritoneal macrophages and highest CD36 expression on inflammatory macrophages during the resolution phase suggest that its role in efferocytosis may be more important for inflammatory responses than for normal homeostasis. Further, we show that acceleration of macrophage clearance from the peritoneum is dependent upon apoptotic cell uptake. Together these data suggest a temporal and context-dependency for ADAM17 modulation of the resolution of inflammation.
To biochemically characterize soluble CD36, we analyzed media collected from cultured WT peritoneal macrophages, and our MS analysis uncovered three probable N-terminal cleavage sites. ADAM17 normally cleaves the extracellular stalk of its substrates proximal to the transmembrane domain (typically within 15 amino acids) with some preferences for particular residues flanking the cleavage site.18 The non-tryptic N-terminus of peptide N1 is estimated to be six amino acids into the putative transmembrane domain, and therefore is less likely to be a direct target of ADAM17. Since we cannot distinguish between ADAM17 involvement in primary and secondary cleavage events, it is possible that initial ADAM17-dependent cleavage of CD36 at N2, N3 or another site leads to activation of regulated intramembrane proteolysis, such as that mediated by γ-secretases,17 or that ADAM17 modulates cleavage by another protease and does not directly target CD36. With a cleavage site 12 amino acids from the transmembrane domain, peptide N2 is more typical of ADAM17 cleavage. In contrast, peptide N3 is 113 amino acids from the transmembrane domain, but 45 amino acids from a hydrophobic region of CD36 that may interact with the plasma membrane,9 and thus may localize the N3 cleavage site in a juxtamembrane position that favors interaction with ADAM17 (Figure 5A). It also contains alanine and valine preferred by ADAM17 in the P1 and P1′ position, respectively.18 Functionally, both N2 and N3 cleavage would likely disrupt the domain of CD36 (amino acids 155-183) implicated in macrophage phagocytosis of apoptotic cells.34 We identified only one C-terminal non-tryptic peptide (C1), and since it is 95 amino acids from the C-terminal transmembrane domain, it likely results from cleavage by an enzyme other than ADAM17. Since ADAM17 deletion only inhibits release of soluble CD36 by 25%, other proteases may be involved in liberation of soluble CD36, such as ADAM10 or multiple other ADAM proteases expressed by peritoneal and bone marrow-derived macrophages (Supplemental Figure 4). Detailed structural data for CD36 and a better understanding of its processing would facilitate interpretation of our data. Although we do not yet understand how soluble CD36 is released from the cell, our data collectively implicate ADAM17 in the shedding of CD36, and establish a role for ADAM17 in the regulation of surface levels of the ditopic transmembrane receptor CD36.
CD36 levels in serum are increased in several chronic inflammatory diseases, and have been found to positively correlate with mortality, type 2 diabetes, and atherosclerotic disease severity.8, 35, 36 However, the biochemical nature of soluble CD36 has not been evaluated in these contexts. A recent study suggested that the soluble CD36 found in the plasma of healthy donors is full-length CD36 and is a component of microparticles.37 Although this study analyzed isolated fractions by Western blot analysis and failed to detect CD36 in the microparticle-depleted platelet-free plasma,37 technical issues with the analysis of plasma may have limited the sensitivity of detection. Given the already robust correlations of soluble levels of CD36 with disease severity and early detection,8, 35 we plan to investigate the relative distribution of soluble CD36 in microparticles and cleaved soluble forms in different disease states, which could significantly enhance their usefulness as biomarkers. Our analysis of WT macrophage conditioned media in vitro showed a relative distribution of 0.48 ± 0.038% in media depleted of microparticles and 0.042 ± 0.001% in the microparticle pellet following ultracentrifugation, both relative to cell lysate CD36 (n=3/group). In the absence of ADAM17, CD36 in media was decreased 25.8% (p =0.011, n=3) and microparticle pellet content was increased by 14.3% (p =0.023, n=3). Our data suggest that under these in vitro conditions, shed CD36 in media is a more significant contributor than microparticle-derived CD36. More detailed biochemical analysis is needed to determine the extent to which soluble CD36 in chronic inflammatory diseases may result from ADAM17-mediated shedding.
