SUMMARY
Allogeneic marrow transplantation offers curative therapy for children with severe aplastic anaemia (SAA). We report the outcomes of 148 children with SAA who received human leucocyte antigen (HLA)-matched related marrow grafts between 1971 and 2010. Patients were divided into 3 groups, reflecting changes in conditioning and graft-versus-host disease (GVHD) prophylaxis regimens that occurred over time. Patients in Group 1 were conditioned with cyclophosphamide (CY; 200 mg/kg) followed by "long" (102 days) methotrexate (MTX). Patients in Groups 2 and 3 received CY alone (Group 2) or combined with anti-thymocyte globulin (Group 3) followed by "short" (days 1, 3, 6, and 11) MTX and ciclosporin (until day 180). With a median follow-up of 25 years, the 5-year survivals were 66%, 95%, and 100% for Groups 1, 2, and 3, respectively (overall p<0.0001). The 3-year estimates of graft rejection were 22%, 32%, and 7%, respectively. The probabilities of grades III-IV acute and 2-year chronic GVHD were 15%, 0%, and 3%, and 21%, 21%, and 10%, respectively. Advances in preparative and GVHD prophylaxis regimens, and supportive care during the past 40 years have led to improved outcomes for children with SAA. These results confirm the use of allogeneic marrow transplantation for children with SAA who have HLA-matched related donors.
Keywords: Bone marrow transplantation, Paediatric aplastic anaemia, Haematopoietic cell transplantation
INTRODUCTION
Acquired severe aplastic anaemia (SAA) is a rare, potentially fatal, haematological disorder characterized by pancytopenia and bone marrow aplasia or hypoplasia (Young, 1995). Allogeneic marrow transplantation offers curative therapy for SAA patients and is the treatment of choice for younger patients with human leucocyte antigen (HLA)-matched related donors (Doney et al, 1997; Fouladi et al, 2000; Pulsipher et al, 2011). Over the past 4 decades, progress has been made in the prevention and treatment of graft rejection and graft-versus-host disease (GVHD), based on preclinical animal studies (Santos & Owens, Jr., 1969; Storb et al, 1970a; Storb et al, 1969; Deeg et al, 1982; Storb et al, 1989; Stucki et al, 1998). This report describes how sequential improvements in marrow transplantation for SAA have led to significantly improved outcomes, particularly for children who have received HLA-matched related marrow grafts at our centre since 1971.
DESIGN AND METHODS
Patients
We retrospectively reviewed the clinical records of 148 paediatric patients with SAA who received HLA-matched related marrow grafts at the Fred Hutchinson Cancer Research Center (FHCRC) between May 1971 and September, 2010 (Table 1). Two patients who received marrow grafts from their identical twins were excluded. Results were analysed as of January, 2011. The median age of patients was 12.8 (range, 1.8–19) years and the median time from diagnosis was 1.2 (range, 0.0–108) months. All patients underwent chromosome fragility testing to exclude underlying inherited marrow failure disorders. Diagnoses established at referring institutions were confirmed at the FHCRC by review of outside marrow specimens and repeat marrow aspirates and biopsies. Patients or their parents signed forms approved by the FHCRC Institutional Review Board documenting informed consent to participate in the clinical trials.
Table 1.
