Abstract
Numerous natural compounds have been extensively investigated for their potential for cancer prevention over decades. Curcumin, from Curcuma longa, is a highly promising natural compound that can be potentially used for chemoprevention of multiple cancers. Curcumin modulates multiple molecular pathways involved in the lengthy carcinogenesis process to exert its chemopreventive effects through several mechanisms: promoting apoptosis, inhibiting survival signals, scavenging reactive oxidative species (ROS), and reducing the inflammatory cancer microenvironment. Curcumin fulfills the characteristics for an ideal chemopreventive agent with its low toxicity, affordability, and easy accessibility. Nevertheless, the clinical application of curcumin is currently compromised by its poor bioavailability. Here we review the potential of curcumin in cancer prevention, its molecular targets, and action mechanisms. Finally, we suggest specific recommendations to improve its efficacy and bioavailability for clinical applications.
Keywords: Chemoprevention, Curcumin, Natural compound, Molecular target, Bioavailability
Introduction
Cancer is a major health problem that can debilitate and destroy human lives. One out of every four deaths in the U.S. is caused by cancer. Over $124.6 billion was spent in direct medical costs for the 13.7 million cancer survivors and 1.5 million newly diagnosed cancer patients in the U.S. in 2010. Increasing human life expectancy will inevitably raise cancer prevalence and the related costs. Consequently, the development of effective cancer prevention strategies is increasingly important. Histologically, the development of cancer involves multiple steps, which occur over several years after the initial carcinogen exposure from normal to hyperplasia, mild, moderate, and severe dysplasia, and carcinoma in situ, before finally progressing to invasive cancer (1). Throughout this long, multi-step developmental course, there is a wide scope of possible preventive approaches that can delay or prevent the development of cancer. Different cancer prevention strategies such as behavioral modification, vaccines, surgical manipulation, and chemoprevention have evolved with tremendous research efforts (2). Many investigations have proven that healthy lifestyles involving balanced diets, regular exercise, smoking cessation, alcohol reduction, weight control, and stress management are beneficial for decreasing cancer risk and can never be overemphasized (3–7). One particular milestone in cancer prevention was the approval by the U.S. Food and Drug Administration (FDA) of the human papilloma virus (HPV) cervical cancer vaccine in 2009 as a result of positive randomized controlled clinical trials.
The term chemoprevention was first coined by M. B. Sporn in 1976 who defined it as a preventive modality in which natural or synthetic agents can be employed to slow, stop, reverse, or prevent the development of cancer. Since then, researchers have investigated numerous agents for the purpose with few successes. The first important translational study of a potentially chemopreventive agent was conducted with 13-cis retinoic acid (13-cRA), which resulted in successful size reduction of the premalignant lesion oral leukoplakia, albeit with some notable toxicities (8). In an attempt to reduce the toxicity, this study was followed by another trial using high dose isotretinoin induction and maintenance with isotretinoin or beta carotene, which suggested that isotretinoin is significantly more effective than beta carotene against leukoplakia (9). Another follow up study using low dose isotretinoin and a large cohort of patients resulted in a negative outcome (10). In contrast, the field of breast cancer chemoprevention research gained considerable momentum after positive large-scale clinical trials of Tamoxifen, a selective estrogen receptor modulator (SERM), led to its FDA approval (11). However, not all cancer types have successful chemoprevention stories. In colorectal cancer, despite positive secondary clinical trials of sulindac, celecoxib, and aspirin, primary prevention using cyclooxygenase-2 (COX-2) inhibitors was shown to have no benefit in the general population and the study was terminated early due to cardiovascular toxicity (12–14). Another disappointment was the recently conducted selenium and vitamin E cancer prevention trial (SELECT), which gave negative results in patients with lung and prostate cancers (15). After several large negative clinical trials were reported, the focus of the new era in chemoprevention has shifted toward molecularly targeted agents and less toxic natural compounds.
In chemoprevention, safety of the participants is the first priority and should be considered of the utmost importance since essentially healthy people will receive the chemopreventive treatment for a long period of time. Moreover, the toxicity of the agents could impact patient accrual in larger scale studies in real clinical practice. To this end, unlike synthetic compounds, the safety of natural compounds present in fruits, vegetables, and spices are well established through their long-term consumption in human history (16). Therefore, taking natural compounds for cancer prevention can be a well justified and effective strategy for people with increased risk for cancer development – such as those with premalignant lesions of intraepithelial neoplasia. Among many such natural compounds, curcumin has drawn special attention for its chemoprevention potential because of its safety, multi-targeted anticancer effects, and easy accessibility (16). The following sections will discuss different aspects of curcumin as a chemopreventive agent, including its safety, efficacy, and mechanism of action.
Curcumin in Chemoprevention
Since 1987, the National Cancer Institute (NCI) has tested over 1,000 different potential agents for chemoprevention activity, of which only about 40 promising agents were moved to clinical trials (17). Curcumin, present in the Indian spice “haldi”, is one such agent that is currently under clinical investigation for cancer chemoprevention. Three polyphenols (Figure 1) were isolated from Curcuma longa, of which curcumin (bis-α,β-unsaturated β-diketone) is the most abundant, potent and extensively investigated (16). Curcumin has been used empirically as a remedy for many illnesses in different cultures. It is only in the last few decades that curcumin’s effects against cancer and cancer therapy-related complications have emerged, through much investigation. The first clinical report of the anticancer properties of curcumin was from Kuttan and coworkers, who used 1% curcumin ointment on skin cancerous lesions with a reduction in smell in 90% of patients (18). 10% patients experienced a reduction in pain and lesion size. In an experimental model of mammary cancer induced by 7,12-dimethylbenz-[a]-anthracene (DMBA) in female rats, the initiation of DMBA-induced mammary adenocarcinoma was significantly decreased by intraperitoneal infusion of curcumin 4 days before DMBA administration (19). In a study of esophageal cancer prevention in curcumin-fed F344 rats, the chemopreventive activity of curcumin was observed not only in the initiation phase but also in post-initiation phases (20). Also, in a familial adenomatous polyposis (FAP)-simulated study in which the APC gene of C57Bl/6J Min/+ mice was mutated to result in the development of numerous adenomas by 15 weeks of age, an oral curcumin diet prevented adenoma development in the intestinal tract, suggesting the chemopreventive effect of curcumin in colorectal cancer with APC mutation (21). Moreover, in a rat model of N-nitrosodiethylamine and phenobarbital-induced hepatic cancer, curcumin reduced lipid peroxidation and salvaged hepatic glutathione antioxidant defense, which eventually may have contributed to hepatic cancer prevention (22). Several studies of cancer prevention at different stages have demonstrated the multi-targeted anticancer and chemopreventive effects of curcumin and have suggested it as a very favorable agent for chemoprevention.
Figure 1.
