Abstract
Background
Approximately 2% to 5% of endometrial cancers may be due to an inherited susceptibility. Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, an autosomal-dominant inherited cancer susceptibility syndrome caused by a germline mutation in one of the DNA mismatch repair genes, accounts for the majority of inherited cases. Lynch syndrome is associated with early onset of cancer and the development of multiple cancer types, particularly colon and endometrial cancer.
Methods
The current status of knowledge regarding Lynch syndrome-associated endometrial cancer and methods for diagnosis, screening and prevention of cancers are reviewed.
Results
The lifetime cumulative risk of endometrial cancer for women with Lynch syndrome is 40% to 60%, which equals or exceeds their risk of colorectal cancer. No current evidence suggests either a survival advantage or disadvantage to endometrial cancer that is associated with Lynch syndrome when these cases are compared with sporadic cases. A combination of family and personal medical history and tumor testing provides an efficient combination for diagnosing Lynch syndrome in women with endometrial cancer. Current gynecologic cancer screening guidelines for women with Lynch syndrome include annual endometrial sampling and transvaginal ultrasonography beginning at age 30 to 35 years.
Conclusions
Diagnosing endometrial cancer patients with Lynch syndrome has important clinical implications for the individual and family members. Screening and prevention practices can decrease the likelihood of developing additional cancers.
Introduction
It is estimated that 5% of endometrial cancer cases may be attributed to a site-specific inherited predisposition to cancer.1 Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, accounts for the majority of inherited endometrial cancers. Mutations in one of the four mismatch repair genes, hMLH1, hMSH2, hMSH6, or hPMS2 have been identified in patients with Lynch syndrome. This paper describes Lynch syndrome and its relevance for physicians and other health care workers caring for endometrial cancer patients, reviews the clinical and pathologic characteristics of Lynch syndrome-associated endometrial cancer, and outlines clinical recommendations for diagnosing individuals with Lynch syndrome and subsequent screening and prevention of secondary cancers.
Lynch Syndrome
Lynch syndrome, or HNPCC, is characterized by an increased risk for colorectal cancer and endometrial cancer. The estimated risk of developing colon cancer in women is 40% to 60% and in men as high as 80%.2 However, for women with Lynch syndrome, the lifetime endometrial cancer risk is also substantially increased, and in multiple studies, the risk of endometrial cancer in women with Lynch syndrome surpasses their risk of colon cancer (Table 1).2-6 For individuals with documented hMLH1 and hMSH2 germline mutations, the lifetime risk of endometrial cancer is estimated to be between 40% to 60%.3,4 In a study specifically evaluating cancer risk in women with hMSH6 mutations, the cumulative risk for endometrial cancer was 71% by 70 years of age.5 Risks of other cancers in Lynch syndrome are lower and include cancers of the renal pelvis, ovary, stomach, small bowel, and ureter.7
Table 1. Lifetime Cumulative Risk* of Lynch Syndrome-Associated Colorectal and Endometrial Cancer.
| Colorectal Cancer | Endometrial Cancer | ||||
|---|---|---|---|---|---|
| Authors | Defective Gene | Lifetime Cumulative Risk | Defective Gene | Lifetime Cumulative Risk | |
| Dunlop et al3 1997 | hMLH1 and hMSH2 | Men | 74% | hMLH1 and hMSH2 | 42% |
| Women (P = .006) |
30% | ||||
| Aarnio et al4 1999 | hMLH1 and hMSH2 | Men | 100% | hMLH1 and hMSH2 | 60% |
| Women | 54% | ||||
| Vasen et al2 1996† | hMLH1 and hMSH2 | Men | 92% | hMLH1 | 42% |
| Women | 83% | hMSH2 | 61% | ||
| Hendriks et al5 2004 | hMLH1 | Men | 65% | hMLH1 | 27% |
| Women | 53% | ||||
| hMSH2 | Men | 65% | hMSH2 | 40% | |
| Women | 53% | ||||
| hMSH6 | Men | 69% | hMSH6 | 71% | |
| Women | 30% | ||||
| Hampel et al6 2005 | hMLH1 and hMSH2 | Men | 69% | hMLH1 and hMSH2 | 54.1% |
| Women | 52% | ||||
Cumulative lifetime risk calculated by age 70.
Cumulative lifetime risk calculated by age 75.
Only significant P values are listed.
Lynch syndrome results from mutations in DNA mismatch repair genes (hMLH1, hMSH2, hMSH6, or hPMS2) that are inherited in an autosomal dominant fashion. Individuals with Lynch syndrome inherit one nonfunctional allele. When subsequent loss of the corresponding allele occurs, repair of genetic DNA is defective in the target tissue. Germline mutations in hMLH1 and hMSH2 account for over 90% of diagnosed Lynch syndrome cases.
