Abstract
Investigation of kinase-related processes often uses pharmacological inhibition to reveal pathways in which kinases are involved. However, one concern about using such kinase inhibitors is their potential lack of specificity. Here, we report that the calcium–calmodulin-dependent kinase II (CaMKII) inhibitor CK59 inhibited multiple voltage-gated calcium channels, including the L-type channel during depolarization in a dose-dependent manner. The use of another CaMKII inhibitor, cell-permeable autocamtide-2 related inhibitory peptide II (Ant-AIP-II), failed to similarly decrease calcium current or entry in hippocampal cultures, as shown by ratiometric calcium imaging and whole-cell patch clamp electrophysiology. Notably, inhibition due to CK59 was reversible; washout of the drug brought calcium levels back to control values upon depolarization. Furthermore, the IC50 for CK59 was approximately 50 μM, which is only fivefold larger than the reported IC50 values for CaMKII inhibition. Similar nonspecific actions of other CaMKII inhibitors KN93 and KN62 have previously been reported. In the case of all three kinase inhibitors, the IC50 for calcium current inhibition falls near that of CaMKII inhibition. Our findings demonstrate that CK59 attenuates activity of voltage-gated calcium channels, and thus provide more evidence for caution when relying on pharmacological inhibition to examine kinase-dependent phenomena.
Keywords: Calcium–calmodulin-dependent kinase II (CaMKII), Calcium channel, Kinase inhibitor, Nonspecific inhibition
Introduction
Calcium–calmodulin-dependent kinase II (CaMKII) is a crucial signaling molecule involved in many of the most fundamental behaviors of neurons, such as gene expression and synaptic plasticity due to long-term potentiation. It follows that the distribution of this protein is widespread throughout the nervous system, and makes up between 1 and 2 % of total protein content in neurons. Given both its importance and its abundance, CaMKII-mediated processes are currently under intense investigation. One of the most common ways to study the activity of CaMKII is to inhibit it with pharmacological agents and then investigate changes in the neuron when CaMKII is no longer active. Unfortunately, a common problem with kinase inhibitors, including CaMKII inhibitors, is their lack of specificity. Therefore, new, more specific inhibitors are always in demand. This demand extends to a clinical setting, where more specific CaMKII inhibitors may help avoid potentially harmful side effects.
Over 30 different isoforms (made up of various alternatively spliced α, β, δ, and γ subunits) of CaMKII have been reported (for review, see Fink and Meyer 2002; Wayman et al. 2011). The primary neuronal subunits are α and β, which are highly homologous. Subunits come together either as homo- or hetero-dodecihedromers, with each subunit containing a catalytic, regulatory, and oligomerization domain. The structure of CaMKII is often described as a petal, where the oligomerization domain makes up the inner core, while the catalytic and regulatory domains form a stack of 2 hexomers (Gaertner et al. 2004; Rosenberg et al. 2006). When calmodulin (CaM) binds to the regulatory domain, autoinhibition is disrupted, and the particular subunit interacting with CaM is free to autophosphorylate neighboring subunits, which causes their catalytic subunits to become active. Kinase inhibitors reduce the activity of the enzyme in different ways. For example, one of the most commonly used inhibitors, KN93 works to compete with CaM binding to the regulatory domain. This is in contrast to an inhibitor such as autocamtide-2 related inhibitory peptide (AIP); unlike KN agents, AIP also inhibits the catalytic function of activated CaMKII (Ishida et al. 1995; Song et al. 2010). Thus, with many different forms of inhibition may come many different off-target effects.
CaMKII phosphorylates a wide variety of proteins to modulate their activity, including voltage-gated calcium channels. Calcium channels are implicated in a diverse array of physiological processes, such as muscle contraction, neurotransmitter release, and gene expression. In neurons, there are three classes of voltage-gated calcium channels, based on their pore-forming α1 subunit (Cav 1.x, Cav 2.x, and Cav 3.x, for review see Catterall 2011). They can further be divided into various isoforms based on their pharmacological and kinetic profiles. For example, Cav 1.2 and Cav 1.3 are sensitive to dihydropyridines, activate at high voltages, and are known as L-type channels for their long-lasting current. Cav 2.1 and Cav 2.2 are known as N- and P/Q-type channels, are sensitive to certain cone snail toxins, and mediate synaptic release. Cav 2.3 was given the name R-type channel due to its resistance to most channel toxins, with a partial exception being the scorpion toxin SNX-482. Calcium channels are extensively regulated, either positively or negatively. Regulation occurs at various sites on the N- and C-terminals, or within the intracellular loops of the channel. G protein βγ subunits (Li et al. 2004) as well as multiple protein kinases (Mitterdorfer et al. 1996; Park et al. 2006; Wang et al. 2009; Bray and Mynlieff 2011) have been shown to interact with all types of voltage-gated calcium channels. Since levels of intracellular calcium often work as a switch for many physiological processes, there is a great deal of interest surrounding how the behavior of these channels can be modified by various signaling molecules.