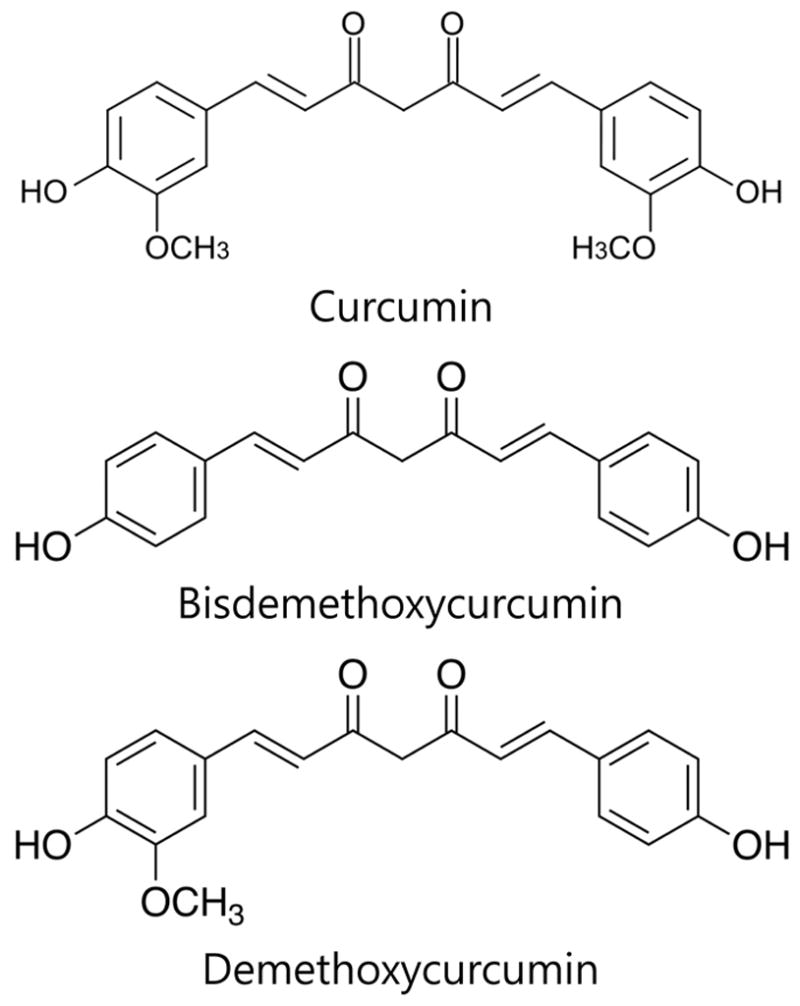
Chemical structure of three polyphenols from Curcuma longa
Mechanisms of Anticancer Effects
According to their mode of action, chemopreventive agents are classified into different subgroups: antiproliferatives, antioxidants, or carcinogen blocking agents. Curcumin belongs to all three subgroups, given its multiple mechanisms of action. The anticancer effects of curcumin mainly result from multiple biochemical mechanisms that are involved in the regulation of programmed cell death and survival signals. The curcumin targets that are involved in signaling pathways include transcription factors, growth factors, inflammatory cytokines, receptors, and enzymes (Figure 2). In different types of cancers, curcumin exhibits anticancer actions through a combination of different mechanisms including; survival signal reduction, proapoptotic promotion, anti-inflammatory actions, and reactive oxygen stress (ROS) scavenging to different degrees. The effects of curcumin on these signaling pathways are expected to be more complicated in the real setting, and the mechanisms of curcumin’s chemopreventive, chemosensitizing, and radiosensitizing effects are more vigorously being studied now.
Figure 2.
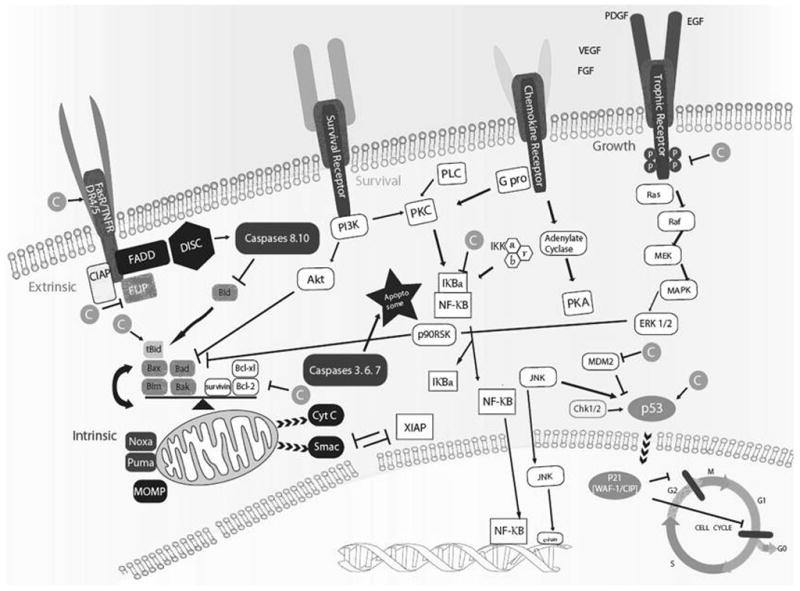
Molecular targets of curcumin. C: Curcumin, CIAP: cleavage inhibitor of apoptosis, FADD: Fas-associated protein with death domain, FLIP: FLICE-like inhibitory protein, DISC: Death-inducing signaling complex, MOMP: Mitochondrial outer membrane permeabilization, PKC: Protein kinase C, PLC: phospholipase C, XIAP: X-linked inhibitor of apoptosis protein, VEGF: vascular endothelial growth factor, FGF: fibroblast growth factor, PDGF: Platelet-derived growth factor, EGF: epidermal growth factor.
Survival signals - nuclear factor-κB (NF–κB)
The survival signals in cancer cells are upregulated to support proliferation and survival against anticancer treatment. The central role players in this process are nuclear factor-κB (NF-κB), Akt, and their downstream cascades that can lead to the upregulation of anti-apoptotic Bcl-2 proteins. Curcumin can modulate these signals by inhibiting the NF-κB pathways at multiple levels (23, 24). Curcumin significantly inhibited the growth of squamous cell carcinoma of head and neck (SCCHN) xenograft tumors in nude mice. Inhibition of nuclear and cytoplasmic IκB-β kinase (IKKβ) in the xenograft tumors decreased NF-κB activity (25). Curcumin was also shown to enhance chemosensitivity in 5-fluorouracil and cisplatin treated esophageal adenocarcinoma as well as in paclitaxel treated breast cancer cells by inhibiting compensatorily upregulated NF-κB (26). Likewise in a colon cancer cell line during radiotherapy, curcumin blocked NF-κB and reduced radioresistance (27).
Apoptotic signals – intrinsic and extrinsic
Curcumin induces programmed cell death (apoptosis) in many cancer cell types. Both the intrinsic and extrinsic apoptotic pathways are activated by curcumin. In the intrinsic pathway, various cell stresses – irreversible DNA damage, defective cell cycle, or loss of growth factors – can generate death signals and ultimately pass them down to mitochondria. Then, depending on the balance of Bcl-2 family members, the destiny of the cell is driven into apoptosis. Curcumin upregulates the p53 modulator of apoptosis (PUMA) and Noxa, which, in turn, activates the proapoptotic multi-domain Bcl-2 family members Bax, Bim, and Bak and downregulates Bcl-2 and Bcl-xl. Loss of balance between pro- and anti-apoptotic Bcl-2 proteins causes calcium influx into mitochondria and decrease in mitochondrial outer membrane permeability (MOMP) which allows cytochrome C and Smac release into the cytoplasm, eventually leading to the activation of a cascade of caspases and formation of the apoptosome, causing apoptosis (28).