Prior to the discovery of the responsible gene mutations, the diagnosis of Lynch syndrome was based on clinical criteria.8-11 The initial Amsterdam I criteria focused on colorectal cancer and were subsequently revised (Amsterdam II) to include all Lynch syndrome-associated cancers. The criteria include (1) three or more relatives with Lynch syndrome-associated cancers, (2) two affected relatives in successive generations, or (3) one or more relatives with a Lynch syndrome-associated cancer diagnosed before the age of 50 years.12
Mismatch Repair Defects and Microsatellite Instability
Defects in the mismatch repair genes hMLH1, hMSH2, hMSH6, or hPMS cause variations in the size of nucleotide repeats throughout the genome. This phenomenon is known as microsatellite instability (MSI) and is the molecular hallmark of DNA mismatch repair defects. High levels of microsatellite instability (MSI-high) measured in the DNA of tumor compared with DNA from normal tissues can be due to one of two causes: (1) Lynch syndrome or (2) a noninherited or sporadic cause via methylation and subsequent transcriptional silencing of the hMLH1 gene promoter. MSI-high tumors secondary to hMLH1 methylation has been well described previously and is estimated to occur in 15% to 20% of sporadic endometrial cancers.13
Clinical and Pathologic Outcomes in Lynch Syndrome-Associated Endometrial Cancer
Currently, there are no data to suggest that the prognosis for women with Lynch syndrome-associated endometrial cancers is either better or worse than for women with sporadic cancers. Vasen et al14 reported on a series of 125 women with endometrial cancer who fulfilled Amsterdam criteria. Genetic testing information was not available. The median age of diagnosis of endometrial cancer was 48 years with a range of 27 to 72 years, and 57% of cases were diagnosed in women under 50 years of age. Ninety-eight percent of cases were diagnosed in women under the age of 65 years. The overall survival rate was high, with only 12% of patients dying as a result of their endometrial cancers. However, 61% of these women developed a second primary cancer, and the majority (54 of 75) were colorectal cancers. In 15% of these women, more than two primary cancers were diagnosed.
A survival analysis was performed in a case-control study of 50 women with Lynch syndrome-associated endometrial cancer (based on either mutation analysis or meeting Amsterdam II criteria) matched to sporadic cases by age at diagnosis and FIGO stage. In this study there was no significant difference in tumor histology.15 The majority of tumors were endometrioid adenocarcinoma (92% for the study group and 88% for the control group). The overall 5-year cumulative survival rate was 88% for patients with Lynch syndrome-associated endometrial cancer and 82% for patients with sporadic endometrial cancer (P = .59).
Although there is general consensus that MSI-high colorectal cancers are associated with a more favorable prognosis, the clinicopathologic impact of MSI in endometrial cancers is not clear. The implication for MSI-high tumors resulting from Lynch syndrome is even less clear as most studies are performed on patients with sporadic MSI-high tumors. There have been conflicting reports on the association of MSI-high endometrial cancers with grade, stage, and clinical outcome.16 While some studies found no association between disease-free survival and MSI status17,18 or a negative prognosis for women with MSI-high tumors,19 studies showing an improved survival have also been reported. Black et al16 retrospectively reviewed 473 patients with endometrial cancer. In this study, 93 (20%) of the tumors were classified as MSI-high. In a multivariate analysis, disease-free survival was improved compared to MSI-negative tumors (hazard ratio [HR] = 0.3; 95% confidence interval [CI] = 0.2–0.7). Fiumicino et al19 reported on a small series of 65 cases of stage I and II primary sporadic endometrioid endometrial adenocarcinoma. In this series, 11 of 65 cases were found to be MSI-high. When compared to the microsatellite-stable (MS) cases, the MSI-high tumors were more likely to be poorly differentiated (50% vs 9%; P = .003). The 5-year disease-free survival rate of MSI-high cases was 63% compared with 96% for the MS controls (P = .0004). A recent large study by Zighelboim et al20 of 446 prospectively collected endometrial cancer cases found no association between MSI-high endometrioid endometrial cancers and disease-free survival (HR = 0.951; 95% CI = 0.554–1.635) or overall survival (HR = 1.011; 95% CI = 0.688–1.48).
Clinical and Histological Comparison of MSI-High Lynch Syndrome and Sporadic Cancers
Most of the research regarding MSI-high endometrial carcinoma has focused on sporadic tumors; thus the relevance to Lynch syndrome is not clear. Broaddus et al21 performed a large study comparing the pathologic features of 26 cases of sporadic MSI-high endometrial carcinoma, 42 cases of sporadic endometrial cancer in women with endometrial cancer diagnosed at age less than 50 years who were Lynch syndrome-negative, and 50 cases of Lynch syndrome-associated MSI-high endometrial carcinoma. In this series of 50 patients with Lynch syndrome, 78% were diagnosed as stage I, 10% as stage II, and 12% with stage III or IV disease. Lymph-vascular space involvement was noted in 24% of cases, and 26% had deep myometrial involvement that was defined as invasion > 50%. In comparison to the sporadic MSI-high endometrial cancer cases and the sporadic cases in women less than 50 years of age, there were no statistical differences in myometrial invasion, presence of lymph-vascular space invasion, and stage.
The histological subtype of the tumors proved to be the most striking difference between these three groups. Two groups — the sporadic group of women younger than age 50 and the sporadic MLH1 methylation group — were almost entirely composed of tumors with endometrioid histology (41/42, 97.6% and 25/26, 96.2%, respectively). However, the histology of Lynch syndrome-associated cancers was more heterogeneous, with 43/50 (86%) of the tumors being endometrioid and the remainder being papillary serous carcinoma, clear cell carcinoma, and malignant mixed Müllerian tumors. Although in the general population non-endometrioid endometrial carcinoma is typically diagnosed in older women, with a mean age of 65 to 68 years, the mean age of diagnosis of the non-endometrioid tumors in the Lynch syndrome patients in this study was 46.4 years, similar to the mean age of endometrial cancer diagnosis in the Lynch syndrome group overall (46.8 years). Interestingly, all of the non-endometrioid tumors in this study occurred in patients with hMSH2 mutations.