Kinase inhibitor studies have been complicated by nonspecificity and off-target effects of compounds used to inhibit CaMKII. An example of this nonspecificity is the interaction of the CaMKII inhibitor KN93 with calcium channels (Gao et al. 2006). Previously regarded as one of the cleanest, most potent CaMKII inhibitors in existence, KN93 was shown to reversibly inhibit only L-type calcium channels. Thus, studies investigating processes or pathways involving both CaMKII and L-type channels will be confounding without the use of other, more specific inhibitors. Recently Konstantopoulos et al. have developed a compound known as CK59 which has been shown to inhibit CaMKII activity (Konstantopoulos et al. 2007). While originally created to investigate the kinase responsible for insulin-mediated GLUT 4 glucose transporter translocation, CK59 also offered an attractive option to those studying CaMKII and calcium channels, due to its potency (IC50 < 10 μM) and cell permeability. However, data here suggest that similar to KN93, CK59 also inhibits calcium channels in a reversible manner, despite being structurally distinct. Furthermore, while L-type channels appear to be dramatically inhibited, it appears to inhibit other voltage-gated calcium channels as well.
Methods
Hippocampal Cell Culture
All animal protocols were approved by the Marquette University Institutional Animal Care and Use Committee and followed the guidelines set forth by the US Public Health Service. Briefly, neurons from the superior region of the neonatal rat hippocampus were cultured as previously described (Mynlieff 1997). Neurons were dissociated from 6–8 day old Sprague–Dawley rat pups, plated on poly-l-lysine coated dishes (1 mg/mL, MW 38,500–60,000; Sigma-Aldrich, St. Louis MO) and kept in culture for 20–24 h to allow recovery from enzymatic digestion before experimentation. Cultures were maintained in Neurobasal A media supplemented with B27 (Life Technologies, Carlsbad CA), 0.02 mg/mL gentamicin (Life Technologies), and 0.5 mM l-glutamine (Sigma-Aldrich).
Calcium Imaging
Cultured neurons were incubated in 5 μM Fura-2 acetoxymethyl ester (Fura-2 AM, Life Technologies, Carlsbad CA) in calcium imaging Ringer’s solution (CIR, 154 mM NaCl, 5.6 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 11 mM glucose, pH 7.4 with NaOH) for 1 h at room temperature in the dark. Cells were rinsed and incubated for 30 min at room temperature in CIR without Fura-2 AM to allow for de-esterification. Depolarization was induced by application of high potassium solution (100 mM NaCl, 50 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 11 mM glucose, pH to 7.4 with NaOH) for 30 s to open voltage-gated calcium channels. Inhibitors were perfused onto the cells in CIR for 15 s, followed by high KCl solution with inhibitor for 35 s. Neurons were excited at 340 and 380 nm, and emissions were measured at 510 nm using Slidebook 5.0 software (Intelligent Imaging Innovations, Denver CO). The ratio of the emissions intensity when Fura-2 is excited at 340 and 380 nm is directly correlated to changes in intracellular calcium (Grynkiewicz et al. 1985). Average data represent the mean ± SEM.
Electrophysiology
Whole-cell electrophysiological recordings were performed as described previously (Carter and Mynlieff 2004; Bray and Mynlieff 2009; Bray and Mynlieff 2011). Data were acquired using a Dagan 3900A patch clamp amplifier (Dagan Corporation, Minneapolis MN), Digidata 1322A acquisition setup, and pClamp 10.0 software (Molecular Devices, Sunnyvale CA). The extracellular solution contained 10 mM CaCl2, 145 mM tetraethylammonium chloride, 10 mM HEPES, and 1 μM tetrodotoxin (Sigma-Aldrich, St. Louis MO), pH 7.4. Recording electrodes with resistances of 3–8 MΩ were pulled from borosilicate glass on a Flaming/Brown Micropipette Puller (model P87, Sutter Instrument Co., Novato CA) and filled with 140 mM Cs-aspartate, 5 mM MgCl2, 10 mM Cs2EGTA, 10 mM HEPES, 2 mM ATP-Na2, and 0.1 mM GTP, pH 7.4. Cells were held at −80 mV and depolarized with a 300 ms pulse to +10 mV. Whole-cell currents were electronically filtered at 1 kHz and digitized at 2 kHz. Linear components of leak current were subtracted posthoc by the passive resistance protocol in pClamp 10.0. CaMKII inhibitors were applied to the cells using a U-tube delivery system, constructed with PE-10 polyethylene tubing housed in a glass tube, which allowed for rapid application and removal of the compound that was gravity fed onto the cell and removed via suction. Averaged data represent the mean ± SEM.