In the extrinsic pathway, death signals are initiated from the exterior environment of the cells via Fas, tumor necrosis factor (TNF), and death receptors (DR) 3–6. When the signal is received, conformational change in the receptors allows Fas-associated death domain (FADD) binding and recruits the death-induced signaling complex (DISC), which activates the formation of initiator caspases 8 and 10. Curcumin was shown to upregulate extrinsic apoptosis pathway signals via the Fas pathway. In TNF-related apoptosis inducing ligand (TRAIL)-resistant cell lines, curcumin also enhanced apoptosis by upregulating the expression of DR 4 and 5 (29). After DISC recruitment, activation of the initiator caspases is regulated by FLICE-like inhibitory protein (FLIP) and curcumin was shown to downregulate c-FLIP in natural killer/T-cell lymphoma (30). Afterwards, the initiator caspase cleaves Bid and the truncated Bid (tBid) provides crosstalk between the intrinsic and extrinsic pathways by delivering death signals from initiator caspases directly to the mitochondrial pathway. In SKOV3 and OVCA429 ovarian carcinoma cells, curcumin showed induction of both intrinsic and extrinsic apoptosis by cleavage of pro-caspase 3, 8, 9 and cytochrome C release followed by tBid formation (31).
p53 plays a major role in tumor development and treatment, however, more than 50% of all cancers have p53 mutations. p53 proofreads DNA and recognizes uncorrectable mutations, at which point it arrests the cell cycle and steers the cell toward programmed cell death. Curcumin was shown to upregulate p53 expression followed by an increase in p21 (WAF-1/CIP-1), resulting in cell cycle arrest at G0/G1 and/or G2/M phases. This is eventually followed by the upregulation of Bax expression, which induces apoptosis (32). On the other hand, curcumin has also showed its p53-independent anticancer effect as an inhibitor of the proteasome pathway by inhibiting ubiquitin isopeptidase (33). In a prostate cancer cell line, curcumin downregulated MDM2, the ubiquitous ligase of p53, and displayed enhanced anticancer effect via PI3K/mTOR/ETS2 pathways in PC3 xenografts in nude mice receiving gemcitabine and radiation therapy (34). In p53 mutant or knockout ovarian cancer cell lines, curcumin induced p53-independent apoptosis which involved p38 mitogen-activated protein kinase (MAPK) activation and inhibited Akt, resulting in decreased expression of Bcl-2 and survivin (31). Taken together, cancers with both deleted/mutant and wild-type p53 can benefit from curcumin treatment to achieve an anticancer effect.
Trophic signals – growth factors and cytokines
Different kinds of trophic factors including growth factors and cytokines can contribute to growth signals in cancer cells. Curcumin inhibits epidermal growth factor receptor (EGFR) kinase phosphorylation and strongly degrades Her2/neu protein, which ultimately inhibits cancer growth (35). In SCCHN, curcumin targets both EGFR and vascular endothelial growth factor (VEGF) to inhibit cell growth (36). Therefore, the multi-targeted activity of curcumin may be potentially more effective. In an estrogen receptor negative breast cancer cell line, curcumin inhibited angiogenesis factors such as VEGF and basic fibroblast growth factor (b-FGF) at the transcriptional level (37). Curcumin was also shown to inhibit expression of pro-inflammatory cytokines such as IL-1β and −6 and exhibited growth inhibitory effects through inhibition of the NF-κB and MAPK pathways (38). In a breast cancer cell line, curcumin was shown to inhibit phosphorylation of Akt within the MAPK/PI3K pathway, which led to proapoptosis (39).
Roles of reactive oxidative stress (ROS)
ROS has opposing effects on cancers: it can be an insult causing DNA mutations in carcinogenesis, and it can also drive mitochondrial apoptosis. Minimizing DNA insult by scavenging ROS is important for the prevention of cancer, whereas generating ROS to drive mitochondrial apoptosis is more important when treating malignancies. In terms of ROS scavenging, curcumin was shown to induce phase II metabolizing enzymes in male mice – glutathione-S-transferase (GST) and quinine reductase, which can neutralize ROS derived from chemical carcinogens (40). Also, curcumin was shown to induce another important ROS scavenging enzyme – hemeoxygenase-1, the redox-sensitive inducible enzyme, via nuclear factor 2-related factor (Nrf-2) regulation (41). Curcumin is a ROS scavenging enzyme inducer but on the other hand, it also uses ROS to kill cancer cells. ROS generated by curcumin in human renal Caki cells downregulated Bcl-xl and inhibitors of apoptosis proteins (IAP), thereby inducing apoptosis (42). In cervical cancer cell lines, curcumin-generated ROS activated extracellular signal-regulated kinase (ERK) which modified radiosensitivity (43). Despite the paradoxical roles of curcumin in scavenging and generating ROS, the overall effect of curcumin is an anticancer activity.
Microenvironments – inflammation
Regarding the cancer microenvironment, the anticancer effect of curcumin is also described as an antagonist to leaky vessels and loss of adhesion, which are closely related to cancer development and invasiveness. The relationship between the proinflammatory enzymes COX-2 and lipoxygenase (LOX) and the possible development of colorectal, lung, and breast cancers has been investigated (44). In colorectal cancer, development of the premalignant lesion aberrant crypt foci (ACF) was shown to be related to upregulated COX-2 level via inducible nitric oxide synthase (iNOS). As a non-specific iNOS inhibitor, curcumin significantly inhibited colonic ACF formation in F344 rats (45). Curcumin also downregulates CXCL-1 and -2 via NF-κB inhibition and, accordingly, downregulates the metastasis-promoting gene CXCR4 in a breast cancer cell line (46). In the normal Wnt pathway, β-catenin participates in the regulation of cell-to-cell adhesion integrity but in certain cancers, aberrant β-catenin accumulation promotes survival through an upregulated Akt pathway. In colon cancer cells, curcumin promoted caspase-3-mediated cleavage of β-catenin and decreased the level of the oncogene c-Myc (47). Additionally, the invasiveness/metastasis of cancers was shown to be related to matrix metalloproteinase-9 (MMP-9) secretion, and treatment of an invasive hepatocellular cancer cell line with curcumin resulted in diminished invasiveness due to inhibition of MMP-9 secretion by curcumin (48).
Cancer stem cells (CSCs) and miRNA
Cancer stem cells (CSCs) are a rare population of cells within the tumor having cell renewal properties and are thought to be responsible for tumor initiation and treatment failures. The cancer stem-cell concept has important implications for cancer therapy and targeting CSCs is a relatively new strategy that can decrease cancer recurrence and relapse and treatment failure. Several studies have suggested that curcumin and its analogs can also target CSCs. In prostate cancer cells under hypoxic conditions, the curcumin analog CDF decreased CSC markers such as Nanog, Oct4, and EZH2 as well as miR-21, which contributed to deregulation of CSC function through the effects of CDF on the hypoxic pathway via HIF-1α (49). In colon cancer cells, STAT3 overexpression was found in ALDH(+)/CD133+ CSCs. The curcumin analog GO-Y030 inhibited the expression of STAT3 expression and suppressed CSC growth in colon cancer cells (50). Also in the rat glioma cell line C6, curcumin was shown to decrease the side population which is known to be associated with stem cell populations (51).
MicroRNAs (miR) also play essential roles in tumorigenesis and anticancer drug development because of their ability to target both tumor suppressor and oncogenes. Curcumin and its analogs also target miR, which contributes to their chemopreventive potential. By controlling epigenetic gene expression via EZH2-miR regulation, CDF increased the levels of tumor-suppressive miR that are mostly absent in pancreatic cancer cells including let-7a, b, c, d, miR-26a, miR-101, miR-146a, and miR-200b, c and resulted in decreased pancreatic cancer cell survival and aggressiveness (49). Curcumin and its cyclohexanone and piperidine analogs inhibited growth of multiple colon cancer cells by targeting Sp transcription factors (52). Induction of the Sp repressors ZBTB10 and ZBTB4 and downregulation of miR-27a, miR-20a and miR-17-5p by these compounds are important for inhibiting Sp transcription factors. miR-203 is also a target of curcumin in bladder cancers that regulates the Src-Akt axis (53).