Together, almost 25% of the patients with Lynch syndrome had pathologic findings for which adjuvant radiation or chemotherapy would be indicated.21
Identification of Lynch Syndrome in Patients With Endometrial Cancer
Diagnosing individuals with Lynch syndrome is important for several reasons. Cancer patients with a germline mutation in one of the mismatch repair genes associated with Lynch syndrome are at significant lifetime risk of developing a second primary malignancy. In addition, once a specific mutation is identified, family members of affected individuals can then be tested. For example, identifying a specific gene mutation in a woman with endometrial cancer would allow her family members to undergo targeted predictive genetic testing for the same mutation.
Because colorectal surgeons, medical oncologists, and gastroenterologists have historically identified individuals with Lynch syndrome, prior emphasis on establishing guidelines to assist physicians in identifying patients with Lynch syndrome has focused on colon cancer patients. The Bethesda criteria, which were established in 1997 and revised in 2004, outline criteria to assist physicians in identifying patients with Lynch syndrome.22 However, as more evidence has accumulated, it is becoming increasingly clear that physicians caring for endometrial cancer patients need to be aware of the importance of Lynch syndrome. In a series of 117 women from families with Lynch syndrome who themselves had a history of both colorectal and either endometrial or ovarian cancer, 16 had a synchronous diagnosis of both cancers.23 For the remaining 101 women, 51% were diagnosed first with a gynecologic malignancy (endometrial or ovarian) and 49% were diagnosed first with a colorectal cancer. This underscores the necessity for gynecologic oncologists to take an active role in the identification of Lynch syndrome among their patients.
Identifying Patients With Lynch Syndrome
General guidelines that can alert health care providers of the presence of a hereditary cancer syndrome include early age of onset, presence of multiple and/or bilateral primary cancers, and multiple affected family members.24 To assist in specifically and more effectively identifying patients at risk for Lynch syndrome, the Society of Gynecologic Oncologists Education Committee recently published guidelines to help practicing clinicians determine which patients may have a 20% to 25% chance of having Lynch syndrome for whom genetic risk assessment is recommended and patients with a slightly lower risk (5% to 10%) for whom genetic risk assessment may be helpful (Table 2).25
Table 2. Society of Gynecologic Oncologists: Guidelines for Genetic Risk Assessment for Lynch Syndrome.
| Patients with greater than approximately 20%–25% chance of having an inherited predisposition to endometrial, colorectal, and related cancers and for whom genetic risk assessment is RECOMMENDED: | Patients with greater than approximately 5%–10% chance of having an inherited predisposition to endometrial, colorectal and related cancers and for whom genetic risk assessment may be HELPFUL: |
|---|---|
| Patients with endometrial or colorectal cancer who meet the revised Amsterdam criteria.12 | Patients with endometrial or colorectal cancer diagnosed < age 50. |
|
|
| Patients with synchronous or metachronous endometrial and colorectal cancer with the first cancer diagnosed < age 50 | Patients with endometrial or ovarian cancer with a synchronous or metachronous colon or other Lynch/HNPCC-associated tumor* at any age. |
| Patients with synchronous or metachronous ovarian and colorectal cancer with the first cancer diagnosed < age 50. | Patients with colorectal or endometrial cancer diagnosed at any age with two or more first- or second-degree relatives with Lynch/HNPCC-associated tumors,* regardless of age. |
| Patients with colorectal or endometrial cancer with evidence of a mismatch repair defect (ie, MSI or IHC loss of expression of MLH1, MSH2, MSH6 or PMS2). | Patients with a first- or second-degree relative that meets the above criteria. |
| Patients with a first- or second-degree relative with a known mismatch repair gene mutation. |
These tumors include colorectal, endometrial, stomach, ovarian, pancreas, ureter and renal pelvis, biliary tract, and brain (glioblastoma in Turcot syndrome), sebaceous gland adenomas, and keratoacanthomas in Muir-Torre syndrome and carcinoma of the small bowel. HNPCC = hereditary nonpolyposis colorectal cancer. Adapted from Lancaster JM, Powell CB, Kauff ND, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions.
In a population-based prevalence study, Hampel et al26 identified a 1.8% prevalence of germline mutations in hMLH1, hMSH2, or hMSH6 among unselected endometrial cancer patients. Two studies evaluating endometrial cancer patients under 50 years of age reported a 9% prevalence of germline mutations in either hMLH1, hMSH2, or hMSH6.27,28 One study found that 18% of individuals with synchronous or metachronous colon and endometrial cancers had Lynch syndrome.29 However, the finding of synchronous endometrial and ovarian cancers does not indicate the same risk. In a study by Soliman et al,30 only 7% of a cohort of women with synchronous endometrial and ovarian cancers met either clinical or molecular criteria for Lynch syndrome. Each of these patients also had a prior history of a Lynch syndrome-associated tumor or a first-degree relative with a history of a Lynch syndrome-associated tumor. The authors concluded that limiting genetic evaluation to women with synchronous endometrial and ovarian cancer who also have a family history suggestive of Lynch syndrome may be more appropriate than testing all women with synchronous endometrial and ovarian cancers.
