Abstract
Background
High levels of outpatient antibiotic use remain observed in many European countries. Several studies have shown a strong relationship between antibiotic use and bacterial resistance.
Aim
To assess the long-term effect of a standardised educational seminar on antibiotic prescriptions by GPs.
Design and setting
Randomised controlled trial of 171 GPs (of 203 initially randomised) in France.
Method
GPs in the control group (n = 99) received no antibiotic prescription recommendation. Intervention group GPs (n = 72) attended an interactive seminar presenting evidence-based guidelines on antibiotic prescription for respiratory infections. The proportion of prescriptions containing an antibiotic in each group and related costs were compared to the baseline up to 30 months following the intervention. Data were obtained from the National Health Insurance System database.
Results
In the intervention group, 4–6 months after the intervention, there was a significant decrease in the proportion of prescriptions containing an antibiotic from 15.2 ± 5.4% to 12.3 ± 5.8% (−2.8% [95% CI = −3.8 to −1.9], P<0.001). By contrast, an increase was observed in controls from 15.3 ± 6.0 to 16.4 ± 6.7% (+1.1% [95% CI = +0.4 to +1.8], P<0.01), resulting in a between-group difference of 3.93% ([95% CI = 2.75 to 5.11], P<0.001). The between-group difference was maintained 30 months after intervention (1.99% [95% CI = 0.56 to 3.42], P<0.01). Persistence of the intervention effect over the entire study period was confirmed in a hierarchical multivariate analysis.
Conclusion
This randomised trial shows that a standardised and interactive educational seminar results in a long-term reduction in antibiotic prescribing and could justify a large-scale implementation of this intervention.
Keywords: anti-bacterial agents, antibiotic prescriptions, education, general practitioners, primary health care
INTRODUCTION
Bacterial resistance to antimicrobial agents has emerged as a major threat for public health.1 The selection pressure induced by antibiotic consumption is one of the main causes of bacterial resistance.1–5 Although debated, several publications have suggested that decreasing antibiotic consumption could contribute to reducing the prevalence of antibiotic-resistant bacteria.6–9
Strong evidence suggests that a substantial proportion of antibiotic prescriptions in general practice could be avoided.10 Respiratory tract infections (RTIs) are the most common reason for antibiotic prescriptions in primary care.11 However, their use in first-line treatment of these diseases is not recommended by international guidelines in patients without underlying conditions.12–14
Ten years ago, antibiotic consumption and bacterial resistance rates in France were among the highest in Europe.4 Since 2002, the national health authorities have developed various programmes to reduce antibiotic consumption mainly through advertising campaigns via radio, television, and newspapers. These nationwide campaigns targeting the general public have led to a decrease in antibiotic consumption.5 Despite their relative efficacy, outpatient antibiotic use in France remains one of the highest in Europe.15 This highlights the need to develop additional strategies to reduce antibiotic use.
Different interventions have been evaluated to improve GP prescribing behaviour:16 mailing campaigns,17,18 group education focusing either on evidence-based medicine19–22 or on communication skills,20,22,23 prescription practice feedback,18,22 private interview with an expert,17,24 and use of education materials.25,26 Recently, Butler et al have reported that a complex education programme reduces antibiotic dispensing in primary care.27 However, the effectiveness of standardised GP educational seminars remains controversial and several studies have shown no significant effect on antibiotic prescription after the training.20,21 Most of the previous studies were of limited size,23, 26 included only selected patients,17,22 or were not randomised.19,20,24 In addition, none of them provided a long-term follow-up of patient antibiotic consumption.
The aim of this large scale randomised trial was to evaluate the effectiveness of a standardised and interactive educational seminar on GP antibiotic prescribing behaviour. This study followed the effect of an evidence-based seminar and of an additional education programme focused on problem-solving strategies with an annual follow-up for 3 consecutive years.
How this fits in
The effectiveness of programmes designed to reduce antibiotic overuse remains controversial and these interventions usually require multifaceted approaches and substantial resources. In addition, few studies have evaluated their long-term effect on the prescribing behaviour of physicians. This randomised controlled trial is one of the first to show a long-term reduction in antibiotic prescribing in general practice after a standardised medical education programme based on interactive methods. The results could serve to build future medical training programmes designed to reduce antibiotic prescribing.
METHOD
Study design
The design of this prospective, randomised controlled trial is described in Figure 1. This study was approved by the institutional review board of Henri Mondor Hospital. All GPs with a practice located in three counties within Paris region (Val de Marne, Hauts-de-Seine, and Seine-et-Marne) were contacted by mail. Two hundred and three GPs signed an informed consent form by June 2004.