Curcumin and host factors: Immunomodulation and metabolism
Due to poor bioavailability, it is practically impossible to reach the in vitro effective dose of curcumin in vivo. Still, curcumin is effective in vivo in inhibiting tumor growth and modulating biomarkers, suggesting that the host factors such as the host immune system and metabolic systems have an effect on its activities. Lack of functional T-cells or T-cell derived cytokines like interferon-γ promotes spontaneous as well as carcinogen-induced tumorigenesis. CD8(+) cytotoxic T lymphocytes (CTLs) are involved in antigen-specific tumor destruction and CD4(+) T cells are essential for helping this CD8(+) T cell-dependent tumor eradication. Curcumin prevented loss of T cells, expanded T cell populations and reversed the type 2 immune bias and attenuated the tumor-induced inhibition of T-cell proliferation in tumor-bearing hosts (54). Moreover, curcumin inhibited the production of immunosuppressive cytokines such as TGF-B and IL-10 in these hosts. Another study suggested that increased CD8+ T cells enhance the production of INF-γ by curcumin (55). Another host effect is on the metabolism of curcumin, which involves two routes: one route transforms curcumin to hexahydrocurmin through successive reductions (probably through the intermediates dihydrocurcumin and tetrahydrocurcumin), the other route involves rapid molecular modification by conjugation to glucuronide, sulfate and glucuronide–sulfate forms (56). Although the main curcumin metabolites remain controversial, both in vivo and in vitro cell free studies suggest that hydrocurcumins are more potent antioxidants than parent curcumin in scavenging free radicals, reducing lipid peroxidation and in enzyme activation (of superoxide dismutase, catalase, GSH peroxidase and GST) (57, 58). These antioxidant effects were shown to be critical for the chemopreventive potential of curcumin. Thus, curcumin displayed efficacy in vivo probably due to the presence of these host effects.
Clinical Trials of Curcumin Use in Cancer
Many positive preclinical cell line and animal model studies have brought curcumin to clinical trials to test its safety and efficacy as a chemopreventive agent. Several clinical trials have already been completed, the results of which are summarized in Table 1 (59–68). In phase I trials, curcumin was tested for its toxicity and tolerability, and was found to be highly tolerable at doses up to 12 g/day with no curcumin-related toxicities (60, 61). However, due to its poor bioavailability, curcumin was not detectable in blood when administered at doses up to 8 g, and was detected at very low levels following 10 g and 12 g doses with a peak concentration at 1~2 hours. Histological improvements of the lesions were observed in most of the treated patients (60). Radiologically stable disease was also demonstrated in five out of fifteen colorectal cancer patients who were refractory to chemotherapy in a second study (61). In a colorectal cancer trial, curcumin was shown to modulate biomarkers such as GST activity, deoxyguanosine adduct M(1)G, and PGE2 (prostaglandin E2) (61–63). A decrease in lymphocytic GST activity of 59% resulted after administration of 440 mg of Curcuma extract (61). The levels of M(1) decreased from 4.8 +/− 2.9 adducts per 107 nucleotides to 2.0 +/− 1.8 adducts per 107 nucleotides after curcumin administration (63). Oral administration of 3.6 g curcumin significantly ((P < 0.05) decreased inducible PEG2 production in blood 1 hour after curcumin administration as compared to the predose level (62). The same study also demonstrated the poor bioavailability and systemic distribution of curcumin. After encapsulated curcumin was administered in different amounts ranging from 0.45 to 3.6 g for 4 months, its biodistribution was examined by biopsy which showed malignant mucosal tissue had a higher concentration of curcumin whereas outside the mucosa, only a negligible amount was found (61). This result may also be very beneficial for colorectal malignancy because any possible toxicity outside of the area of interest can be minimized. In one study where five FAP patients who had prior colectomy were treated with the combination of curcumin and quercetin for 6 months, the size and number of adenomas were reduced significantly, supporting the use of curcumin for FAP colorectal cancer prevention (66). Administration of 4 g curcumin per day for 30 days significantly inhibited (40%) the number of ACF although the 2 g per day dose was found to be ineffective (68). Several patients with advanced pancreatic cancers also responded to 8 g/day of curcumin treatment (69). These promising results are very convincing but need to be validated with further larger-scale randomized trials. Table 2 shows ongoing clinical studies with curcumin.
Table 1.
Completed clinical trials using curcumin
| Type | Method and material | Results and Conclusion | Reference |
|---|---|---|---|
| Phase I Safety trial | Patients: 10, 2000 mg/day + piperine 20 mg/kg; | Piperine, a known inhibitor of hepatic and intestinal glucuronidation enhanced serum concentration, extent of absorption, and bioavailability. Much higher concentration with piperine at 1/4 to 1 h post drug (P < 0.01 at 0.25 h and 0.5 h; P < 0.001 at 1 h) |
Shoba et al. 1998 (58) |
| Phase I Safety trial | Patients: 25, Oral 500–12,000 mg/d for 90 days Bx done after treatment |
Oral curcumin is not toxic to humans up to 8,000 mg/d for 3 months. Histologic improvement of precancerous lesions were observed in bladder cancer, oral leukoplakia, intestinal metaplasia of the stomach, CIN, and Bowen’s disease |
Cheng et al. 2001 (59) |
| Phase I Colon cancer | Patients: 15, Oral curcumin extract of 440–2200 mg/d for 120 days. Activity of GST and levels of M1G were measured. |
Safe administration of curcumin extract at doses up to 2.2 g daily, equivalent to 180 mg of curcumin. Curcumin has low oral bioavailability in humans and may undergo intestinal metabolism. Lowered GST (Glutathione-S-transferase) with constant M1G. |
Sharma et al. 2001 (60) |
| Phase I Colorectal cancer | Patients: 15, Oral 450–3600 mg/d for 120 days. Dose-escalation study. Levels of curcumin and its metabolites in plasma, urine, and feces were measured. |
Lowered inducible serum PGE2 levels (P < 0.05). No dose-limiting toxicity. A daily oral dose of 3.6 g of curcumin is advocated for Phase II evaluation in the cancer prevention outside the gastrointestinal tract. Levels of curcumin and its metabolites in the urine can be used to assess general compliance. |
Sharma et al. 2004 (61) |
| Phase I Colorectal cancer | Patients: 12, Oral 450–3600 mg/d for 7 days. Bx samples of normal and malignant colorectal tissue, at diagnosis and at 6 to 7 hours after last dose of curcumin. |
M1G levels were 2.5-fold higher in malignant tissue as compared with normal tissue (P < 0.05 by ANOVA). The concentrations in normal and malignant colorectal tissue of patients receiving 3,600 mg of curcumin were 12.7±5.7 and 7.7±1.8 nmol/g, respectively. The daily dose of 3.6 g curcumin achieves pharmacological efficacy in the colorectum with negligible distribution of curcumin outside the gut. |
Garcea et al. 2005 (62) |
| Phase I Safety trial | Patients: 24, Oral 500–12,000 mg/day. Dose-escalation study for MTD and safety | Seven of 24 subjects (30%) experienced only minimal toxicity. Systemic bioavailability of curcumin or its metabolites may not be essential for CRC chemoprevention because CRC can still benefit from curcumin. |
Lao et al. 2006 (63) |
| Phase I Open-label Advanced metastatic breast cancer | Patients: 14, Docetaxel (100 mg/m2) 1 h i.v. every 3 wk on d 1 x six cycles + Oral 500 mg/d for 7 consecutive days and escalated the dose until toxicity. VEGF, and tumor markers measured |
MTD at 8,000 mg/d 8/14 patients had measurable lesions, with 5 PR and 3 SD. Some biological and clinical responses were observed in most patients. The recommended dose of curcumin is 6,000 mg/d for seven consecutive d every 3 wk in combination with a standard dose of docetaxel. |
Bayet-Robert et al. 2010 (64) |
| Phase II Efficacy trial Skin lesion | Patients: 62, 1% ointment, several months for “External cancerous lesion” | The first clinical study. Reduction in smell in 90% patients, reduction of itching in all cases, dry lesions in 70% patients, reduction in lesion size and pain in 10% patients. |
Kuttan et al. 1987 (17) |
| Phase II FAP | Patients: 5, Oral curcumin 480g + quercetin 20 mg tid for 180 days. Polyps size and # assessed |
Decrease in the number of polyps was seen in 60.4% Decrease in the size of polyps was 50.9% in FAP patients. RCT in the future are necessary |
Cruz-Correa et al. 2006 (65) |
| Cohort study PIN | Patients: 24 | Zyflamend, a novel herbal anti-inflammatory mixture, as a potential chemoprevention agent in a phase I trial for patients diagnosed with PIN. | Rafailov et al. 2007 (66) |
| Phase IIa | Patients: 44 | 40% reduction in ACF numbers with 4g dose | Carroll et al. (67) |
Table 2.