Our studies have focused on enhanced uptake of apoptotic cells in the absence of macrophage ADAM17 and identified CD36 as the primary apoptotic cell receptor targeted by ADAM17. The increased ADAM17-mediated CD36 shedding uncovered in our study may provide a mechanistic link between the non-resolving nature of certain diseases and defective apoptotic cell phagocytosis. However, in addition to apoptotic cell uptake, CD36 has a number of other functions, such as uptake of pathogens and modified low-density lipoproteins important in inflammatory responses including atherosclerosis, mediation of long-chain fatty acid uptake and transport into cells involved in metabolic disorders, and binding thrombospondin and related proteins to inhibit angiogenesis in wound healing and various pathologies. Essential to these 9other functions is CD36 assembly of signaling complexes, most likely mediated by the C-terminal cytoplasmic domain.38, 39 Proteolysis would disable this downstream pathway, and thus it is likely that this mechanism has even broader implications for inflammation and disease pathogenesis than has been uncovered in our investigations. Therefore, it will be important for future studies to define the role of proteolysis in the multiple additional functions of CD36 in normal homeostasis and pathology.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Uptake of dying cells decreases inflammation and promotes its resolution.
In chronic inflammation, uptake of dying cells is impaired and the levels of the soluble forms of their receptors are elevated.
The proteolysis of cell surface receptors for dying cells can regulate the levels of these receptors on the surface of macrophages .
What New Information Does This Article Contribute?
Deficiency of the transmembrane protease ADAM17 in macrophages increases in vivo uptake of dying cells.
ADAM17 mediates the cleavage of the dual-pass apoptotic receptor CD36 and in Adam17-deficient mice enhanced uptake of dying cells is CD36-dependent.
ADAM17 limits the uptake of dying cells, and thereby the resolution of inflammation.
Dysfunctional uptake of dying cells during chronic inflammation impairs resolution of inflammation, but the underlying mechanisms are poorly understood. Here we show that in vivo proteolysis by ADAM17 controls the levels of the scavenger receptor CD36 on macrophage surface and therefore limits the uptake of dying cells by this receptor. As a post-translational mechanism, ADAM17 proteolysis can rapidly regulate the inflammatory response, but our studies suggest that ADAM17-mediated effects are dependent upon both temporal and contextual modulation of CD36 expression. These findings suggest a potential mechanistic link between proteolysis, chronic inflammation and defective uptake of dying cells.
ACKNOWLEDGMENTS
We thank Roy L. Silverstein (Medical College of Wisconsin) and Debra Rateri (University of Kentucky) for providing of antibodies to CD36 (Clone CRF-D 2717) and SR-A (guinea pig polyclonal), respectively. We also thank Cindy Chang for excellent technical assistance.
SOURCES OF FUNDING
This work was partially supported by grants from the NIH P01 HL018645 to EWR, TV by P01 HL092969, and WSD by an American Heart Association Predoctoral Fellowship and NIH Training Grant T32 HL07312. Mass spectrometric analysis was funded by NIH P30 DK017047 to the Diabetes Research Center and the University of Washington’s Proteomics Resource (UWPR95794).