Figure 1.
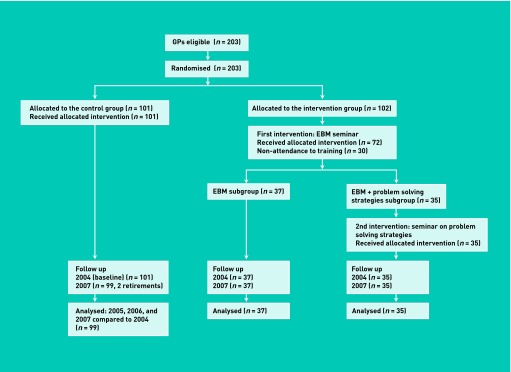
Design of the randomised controlled trial (EBM = evidence based medicine, R= randomisation).
Enrolled GPs were randomly assigned either to the control or intervention groups (1:1), with stratification according to sex and professional setting. The intervention group was then randomly divided into two subgroups (Figure 1). The randomisation procedure was entirely performed before the initiation of the study. The random allocation sequences were generated using a random number table.
GPs who were randomly enrolled in the control group (n = 101) received no specific recommendations about antibiotic prescriptions. Two GPs were excluded from this group after randomisation due to retirement during the follow-up period (n = 99).
All GPs assigned to the intervention group attended a 2-day didactic educational seminar focusing on evidence-based guidelines for diagnosis and treatment of acute RTIs (see below). The intervention in the first subgroup was limited to this 2-day seminar (evidence-based medicine subgroup). In the second subgroup, GPs attended an additional 1-day seminar focused on problem-solving strategies (evidence-based medicine plus problem-solving strategies subgroup).
Of 102 GPs initially assigned to the intervention group, 30 could not attend the seminar due to busy schedules and were not considered for analysis. Finally, 72 GPs were included in the intervention group (37 and 35 GPs in the evidence-based medicine and evidence-based medicine plus problem-solving strategies subgroups respectively).
Educational meeting (September 2004)
The educators responsible for the evidence-based medicine programme content were GPs with expertise in continuing medical education and experts in infectious diseases. The programme alternated between small group discussions and plenary sessions. The programme presented differences in European antibiotic prescription rates compared to currently observed antibiotic resistance in bacteria. GPs were trained in evidence-based guidelines for the diagnosis and the treatment of upper and lower RTIs. Specific criteria for antibiotic prescription and the first choice antibiotic were described. French guidelines and references were provided to the attendees at the end of the session.28,29
GPs who were randomly assigned to the evidence-based medicine plus problem-solving strategies subgroup had to attend another didactic seminar (7 hours) that focused on motivational enhancement and problem-solving strategies. Programme content was based on the results of a previous study performed by the group.30 This preliminary study described the reasons put forward by GPs for inappropriate antibiotic prescription for patients with upper or lower RTIs. It also analysed the problem-solving strategies used by physicians in these clinical settings. The seminar was illustrated by four role-play sessions performed by attendees. In addition, physicians completed six case report forms after the training, designed to assist them in reflecting on their own medical practice.
A detailed description of both educational seminars is available from the authors.
Data collection
In France, the prescriptions of physicians are reimbursed by the NHIS. From January 2004 to March 2004, defined as the baseline, antibiotic prescriptions from all enrolled GPs were extracted from the National Health Insurance System (NHIS)database. The analysis was limited to a pre-established list of medications used in the treatment of RTIs and available for outpatient prescription (antibiotics and symptomatic drugs: analgesics, antihistamines, decongestants, mucolytics and cough remedies). From 2005 to 2007, the same data were collected each year from January to March to reduce variability in antibiotic prescription induced by epidemic viral infections. For each GP, the proportion of their prescriptions containing an antibiotic was determined, that is, the number of their prescriptions containing an antibiotic divided by the total number of all their prescriptions. Symptomatic drugs were presented in a similar way. No feedback was provided to the enrolled GPs during the study.
The cost analysis was performed based on the cost of prescriptions for the French NHIS. The analysis was based on prices and reimbursement rates determined by the national health authorities at the national level. For each GP included in the study, the NHIS calculated the cost of their prescriptions of antibiotics and symptomatic drugs used in the treatment of respiratory tract infections (Appendix 1) at baseline and during the follow-up periods.
The primary outcomes of this study were the comparison of the change in the proportion of prescriptions containing an antibiotic in 2005 (compared to the baseline) between the group of physicians attending the training and control GPs, as well as the change in the related costs.