Ongoing clinical trials with curcumin
| Trial type | Official title | Cancer type | Identifier number | |
|---|---|---|---|---|
| Phase 1 Non-randomized | Phase I Study of Surface-Controlled Water Soluble Curcumin (THERACURMIN CR-011L) in Patients With Advanced Malignancies | Advanced malignancies | Safety/Efficacy Study Single Group Assignment Open Label |
NCT 01201694 |
| Phase 1 Non-randomized | Phase I Pharmacokinetic Trial of Curcuminoids Administered in a Capsule Formulation | Colon cancer | Pharmacokinetics study Single Group Assignment Open Label |
NCT 00027495 |
| Phase 1 Randomized controlled trial Recruiting | Phase I Clinical Trial Investigating the Ability of Plant Exosomes to Deliver Curcumin to Normal and Malignant Colon Tissue | Colon cancer | Bioavailability Study Factorial Assignment Open Label |
NCT 01294072 |
| Phase 1 Randomized controlled trial | Crossover, Multiple Dose Pharmacokinetics of Two Curcumin Formulations in Healthy Volunteers | Healthy volunteers | Pharmacokinetics Study Crossover Assignment Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor) |
NCT 01330810 |
| Phase 1 Non-randomized | Curcumin Chemoprevention of Colorectal Neoplasia (Curcumin biomarker) | Colorectal cancer | Pharmacodynamics Study Single Group Assignment Intervention Open Label |
NCT 01333917 |
| Phase 1 Randomized controlled trial | Pilot Study of Curcumin, Vorinostat, and Sorafenib in Patients With Advanced Solid Tumors | Advanced solid tumor | Safety/Efficacy Study Single Group Assignment Open Label |
NCT 01608139 |
| Phase 2 Randomized controlled trial | Phase II Double Blind Placebo-Controlled Trial of Curcuminoids’ Effect on Cellular Proliferation, Apoptosis and COX-2 Expression in the Colorectal Mucosa of Subjects With Recently Resected Sporadic Adenomatous Polyps | Colorectal cancer | Safety/Efficacy Study Parallel Assignment Double Blind (Subject, Investigator) |
NCT 00118989 |
| Phase 2 Non-randomized | Phase II Trial of Curcumin in Cutaneous T-cell Lymphoma Patients | Cutaneous T-Cell Lymphoma | Efficacy Study Single Group Assignment Open Label. |
NCT 00969085 |
| Phase 2 Non-randomized | Phase II Trial of Curcumin in Patients With Advanced Pancreatic Cancer | Advanced pancreatic cancer | Safety/Efficacy Study Single Group Assignment Open Label. |
NCT 00094445 |
| Phase 2 Randomized controlled trial Recruiting | Curcumin for Treatment of Intestinal Adenomas in Familial Adenomatous Polyposis (FAP) | Colorectal cancer | Parallel Assignment Double Blind (Subject, Investigator) |
NCT 00641147 |
| Phase 2 Randomized controlled trial | Curcumin With Pre-operative Capecitabine and Radiation Therapy Followed by Surgery for Rectal Cancer | Rectal cancer | Safey/Efficacy Study Single group Assignment Double Blind (Subject, Caregiver) |
NCT 00745134 |
Biomarkers in Curcumin Chemoprevention Trials
Biomarkers can be very useful in identifying high risk subjects for intervention, monitoring the effects of treatment, predicting outcome and selecting patients who may benefit most from a given intervention. A well validated biomarker may also serve as a surrogate endpoint to replace the current use of size reduction or histologic improvement of the precancerous lesion as the sole measure of success of chemoprevention; the use of a surrogate marker would potentially provide a more accurate assessment of outcome and would resolve the current difficulty in patient accrual due to the requirement for biopsy in chemoprevention clinical trials.
Although biomarkers for chemoprevention by curcumin have been extensively studied in cell culture and rodent models, only a few clinical studies have focused on biomarker modulation and attempted to correlate these with outcomes. In a recent pilot study, IKKβ kinase activity and the levels of proinflammatory cytokine IL-8 in the saliva of SCCHN patients were measured and the results suggested that IKKβ kinase activity could be used to detect the effect of curcumin treatment in SCCHN (70). In a double blind randomized trial, curcumin was found to significantly decrease the levels of serum calcitonin gene-related peptide (CGRP) as compared to placebo (71). There were also significant decreases in serum IL-8 and high-sensitivity C-reactive protein (hs-CRP) in both curcumin and placebo group with a higher magnitude in the curcumin group. In a placebo-controlled study, oral administration of curcumin significantly reduced erythrocyte malonaldehyde (MDA) and increased GSH levels in patients with tropical pancreatitis (72). Curcumin was also found to significantly decrease the serum levels of markers of oxidative damage (MDA, 8-hydroxydeoxyguanosine) and increase those of antioxidants (vitamins C and E) in patients with oral leukoplakia, oral submucous fibrosis or lichen planus along with a significant decrease in pain and lesion size (73). Another clinical study suggested that cytokines and NF-kB pathway markers are important targets for curcumin chemoprevention (69). Other enzymes including COX-2 and hepatic GST nucleotidase have also been suggested for use in monitoring the effect of curcumin in chemoprevention studies (74). Also, in a recent phase IIa clinical trial of curcumin chemoprevention in colorectal neoplasia, although the levels of PGE2 and 5-HETE did not significantly correlate with curcumin treatment, the amount of the premalignant lesion ACF was decreased (68), while other studies showed marked modulation of PGE2 (62). To clarify these ambiguous results, many more clinical studies testing different surrogate biomarkers in larger patient numbers must be performed to overcome the limitations to the study of surrogate monitoring biomarkers. Based on these previous results, however, biomarkers of oxidative stress, NF-kB pathway markers and cytokine levels in serum and tissues appear to be promising markers that new studies should be designed to measure.
Hurdles: Pharmacokinetics and Pharmacodynamics
Phase I/II clinical trials have clearly shown that curcumin exhibits poor bioavailability in humans, ~1% after oral administration, a major barrier for its use in the clinic. The major factors contributing to the low plasma and tissue levels of curcumin appear to be its poor absorption due to insolubility in water, rapid systemic elimination in the bile and urine due to extensive enterohepatic recirculation and fast metabolism (56). In fact, 40% of orally administered curcumin is excreted unchanged in the feces. To circumvent the bioavailability problem, numerous approaches have been considered, including structural modification or modification of the delivery system such as adding adjuvant, liposomal curcumin, curcumin nanoparticles and phospholipid complex.
Curcumin analogs
Studies suggest that the β-diketone moiety is responsible for the instability and weak pharmacokinetic profile of curcumin. Modifications of the structure of natural curcumin significantly improved solubility, stability and bioavailability. James Snyder’s group at Emory University has synthesized a series of curcumin analogs by modifying the diketone moiety and the side chains of the benzene rings. Many of these compounds showed increased water solubility and improved pharmacokinetic properties including tissue distribution and terminal elimination half life (75). Analog HO3867 also showed tremendous improvement in cellular uptake and tissue distribution as compared to its natural counterpart (76). Gagliardi et al., (77) synthesized more than 40 curcumin analogs and studied the bioavailability of some of these compounds in mice. One particular compound with a valine substitution at the phenyl ring showed more than 50-fold greater bioavailability than natural curcumin. A Japanese group also synthesized 86 different analogs of curcumin and determined their IC50 against 16 cancer cell lines. Many of these analogs, namely GO-Y078, 079, 030, 097, and 098, were at least 10-fold more potent than natural curcumin. This set of compounds is also more soluble in water suggesting that they might show better bioavailability also (78). Another synthetic curcumin known as dimethoxycurcumin exhibited significantly higher stability in vivo and against microsomal metabolism (79). In attempts to overcome the poor bioavailability of curcumin and to increase its tumor-specificity, many more innovative analogs have also been studied (Table 3) (80–93).