Chemicals and Reagents
The cell-permeable CaMKII inhibitor 2-(2-Hydroxyethylamino)-6-aminohexylcarbamic acid tert-butyl ester-9-isopropylpurine (CK59, EMD Millipore, IC50 < 10 μM) was dissolved in DMSO and diluted 1:200,000–1:250 to produce final concentrations between 500 nM and 250 μM. A second CaMKII inhibitor, cell-permeable autocamtide-2 related inhibitory peptide II (Ant-AIP-II, EMD Millipore catalog #189485), was dissolved in distilled water and diluted 1:1,000 to produce a final concentration of 50 nM. This compound is an autocamtide-2 related inhibitory peptide (AIP) analog that contains the Antennapedia transport peptide sequence fused to the amino terminus of autocamtide-2 related inhibitory peptide II (AIP-II, EMD Millipore catalog #189484, IC50 = 4 nM) to enhance cell permeability. Ant-AIP-II has been demonstrated to successfully enter both glia and neurons in culture (Watterson et al. 2001; Mauceri et al. 2004). In electrophysiological experiments designed to block N and P/Q channel activity, 2 μM ω-conotoxin MVIIC (Sigma-Aldrich, St. Louis MO) was added to the bath and U-tube. In experiments where L-channel activity was blocked, 20 μM nimodipine (Tocris Bioscience, Minneapolis, MN) was added to the bath and U-tube in electrophysiological experiments, or perfused onto the cells in calcium imaging experiments.
Results
CK59 Inhibits Depolarization-Induced Calcium Entry
The effects of CaMKII inhibitors CK59 and Ant-AIP-II were first explored with the use of ratiometric calcium imaging. Cells were depolarized with a high KCl solution in the presence and absence of various CaMKII inhibitors. Cells were treated with CK59 for 15 s prior to the depolarization with high KCl. There was no change in the fluorescence ratio during this pretreatment suggesting that CK59 does not affect the baseline extrusion levels of calcium. It is clear that in the presence of CK59 (50 μM) the high KCl solution was not able to elicit as large an increase in intracellular calcium (Fig. 1a). This effect was reversible, as the response to a KCl-induced depolarization after washout of CK59 was restored to the pre-CK59 level. In contrast, the increase in intracellular calcium with the high KCl solution was not affected by the inclusion of the second CaMKII inhibitor, Ant-AIP-II (50 nM, Fig. 1b). On average, the increase in intracellular calcium with high KCl stimulation was reduced to 44.83 ± 1.88 % of control with CK59 (N = 128; paired t test, p < 0.001) and only reduced to 94.68 ± 1.29 % of control with Ant-AIP-II (N = 255, Fig. 1c). This result together with the lack of effect on baseline extrusion suggests that the novel CaMKII inhibitor CK59 shows off-target inhibition of voltage-gated calcium channels. However, the data do not exclude the possibility that there may be effects on extrusion in the presence of high intracellular calcium.
Fig. 1.