Predetermined secondary outcomes were changes (compared to baseline) in: (1) the proportion of prescriptions containing an antibiotic in the following years (2006, 2007), that is, testing the persistence of the effect of intervention; (2) the proportion of prescriptions containing a symptomatic drug for respiratory infections, and (3) the cost of symptomatic drug prescriptions. In addition, the impact of the both interventions on antibiotic prescribing was also analysed.
Sample size determination
Sample size determination showed that 60 GPs per group were required to be able to detect a 10% relative decrease in the primary outcome (change in the proportion of prescriptions containing an antibiotic compared to 2004) between intervention and control groups (for α = 0.05 and β = 0.05), assuming a standard deviation in both groups of 15%. Due to the risk of GPs lost to follow-up, 100 GPs per group were included.
For the comparison between the two intervention subgroups (evidence-based medicine versus evidence-based medicine plus problem-solving strategies), the randomisation was balanced (1:1). This number of GPs allowed the detection of a 20% difference in the primary outcome between both intervention subgroups (for α = 0.05 and β = 0.05).
Statistical analysis
Results are presented as absolute differences between data in 2004 and data the following years. Changes in the proportion of prescriptions containing an antibiotic or a symptomatic drug over time were expressed as absolute variations compared to baseline (that is, data during the 2005/2006/2007 period minus data in 2004) with their 95% CIs. All quantitative variables were compared using a non-parametric Mann-Whitney test for two groups or a Kruskal-Wallis test for three groups. Analyses concerning prescriptions were performed at GP level.
A hierarchical discrete-time linear model was performed to take into account the repeated measures (2004, 2005, 2006, and 2007) nature of the data, with the time at level 1 and the GP at level 2. The dependent variable was the proportion of prescriptions containing antibiotic. An empty model was built and then a second model adjusted for sex of the GP, time, educational intervention and an interaction term between time and educational intervention. The GP effect was random on the intercept. ANOVA were performed for secondary analysis.
Results were considered to be significantly different for P-values <0.05.
RESULTS
Demographic and professional characteristics of GPs are shown in Table 1. GP activity in terms of consultation and prescribing were similar.
Table 1.
GPs characteristics
| GP characteristics | Control group | Intervention group |
|---|---|---|
| N | 99 | 72 |
|
| ||
| Sex: n (%) of male | 70 (71) | 58 (81) |
|
| ||
| Practice setting: solo/group practice, n (%) | 42 (42)/57 (58) | 28 (39)/44 (61) |
|
| ||
| Experience (years) | 19.4 ± 8.1 | 20.8 ± 8.3 |
|
| ||
| Practice location: rural/urban, n (%) | 28 (28)/71 (72) | 18 (25)/54 (75) |
|
| ||
| Distribution of patients treated by antibiotic by age (baseline period) | ||
| Children (<15 years old) (%) | 30 | 29 |
| Adults (%) | 55 | 54 |
| Seniors (>60 years old) (%) | 15 | 17 |
Effect of the intervention on antibiotic prescriptions
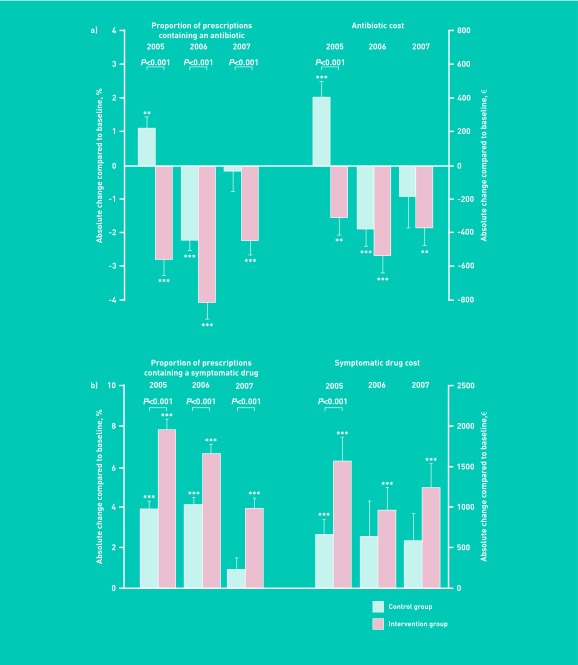
From 2004 to 2005, a significant decrease was observed in the proportion of prescriptions containing an antibiotic in the intervention group from 15.2% ± 5.4% to 12.3% ± 5.8% (that is, −2.8% [95% CI = −3.8 to −1.9], P<0.001). By contrast, an increase was observed in the control group, from 15.3 ± 6.0 to 16.4 ± 6.7% (+1.1% [95% CI = +0.4 to +1.8], P<0.01), resulting in a between-group difference of 3.93% ([95% CI = 2.75 to 5.11], P<0.001). In 2006, both groups showed a decrease in the proportion of prescriptions containing an antibiotic compared to 2004 (−4.1% [95% CI = −5.1 to −3.2] versus −2.2% [95% CI = −2.9 to −1.6] in controls, both P<0.001). However, the decrease was larger in the intervention group than in controls, with a between group difference of 1.91% ([95% CI = 0.79 to 3.03], P<0.001). The difference between intervention and control groups was maintained in 2007 (1.99% [95% CI = 0.56 to 3.42], P<0.01) (Figure 2).