Characteristics (n=148)
| Group denomination, number of patients |
Group 1 (n=98) | Group 2 (n=19) | Group 3 (n=31) |
|---|---|---|---|
| Years of transplantation | 1971–1984 | 1981–1988* | 1989–2010 |
| Age, years | |||
| Median (range) | 13.1 (1.9–19) | 12.5 (1.8–18.8) | 10.9 (2.0–18.3) |
| Gender, n (%) | |||
| Male | 56 (57%) | 8 (42%) | 20 (65%) |
| Female | 42 (43%) | 11 (58%) | 11 (35%) |
| Aetiology of aplastic anaemia, (n) | |||
| Unknown | 81 | 19 | 26 |
| Hepatitis | 7 | 0 | 5 |
| Drugs/chemicals | 8 | 0 | 0 |
| Paroxysmal nocturnal haemoglobinuria | 2 | 0 | 0 |
| Number of patients who received transfusions before marrow transplantation, n (%) | |||
| Red blood cell | 75 (77%) | 15 (79%) | 28 (90%) |
| Platelet | 71 (72%) | 14 (74%) | 28 (90%) |
| Number of patients who received treatment before marrow transplantation, n (%) | |||
| Androgens | 35 (36%) | 3 (16%) | 0 (0%) |
| Steroids | 55 (56%) | 7 (37%) | 6 (19%) |
| ATG or other agent | 0 (0%) | 1 (5%) | 3 (10%) |
| Months from diagnosis to marrow transplantation | |||
| Median, (range) | 1 (0–108) | 1.4 (0.3–5.8) | 1.2 (0.4–24.0) |
| Preparative regimen | CY | CY | CY/ATG |
| GVHD prophylaxis | MTX (n=95) CSP (n=3) |
MTX/CSP (n=19) | MTX/CSP (n=31) |
| Nucleated marrow cell dose (× 108/kg) | |||
| Median, (range) | 3.7 (0.6–15.5) | 2.7 (0.7–9.6) | 2.8 (0.9–6.2) |
| Follow-up living patients, years | |||
| Median, (range) | 31.1 (11–37.2) | 23 (15.2–27.1) | 6.1 (0.3–21.5) |
ATG=anti-thymocyte globulin; CSP=ciclosporin; CY = cyclophosphamide, GVHD=graft versus host disease; kg=kilogram; MTX=methotrexate; n=number
There was one patient included in group 2 who received a transplant in 1995 following conditioning with CY and CSP/MTX for GVHD prevention. This patient had a positive skin test to ATG and was therefore unable to receive ATG.
Preparative regimen and postgrafting immunosuppression
For purposes of the analysis, patients were divided into 3 groups, reflecting changes in conditioning and GVHD prophylaxis regimens that occurred over time. All patients were conditioned with cyclophosphamide (CY; 50 mg/kg intravenously (IV) × 4 days). Group 1 included 98 patients (1971–1984) given CY followed by “long” methotrexate (MTX; n=95; 15 mg/m2 day 1, 10 mg/m2 days 3, 6, and 11, then weekly until 102 days following marrow infusion) or ciclosporin (CSP; n=3) (Storb et al, 1977). Group 2 included 19 patients (1981–1988) who received CY followed by “short” (15 mg/m2 day 1, 10 mg/m2 days 3, 6, and 11) MTX and CSP (until day +180) (Storb et al, 1986a). There was one additional patient included in Group 2 who received a transplant in 1995 with CY alone due to a positive skin test to antithymocyte globulin (ATG). Group 3 included 31 patients (1989–2010) who received CY and horse ATG (30 mg/kg/day × 3 days) followed by “short” MTX and CSP (Storb et al, 1994). From 1981–1984 patients were randomized between MTX (Group 1) and MTX/CSP (Group 2) (Storb et al, 1986a). MTX/CSP was shown to be superior to MTX alone, and therefore all patients received MTX/CSP from that point on. Thirty multiply-transfused patients were given donor buffy coat (non-mobilized peripheral blood leucocytes) infusions in addition to marrow as part of a prospective study aimed at reducing the incidence of graft rejection (Storb et al, 1982).
GVHD grading and treatment
Diagnosis and clinical grading of acute and chronic GVHD were performed according to established criteria and both complications were treated as previously described (Przepiorka et al, 1995; Sullivan et al, 1991; Koc et al, 2002). Patients were not evaluated for acute GVHD if they died before engraftment or for chronic GVHD if they died before day 80 after marrow transplantation.