CaMKII inhibitor CK59 but not Ant-AIP-II significantly attenuates the amount of high KCl-induced increase in intracellular calcium when measured with Fura-2 ratiometric imaging. a Example of 340/380 ratio obtained with high KCl solution alone or high KCl solution in the presence of 50 μM CK59. Each line shown represents an individual cell (N = 6). b The same conditions as in A, but using 50 nM Ant-AIP-II (N = 6). c Average change in intracellular calcium as determined by the 340/380 ratios with KCl alone (solid bars), KCl with CK59 (crossed hash bar, N = 128), or KCl with Ant-AIP-II (diagonal hashed bar, N = 255). *Paired t test, p < 0.001)
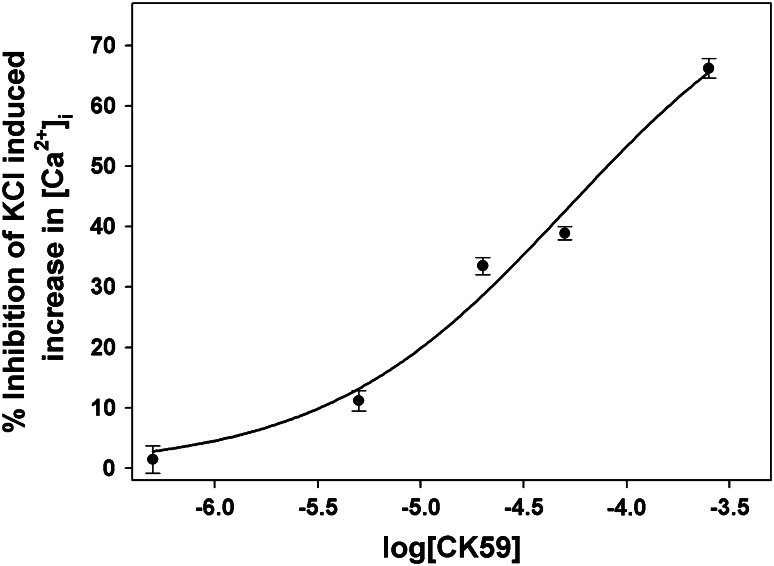
High KCl-induced increases in intracellular calcium were measured in the presence of 500 nM–250 μM CK59 (Fig. 2). The solubility of CK59 in DMSO limited the highest concentration used to 250 μM. Control experiments with DMSO, diluted 1:250 in CIR with no CK59, verified that DMSO alone did not affect high KCl-induced calcium influx at this concentration (data not shown). The dose–response curve data were fit with a 3-parameter sigmoidal curve that assumed if the concentration was increased to high enough levels, all calcium entries would be blocked. The curve generated an IC50 of 52 μM. It is possible that this is an overestimate; if all calcium entries are not completely inhibited and instead 70 % CK59-mediated inhibition is the actual maximum, then the IC50 would be closer to 22 μM. CK59’s IC50 for inhibition of CaMKII activity is <10 μM. Other nonspecific CaMKII inhibitors that inhibit L-type calcium channels are more potent, both in their primary and off-target effects. For example, the IC50 for KN93’s effect on CaMKII is 0.37 μM (Sumi et al. 1991), whereas its IC50 for L-type channel inhibition is approximately 1 μM (Gao et al. 2006). The IC50 of a related inhibitor, KN62, is 0.9 μM for CaMKII activity; the IC50 for its off-target effects on calcium entry is approximately 0.5 μM (Sihra and Pearson 1995). Given the relatively close IC50 values for primary and off-target effects of these CaMKII inhibitors, one must be extremely cautious when selecting a compound to inhibit CaMKII activity.
Fig. 2.
Various doses (0.5, 5, 20, 50, and 250 μM; N = 122, 51, 166, 326, and 122, respectively) of CK59 were applied to cells during high KCl-induced depolarization. The percent inhibition of high KCl-induced intracellular calcium by CK59 was calculated as Data were fit with a 3-parameter sigmoidal curve (r 2 = 0.983). This allowed calculation of the IC50 value for CK59, 52 μM
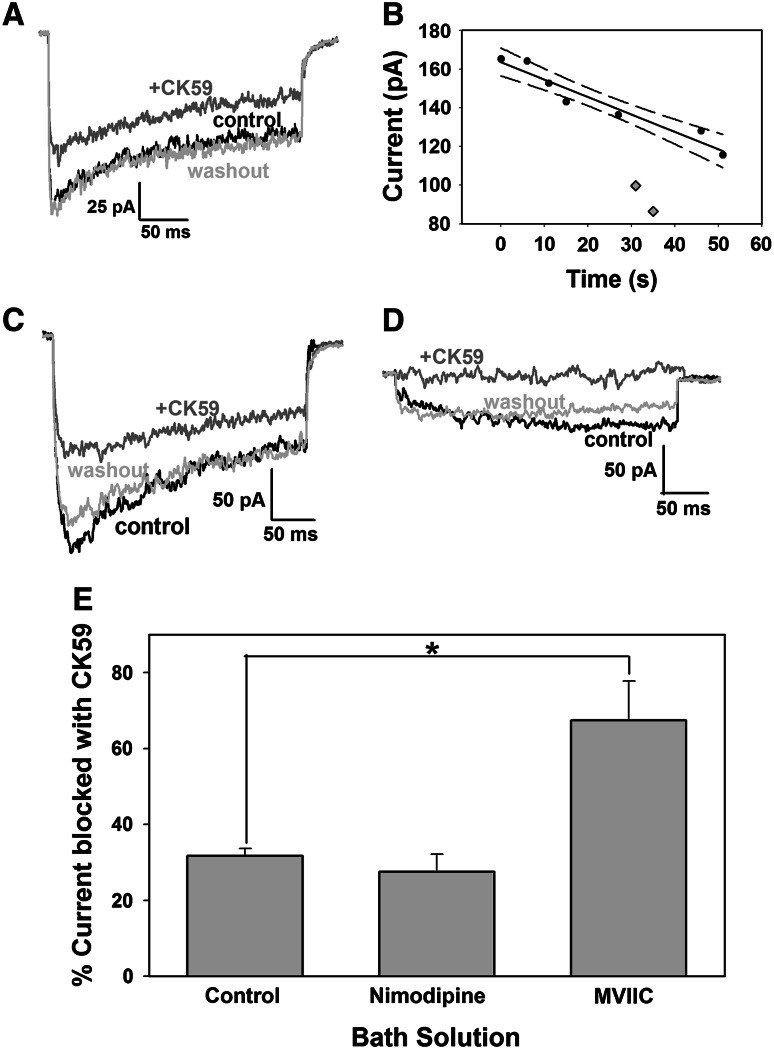