Figure 2.
Effects of an educational meeting on GP prescriptions. Absolute change from 2005 to 2007 compared to baseline in (a) proportion of prescriptions containing an antibiotic and related cost and (b) proportion of prescriptions containing a symptomatic drug and related cost. Data are expressed as mean ± SEM. Asterisks indicate the level of statistical significance for intra-group comparisons (compared to baseline) (*P<0.05, **P<0.01, ***P<0.001). P-values represent between-group differences (control group versus intervention).
In a second step, a hierarchical multivariate analysis was performed taking into account an interaction between time and educational intervention, using absolute proportions of prescriptions containing an antibiotic. The educational intervention was associated over the 2005–2007 period with a significant reduction of 7.7% of the proportion of prescriptions containing an antibiotic compared to controls when adjusted for time and sex. Female GPs were independently associated with a 2% increase in the proportion of prescriptions containing an antibiotic. A non-significant decrease of 1.3% was observed over time.
Data on absolute proportions of prescriptions containing an antibiotic in 2004, 2005, 2006, and 2007 are available from the authors.
The effect of the intervention on the volumes of antibiotic prescriptions was similar to the effect on the proportions of prescriptions containing an antibiotic. For a similar volume of prescriptions at baseline (n = 136 [95% CI = 118 to 154] versus n = 150 [95% CI = 130 to 170] prescriptions of antibiotics per GP over 3 months in intervention and control groups respectively, P = 0.31), the volume of prescriptions decreased after the educational meeting in the intervention group (n = 121 [95% CI = 103 to 139], n = 109 [95% CI = 95 to 123] and n = 120 [95% CI = 104 to 136] in 2005, 2006 and 2007, respectively) whereas fluctuations around the baseline were observed in the control group (n = 171 [95% CI = 149 to 193]; n = 134 [95% CI = 116 to 152] and n = 146 [95% CI = 128 to 164]). ANOVA showed a significant group effect between intervention and control groups (P<0.01), time effect (P<0.01) and interaction between time and group (P<0.01).
Effect of the intervention on symptomatic drug prescriptions
The opposite effect was observed in symptomatic drug prescriptions (Figure 2b). From 2004 to 2005, both groups showed an increase in the proportion of prescriptions containing a symptomatic medication, but the increase was larger in the intervention group than in controls (+7.8% [95% CI = +6.8 to +8.8] versus +3.9% [95% CI = +3.2 to +4.7], respectively, P<0.001 between groups). Similar results were observed in 2006 and 2007. Analysis for repeated measures showed over the study period (2005–2007) a significant group effect (intervention group versus control, P = 0.05), time effect (P = 0.001) and interaction between time and group P = 0.004).
Cost analysis
The cost analysis showed that the decrease in antibiotic prescription was associated with a significant reduction in antibiotic prescription costs in the intervention group between 2004 and 2005 (−313€ [95% CI = −512 to −113] versus +393€ [95% CI = +201 to +585] in controls, P<0.001) (Figure 2b). Analysis for repeated measures showed significant group effect (P = 0.044), time effect (P<0.001) and interaction between time and group (P<0.001) over the study period (2005–2007).
By contrast, a larger increase was observed in the cost of symptomatic drug prescription in the intervention group than in controls between 2004 and 2005 (P<0.01) (Figure 2b). However, the ANOVA showed no group effect over the entire study period for this parameter.