Analyses of donor engraftment
Graft rejection was defined as either failure to reach a granulocyte count > 1 × 109/l for at least 3 consecutive days or by a progressive decrease in peripheral blood counts after initial engraftment, together with recurrent marrow aplasia. In addition, the disappearance of donor haematopoietic cells and reappearance of T lymphocytes of host origin were interpreted to represent graft rejection. In patients with sex-mismatched grafts, donor chimerism was evaluated by marrow cytogenetics and, more recently, using fluorescence in situ hybridization (FISH). In all other patients, informative erythrocyte antigens, erythrocyte enzyme polymorphisms, and leucocyte enzyme polymorphisms served as donor and recipient markers through the 1980s. Beginning in the 1990s, variable number tandem repeat polymorphisms were used to monitor donor chimerism (Martin, 2009).
Statistical methods
Median, range, and proportions were used to summarize descriptive data, as appropriate. Overall survival was estimated by the Kaplan-Meier method with time to death as the primary outcome and censoring at last follow-up. Cumulative incidence estimates were calculated for the probabilities of acute and chronic GVHD, and rejection. Prevalence of chronic GVHD was estimated according to methods previously described (Pepe et al, 1991). Death was treated as a competing risk event for all outcomes (Kalbfleisch & Prentice, 1980). Comparisons among groups for time-to-event endpoints were based on likelihood ratio tests from Cox regression models. Comparisons of time to rejection among patients who rejected, and time between first and second marrow transplantation among patients receiving a second transplant for rejection were by Wilcoxon two-sample test. All reported P-values are 2-sided and were considered statistically significant when ≤ 0.05.
RESULTS
Engraftment
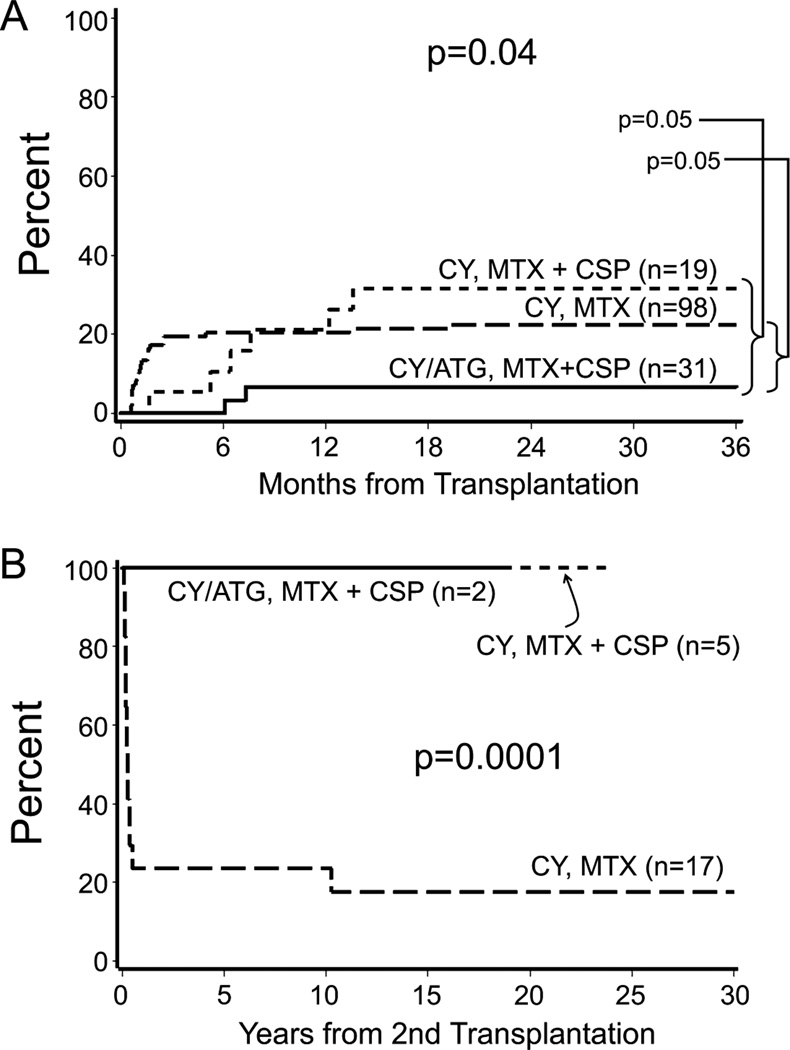
Overall, 30 patients (20%) rejected their first marrow graft. The 3-year estimates of graft rejection were 22% for Group 1, 32% for Group 2, and 7% for Group 3 (overall p = 0.04; Fig 1A). Of the 30 patients who rejected their first grafts, 24 received second marrow grafts (Group 1, n=17; Group 2, n=5; Group 3, n=2) and 3 received third marrow grafts (Group 1, n=1; Group 2, n=1; Group 3; n=1) due to rejections of first and then second grafts, respectively. No graft rejections have occurred in patients who had marrow transplantation after 1993 and no deaths have occurred due to graft rejection since 1987.