Table 3.
Curcumin analogs and their benefits
| Analog | Study conclusion | Benefits and aims | Reference |
|---|---|---|---|
| EF24 | In ovarian cancer cells, VEGF was dose-dependently reduced with EF24 demonstrating ~8-fold greater potency than curcumin (P < .05). Synergism with cisplatin. | Enhanced potency | Tan et al. (79) |
| Novel strategy curcumin analog EF24 with a p38 inhibitor for lung cancer | Enhanced potency | Thomas et al. (80) | |
| In MDA-MB231 and PC3, EF-24 inhibits HIF-1 and genuinely disrupts the microtubule cytoskeleton unlike curcumin | Mechanism | Thomas et al. (81) | |
| EF24 shows anticancer potency 10 times higher than curcumin, against lung, breast, ovarian, and cervical cancer cells by blocking the nuclear translocation of NF-kB | Enhanced potency | Kasinski et al. (82) | |
| EF31 | EF31 has greater potency in NF-kB activity inhibition compared to curcumin and another analog EF24 and its action mechanism is based on its anti-inflammatory and antisurvival activities. | Enhanced potency | Olivera et al. (83) |
| BDMCA | Chemopreventive effect through prevention of circulatory oxidative stress is not by methoxy group but by the terminal phenolic moieties or the central 7-carbon chain | Mechanism, Structure, roles | Devasena et al. (84). |
| BDMCA is antioxidant and lipid peroxidation and antioxidant status could be used as markers for colon cancer chemoprevention using BDMCA | Mechanism, Biomarker | Devasena et al. (85). | |
| CDF | Combination of CDF and conventional 5-FU+Oxaliplatin could be an strategy for preventing the emergence of chemoresistant colon cancer cells | Overcoming resistance | Kanwar et al. (86) |
| CDF had better retention and bioavailability and the concentration of CDF in the pancreas tissue was 10-fold higher compared to curcumin | Improved bioavailability | Padhye et al. (87) | |
| FLLL32 | FLLL32 has biochemically superior properties and more specifically targets STAT3, a transcription factor | Enhanced specificity | Fossey et al. (88) |
| FLLL32 reduced expression of STAT3-target genes | Enhanced specificity | Bill et al. (89). | |
| GO-Y030 | GO-Y030 has 30-fold higher potency in suppressing tumor cell growth compared with curcumin by inhibition of IKKβ | Enhanced potency | Sato et al. (90) |
| Improved chemopreventive effect with GO-Y030 compared with curcumin (191 days). Diminished polyp incidence in Apc(580D/+) mice fed GO-Y030. | Enhanced prevention | Shibata et al. (91) | |
| DAP | High levels of HO-3867 were detected in the liver, kidney, stomach, and blood 3 hours after DAP i.p. injection. Higher bioabsorption | Improved bioavailability | Dayton et al. (74) |
| [DLys(6)]-LHRH-Curcumin | The analog inhibited the proliferation of pancreatic cancer cell lines (p < 0.05) by inducing apoptosis. Water soluble and i.v. infusible. i.v. infusion could achieve significant tumor weight and volume (p < 0.01) | Targeted delivery | Aggarwarl et al. (92) |
Curcumin nanoparticles
Delivery of drugs via their formulation as nanoparticles is an emerging platform for an efficient approach to improve pharmacokinetic properties such as solubility and stability, and thus bioavailability of poorly bioavailable drugs. This approach has been extensively used for curcumin with success in preclinical studies. Formulation of curcumin by encapsulation in polymeric micelles, liposomes, polymeric nanoparticles, lipid-based nanoparticles and hydrogels makes the formulation aqueous soluble (56). Many of these formulations also showed improved bioavailability and pharmacokinetic properties in vivo. Encapsulation of curcumin in polylactic-co-glycolic acid (PLGA) and PLGA-polyethylene glycol (PEG) (PLGA-PEG) blend nanoparticles by a single-emulsion solvent-evaporation technique increased its mean half-life to approximately 4 and 6h, respectively, C(max) by 2.9- and 7.4-fold and bioavailability by 15.6- and 55.4-fold, respectively (94). Encapsulation of curcumin in poly(butyl) cyanoacrylate (PBCA) nanoparticles led to a 52-fold increase in elimination half-life and 2-fold increase in AUC (95). Curcumin encapsulated in MePEO-b-PCL micelles also showed similar improvements in pharmacokinetics parameters (96). Formulation of curcumin in solid-lipid nanoparticles also tremendously increased its bioavailability (more than 80-fold higher concentration in blood) (97).
Curcumin conjugates
Conjugation of curcumin with polymers or other lipophilic compounds is another widely used approach to improve the water solubility and stability of curcumin. Conjugation of curcumin with hyaluronic acid or polyvinylpyrrolidone forms water soluble micelles with improved stability at physiological pH and cytotoxic activities (98, 99). Polymerization of curcumin using diacid also produced a water soluble curcumin polymer with improved anti-cancer activity (100). Complexation of curcumin with phosphatidylcholine also significantly (3-20–fold) improved its pharmacokinetic parameters, including bioavailability in animal models (101).
Adding adjuvant
One of the major reasons for the poor bioavailability of curcumin is its rapid glucuronidation. Protection of curcumin from such metabolic conversion using an adjuvant was found to be successful in improving its bioavailability. Piperine is an inhibitor of intestinal and hepatic glucuronidation. Concomitant administration of curcumin with piperine increased the bioavailability of curcumin by 1100% in human volunteers and 154% in rats (59).
Future Possibilities
High risk individuals and cancer survivors alike may benefit from chemoprevention, not only because primary cancer chemoprevention is beneficial for high risk groups but also because of the devastating nature of the disease course when patients experience SPT or recurrence. As curcumin is a non-prescription dietary derivative that has multiple targets at different levels in multiple pathways, it has great potential in the prevention of cancer and SPT. When its systemic bioavailability is increased through the development of different analogs and formulations, the promise of curcumin in chemoprevention may be feasible in many cancer types, not necessarily limited only to gastrointestinal cancers. A number of new analogs and formulations have already been developed with higher systemic bioavailability and potency. More standardized clinical trials for bioavailability and randomized control trials for efficacy should validate the potential of these newer agents and formulations. First, specific trials can improve the application of curcumin through changing the route of administration, achieve targeted delivery straight to the lesion sites by increasing tumor-specific affinity, and develop different analogs that can bypass or minimize the first-pass metabolism occurring in the gastrointestinal mucosa and liver. Second, to minimize its metabolism before reaching the targeted site, different preparations of curcumin may improve its delivery to the target and therefore increase its bioavailability. Third, formulating curcumin using nanoparticles and microparticles, which are among the most innovative modalities that can maximize delivery to a target tissue and increase sensitivity and specificity, may enhance its therapeutic index.
Defining the optimal precancerous candidates and surrogate endpoints to properly assess chemopreventive response is mandatory in chemoprevention research. Although we expect the network of signaling pathways to be considerably more complicated than we currently understand, further studies will better dissect the molecular effects of curcumin in different cancers. Specifically, microarray or recently developed RNASeq studies may be particularly valuable in defining unknown positive and negative signaling loops, and may represent a new field of future research directed at understanding the critical factors necessary for chemoprevention. In the future, targeting specific patient populations with certain biomarkers, so-called tailored chemoprevention, is necessary. Defining critical biomarkers will help to better design a personalized plan for tailored chemoprevention. Progress in personal genome-based risk assessment and profiling of individual patients may also help to identify the patient population best suited to curcumin chemoprevention in the future.