Subgroup analysis
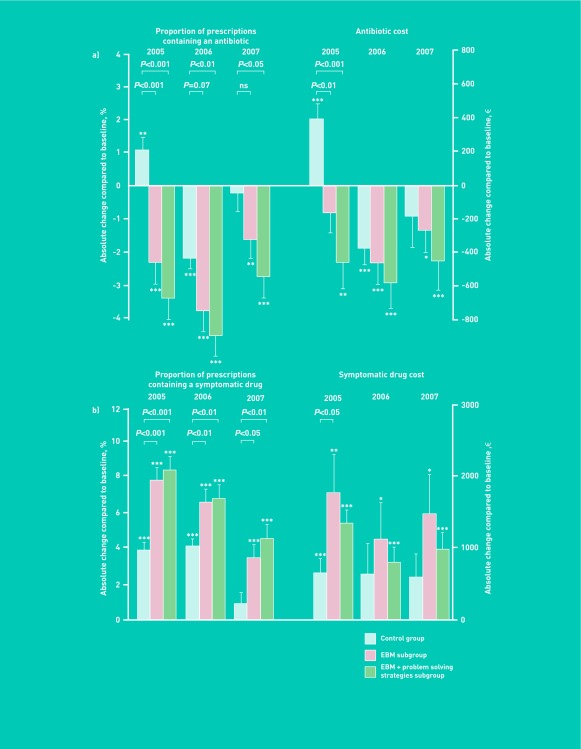
As predetermined, the modification in prescribing behaviour was analysed in both intervention subgroups. Compared to baseline, a reduction was observed in the proportion of prescriptions containing an antibiotic in both intervention subgroups during the entire study period (Figure 3). The decrease in the proportion of prescriptions containing an antibiotic was significantly larger in the evidence-based medicine plus problem-solving strategies subgroup than in controls in 2005, 2006, and 2007. Similarly, the decrease was significantly larger in the evidence-based medicine subgroup than in controls in 2005 and a similar trend was observed in 2006. The effects of the intervention on prescription costs and symptomatic drug prescriptions in both intervention subgroups are presented in Figure 3.
Figure 3.
Comparison of the effect of two educational strategies: evidence based medicine subgroup (evidence-based medicine subgroup) and evidence-based medicine plus problem-solving strategies subgroup. Absolute change from 2005 to 2007 compared to baseline in (a) Proportion of prescriptions containing an antibiotic and related cost, (b) Proportion of prescriptions containing a non-antibiotic symptomatic drug and related cost. Data are expressed as mean ± SEM. Asterisks indicate the level of statistical significance for intra-group comparisons (compared to baseline) (*P<0.05, **P<0.01, ***P<0.001). P-values represent between-group differences (control group versus intervention).
DISCUSSION
Summary
This study showed that a standardised medical education programme based on interactive methods significantly reduces antibiotic prescribing in general practice. The modifications of GP prescribing behaviour were maintained for up to 30 months following intervention.
Strengths and limitations
The study has several strengths. First, the analysis was based on a large number of prescriptions (around 160 000 prescriptions per year) and changes in the prescribing behaviour of GPs were followed for a long period of time. Secondly, GPs’ prescriptions were collected from the database of the NHIS to obtain comprehensive and comparable data throughout the study. Finally, data in the control group, showing an increase in antibiotic prescriptions in 2005 after a severe flu epidemic and a decrease in 2006, were consistent with national fluctuations. This confirmed how representative the sample is.
The aim of this trial was to evaluate the effectiveness of the intervention on GP prescribing behaviour. The study was therefore not designed to analyse patient outcome, since previous studies have shown that a reduction of antibiotic prescribing has no effect on patient outcome.22,31,32
Thirty GPs randomly assigned to the intervention group could not attend the educational meeting due to busy working schedules despite their interest in the seminar. This is inherent in pragmatic studies conducted in general practice during daily patient care. Since the aim of this study was to assess the impact of participation in an educational seminar, the intervention group included only GPs who actually attended the training. Data were analysed on a per protocol basis.
Comparison with existing literature
Various interventions have been evaluated for their impact on reducing antibiotic prescriptions. Distributing guidelines, educational materials, auditing, and feedback do not change physician’s behaviour when they are used individually.16 Multifaceted educational campaigns have also reported inconsistent results. In a Wisconsin study, a combination of media advertisements, educational materials and conferences was followed by fewer requests by patients for antibiotics.33 However, physician prescribing strategies were not modified and antibiotic consumption was not reduced after this large campaign.34 Similarly, another randomised community-wide educational intervention in Massachusetts showed no effect on physician beliefs concerning antibiotic overuse and bacterial resistance.35 By contrast, more favourable results have been reported in France and Belgium after nationwide campaigns.5,6 Furthermore, a complex intervention has shown a reduction in antibiotic prescribing with a 1-year follow-up in Wales.27 The results from all these studies strongly suggest that significant resources must be mobilised to obtain a moderate change in antibiotic prescribing.