Tissue Testing for Identifying Women With Lynch Syndrome
While family history information is important in identifying individuals who may benefit from genetic counseling and predictive genetic testing, the presence of MSI in tumors of patients with Lynch syndrome provides a useful adjunct for triaging which patients who may be at risk for having one of the germline DNA mismatch repair mutations. Tissue testing (immunohistochemistry [IHC] or MSI analysis) has emerged as a practical first step in the evaluation of women thought to be at risk for having Lynch syndrome. Tissue testing may be especially helpful in cases where, due to family or personal cancer history, individuals fall into the lower risk category of 5% to 10% risk of Lynch syndrome, such as individuals diagnosed with endometrial cancer before the age of 50 years. For both IHC and MSI, the health care provider needs to initiate the investigation by specifically ordering these tests.
At our institution, we perform IHC analysis for hMLH1, hMSH2, hMSH6, and hPMS2 and MSI analysis on formalin-fixed, paraffin-embedded tissues. The above antibodies are commercially available, and there is no need for special handling of tissue or extra fresh or frozen specimens. For the IHC tests, it is important that the pathologist choose sections of tumor that have some normal cells present because the nontumor cells behave as internal controls. Fig 1A-C images demonstrate positive staining for hMLH in an endometrioid endometrial carcinoma from a patient with Lynch syndrome. This tumor did not stain for hMSH2.
Fig 1A-C.
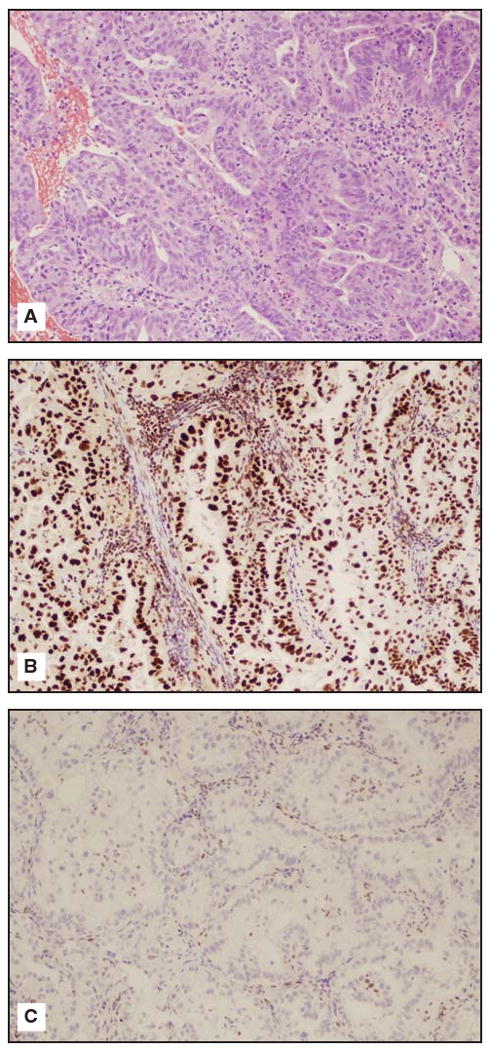
(A) Endometrioid endometrial carcinoma hematoxylin-eosin stain from a patient with Lynch Syndrome/hereditary nonpolyposis colorectal cancer (10 × power). (B) Endometrioid endometrial carcinoma from a patient with Lynch syndrome/HNPCC staining positively for hMLH1. (C) Endometrioid endometrial carcinoma from a patient with Lynch syndrome/HNPCC staining negatively for hMSH2. Courtesy of Russell R. Broaddus, MD, PhD.
We also perform MSI analysis in parallel with IHC at our institution. For MSI analysis, tumor and normal nontumor tissues are required. Any normal tissues from the hysterectomy specimen can be used, including cervix, benign fallopian tube, or benign lymph nodes. The pathologist maps on hematoxylin-eosin (H&E)-stained slides the areas of tumor and normal to be microdissected. Approximately 5 to 10 unstained slides of normal and tumor are needed to provide enough DNA to perform the polymerase chain reaction (PCR)-based MSI analysis. A panel of 7 markers recommended by the National Cancer Institute (BAT25, BAT26, BAT40, D2S123, D5S346, D173250, and TGF- R2) is used to detect changes in the number of microsatellite repeats in the tumor compared with normal tissue.31 The amplified DNA is analyzed on an ABI Genetic Analyzer (Applied Biosystems, Foster City, California) using capillary electrophoresis. Tumors with allelic shift in 2 or more microsatellites in the panel are considered MSI-high. Tumors with no allelic shift in all 7 microsatellites are considered microsatellite-stable. Tumors with allelic shift in only 1 microsatellite are considered MSI-low. The significance, if any, of MSI-low in endometrial and ovarian tumors is not known. A representative MSI chromatogram for the microsatellites BAT25, BAT26, and BAT40 is shown in Fig 2. The tumor DNA (lower tracing for each microsatellite) demonstrates an increased number of peaks compared with the DNA extracted from nonneoplastic tissue from the same patient (upper tracing for each microsatellite). Therefore, allelic shift is present for these 3 microsatellites. If a tumor exhibits such allelic shift in 2 or more of the panel of 7 microsatellites, the tumor is considered MSI-high.
Fig 2.