Acknowledgments
We thank Anthea Hammond for editorial assistance. This work was supported, in whole or in part, by National Institutes of Health Grants P50 CA128613 (DMS) and R03 CA159369 (ARA). ARA is a recipient of Robbins Scholar Award.
Footnotes
Conflict of Interest: None
References
- 1.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–54. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 2.Lippman SM, Hawk ET. Cancer prevention: from 1727 to milestones of the past 100 years. Cancer Res. 2009;69:5269–84. doi: 10.1158/0008-5472.CAN-09-1750. [DOI] [PubMed] [Google Scholar]
- 3.Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–7. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 4.Pollack ES, Nomura AM, Heilbrun LK, Stemmermann GN, Green SB. Prospective study of alcohol consumption and cancer. N Engl J Med. 1984;310:617–21. doi: 10.1056/NEJM198403083101003. [DOI] [PubMed] [Google Scholar]
- 5.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 6.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–32. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 8.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 10.Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, Benner SE, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–50. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 12.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 13.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 14.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 15.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.http://prevention.cancer.gov/programs-resources/resources/agents
- 18.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 19.Singletary K, MacDonald C, Wallig M, Fisher C. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumorigenesis and DMBA-DNA adduct formation by curcumin. Cancer Lett. 1996;103:137–41. doi: 10.1016/0304-3835(96)04224-3. [DOI] [PubMed] [Google Scholar]
- 20.Ushida J, Sugie S, Kawabata K, Pham QV, Tanaka T, Fujii K, et al. Chemopreventive effect of curcumin on N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Jpn J Cancer Res. 2000;91:893–8. doi: 10.1111/j.1349-7006.2000.tb01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, et al. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–40. [PubMed] [Google Scholar]
- 22.Sreepriya M, Bali G. Effects of administration of Embelin and Curcumin on lipid peroxidation, hepatic glutathione antioxidant defense and hematopoietic system during N-nitrosodiethylamine/Phenobarbital-induced hepatocarcinogenesis in Wistar rats. Mol Cell Biochem. 2006;284:49–55. doi: 10.1007/s11010-005-9012-7. [DOI] [PubMed] [Google Scholar]
- 23.Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv Exp Med Biol. 2007;595:127–48. doi: 10.1007/978-0-387-46401-5_4. [DOI] [PubMed] [Google Scholar]
- 24.Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D’Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 25.Duarte VM, Han E, Veena MS, Salvado A, Suh JD, Liang LJ, et al. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKbeta protein of the NFkappaB pathway. Mol Cancer Ther. 2010;9:2665–75. doi: 10.1158/1535-7163.MCT-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartojo W, Silvers AL, Thomas DG, Seder CW, Lin L, Rao H, et al. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor kappaB in esophageal adenocarcinoma. Transl Oncol. 2010;3:99–108. doi: 10.1593/tlo.09235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandur SK, Deorukhkar A, Pandey MK, Pabon AM, Sentu S, Guha S, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009;75:534–42. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–18. [PubMed] [Google Scholar]
- 29.Moragoda L, Jaszewski R, Majumdar AP. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001;21:873–8. [PubMed] [Google Scholar]
- 30.Kim K, Ryu K, Ko Y, Park C. Effects of nuclear factor-kappaB inhibitors and its implication on natural killer T-cell lymphoma cells. Br J Haematol. 2005;131:59–66. doi: 10.1111/j.1365-2141.2005.05720.x. [DOI] [PubMed] [Google Scholar]
- 31.Watson JL, Greenshields A, Hill R, Hilchie A, Lee PW, Giacomantonio CA, et al. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and downregulation of Bcl-2 and survivin expression and Akt signaling. Mol Carcinog. 2010;49:13–24. doi: 10.1002/mc.20571. [DOI] [PubMed] [Google Scholar]
- 32.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–40. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 33.Mullally JE, Fitzpatrick FA. Pharmacophore model for novel inhibitors of ubiquitin isopeptidases that induce p53-independent cell death. Mol Pharmacol. 2002;62:351–8. doi: 10.1124/mol.62.2.351. [DOI] [PubMed] [Google Scholar]
- 34.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–96. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 35.Tikhomirov O, Carpenter G. Identification of ErbB-2 kinase domain motifs required for geldanamycin-induced degradation. Cancer Res. 2003;63:39–43. [PubMed] [Google Scholar]
- 36.Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, Lin JP, et al. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF) Cancer Lett. 2009;285:127–33. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 37.Shao ZM, Shen ZZ, Liu CH, Sartippour MR, Go VL, Heber D, et al. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int J Cancer. 2002;98:234–40. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- 38.Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19:469–74. [PubMed] [Google Scholar]
- 39.Squires MS, Hudson EA, Howells L, Sale S, Houghton CE, Jones JL, et al. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem Pharmacol. 2003;65:361–76. doi: 10.1016/s0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal M, Sharma SD, Okazaki Y, Fujisawa M, Okada S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol. 2003;92:33–8. doi: 10.1034/j.1600-0773.2003.920106.x. [DOI] [PubMed] [Google Scholar]
- 41.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresty R, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–95. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 43.Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grosser T. The pharmacology of selective inhibition of COX-2. Thromb Haemost. 2006;96:393–400. [PubMed] [Google Scholar]
- 45.Rao CV, Kawamori T, Hamid R, Reddy BS. Chemoprevention of colonic aberrant crypt foci by an inducible nitric oxide synthase-selective inhibitor. Carcinogenesis. 1999;20:641–4. doi: 10.1093/carcin/20.4.641. [DOI] [PubMed] [Google Scholar]
- 46.Bachmeier BE, Mohrenz IV, Mirisola V, Schleicher E, Romeo F, Hohneke C, et al. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008;29:779–89. doi: 10.1093/carcin/bgm248. [DOI] [PubMed] [Google Scholar]
- 47.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–27. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 48.Lin LI, Ke YF, Ko YC, Lin JK. Curcumin inhibits SK-Hep-1 hepatocellular carcinoma cell invasion in vitro and suppresses matrix metalloproteinase-9 secretion. Oncology. 1998;55:349–53. doi: 10.1159/000011876. [DOI] [PubMed] [Google Scholar]
- 49.Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li Y, et al. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PloS One. 2012;7:e43726. doi: 10.1371/journal.pone.0043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin L, Liu Y, Li H, Li PK, Fuchs J, Shibata H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer. 2011;105:212–20. doi: 10.1038/bjc.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fong D, Yeh A, Naftalovich R, Choi TH, Chan MM. Curcumin inhibits the side population (SP) phenotype of the rat C6 glioma cell line: towards targeting of cancer stem cells with phytochemicals. Cancer Lett. 2010;293:65–72. doi: 10.1016/j.canlet.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gandhy SU, Kim K, Larsen L, Rosengren RJ, Safe S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saini S, Arora S, Majid S, Shahryari V, Chen Y, Deng G, et al. Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev Res (Phila) 2011;4:1698–709. doi: 10.1158/1940-6207.CAPR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhattacharyya S, Md Sakib Hossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 2010;7:306–15. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo F, Song X, Zhang Y, Chu Y. Low-dose curcumin leads to the inhibition of tumor growth via enhancing CTL-mediated antitumor immunity. Int Immunopharmacol. 2011;11:1234–40. doi: 10.1016/j.intimp.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Belkacemi A, Doggui S, Dao L, Ramassamy C. Challenges associated with curcumin therapy in Alzheimer disease. Expert Rev Mol Med. 2011;13:e34. doi: 10.1017/S1462399411002055. [DOI] [PubMed] [Google Scholar]