Physician-level interventions could contribute to improve GP antibiotic prescribing behaviour. In Finland, a controlled study analysed the effect of guideline dissemination through local interactive discussion groups: no reduction in antibiotic prescription was observed during the 5-year follow-up.36 In Switzerland, a similar lack of efficacy was reported for an interactive seminar on guidelines with and without a patient-centred communication conference.20 However, other authors have reported more positive results. In Michigan, a small non-randomised controlled trial associating half-day educational sessions with distribution of patient educational materials showed a 25% reduction in antibiotic prescribing 5 months after the intervention.32 Another study from Colorado reported a reduction in antibiotic prescriptions in patients with acute bronchitis after an intervention based on physician communication training, evidence-based conferences and distribution of educational materials.31 Finally, during a randomised study in Belgium, patients were less likely to receive antibiotics in the group of GPs who received an educational visit based on guidelines, than in controls.37
Few randomised studies have evaluated the impact of medical education programmes on medical practice using an appropriate methodology.38,39 It was recently pointed out that multiple interventions are more effective than interventions limited to a single element.40 Interestingly, the results show that a significant effect can also be obtained with a one-time intervention based on an appropriate programme. Several key factors could have contributed to the efficacy of the programme.38,41,42 The standardised intervention meets criteria recommended for medical education programmes and adult learning: a seminar lasting several days, specifically targeting GPs, and based on interactive methods (case-based learning in small groups, feedback in plenary sessions). In addition, sessions were led by experts in general practice and infectious diseases.42 One of the more interesting results of the study was that its effect was maintained up to 30 months after the intervention, despite fluctuations observed in antibiotic prescribing at national level. In most previous studies, follow-up rarely extended beyond 1 year after intervention,38,39,43 and long-term outcome assessment was negatively correlated with the effect of medical education programmes.42 The maintained effects in the trial of more than 2 years after intervention show that long-term change in GP behaviour is possible.43 In a subgroup of GPs, the efficacy of an additional intervention focused on problem-solving strategies and based on the results of a preliminary qualitative study was evaluated.30 Some of the results suggest that the effect of the intervention on antibiotic prescriptions could last longer in the evidence-based medicine plus problem-solving strategies subgroup. However, the difference between both education subgroups was not significant, in accordance with the study design that was not empowered to detect the observed differences between both subgroups.
Implications for research and practice
This randomised controlled trial shows that a standardised medical education programme reduces antibiotic prescribing whatever the winter epidemic context. Its efficacy was maintained for at least 30 months after intervention. These results could justify the large-scale implementation of this intervention.
Acknowledgments
The authors thank Cedric Viallette (URC-Mondor) for technical support.
Appendix 1. List of generic names of all antibiotics included in the analysis
Penicillins
Phenoxymethylpenicillins
Penicillin V
Aminopenicillins
Amoxicillin
Ampicillin
Bacampicillin
Pivampicillin
Aminopenicillins combined with betalactamase inhibitors
Amoxicillin / Clavulanate potassium
Ampicillin / Sulbactam sodium
Macrolides
Josamycin
Spiramycin
Midecamycin
Roxithromycin
Dirithromycin
Erythromycin
Clarithromycin
Telithromycin
Azithromycin
Macrolides combined with another antibiotic class
Spiramycin / Metronidazole
Erythromycin / Sulfisoxazole
Streptogramins
Virginiamycin
Cephalosporins
- First generation
- Cefaclor
- Cefatrizine
- Cefadroxil
- Cephalexin
- Cephradrine
- Second generation
- Cefuroxime
- Third generation
- Cefixime
- Cefpodoxime proxetil
- Cefotiam
- Ceftriaxone
Quinolones
Ciprofloxacin
Levofloxacin
Ofloxacin
Lomefloxacin
Pefloxacin
Moxifloxacin
Sparfloxacin
Tetracyclines
Doxycycline
Minocycline
Methacycline
Lymecycline
Tetracycline
Lincosamides
Clindamycin
Lincomycin
Funding