Fig 1.
Incidence of (A) graft rejection according to conditioning regimen and graft-versus-host disease (GVHD) prophylaxis over time in 148 children with severe aplastic anaemia (SAA) and (B) survival following second marrow transplantation for graft rejection according to the conditioning regimen and GVHD prophylaxis over time in 24 patients with SAA.
ATG=anti-thymocyte globulin; CSP=ciclosporin; CY = cyclophosphamide; MTX=methotrexate.
The median number of days to first graft rejection was 35 (range, 18–588) for Group 1 and 210 (range, 51–414) days for Groups 2 and 3 (P = 0.001). As a result, the time between first and second marrow transplantation was longer for patients in Groups 2 and 3 (median days to second marrow transplantation = 237) compared to Group 1 (median days to second marrow transplantation = 39 days; P = 0.004). Of note, three patients experienced pancytopenia at 25, 11, and 14 years following marrow transplantation. All had donor engraftment. The aetiology of the pancytopenia was unknown in two patients. One patient died of hepatitis C-related hepatocellular carcinoma with continued pancytopenia and the other patient was given a course of immunosuppression with resolution of the cytopenia. The third patient had recurrence of paroxysmal nocturnal haemoglobinuria (PNH) and received a second transplant following nonmyeloablative conditioning with resolution of the PNH.
GVHD
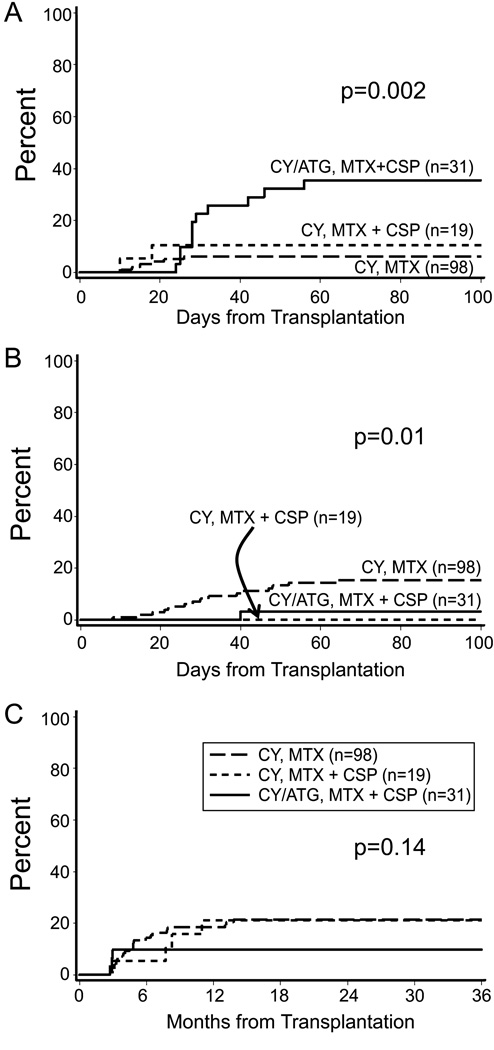
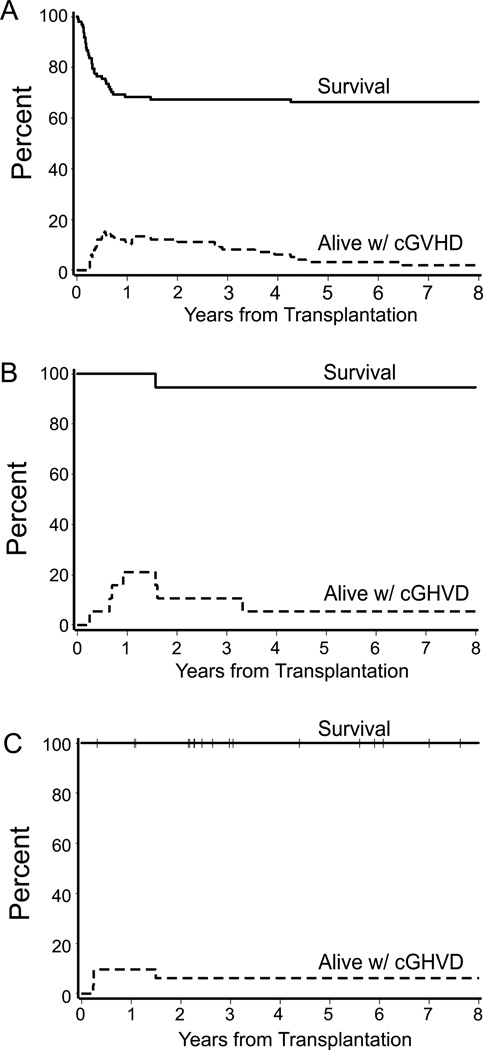