Nonstandard Abbreviations
- acLDL
acetyl low-density lipoprotein
- iNOS
inducible nitric oxide synthase
- LCMS
liquid chromatography-mass spectrometry
- LOX-1
lectin-type oxidized low-density lipoprotein receptor
- MerTK
Mer tyrosine kinase
- MS
mass spectrometry
- PS
phosphatidylserine
- SR
scavenger receptor
- SR-PSOX
scavenger receptor for phosphatidylserine and oxidized LDL
- TNF
tumor necrosis factor
- WT
wildtype
Footnotes
DISCLOSURES
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Devitt A, Marshall LJ. The innate immune system and the clearance of apoptotic cells. J Leukoc Biol. 2011;90:447–457. doi: 10.1189/jlb.0211095. [DOI] [PubMed] [Google Scholar]
- 2.Huynh M-L, Fadok V, Henson P. Phosphatidylserine-dependent ingestion of apoptotic cells promotes tgf-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merched A, Ko K, Gotlinger K, Serhan C, Chan L. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. Faseb J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Ekman C, Jonsen A, Sturfelt G, Bengtsson AA, Gottsater A, Lindblad B, Lindqvist E, Saxne T, Dahlback B. Increased plasma levels of the soluble mer tyrosine kinase receptor in systemic lupus erythematosus relate to disease activity and nephritis. Arthritis Res Ther. 2011;13:R62. doi: 10.1186/ar3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kume N, Mitsuoka H, Hayashida K, Tanaka M, Kominami G, Kita T. Soluble lectin-like oxidized ldl receptor-1 (slox-1) as a sensitive and specific biomarker for acute coronary syndrome--comparison with other biomarkers. J Cardiol. 2010;56:159–165. doi: 10.1016/j.jjcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Handberg A, Norberg M, Stenlund H, Hallmans G, Attermann J, Eriksson JW. Soluble cd36 (scd36) clusters with markers of insulin resistance, and high scd36 is associated with increased type 2 diabetes risk. J Clin Endocrinol Metab. 2010;95:1939–1946. doi: 10.1210/jc.2009-2002. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein RL, Febbraio M. Cd36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham DK, Bowman GW, Dawson TL, Stanford WL, Earp HS, Snodgrass HR. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene. 1995;10:2349–2359. [PubMed] [Google Scholar]
- 11.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 12.Thorp E, Vaisar T, Subramanian M, Mautner L, Blobel C, Tabas I. Shedding of the mer tyrosine kinase receptor is mediated by adam17 protein through a pathway involving reactive oxygen species, protein kinase cdelta, and p38 mitogen-activated protein kinase (mapk) J Biol Chem. 2011;286:33335–33344. doi: 10.1074/jbc.M111.263020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao XQ, Zhang MW, Wang F, Zhao YX, Li JJ, Wang XP, Bu PL, Yang JM, Liu XL, Zhang MX, Gao F, Zhang C, Zhang Y. Crp enhances soluble lox-1 release from macrophages by activating tnf-alpha converting enzyme. J Lipid Res. 2011;52:923–933. doi: 10.1194/jlr.M015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 15.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 16.Garton KJ, Gough PJ, Raines EW. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J Leukoc Biol. 2006;79:1105–1116. doi: 10.1189/jlb.0106038. [DOI] [PubMed] [Google Scholar]
- 17.Murphy G, Murthy A, Khokha R. Clipping, shedding and ripping keep immunity on cue. Trends Immunol. 2008;29:75–82. doi: 10.1016/j.it.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Caescu CI, Jeschke GR, Turk BE. Active-site determinants of substrate recognition by the metalloproteinases tace and adam10. Biochem J. 2009;424:79–88. doi: 10.1042/BJ20090549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang J, Zarbock A, Gomez I, Wilson CL, Lefort CT, Stadtmann A, Bell B, Huang LC, Ley K, Raines EW. Adam17-dependent shedding limits early neutrophil influx but does not alter early monocyte recruitment to inflammatory sites. Blood. 2011;118:786–794. doi: 10.1182/blood-2010-11-321406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson C, Gough P, Chang C, Chan C, Frey J, Liu Y, Braun K, Chin M, Wight T, Raines E. Endothelial deletion of adam17 in mice results in defective remodeling of the semilunar valves and cardiac dysfunction in adults. Mech Dev. 2013 doi: 10.1016/j.mod.2013.01.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Underdown BJ, Dorrington KJ. Studies on the structural and conformational basis for the relative resistance of serum and secretory immunoglobulin a to proteolysis. J Immunol. 1974;112:949–959. [PubMed] [Google Scholar]