This work was supported by the French National Health Insurance (‘Fonds d’aide à la qualité des soins de ville’, ‘Fonds d’intervention pour la qualité et la coordination des soins’); Union des Medecins Liberaux – Ile de France and GlaxoSmithKline. The funders have not played any decision-making role in the research.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and received ethical approval from the institutional review board of Henri Mondor University Hospital.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 2.Albrich WC, Monnet DL, Harbarth S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis. 2004;10(3):514–517. doi: 10.3201/eid1003.030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 4.Elseviers MM, Ferech M, Vander Stichele RH, et al. Antibiotic use in ambulatory care in Europe (ESAC data 1997–2002): trends, regional differences and seasonal fluctuations. Pharmacoepidemiol Drug Saf. 2007;16(1):115–123. doi: 10.1002/pds.1244. [DOI] [PubMed] [Google Scholar]
- 5.Sabuncu E, David J, Bernède-Bauduin C, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med. 2009;6(6):e1000084. doi: 10.1371/journal.pmed.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goossens H, Coenen S, Costers M, et al. Achievements of the Belgian Antibiotic Policy Coordination Committee (BAPCOC) Euro Surveill. 2008;13(46):19036. pii. [PubMed] [Google Scholar]
- 7.Nasrin D, Collignon PJ, Roberts L, et al. Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ. 2002;324(7328):28–30. doi: 10.1136/bmj.324.7328.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337(7):441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 9.Waterer GW, Buckingham SC, Kessler LA, et al. Decreasing beta-lactam resistance in Pneumococci from the Memphis region: analysis of 2152 isolates From 1996 to 2001. Chest. 2003;124(2):519–525. doi: 10.1378/chest.124.2.519. [DOI] [PubMed] [Google Scholar]
- 10.Petursson P. GPs’ reasons for ‘non-pharmacological’ prescribing of antibiotics. A phenomenological study. Scand J Prim Health Care. 2005;23(2):120–125. doi: 10.1080/02813430510018491. [DOI] [PubMed] [Google Scholar]
- 11.Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289(6):719–725. doi: 10.1001/jama.289.6.719. [DOI] [PubMed] [Google Scholar]
- 12.Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med. 2001;134(6):479–486. doi: 10.7326/0003-4819-134-6-200103200-00013. [DOI] [PubMed] [Google Scholar]
- 13.Wong DM, Blumberg DA, Lowe LG. Guidelines for the use of antibiotics in acute upper respiratory tract infections. Am Fam Physician. 2006;74(6):956–966. [PubMed] [Google Scholar]
- 14.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26(6):1138–1180. doi: 10.1183/09031936.05.00055705. [DOI] [PubMed] [Google Scholar]
- 15.Van de Sande-Bruinsma N, Grundmann H, Verloo D, et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14(11):1722–1730. doi: 10.3201/eid1411.070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev. 2005;(4):CD003539. doi: 10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Santis G, Harvey KJ, Howard D, et al. Improving the quality of antibiotic prescription patterns in general practice. The role of educational intervention. Med J Aust. 1994;160(8):502–505. [PubMed] [Google Scholar]
- 18.Zwar N, Wolk J, Gordon J, et al. Influencing antibiotic prescribing in general practice: a trial of prescriber feedback and management guidelines. Fam Pract. 1999;16(5):495–500. doi: 10.1093/fampra/16.5.495. [DOI] [PubMed] [Google Scholar]
- 19.Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108(3):575–583. doi: 10.1542/peds.108.3.575. [DOI] [PubMed] [Google Scholar]
- 20.Briel M, Langewitz W, Tschudi P, et al. Communication training and antibiotic use in acute respiratory tract infections. A cluster randomised controlled trial in general practice. Swiss Med Wkly. 2006;136(15–16):241–247. doi: 10.4414/smw.2006.11342. [DOI] [PubMed] [Google Scholar]
- 21.Poses RM, Cebul RD, Wigton RS. You can lead a horse to water--improving physicians’ knowledge of probabilities may not affect their decisions. Med Decis Making. 1995;15(1):65–75. doi: 10.1177/0272989X9501500110. [DOI] [PubMed] [Google Scholar]
- 22.Welschen I, Kuyvenhoven MM, Hoes AW, et al. Effectiveness of a multiple intervention to reduce antibiotic prescribing for respiratory tract symptoms in primary care: randomised controlled trial. BMJ. 2004;329(7463):431. doi: 10.1136/bmj.38182.591238.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cals JW, Butler CC, Hopstaken RM, et al. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ. 2009;338:b1374. doi: 10.1136/bmj.b1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaffner W, Ray WA, Federspiel CF, et al. Improving antibiotic prescribing in office practice. A controlled trial of three educational methods. JAMA. 1983;250(13):1728–1732. [PubMed] [Google Scholar]