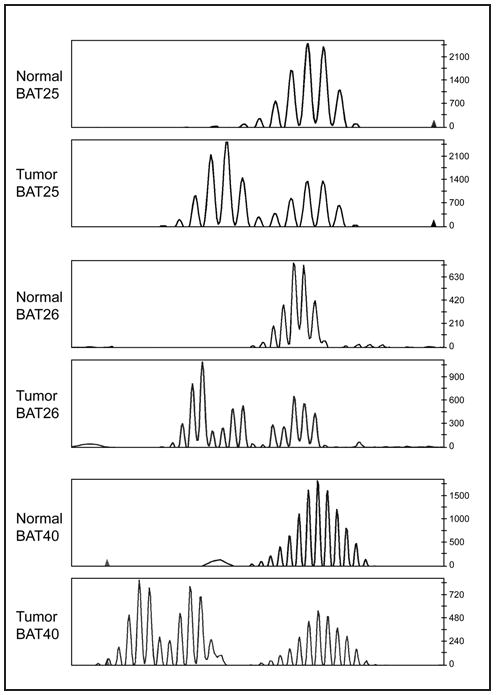
Representative MSI chromatogram for the microsatellites BAT25, BAT26, and BAT40. The tumor DNA (lower tracing for each microsatellite) demonstrates an increased number of peaks compared with the DNA extracted from nonneoplastic tissue from the same patient (upper tracing for each microsatellite). Therefore, allelic shift is present for these 3 microsatellites. If a tumor exhibits such allelic shift in 2 or more of the panel of 7 microsatellites, the tumor is considered MSI-high. Courtesy of Russell R. Broaddus, MD, PhD.
In addition, for MSI-high tumors with loss of MLH1 by IHC, we also perform methylation-specific PCR for MLH1 proximal promoter region −248 to −178 to detect possible methylation of the MLH1 promoter. Research studies have shown that this small proximal region in the MLH1 promoter located −248 to −178 relative to the gene transcription start site invariably correlates with the loss of MLH1 expression.32,33 If methylation is present, the patient most likely has a sporadic carcinoma rather than a Lynch syndrome-associated tumor. The MLH1 methylation assay can be performed using the same DNA extracted for the MSI analysis.
Genetic Testing of At-Risk Individuals
Whether identified on the basis of family or personal cancer history or tumor studies alone, or a combination of the above, genetic testing is important. Referral to a genetic counselor can be helpful for pretest counseling and information on the implications of genetic testing. Genetic testing can definitively diagnose individuals with Lynch syndrome; however, there are families who meet clinical criteria for Lynch syndrome for whom a specific mutation cannot be identified. Genetic testing for this syndrome is only currently available for mutations in hMLH1, hMSH2, or hMSH6 and is expensive (up to $3,000). As the prevalence of germline DNA mismatch repair genes is not thought to be greater than 5%, it is not reasonable to test all patients with endometrial cancer. After tissue testing, patients can have a more targeted approach to genetic testing. For example, a patient with an MSI-high tumor and loss of hMSH2 by IHC analysis can have focused testing of only the hMSH2 gene. If a specific germline mutation in one of the DNA mismatch repair genes is identified, then at-risk family members can also be evaluated for the same mutation at a substantially reduced cost (approximately $300 for “site specific” testing).
Screening and Prevention for Individuals With Lynch Syndrome
Diagnosis of Lynch syndrome in cancer patients and their relatives who have not yet developed a malignancy is important so that tailored screening and prevention can be offered. Lindor et al34 compiled a review of recommendations for the care of individuals with Lynch syndrome (Table 3). Cancer patients with Lynch syndrome are at high lifetime risk for a second malignancy and should be counseled regarding available screening and preventive measures. For example, Lynch-associated endometrial cancer survivors should undergo colorectal cancer screening. Women with Lynch syndrome-associated colorectal cancer may be counseled regarding the limited information on screening for endometrial cancer but offered screening ultrasound and in-office endometrial biopsy or prophylactic hysterectomy and bilateral salpingo-oophorectomy if fertility is no longer desirable. Family members who have not yet been diagnosed with any cancers should undergo screening for both endometrial cancer and colorectal cancer and may also benefit from prophylactic hysterectomy and bilateral salpingo-oophorectomy if child-bearing has been completed.
Table 3. Recommended Screening and Prevention Options for Women With Lynch Syndrome.
| Intervention | Recommendation |
|---|---|
| Colonoscopy | Every 1–2 years beginning at age 20–25 years or 10 years prior to the youngest age of cancer diagnosis in the family, whichever comes first. For MSH6 families, begin at age 30. |
| Endometrial sampling | Every year beginning at age 30–35 years. |
| Transvaginal ultrasound | Every year beginning at age 30–35 years. |
| Urinalysis with cytology | Every 1–2 years beginning at age 25–35 years. |
| History and physical examination | Every year beginning at age 21 with review of systems, education, and counseling. |
| Colorectal resection | Generally not recommended for primary prophylaxis, but if cancer is diagnosed, the preferred procedure is a subtotal colectomy. |
| Hysterectomy with bilateral salpingo-oophorectomy | Discuss as an option after childbearing is complete. |
Adapted from Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review.