- 24.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishikawa K, Arai H, Inoue K. Scavenger receptor-mediated uptake and metabolism of lipid vesicles containing acidic phospholipids by mouse peritoneal macrophages. J Biol Chem. 1990;265:5226–5231. [PubMed] [Google Scholar]
- 26.Momen-Heravi F, Balaj L, Alian S, Trachtenberg A, Hochberg F, KSkog J, Kuo W. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 2012;3:162. doi: 10.3389/fphys.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigotti A, Acton SL, Krieger M. The class b scavenger receptors sr-bi and cd36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, Leesnitzer MA, Becherer JD. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases adam10 and adam17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen. 2005;8:161–171. doi: 10.2174/1386207053258488. [DOI] [PubMed] [Google Scholar]
- 29.Gomez I, Tang J, Wilson C, Yan W, Heinecke J, Harlan J, Raines E. Metalloproteinase-mediated shedding of integrin β2 promotes macrophage efflux from inflammatory sites. J Biol Chem. 2012;287:4581–4589. doi: 10.1074/jbc.M111.321182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato O, Kuriki C, Fukui Y, Motojima K. Dual prmoter structure of mouse and human fatty acid translocase/cd36 genes and unique transcriptional activation by peroxisome proliferator-activated receptor α and β. J Biol Chem. 2002;277:15703–15711. doi: 10.1074/jbc.M110158200. [DOI] [PubMed] [Google Scholar]
- 31.Finnemann SC, Silverstein RL. Differential roles of cd36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J Exp Med. 2001;194:1289–1298. doi: 10.1084/jem.194.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-cd36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas M, Stuart LM, Zhang A, Hodivala-Dilke K, Febbraio M, Silverstein R, Savill J, Lacy-Hulbert A. Requirements for apoptotic cell contact in regulation of macrophage responses. J Immunol. 2006;177:4047–4054. doi: 10.4049/jimmunol.177.6.4047. [DOI] [PubMed] [Google Scholar]
- 34.Navazo M, Daviet L, Savill J, Ren Y, Leung L, McGregor J. Identification of a domain (155-183) on cd36 implicated in the phagocytosis of apoptotic neutrophils. J Biol Chem. 1996;271:15381–15385. doi: 10.1074/jbc.271.26.15381. [DOI] [PubMed] [Google Scholar]
- 35.Chmielewski M, Bragfors-Helin AC, Stenvinkel P, Lindholm B, Anderstam B. Serum soluble cd36, assessed by a novel monoclonal antibody-based sandwich elisa, predicts cardiovascular mortality in dialysis patients. Clin Chim Acta. 2010;411:2079–2082. doi: 10.1016/j.cca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Handberg A, Hojlund K, Gastaldelli A, Flyvbjerg A, Dekker JM, Petrie J, Piatti P, Beck-Nielsen H. Plasma scd36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J Intern Med. 2011;271:294–304. doi: 10.1111/j.1365-2796.2011.02442.x. [DOI] [PubMed] [Google Scholar]
- 37.Alkhatatbeh MJ, Mhaidat NM, Enjeti AK, Lincz LF, Thorne RF. The putative diabetic plasma marker, soluble cd36, is non-cleaved, non-soluble and entirely associated with microparticles. J Thromb Haemost. 2011;9:844–851. doi: 10.1111/j.1538-7836.2011.04220.x. [DOI] [PubMed] [Google Scholar]
- 38.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A cd36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuart L, Deng J, Silver M, Takahashi K, Tseng A, Hennessy E, Ezekowitz R, Moore K. Response to staphylococcus aureus requires cd36-mediated phagocytosis triggered by the cooh-terminal cytoplasmic domain. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen J, Berglund L, Rasmussen M, Petersen T. Assignment of disulfide bridges in bovine cd36. Eur J Biochem. 1998;257:488–494. doi: 10.1046/j.1432-1327.1998.2570488.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.