The effects of CK59 on voltage-gated calcium currents were measured directly using whole-cell patch clamp recording. Figure 3 illustrates the calcium current elicited in the presence and absence of CK59 (50 μM). The total current was reduced with CK59 in a reversible manner (Fig. 3a). Calcium currents often change during recording so that the data were adjusted for run-up or run-down prior to measuring the magnitude of current inhibited (Carter and Mynlieff 2004; Bray and Mynlieff 2009, 2011). Typically a few control currents were measured both before and after CK59 application. These currents were fit with a linear regression and the current in the presence of CK59 was compared back to the linear regression line (“expected current in the absence of inhibitor”; Fig. 3b). A change in the current magnitude was only considered significant if it fell outside of the 95 % confidence interval for the linear regression (dashed line in Fig. 3b). In all 15 cells tested, CK59 significantly reduced the current with an average reduction of 32.05 ± 1.86 % when compared to the linear regression line (Fig. 3e). The difference in reduction of calcium entry observed when using ratiometric calcium imaging versus whole-cell patch clamp electrophysiology likely stems from the length of time neurons are stimulated. In calcium imaging experiments, neurons were depolarized for 30 s so that the majority of calcium influx is through long-lasting L-type channels, whereas electrophysiological experiments depolarize neurons for a relatively short 300 ms. The currents measured in response to a short depolarization to +10 mV are likely composed of L-, N-, P/Q-, and R-type currents. As seen with ratiometric calcium imaging, electrophysiological recordings illustrate the nonspecificity of CaMKII inhibitor CK59, as it decreases voltage-gated calcium current.
Fig. 3.
Calcium currents elicited by a 300 ms depolarizing pulse to +10 mV from a holding potential of −80 mV demonstrates the nonspecific effects of CK59 on calcium channel types. a CK59 application reduces current in normal recording solution. Voltage-gated calcium currents were recorded before (black trace), during (gray trace), and after (light gray trace) application of CK59. b Control calcium currents were measured before and after CK59 application (filled circles) and fit with a linear regression line and 95 % confidence interval (dashed line). Calcium currents elicited in the presence of CK59 (gray diamonds) fell outside of the confidence interval for the control currents. The percent of current blocked by CK59 was calculated by comparing the current in the presence of CK59 to the regression line for that specific time point. c 20 μM nimodipine was bath applied and voltage-gated calcium currents were recorded before (black trace), during (gray trace), and after (light gray trace) application of CK59 (50 μM). d 2 μM ω-conotoxin MVIIC was bath applied and voltage-gated calcium currents were recorded before (black trace), during (gray trace), and after (light gray trace) application of CK59 (50 μM). The traces illustrated in a, c, and d were recorded in different neurons. e Average decrease in voltage-gated calcium current seen with CK59 application (50 μM) in control bathing solution (N = 15), with 20 μM nimodipine (N = 7), and with 2 μM ω-conotoxin MVIIC (N = 5) in the bath. *Unpaired t test, p < 0.001