Colorectal Cancer Screening
Järvinen et al35 reported that individuals from Lynch syndrome families undergoing prospective asymptomatic screening (colonoscopy or barium enema) had a 62% reduction in the incidence of invasive colorectal carcinoma compared with individuals from the same families who did not receive routine screening. Subsequent follow-up revealed that the group who underwent prospective colorectal cancer screening also had reduced mortality by approximately 65%.36 The current recommendations for colorectal cancer screening for individuals with known or suspected mutations in a DNA mismatch repair gene is colonoscopy every 1 to 2 years, starting at the age of 20 to 25 years (or age 30 years in families with known hMSH6 mutations).34 The age to begin screening is increased for families with hMSH6 mutations because some evidence suggests that the age of onset of colon cancer for such individuals is on average 10 years later than with mutations in the other genes associated with Lynch syndrome.37 Colonoscopy is the preferred method of screening as individuals with Lynch syndrome are more likely than those who develop sporadic colon cancer to have proximal tumors that may be missed by more limited screening. In addition, the majority of data on cancer incidence and mortality reduction following screening in this high-risk population was collected using colonoscopy for screening.34
Endometrial Cancer Screening
The data for endometrial cancer screening are less convincing for a variety of reasons. Because of the low prevalence of disease in the general population, the presence of early presenting symptoms such as vaginal bleeding, and the overall good survival rates for early disease, endometrial cancer screening programs for the general population do not exist and thus background data on sensitivity and specificity for screening in the general population are not available. Two studies were performed evaluating transvaginal ultrasound (TVU) and measurement of the endometrial lining in this high-risk population and reported that screening had a high false-positive rate and lacked efficacy.38,39 In one study of 41 women (35 premenopausal and 6 postmenopausal) diagnosed with Lynch syndrome via gene mutation or by fulfilling Amsterdam criteria, annual TVU and serum level CA-125 analysis were performed for screening for gynecologic malignancies.38 After a median follow-up of 5 years, the results from 17 of 179 ultrasounds (0.9%) suggested further evaluation be performed via endometrial sampling. From this, only 3 premalignant lesions were discovered. One interval endometrial cancer was detected after clinical symptoms were manifest. Dove-Edwin et al39 performed a study of TVU screening in 269 women who either had Lynch syndrome or came from Lynch syndrome-like families. During the study period, which incorporated 825.7 years of risk, only two cases of endometrial cancer were reported; both presented symptomatically as opposed to identified via screening ultrasound.
No studies to date have evaluated the efficacy of prospective in-office endometrial biopsy alone as a screening tool for women with Lynch syndrome. However, a Finnish study40 studied the combination of endometrial sampling and ultrasound in 175 women with germline mutations in MLH1, MSH2, or MSH6. Although there was no significant difference in long-term outcomes in the 11 patients with screen-detected endometrial cancer compared to 83 women from the same families who were diagnosed with symptom-detected endometrial cancer, this study revealed stage migration with 7% of women in the surveillance group presenting with stage III/IV disease vs 17% of women who presented symptomatically.
Despite the lack of convincing data of current screening methods for endometrial cancer, women with Lynch syndrome are at an increased lifetime risk of endometrial cancer and often develop it at a younger age when it may be more difficult to recognize bleeding as a warning sign. Additionally, TVU may be helpful in identifying ovarian abnormalities for this group of women who have a lifetime ovarian cancer risk of 6% to 12%.7,38 Therefore, screening is a reasonable option. For these reasons, TVU and in-office endometrial biopsy are currently offered to women with Lynch syndrome and are recommended annually for women over the age of 30 to 35 years.34 This recommendation is based on expert consensus.34,41 However, given the paucity of data, further studies evaluating ultrasound and endometrial biopsy or studies evaluating novel screening techniques for endometrial cancer are needed.
Prophylactic Surgery
Schmeler et al42 reported on the efficacy of prophylactic hysterectomy in reducing incidence of endometrial cancer in a cohort of women with a documented germline mutation in one of the DNA mismatch repair genes associated with Lynch syndrome. In their study, 61 women who underwent a prophylactic hysterectomy were matched with 210 women who did not undergo risk-reducing surgery. None of the women who underwent prophylactic hysterectomy developed endometrial cancer compared with 33% of the women in the comparison group. Thus, women with Lynch syndrome should be offered prophylactic hysterectomy and bilateral salpingo-oophorectomy as a reasonable prevention strategy with careful discussion of the potential risks, benefits, and alternatives to surgery.
The mean age at diagnosis for endometrial cancer in Lynch syndrome patients is 50 years.4 Because individuals with Lynch syndrome are at increased risk for endometrial cancer at a younger age than the general population, it is recommended that prophylactic surgery be performed after a woman no longer desires fertility as opposed to waiting until the onset of menopause. After childbearing is complete, women may have the procedure done through a traditional laparotomy (abdominal hysterectomy) or via a laparoscopic approach. Over the last decade, laparoscopic hysterectomy and bilateral salpingo-oophorectomy have been increasingly performed for both benign gynecologic indications and endometrial cancer.43,44 Benefits of a minimally invasive approach over abdominal hysterectomy include lower intraoperative blood loss, shorter hospital stay, faster return to normal activity, and decreased wound infections at the expense of an increased risk of urinary tract injuries (odds ratio = 2.61, 95% CI = 1.22–5.6).45 Additionally, women with Lynch syndrome who have been diagnosed with colorectal carcinoma may elect to have this procedure done in combination with colon surgery.46
Occult malignancy at the time of prophylactic hysterectomy has been reported in individuals with Lynch syndrome.47 Because of the risk of occult malignancy in either the endometrium or the ovaries at the time of prophylactic surgery, preoperative endometrial biopsy should be performed, ideally by a gynecologic oncologist or with arranged surgical back-up in case staging is necessary. In addition, at the time of surgery, communication with the pathologist is important. The pathologist should be aware that the patient has Lynch syndrome and the hysterectomy specimen opened with careful examination of both the endometrial cavity and ovaries. Any suspicious masses should undergo microscopic evaluation by frozen section. If no gross abnormalities are seen, then routine pathologic sampling and microscopic examination of the ovaries and endometrium can be performed.
