Abstract
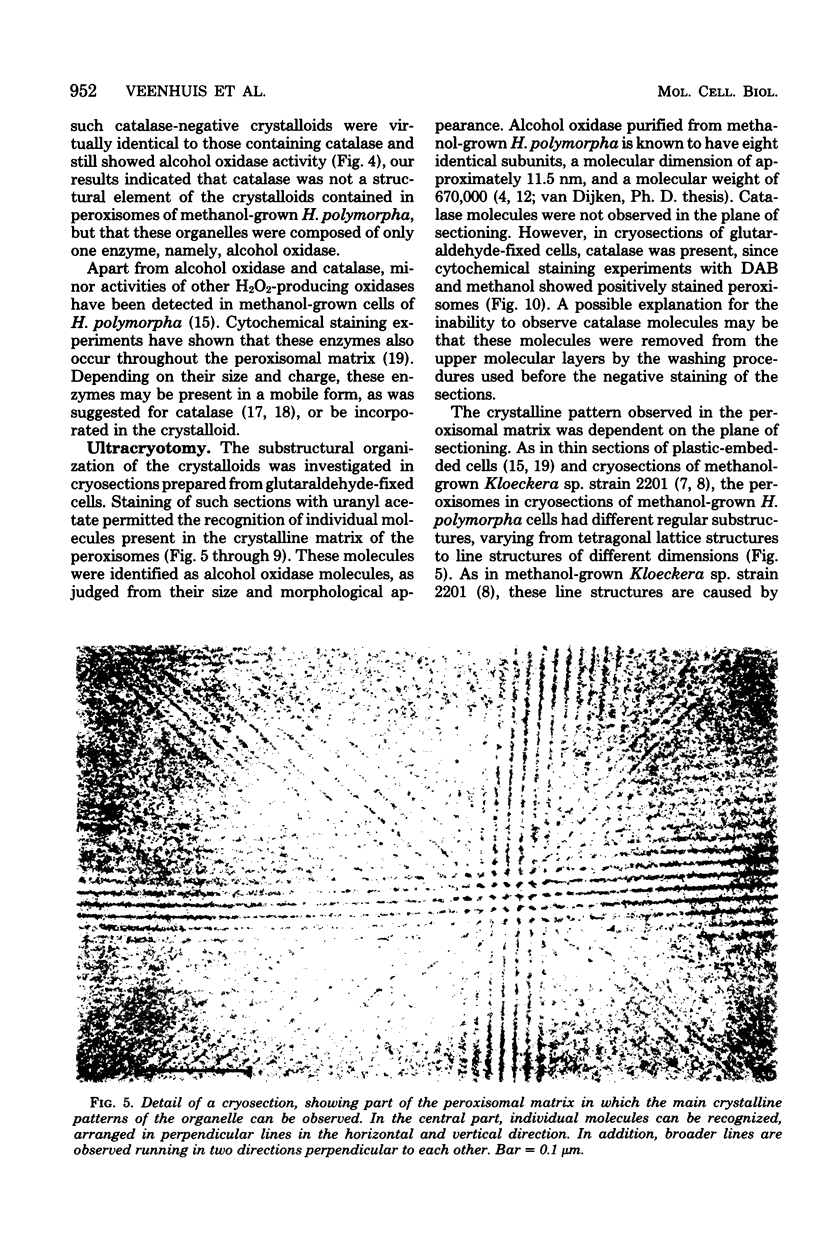
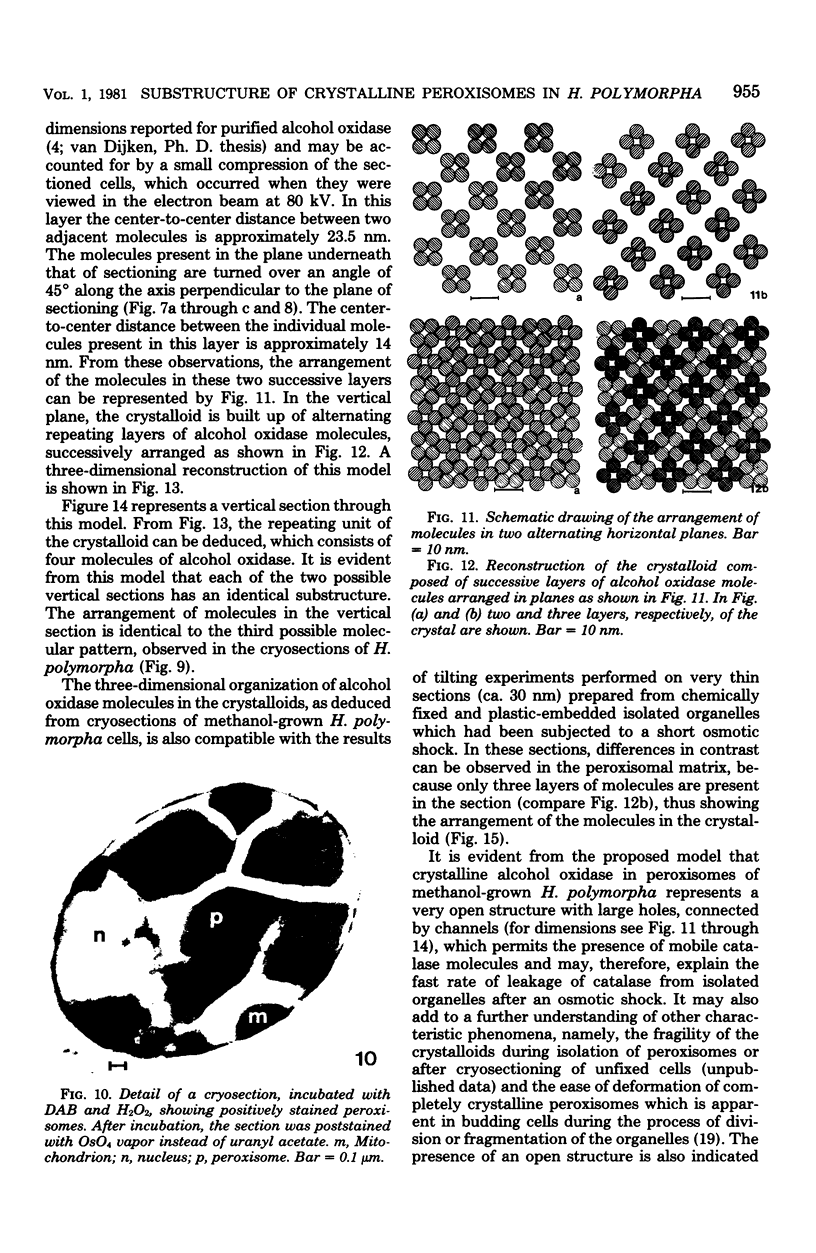
The substructural organization of completely crystalline peroxisomes present in Hansenula polymorpha cells grown under methanol limitation in a chemostat was investigated by different cytochemical and ultrastructural techniques. Time-dependent cytochemical staining experiments indicated that activities of the two main constituents of these organelles, namely, alcohol oxidase and catalase, were present throughout the crystalline matrix. Catalase was completely removed from isolated peroxisomes by osmotic shock treatment. After such treatment, the ultrastructure of the crystalline matrix of the organelles remained virtually intact. Because alcohol oxidase activity was still present in this matrix, it was concluded that alcohol oxidase protein is the only structural element of the peroxisomal crystalloids. The molecular architecture of the crystalloids was investigated in ultrathin cryosections which permitted recognition of individual molecules in the crystalline matrix. Depending on the plane of sectioning, different crystalline patterns were observed. Tilting experiments indicated that these images were caused by superposition of octameric alcohol oxidase molecules arranged in a tetragonal lattice. A three-dimensional model of the crystalloid is presented. The repeating unit of this structure is composed of four alcohol oxidase molecules. The crystalloid represents an open structure, which may explain the observed free mobility of catalase molecules.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fukui S., Tanaka A., Kawamoto S., Yasuhara S., Teranishi Y., Osumi M. Ultrastructure of methanol-utilizing yeast cells: appearance of microbodies in relation to high catalase activity. J Bacteriol. 1975 Jul;123(1):317–328. doi: 10.1128/jb.123.1.317-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeu W., Batenburg-Van der Vegte W. H., Nieuwdorp P. J. The fine structure of microbodies in the yeast Pichia pastoris. Experientia. 1975 Aug 15;31(8):926–927. doi: 10.1007/BF02358855. [DOI] [PubMed] [Google Scholar]