CK59 Decreases Calcium Entry Through More Than One Voltage-Gated Calcium Channel
Since it had previously been reported that the CaMKII inhibitor KN93 reduced L-type calcium current, we sought to assess whether L-type calcium channels were similarly inhibited by CK59 with whole-cell recording. Calcium currents were measured during the final 50 ms of a 300 ms depolarizing pulse to +10 mV to minimize the contribution of fast (T-type) current while maximizing the long-lasting (L-type) component of the current. Notably, N-, P/Q-, and R-type currents are still present during this period, as demonstrated in Fig. 3c, where current still remains after the L-type calcium channel antagonist nimodipine (20 μM) was used. Thus, the current remaining in the presence of nimodipine should primarily be composed of N-, P/Q-, and R-types of calcium current. If CK59 only inhibited L-type channels, then the addition of CK59 in the presence of nimodipine should not further inhibit the total calcium current because those channels would already be blocked. Figure 3c illustrates the calcium current elicited in the presence and absence of CK59 (50 μM) in a cell pretreated with nimodipine (20 μM). The current was reduced with CK59 in a reversible manner similar to the non-nimodipine treated cells. CK59 caused a significant decrease in calcium current with an average reduction of 27.54 ± 4.66 % (N = 7, Fig. 3e). The reduction in voltage-gated calcium current elicited in the presence of the L-type calcium channel antagonist implicates that CK59 may inhibit N-, P/Q-, and/or R-type current(s).
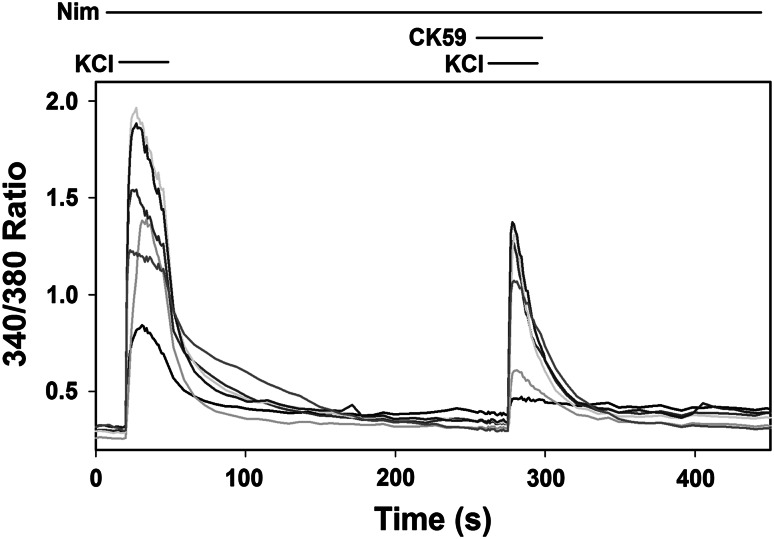
Ratiometric calcium imaging experiments were done to confirm that CK59 can reduce calcium entry in the presence of an L-type calcium channel antagonist. Nimodipine (20 μM) was present in the bath for the entire time course of the experiment to block L-type calcium channels, and high KCl solution alone or high KCl solution with CK59 (50 μM) were applied acutely (Fig. 4). Application of CK59 under these conditions reduced high KCl-induced calcium entry to 43.73 ± 1.38 % (N = 185) of the average calcium entry elicited by high KCl application alone. Given that both calcium current and entry decreased in the presence of the L-type calcium channel blocker nimodipine, it was hypothesized that CK59 exerts its effects by inhibiting another calcium channel type than L-type calcium channels, suggesting a different nonspecific effect from that of CaMKII inhibitor KN93 (Gao et al. 2006). However, the effect of CK59 on L-type calcium channels may have been masked because nimodipine would already be blocking L-type channels in these experiments. Therefore, experiments were performed to address the effect of CK59 more specifically on L-type channels.
Fig. 4.
