Abstract
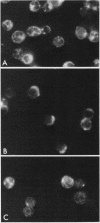
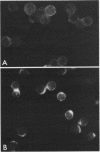
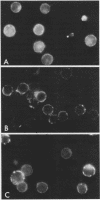
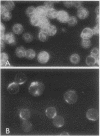
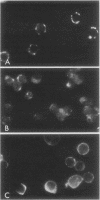
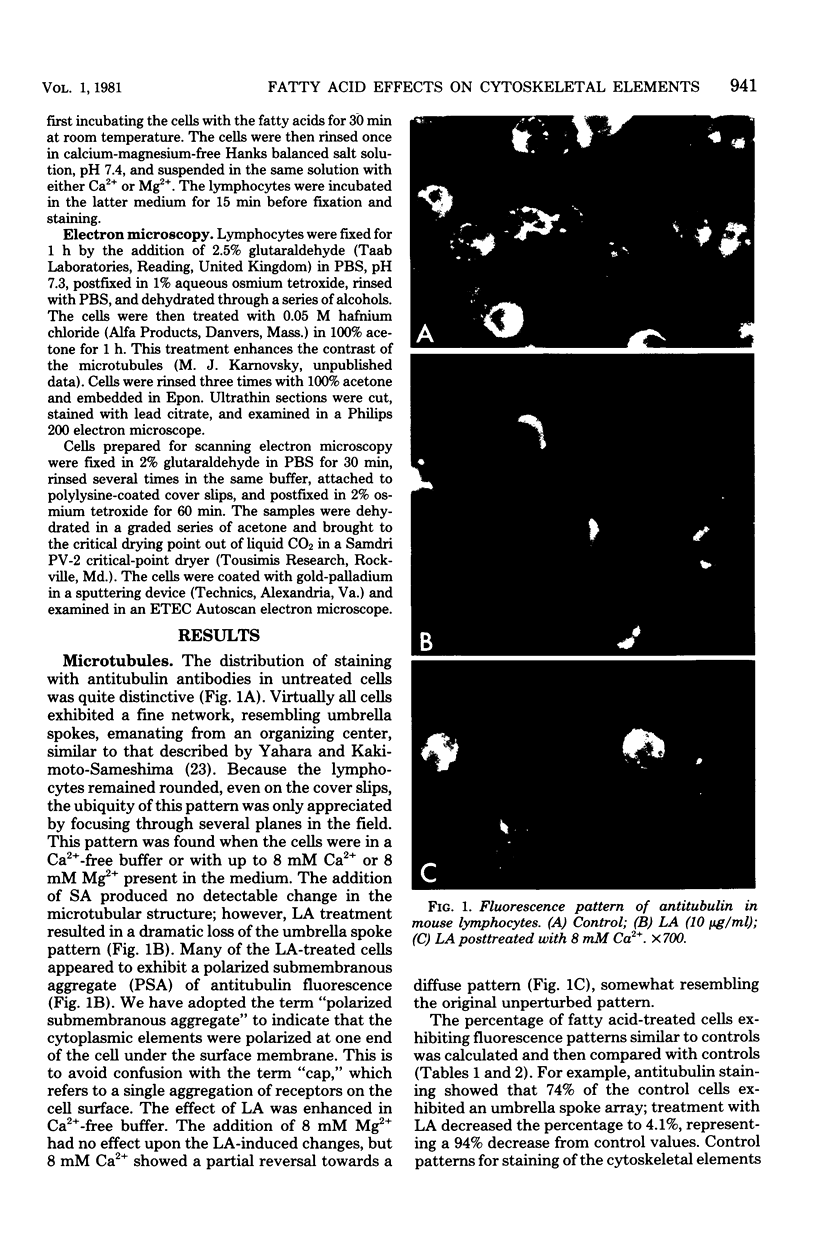
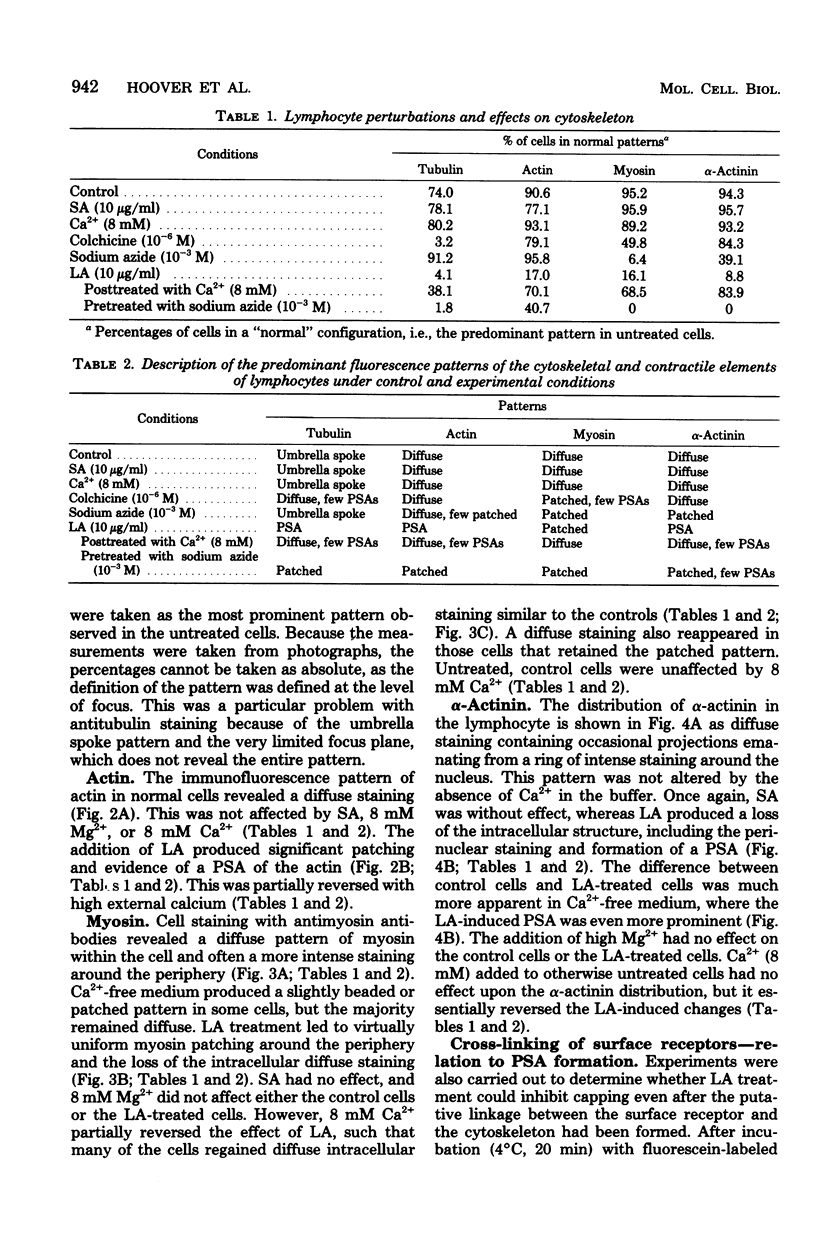
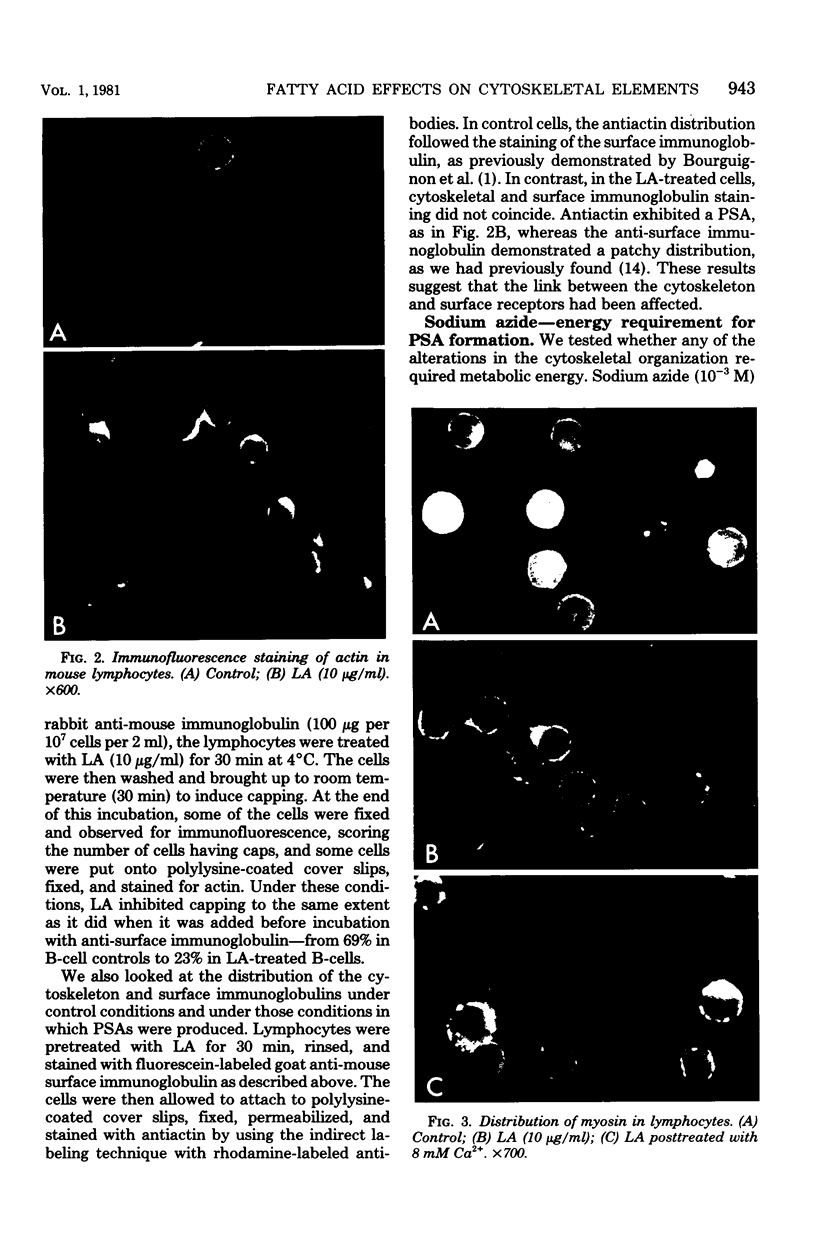
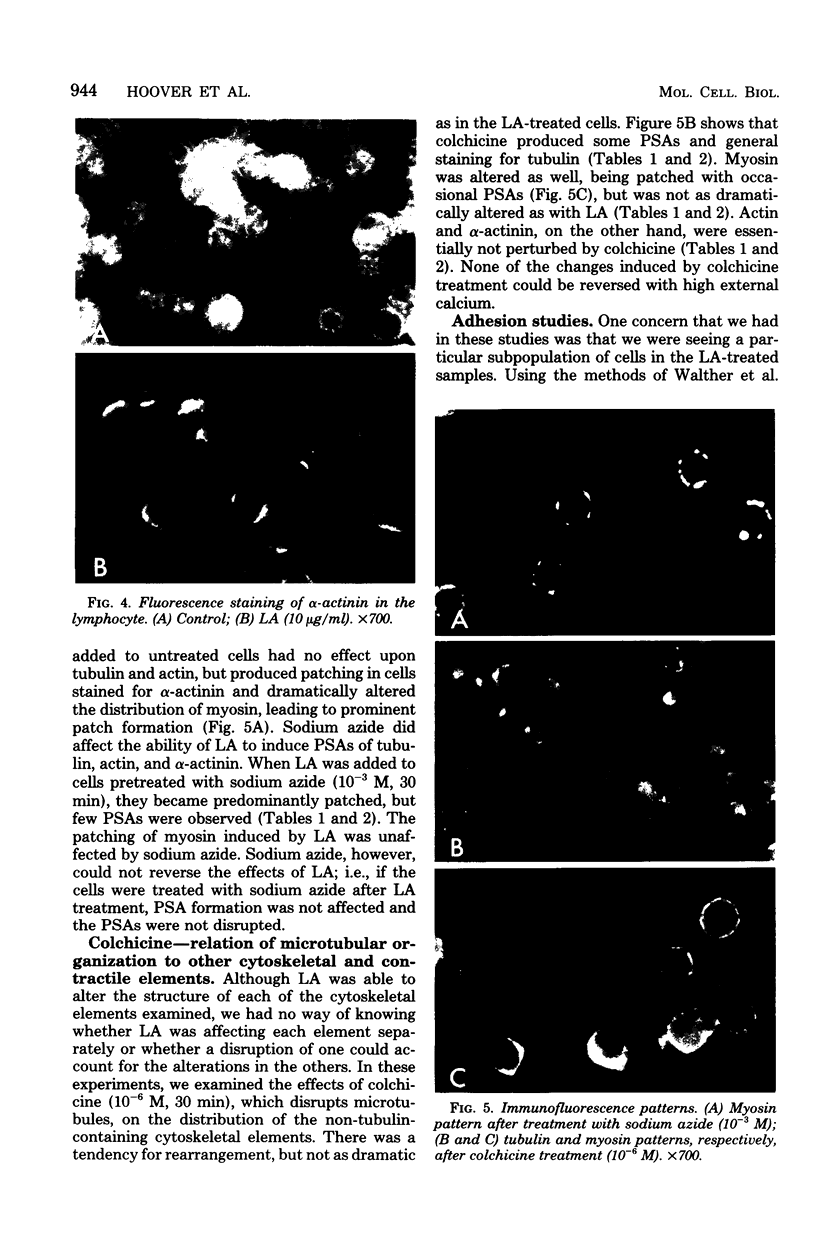
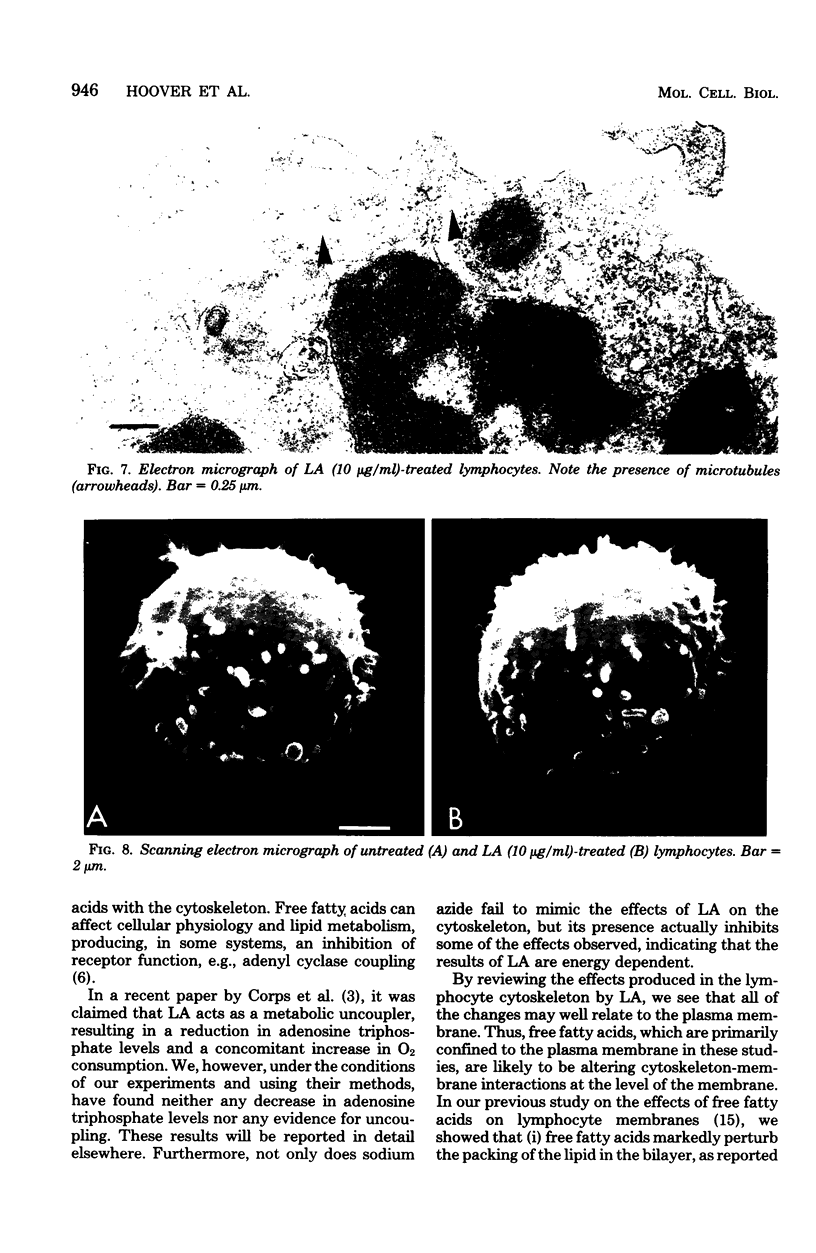
Treatment of mouse lymphocytes with cis-unsaturated free fatty acids produced alterations in the immunofluorescence patterns of the cytoskeleton and contractile proteins. Saturated free fatty acids and trans-unsaturated free fatty acids had no effect. In untreated cells, the microtubular pattern exhibited radiation from an organizing center, resembling the spokes of an umbrella. The addition of linoleic acid produced a polarized submembranous aggregate. Under control conditions, staining for actin revealed a diffuse pattern over the entire cell, but the addition of linoleic acid caused the formation of a single large patch, or polarized submembranous aggregate. The pattern for alpha-actinin normally revealed intense perinuclear staining on a diffuse background. Linoleic acid caused the loss of this pattern and the formation