- 25.Francis NA, Butler CC, Hood K, et al. Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on reconsulting and antibiotic prescribing: a cluster randomised controlled trial. BMJ. 2009;339:b2885. doi: 10.1136/bmj.b2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macfarlane J, Holmes W, Gard P, et al. Reducing antibiotic use for acute bronchitis in primary care: blinded, randomised controlled trial of patient information leaflet. BMJ. 2002;324(7329):91–94. doi: 10.1136/bmj.324.7329.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler CC, Simpson SA, Dunstan F, et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ. 2012;344:d8173. doi: 10.1136/bmj.d8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agence Française de Sécurité Sanitaire des Produits de Santé Systemic antibiotic treatment in upper and lower respiratory tract infections: official French guidelines. Clin Microbiol Infect. 2003;9(12):1162–1178. doi: 10.1111/j.1469-0691.2003.00798.x. [DOI] [PubMed] [Google Scholar]
- 29.Trémolières F, Perronne C. Practice guidelines for antibiotic therapy of respiratory tract infections in routine practice. Presse Med. 2001;30(36):1764–1765. [PubMed] [Google Scholar]
- 30.Attali C, Amade-Escot C, Ghadi V. Limiting antibiotics prescriptions in so-called viral respiratory infections. Rev Prat Med Gen. 2003;17:155–160. [Google Scholar]
- 31.Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. JAMA. 1999;281(16):1512–1519. doi: 10.1001/jama.281.16.1512. [DOI] [PubMed] [Google Scholar]
- 32.Juzych NS, Banerjee M, Essenmacher L, et al. Improvements in antimicrobial prescribing for treatment of upper respiratory tract infections through provider education. J Gen Intern Med. 2005;20(10):901–905. doi: 10.1111/j.1525-1497.2005.0198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiang KM, Kieke BA, Como-Sabetti K, et al. Clinician knowledge and beliefs after statewide program to promote appropriate antimicrobial drug use. Emerg Infect Dis. 2005;11(6):904–911. doi: 10.3201/eid1106.050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belongia EA, Knobloch MJ, Kieke BA, et al. Impact of statewide program to promote appropriate antimicrobial drug use. Emerg Infect Dis. 2005;11(6):912–920. doi: 10.3201/eid1106.050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stille CJ, Rifas-Shiman SL, Kleinman K, et al. Physician responses to a community-level trial promoting judicious antibiotic use. Ann Fam Med. 2008;6(3):206–212. doi: 10.1370/afm.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rautakorpi UM, Huikko S, Honkanen P, et al. The Antimicrobial Treatment Strategies (MIKSTRA) program: a 5-year follow-up of infection-specific antibiotic use in primary health care and the effect of implementation of treatment guidelines. Clin Infect Dis. 2006;42(9):1221–1230. doi: 10.1086/503036. [DOI] [PubMed] [Google Scholar]
- 37.Coenen S, Van Royen P, Michiels B, Denekens J. Optimizing antibiotic prescribing for acute cough in general practice: a cluster-randomized controlled trial. J Antimicrob Chemother. 2004;54(3):661–672. doi: 10.1093/jac/dkh374. [DOI] [PubMed] [Google Scholar]
- 38.Davis D, Galbraith R, American College of Chest Physicians Health and Science Policy Committee Continuing medical education effect on practice performance: effectiveness of continuing medical education: American College of Chest Physicians Evidence-Based Educational Guidelines. Chest. 2009;135(3 Suppl):42S–48S. doi: 10.1378/chest.08-2517. [DOI] [PubMed] [Google Scholar]
- 39.Mazmanian PE, Davis DA, Galbraith R, American College of Chest Physicians Health and Science Policy Committee Continuing medical education effect on clinical outcomes: effectiveness of continuing medical education: American College of Chest Physicians Evidence-Based Educational Guidelines. Chest. 2009;135(3 Suppl):49S–55S. doi: 10.1378/chest.08-2518. [DOI] [PubMed] [Google Scholar]
- 40.van der Velden AW, Pijpers EJ, Kuyvenhoven MM, et al. Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br J Gen Pract. 2012;62(605):801–807. doi: 10.3399/bjgp12X659268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis D, O’Brien MA, Freemantle N, et al. Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes? JAMA. 1999;282(9):867–874. doi: 10.1001/jama.282.9.867. [DOI] [PubMed] [Google Scholar]
- 42.Mansouri M, Lockyer J. A meta-analysis of continuing medical education effectiveness. J Contin Educ Health Prof. 2007;27(1):6–15. doi: 10.1002/chp.88. [DOI] [PubMed] [Google Scholar]
- 43.Tian J, Atkinson NL, Portnoy B, et al. A systematic review of evaluation in formal continuing medical education. J Contin Educ Health Prof. 2007;27(1):16–27. doi: 10.1002/chp.89. [DOI] [PubMed] [Google Scholar]