CaMKII inhibitor CK59 attenuates the amount of high KCl-induced increase in intracellular calcium in the presence of the L-type channel blocker, nimodipine when measured with Fura-2 ratiometric imaging. Nimodipine (20 μM) was perfused during the entire measurement period. The 340/380 ratio was measured in response to high KCl solution before and during CK59 (50 μM) application and representative cells are shown (N = 7). Each line represents an individual cell
To clarify if L-type calcium channels could also be blocked by CK59, ω-conotoxin MVIIC (2 μM) was added to the bath during electrophysiological studies to block N- and P/Q-type calcium channels. The current in Fig. 3d is reduced due to the presence of MVIIC. On average, cells treated with MVIIC displayed 74.25 % less calcium current than control cells (data not shown). Comparing the raw current in 3c with the L-type channel blocker nimodipine with the raw current in 3d with ω-conotoxin MVIIC suggests that while L-type current is certainly present in these neurons, currents sensitive to ω-conotoxin MVIIC (N- and P/Q-type) make up the bulk of the high voltage-activated whole-cell current in neonatal neurons of the superior hippocampus when a 300 ms depolarizing pulse is applied. Addition of CK59 (50 μM) reduced the current even further, so that very little current remained when MVIIC and CK59 were simultaneously applied (Fig. 3d). Calcium currents in these studies were decreased on an average of 67.47 ± 10.38 % (N = 5; Fig. 3e), which was a significantly larger decrease by CK59 than that seen when ω-conotoxin MVIIC was not present (unpaired t test, p < 0.001, Fig. 3e). Furthermore, total calcium current measured in 4 of 5 cells was nearly completely abolished with measurements below 10 pA. These data suggest that CaMKII nonspecifically inhibits N-, P/Q-, L-, or R-type current, or some combination of these currents collectively.
Discussion
Data presented here clearly show the off-target action of novel CaMKII inhibitor CK59 on voltage-gated calcium channels. Both electrically stimulated currents in whole-cell recording and high KCl-induced calcium influx were significantly reduced by CK59 in a dose-dependent manner. However, determining the exact channel(s) targeted by CK59 is more difficult. Studies done in the presence of ω-conotoxin MVIIC strongly argue for L-type channel inhibition due to the drastic reduction in expected current as well as the nearly complete extinction of L-type current in the majority of cells tested when CK59 was applied. However, in the presence of nimodipine, both calcium current and calcium entry decrease upon application of CK59. This may seem to suggest that L-type channels are not affected; however, when nimodipine is present, L-type current is already being blocked. Therefore, any effect of CK59 on L-type channels would be masked. The decrease in calcium levels in the presence of nimodipine after CK59 application does suggest inhibition of additional channels besides only the L-type channel. One possibility is that R-type calcium channels are inhibited by CK59. In a report by Castelli and Magistretti (Castelli and Magistretti, 2006), R-type current in the entorhinal cortex made up only 11–13 % of total calcium current. The small amount of current seen in the cells treated with ω-conotoxin MVIIC also suggests that a large component of calcium influx is through N- and P/Q-type channels. Even if complete blockade of R-type current was assumed, this would likely not make up for the amount of inhibition seen in nimodipine-treated cells. There was 27.54 % reduction in whole-cell calcium current and 56.27 % reduction in high KCl-mediated increase in intracellular calcium when nimodipine was present, suggesting that even if R-type channels are affected along with L-type calcium channels, there must be another channel that is inhibited by CK59. A second possibility is that L-type channels were not completely blocked. This seems unlikely due to the high concentration of nimodipine used in these studies (IC50 = 139 nM for CaV 1.2 and 3 μM for CaV 1.3; Catterall et al. 2005). It is more likely that at least one of the channels blocked by ω-conotoxin MVIIC is susceptible to inhibition by CK59. In either case, it seems that CK59 inhibits L-type channels as well as others. While the identity of the other channels CK59 may inhibit in addition to L-type channels is not completely clear, it is evident that CK59 has off-target effects that inhibit the entry of calcium into neurons.
Acknowledgments
We thank Amanda Larson for assistance in collection of data. This research was supported by NIH 2R15NS048900-02A1.