- Kato N., Omori Y., Tani Y., Ogata K. Alcohol oxidases of Kloeckera sp. and Hansenula polymorpha. Catalytic properties and subunit structures. Eur J Biochem. 1976 May 1;64(2):341–350. doi: 10.1111/j.1432-1033.1976.tb10307.x. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kaneko T., Yamamoto Y. Lysis of viable yeast cells by enzymes of Arthrobacter luteus. Arch Biochem Biophys. 1971 Jul;145(1):402–404. doi: 10.1016/0003-9861(71)90053-1. [DOI] [PubMed] [Google Scholar]
- Osumi M., Sato M. Further studies on the localization of catalase in yeast microbodies by ultrathin frozen sectioning. J Electron Microsc (Tokyo) 1978;27(2):127–136. [PubMed] [Google Scholar]
- Roels F., Goldfischer S. Cytochemistry of human catalase. The demonstration of hepatic and renal peroxisomes by a high temperature procedure. J Histochem Cytochem. 1979 Nov;27(11):1471–1477. doi: 10.1177/27.11.92501. [DOI] [PubMed] [Google Scholar]
- Roggenkamp R., Sahm H., Hinkelmann W., Wagner F. Alcohol oxidase and catalase in peroxisomes of methanol-grown Candida boidinii. Eur J Biochem. 1975 Nov 1;59(1):231–236. doi: 10.1111/j.1432-1033.1975.tb02446.x. [DOI] [PubMed] [Google Scholar]
- Tani Y., Kato N., Yamada H. Utilization of methanol by yeasts. Adv Appl Microbiol. 1978;24:165–186. doi: 10.1016/s0065-2164(08)70639-7. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A study of positive staining of ultrathin frozen sections. J Ultrastruct Res. 1978 Jun;63(3):287–307. doi: 10.1016/s0022-5320(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Harder W. Cytochemical studies on the localization of methanol oxidase and other oxidases in peroxisomes of methanol-grown Hansenula polymorpha. Arch Microbiol. 1976 Dec 1;111(1-2):123–135. doi: 10.1007/BF00446559. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Pilon S. A., Harder W. Development of crystalline peroxisomes in methanol-grown cells of the yeast Hansenula polymorpha and its relation to environmental conditions. Arch Microbiol. 1978 May 30;117(2):153–163. doi: 10.1007/BF00402303. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Otto R., Harder W. Growth of Hansenula polymorpha in a methanol-limited chemostat. Physiological responses due to the involvement of methanol oxidase as a key enzyme in methanol metabolism. Arch Microbiol. 1976 Dec 1;111(1-2):137–144. doi: 10.1007/BF00446560. [DOI] [PubMed] [Google Scholar]