- 57.Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull. 2007;30:74–8. doi: 10.1248/bpb.30.74. [DOI] [PubMed] [Google Scholar]
- 58.Murugan P, Pari L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006;79:1720–8. doi: 10.1016/j.lfs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 60.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 61.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–900. [PubMed] [Google Scholar]
- 62.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 63.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–5. [PubMed] [Google Scholar]
- 64.Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- 66.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–8. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 67.Rafailov S, Cammack S, Stone BA, Katz AE. The role of Zyflamend, an herbal anti-inflammatory, as a potential chemopreventive agent against prostate cancer: a case report. Integr Cancer Ther. 2007;6:74–6. doi: 10.1177/1534735406298843. [DOI] [PubMed] [Google Scholar]
- 68.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–64. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 70.Kim SG, Veena MS, Basak SK, Han E, Tajima T, Gjertson DW, et al. Curcumin treatment suppresses IKKbeta kinase activity of salivary cells of patients with head and neck cancer: a pilot study. Clin Cancer Res. 2011;17:5953–61. doi: 10.1158/1078-0432.CCR-11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panahi Y, Sahebkar A, Parvin S, Saadat A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann Clin Biochem. 2012;49:580–8. doi: 10.1258/acb.2012.012040. [DOI] [PubMed] [Google Scholar]
- 72.Durgaprasad S, Pai CG, Vasanthkumar, Alvres JF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122:315–8. [PubMed] [Google Scholar]
- 73.Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci. 2010;52:251–6. doi: 10.2334/josnusd.52.251. [DOI] [PubMed] [Google Scholar]
- 74.Shahani K, Panyam J. Highly loaded, sustained-release microparticles of curcumin for chemoprevention. J Pharm Sci. 2011;100:2599–609. doi: 10.1002/jps.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu S, Moore TW, Lin X, Morii N, Mancini A, Howard RB, et al. Synthetic curcumin analog EF31 inhibits the growth of head and neck squamous cell carcinoma xenografts. Integr Biol (Camb) 2012;4:633–40. doi: 10.1039/c2ib20007d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dayton A, Selvendiran K, Kuppusamy ML, Rivera BK, Meduru S, Kalai T, et al. Cellular uptake, retention and bioabsorption of HO-3867, a fluorinated curcumin analog with potential antitumor properties. Cancer Biol Ther. 2010;10:1027–32. doi: 10.4161/cbt.10.10.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gagliardi S, Ghirmai S, Abel KJ, Lanier M, Gardai SJ, Lee C, et al. Evaluation in vitro of synthetic curcumins as agents promoting monocytic gene expression related to beta-amyloid clearance. Chem Res Toxicol. 2012;25:101–12. doi: 10.1021/tx200246t. [DOI] [PubMed] [Google Scholar]
- 78.Kudo C, Yamakoshi H, Sato A, Nanjo H, Ohori H, Ishioka C, et al. Synthesis of 86 species of 1,5-diaryl-3-oxo-1,4-pentadienes analogs of curcumin can yield a good lead in vivo. BMC Pharmacol. 2011;11:4. doi: 10.1186/1471-2210-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu ZL, et al. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin Cancer Res. 2007;13:1269–77. doi: 10.1158/1078-0432.CCR-06-1839. [DOI] [PubMed] [Google Scholar]
- 80.Tan X, Sidell N, Mancini A, Huang RP, Shenming W, Horowitz IR, et al. Multiple anticancer activities of EF24, a novel curcumin analog, on human ovarian carcinoma cells. Reprod Sci. 2010;17:931–40. doi: 10.1177/1933719110374239. [DOI] [PubMed] [Google Scholar]
- 81.Thomas SL, Zhao J, Li Z, Luo B, Du Y, Purcell J, et al. Activation of the p38 pathway by a novel monoketone curcumin analog, EF24, suggests a potential combination strategy. Biochem Pharmacol. 2010;80:1309–16. doi: 10.1016/j.bcp.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas SL, Zhong D, Zhou W, Malik S, Liotta D, Snyder JP, et al. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle. 2008;7:2409–17. doi: 10.4161/cc.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kasinski AL, Du Y, Thomas SL, Zhao J, Sun SY, Khuri FR, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–61. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Olivera A, Moore TW, Hu F, Brown AP, Sun A, Liotta DC, et al. Inhibition of the NF-kappaB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. Int Immunopharmacol. 2012;12:368–77. doi: 10.1016/j.intimp.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Devasena T, Menon VP, Rajasekharan KN. Prevention of 1,2-dimethylhydrazine-induced circulatory oxidative stress by bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione during colon carcinogenesis. Pharmacol Rep. 2006;58:229–35. [PubMed] [Google Scholar]
- 86.Devasena T, Menon Venugopal VP, Rajasekaran KN. Chemoprevention of colon cancer by a synthetic curcumin analog involves amelioration of oxidative stress. Toxicol Mech Methods. 2005;15:355–9. doi: 10.1080/15376520500195947. [DOI] [PubMed] [Google Scholar]
- 87.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Padhye S, Sarkar FH, et al. Difluorinated-curcumin (CDF): a novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm Res. 2011;28:827–38. doi: 10.1007/s11095-010-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, et al. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res. 2009;26:2438–45. doi: 10.1007/s11095-009-9955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fossey SL, Bear MD, Lin J, Li C, Schwartz EB, Li PK, et al. The novel curcumin analog FLLL32 decreases STAT3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer. 2011;11:112. doi: 10.1186/1471-2407-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bill MA, Fuchs JR, Li C, Yui J, Bakan C, Benson DM, Jr, et al. The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with anti-tumor activity. Mol Cancer. 2010;9:165. doi: 10.1186/1476-4598-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato A, Kudo C, Yamakoshi H, Uehara Y, Ohori H, Ishioka C, et al. Curcumin analog GO-Y030 is a novel inhibitor of IKKbeta that suppresses NF-kappaB signaling and induces apoptosis. Cancer Sci. 2011;102:1045–51. doi: 10.1111/j.1349-7006.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 92.Shibata H, Yamakoshi H, Sato A, Ohori H, Kakudo Y, Kudo C, et al. Newly synthesized curcumin analog has improved potential to prevent colorectal carcinogenesis in vivo. Cancer Sci. 2009;100:956–60. doi: 10.1111/j.1349-7006.2009.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aggarwal S, Ndinguri MW, Solipuram R, Wakamatsu N, Hammer RP, Ingram D, et al. [DLys(6)]-luteinizing hormone releasing hormone-curcumin conjugate inhibits pancreatic cancer cell growth in vitro and in vivo. Int J Cancer. 2011;129:1611–23. doi: 10.1002/ijc.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khalil NM, do Nascimento TC, Casa DM, Dalmolin LF, de Mattos AC, Hoss I, et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013;101:353–60. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 95.Duan J, Zhang Y, Han S, Chen Y, Li B, Liao M, et al. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm. 2010;400:211–20. doi: 10.1016/j.ijpharm.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 96.Ma Z, Shayeganpour A, Brocks DR, Lavasanifar A, Samuel J. High-performance liquid chromatography analysis of curcumin in rat plasma: application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed Chromatogr. 2007;21:546–52. doi: 10.1002/bmc.795. [DOI] [PubMed] [Google Scholar]
- 97.Wang W, Zhu R, Xie Q, Li A, Xiao Y, Li K, et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine. 2012;7:3667–77. doi: 10.2147/IJN.S30428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manju S, Sreenivasan K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J Colloid Interface Sci. 2011;359:318–25. doi: 10.1016/j.jcis.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 99.Manju S, Sreenivasan K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J Pharm Sci. 2011;100:504–11. doi: 10.1002/jps.22278. [DOI] [PubMed] [Google Scholar]
- 100.Tang H, Murphy CJ, Zhang B, Shen Y, Van Kirk EA, Murdoch WJ, et al. Curcumin polymers as anticancer conjugates. Biomaterials. 2010;31:7139–49. doi: 10.1016/j.biomaterials.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Gupta NK, Dixit VK. Bioavailability enhancement of curcumin by complexation with phosphatidyl choline. J Pharm Sci. 2011;100:1987–95. doi: 10.1002/jps.22393. [DOI] [PubMed] [Google Scholar]