References
- Bray JG, Mynlieff M (2009) Influx of calcium through L-type calcium channels in early postnatal regulation of chloride transporters in the rat hippocampus. Dev Neurobiol 69:885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JG, Mynlieff M (2011) Involvement of protein kinase C and protein kinase A in the enhancement of L-type calcium current by GABAB receptor activation in neonatal hippocampus. Neuroscience 179:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TJ, Mynlieff M (2004) Gamma-aminobutyric acid type B receptors facilitate L-type and attenuate N-type Ca(2+) currents in isolated hippocampal neurons. J Neurosci Res 76:323–333 [DOI] [PubMed] [Google Scholar]
- Castelli L, Magistretti J (2006) High-voltage-activated Ca2+ currents show similar patterns of expression in stellate and pyramidal cells from rat entorhinal cortex layer II. Brain Res 1090:76–88 [DOI] [PubMed] [Google Scholar]
- Catterall WA (2011) Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3:003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (2005) International Union of Pharmacology. XLVIII. Nomenclature and structure–function relationships of voltage-gated calcium channels. Pharmacol Rev 57:411–425 [DOI] [PubMed] [Google Scholar]
- Fink CC, Meyer T (2002) Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol 12:293–299 [DOI] [PubMed] [Google Scholar]
- Gaertner TR, Kolodziej SJ, Wang D, Kobayashi R, Koomen JM, Stoops JK, Waxham MN (2004) Comparative analyses of the three-dimensional structures and enzymatic properties of alpha, beta, gamma and delta isoforms of Ca2+-calmodulin-dependent protein kinase II. J Biol Chem 279:12484–12494 [DOI] [PubMed] [Google Scholar]
- Gao L, Blair LA, Marshall J (2006) CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun 345:1606–1610 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450 [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H (1995) A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun 212:806–812 [DOI] [PubMed] [Google Scholar]
- Konstantopoulos N, Marcuccio S, Kyi S, Stoichevska V, Castelli LA, Ward CW, Macaulay SL (2007) A purine analog kinase inhibitor, calcium/calmodulin-dependent protein kinase II inhibitor 59, reveals a role for calcium/calmodulin-dependent protein kinase II in insulin-stimulated glucose transport. Endocrinology 148:374–385 [DOI] [PubMed] [Google Scholar]
- Li B, Zhong H, Scheuer T, Catterall WA (2004) Functional role of a C-terminal Gbetagamma-binding domain of Ca(v)2.2 channels. Mol Pharmacol 66:761–769 [DOI] [PubMed] [Google Scholar]
- Mauceri D, Cattabeni F, Di LM, Gardoni F (2004) Calcium/calmodulin-dependent protein kinase II phosphorylation drives synapse-associated protein 97 into spines. J Biol Chem 279:23813–23821 [DOI] [PubMed] [Google Scholar]
- Mitterdorfer J, Froschmayr M, Grabner M, Moebius FF, Glossmann H, Striessnig J (1996) Identification of PK-A phosphorylation sites in the carboxyl terminus of L-type calcium channel alpha 1 subunits. Biochemistry 35:9400–9406 [DOI] [PubMed] [Google Scholar]
- Mynlieff M (1997) Dissociation of postnatal hippocampal neurons for short term culture. J Neurosci Methods 73:35–44 [DOI] [PubMed] [Google Scholar]
- Park JY, Kang HW, Moon HJ, Huh SU, Jeong SW, Soldatov NM, Lee JH (2006) Activation of protein kinase C augments T-type Ca2+ channel activity without changing channel surface density. J Physiol 577:513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg OS, Deindl S, Comolli LR, Hoelz A, Downing KH, Nairn AC, Kuriyan J (2006) Oligomerization states of the association domain and the holoenzyme of Ca2+/CaM kinase II. FEBS J 273:682–694 [DOI] [PubMed] [Google Scholar]
- Sihra TS, Pearson HA (1995) Ca/calmodulin-dependent kinase II inhibitor KN62 attenuates glutamate release by inhibiting voltage-dependent Ca(2+)-channels. Neuropharmacology 34:731–741 [DOI] [PubMed] [Google Scholar]
- Song YH, Cho H, Ryu SY, Park SH, Noh CI, Lee SH, Ho WK (2010) L-type Ca2+ channel facilitation mediated by H2O2-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol 48:773–780 [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H (1991) The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12 h cells. Biochem Biophys Res Commun 181:968–975 [DOI] [PubMed] [Google Scholar]
- Wang WY, Hao LY, Minobe E, Saud ZA, Han DY, Kameyama M (2009) CaMKII phosphorylates a threonine residue in the C-terminal tail of Cav1.2 Ca(2+) channel and modulates the interaction of the channel with calmodulin. J Physiol Sci 59:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson DM, Mirzoeva S, Guo L, Whyte A, Bourguignon JJ, Hibert M, Haiech J, Van Eldik LJ (2001) Ligand modulation of glial activation: cell permeable, small molecule inhibitors of serine–threonine protein kinases can block induction of interleukin 1 beta and nitric oxide synthase II. Neurochem Int 39:459–468 [DOI] [PubMed] [Google Scholar]
- Wayman GA, Tokumitsu H, Davare MA, Soderling TR (2011) Analysis of CaM-kinase signaling in cells. Cell Calcium 50:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]