Conclusions
Women with Lynch syndrome have a high lifetime risk of endometrial cancer and second primary tumors. As such, it is paramount that physicians caring for women with endometrial cancer maintain a high index of suspicion when obtaining personal and family history information when evaluating their patients so that an appropriate and timely diagnosis can be made. Tumor studies provide clinicians with an intermediate step prior to performing germline mutational analysis in evaluating individuals with Lynch syndrome related malignancies. They can rule out Lynch syndrome in these patients and can simplify genetic testing by targeting particular genes in individuals with positive results. A diagnosis of Lynch syndrome provides an opportunity to utilize screening and prevention strategies that may decrease the incidence and mortality of colorectal cancer. Further research is needed to determine the efficacy of screening methods compared with prophylactic surgery for the reduction of endometrial cancer morbidity and mortality in women with Lynch syndrome. Additional research is needed to identify possible chemoprevention strategies and to assess the effect of prophylactic surgery on survival and gynecologic cancer-related deaths. In the meantime, we recommend that individuals diagnosed with Lynch syndrome be counseled by their health care providers to follow the current screening recommendations34,41 and be offered the choice of prophylactic surgery.
Abbreviations used in this paper
- MSI
microsatellite instability
- IHC
immunohistochemistry
Footnotes
Disclosures: No significant relationship exists between the authors and the companies/organizations whose products or services may be referenced in this article.
References
- 1.Gruber SB, Thompson WD. A population-based study of endometrial cancer and familial risk in younger women. Cancer and Steroid Hormone Study Group. Cancer Epidemiol Biomarkers Prev. 1996;5(6):411–417. [PubMed] [Google Scholar]
- 2.Vasen HF, Wijnen JT, Menko FH, et al. Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology. 1996;110(4):1020–1027. doi: 10.1053/gast.1996.v110.pm8612988. Erratum in: [DOI] [PubMed] [Google Scholar]; Gastroenterology. 1996;111(5):1402. [Google Scholar]
- 3.Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6(1):105–110. doi: 10.1093/hmg/6.1.105. [DOI] [PubMed] [Google Scholar]
- 4.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81(2):214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Hendriks YM, Wagner A, Morreau H, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127(1):17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 6.Hampel H, Stephens JA, Pukkala E, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology. 2005;129(2):415–421. doi: 10.1016/j.gastro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123(2):444–449. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolaides NC, Papadopoulos N, Liu B, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos N, Nicolaides NC, Wei YF, et al. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- 10.Fishel R, Lescoe MK, Rao MR, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. Erratum in: [DOI] [PubMed] [Google Scholar]; Cell. 1994;77(1):167. [Google Scholar]
- 11.Bronner CE, Baker SM, Morrison PT, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 12.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 13.Esteller M, Levine R, Baylin SB, et al. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998;17(18):2413–2417. doi: 10.1038/sj.onc.1202178. [DOI] [PubMed] [Google Scholar]
- 14.Vasen HF, Watson P, Mecklin JP, et al. The epidemiology of endometrial cancer in hereditary nonpolyposis colorectal cancer. Anticancer Res. 1994;14(4B):1675–1678. [PubMed] [Google Scholar]
- 15.Boks DE, Trujillo AP, Voogd AC, et al. Survival analysis of endometrial carcinoma associated with hereditary nonpolyposis colorectal cancer. Int J Cancer. 2002;102(2):198–200. doi: 10.1002/ijc.10667. [DOI] [PubMed] [Google Scholar]
- 16.Black D, Soslow RA, Levine DA, et al. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006;24(11):1745–1753. doi: 10.1200/JCO.2005.04.1574. Epub 2006 Mar 20. [DOI] [PubMed] [Google Scholar]
- 17.Basil JB, Goodfellow PJ, Rader JS, et al. Clinical significance of microsatellite instability in endometrial carcinoma. Cancer. 2000;89(8):1758–1764. doi: 10.1002/1097-0142(20001015)89:8<1758::aid-cncr16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald ND, Salvesen HB, Ryan A, et al. Frequency and prognostic impact of microsatellite instability in a large population-based study of endometrial carcinomas. Cancer Res. 2000;60(6):1750–1752. [PubMed] [Google Scholar]
- 19.Fiumicino S, Ercoli A, Ferrandina G, et al. Microsatellite instability is an independent indicator of recurrence in sporadic stage I-II endometrial adenocarcinoma. J Clin Oncol. 2001;19(4):1008–1014. doi: 10.1200/JCO.2001.19.4.1008. [DOI] [PubMed] [Google Scholar]
- 20.Zighelboim I, Goodfellow PJ, Gao F, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25(15):2042–2048. doi: 10.1200/JCO.2006.08.2107. [DOI] [PubMed] [Google Scholar]