of a polarized submembranous aggregate. Linoleic acid treatment also caused the pattern for myosin to change from diffuse to uniform submembranous patching around the periphery of the cell. For all of these proteins, calcium (8 mM), but not magnesium, partially reversed the effects of linoleic acid. Sodium azide had little effect on the normal distribution of actin, tubulin, and alpha-actinin; however, myosin staining revealed prominent patch formation. Colchicine treatment caused diffuse staining, some polarized submembranous aggregate formation of tubulin, and some patching of myosin, but not as extensively as did treatment with linoleic acid. Actin and alpha-actinin were unaffected. These results, in view of the previously shown facts that pretreatment of cells with linoleic acid followed by anti-immunoglobulin inhibits capping of surface immunoglobulin (Klausner, et al., Proc. Natl. Acad. Sci. U.S.A. 77:437-441, 1980) and that free fatty acids partition into the surface membrane (Klausner et al., J. Biol. Chem. 255:1286-1295, 1980), suggest that the perturbation of the plasma membrane with unsaturated free fatty acids alters the interaction of surface receptors with the cytoskeleton, which in turn affects cytoplasmic distribution of the proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon L. Y., Tokuyasu K. T., Singer S. J. The capping of lymphocytes and other cells, studied by an improved method for immunofluorescence staining of frozen sections. J Cell Physiol. 1978 Jun;95(3):239–257. doi: 10.1002/jcp.1040950302. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Corps A. N., Pozzan T., Hesketh T. R., Metacalfe J. C. cis-Unsaturated fatty acids inhibit cap formation on lymphocytes by depleting cellular ATP. J Biol Chem. 1980 Nov 25;255(22):10566–10568. [PubMed] [Google Scholar]
- De Petris S. Inhibition and reversal of capping by cytochalasin B, vinblastine and colchicine. Nature. 1974 Jul 5;250(461):54–56. doi: 10.1038/250054a0. [DOI] [PubMed] [Google Scholar]
- Dragsten P., Henkart P., Blumenthal R., Weinstein J., Schlessinger J. Lateral diffusion of surface immunoglobulin, Thy-1 antigen, and a lipid probe in lymphocyte plasma membranes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5163–5167. doi: 10.1073/pnas.76.10.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard V. H., Esko J. D., Storm D. R., Glaser M. Modification of adenylate cyclase activity in LM cells by manipulation of the membrane phospholipid composition in vivo. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4482–4486. doi: 10.1073/pnas.73.12.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J., Koch G. L. Cross-linked surface Ig attaches to actin. Nature. 1978 May 25;273(5660):278–281. doi: 10.1038/273278a0. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Simultaneous localization of myosin and tubulin in human tissue culture cells by double antibody staining. J Cell Biol. 1978 Apr;77(1):182–195. doi: 10.1083/jcb.77.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Porter M. E., Pollard T. D. Alpha-actinin localization in the cleavage furrow during cytokinesis. J Cell Biol. 1978 Oct;79(1):268–275. doi: 10.1083/jcb.79.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Chaponnier C., Zumbe A., Vassalli P. Actin and tubulin co-cap with surface immunoglobulins in mouse B lymphocytes. Nature. 1977 Oct 20;269(5630):697–698. doi: 10.1038/269697a0. [DOI] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. The participation of alpha-actinin in the capping of cell membrane components. Cell. 1979 Jan;16(1):213–222. doi: 10.1016/0092-8674(79)90202-2. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Bhalla D. K., Yanovich S., Inbar M., Karnovsky M. J. Effects of linoleic acid on capping, lectin mediated mitogenesis, surface antigen expression, and fluorescent polarization in lymphocytes and BHK cells. J Cell Physiol. 1980 Jun;103(3):399–406. doi: 10.1002/jcp.1041030305. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Bhalla D. K., Dragsten P., Hoover R. L., Karnovsky M. J. Model for capping derived from inhibition of surface receptor capping by free fatty acids. Proc Natl Acad Sci U S A. 1980 Jan;77(1):437–441. doi: 10.1073/pnas.77.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Fujiwara K., Pollard T. D., Unanue E. R. Redistribution of myosin accompanying capping of surface Ig. J Exp Med. 1977 May 1;145(5):1393–1398. doi: 10.1084/jem.145.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Stephens R. E., Edds K. T. Microtubules: structure, chemistry, and function. Physiol Rev. 1976 Oct;56(4):709–777. doi: 10.1152/physrev.1976.56.4.709. [DOI] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihing R. R. The cytoskeleton and plasma membrane. Methods Achiev Exp Pathol. 1979;8:42–109. [PubMed] [Google Scholar]
- Yakara I., Kakimoto-Sameshima F. Microtubule organization of lymphocytes and its modulation by patch and cap formation. Cell. 1978 Sep;15(1):251–259. doi: 10.1016/0092-8674(78)90100-9. [DOI] [PubMed] [Google Scholar]