- 21.Broaddus RR, Lynch HT, Chen LM, et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer. 2006;106(1):87–94. doi: 10.1002/cncr.21560. [DOI] [PubMed] [Google Scholar]
- 22.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu KH, Dinh M, Kohlmann W, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105(3):569–574. doi: 10.1097/01.AOG.0000154885.44002.ae. [DOI] [PubMed] [Google Scholar]
- 24.Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23(2):276–292. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Lancaster JM, Powell CB, Kauff ND, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2007;107(2):159–162. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66(15):7810–7817. doi: 10.1158/0008-5472.CAN-06-1114. [DOI] [PubMed] [Google Scholar]
- 27.Lu KH, Schorge JO, Rodabaugh KJ, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25(33):5158–5164. doi: 10.1200/JCO.2007.10.8597. Epub 2007 Oct 9. [DOI] [PubMed] [Google Scholar]
- 28.Berends MJ, Wu Y, Sijmons RH, et al. Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol. 2003;21(23):4364–4370. doi: 10.1200/JCO.2003.04.094. [DOI] [PubMed] [Google Scholar]
- 29.Millar AL, Pal T, Madlensky L, et al. Mismatch repair gene defects contribute to the genetic basis of double primary cancers of the colorectum and endometrium. Hum Mol Genet. 1999;8(5):823–829. doi: 10.1093/hmg/8.5.823. [DOI] [PubMed] [Google Scholar]
- 30.Soliman PT, Broaddus RR, Schmeler KM, et al. Women with synchronous primary cancers of the endometrium and ovary: do they have Lynch syndrome? J Clin Oncol. 2005;23(36):9344–9350. doi: 10.1200/JCO.2005.03.5915. [DOI] [PubMed] [Google Scholar]
- 31.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. [PubMed] [Google Scholar]
- 32.Deng G, Chen A, Hong J, et al. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59(9):2029–2033. [PubMed] [Google Scholar]
- 33.Kang GH, Lee S, Shim YH, et al. Profile of methylated CpG sites of hMLH1 promoter in primary gastric carcinoma with microsatellite instability. Pathol Int. 2002;52(12):764–768. doi: 10.1046/j.1440-1827.2002.01423.x. [DOI] [PubMed] [Google Scholar]
- 34.Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296(12):1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 35.Järvinen HJ, Mecklin JP, Sistonen P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 1995;108(5):1405–1411. doi: 10.1016/0016-5085(95)90688-6. [DOI] [PubMed] [Google Scholar]
- 36.Järvinen HJ, Aarnio M. Surveillance on mutation carriers of DNA mismatch repair genes. Ann Chir Gynaecol. 2000;89(3):207–210. [PubMed] [Google Scholar]
- 37.Plaschke J, Engel C, Krüger S, et al. Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German Hereditary Nonpolyposis Colorectal Cancer Consortium. J Clin Oncol. 2004;22(22):4486–4494. doi: 10.1200/JCO.2004.02.033. Epub 2004 Oct 13. [DOI] [PubMed] [Google Scholar]
- 38.Rijcken FE, Mourits MJ, Kleibeuker JH, et al. Gynecologic screening in hereditary nonpolyposis colorectal cancer. Gynecol Oncol. 2003;91(1):74–80. doi: 10.1016/s0090-8258(03)00371-8. [DOI] [PubMed] [Google Scholar]
- 39.Dove-Edwin I, Boks D, Goff S, et al. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary non-polyposis colorectal carcinoma and familial colorectal carcinoma. Cancer. 2002;94(6):1708–1712. doi: 10.1002/cncr.10380. [DOI] [PubMed] [Google Scholar]
- 40.Renkonen-Sinisalo L, Bützow R, Leminen A, et al. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007;120(4):821–824. doi: 10.1002/ijc.22446. [DOI] [PubMed] [Google Scholar]
- 41.Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer) J Med Genet. 2007;44(6):353–362. doi: 10.1136/jmg.2007.048991. Epub 2007 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354(3):261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 43.Barakat RR, Lev G, Hummer AJ, et al. Twelve-year experience in the management of endometrial cancer: a change in surgical and postoperative radiation approaches. Gynecol Oncol. 2007;105(1):150–156. doi: 10.1016/j.ygyno.2006.11.007. Epub 2007 Jan 2. [DOI] [PubMed] [Google Scholar]
- 44.Kluivers KB, Hendriks JC, Mol BW, et al. Quality of life and surgical outcome after total laparoscopic hysterectomy versus total abdominal hysterectomy for benign disease: a randomized, controlled trial. J Minim Invasive Gynecol. 2007;14(2):145–152. doi: 10.1016/j.jmig.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Johnson N, Barlow D, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2006;2 doi: 10.1002/14651858.CD003677.pub3. CD003677. [DOI] [PubMed] [Google Scholar]
- 46.Lynch HT, Lanspa SJ, Boman BM, et al. Hereditary nonpolyposis colorectal cancer: Lynch syndromes I and II. Gastroenterol Clin North Am. 1988;17(4):679–712. [PubMed] [Google Scholar]
- 47.Chung L, Broaddus R, Crozier M, et al. Unexpected endometrial cancer at prophylactic hysterectomy in a woman with hereditary nonpolyposis colon cancer. Obstet Gynecol. 2003;102(5 Pt 2):1152–1155. doi: 10.1016/s0029-7844(03)00699-9. [DOI] [PubMed] [Google Scholar]