The estimated probabilities of Grade II acute GVHD were 6%, 11%, and 36% for Groups 1, 2, and 3, respectively (overall P = 0.002) and the estimated probabilities of Grades III-IV acute GVHD were 15%, 0%, and 3 % for Groups 1, 2, and 3, respectively (overall P = 0.01; Fig 2A–B). The median onset of grades II–IV acute GVHD was day 24 (range, 8–64) in Group 1 and day 29 (range, 24–56) in Group 3. Two patients in Group 2 developed grade II–IV acute GVHD at day 10 and 18 following marrow transplantation. The 2-year estimates of extensive chronic GVHD were 21%, 21%, and 10% for Groups 1, 2, and 3, respectively (overall P = 0.14; Fig 2C). The median onset of extensive chronic GVHD was day 146 (range, 84–421) in Group 1 and day 245 (range, 88–335) in Group 2. Three patients in Group 3 were diagnosed with extensive chronic GVHD at days 83, 89, and 91, respectively, following marrow transplantation. The prevalence curves (Fig 3A–C) describe extensive chronic GVHD onset, its resolution, and eventual discontinuation of all immunosuppressive therapy, using previously described methods (Pepe et al, 1991). Of the 30 patients with donor buffy coat infusions following bone marrow transplantation, 27 were in Group 1. Within Group 1, the cumulative incidence of chronic GVHD was 14% without donor buffy coat and 41% with donor buffy coat (p=0.01).
Fig 2.
Cumulative incidence of (A) Grade II acute graft-versus-host disease (GVHD) (B) Grade III–IV acute GVHD (C) and chronic GVHD in 148 children with severe aplastic anaemia.
ATG=anti-thymocyte globulin; CSP=ciclosporin; CY = cyclophosphamide; MTX=methotrexate.
Fig 3.
Survival (upper curves) and prevalence of chronic graft-versus-host disease (lower curves) among patients in (A) Group 1 (CY, MTX n=98) (B) Group 2 (CY, MTX + CSP n= 19) and (C) Group 3 (CY/ATG, MTX + CSP n=31) in 148 children with severe aplastic anaemia.
ATG=anti-thymocyte globulin; CSP=ciclosporin; CY = cyclophosphamide; MTX=methotrexate.
Malignancy after transplantation
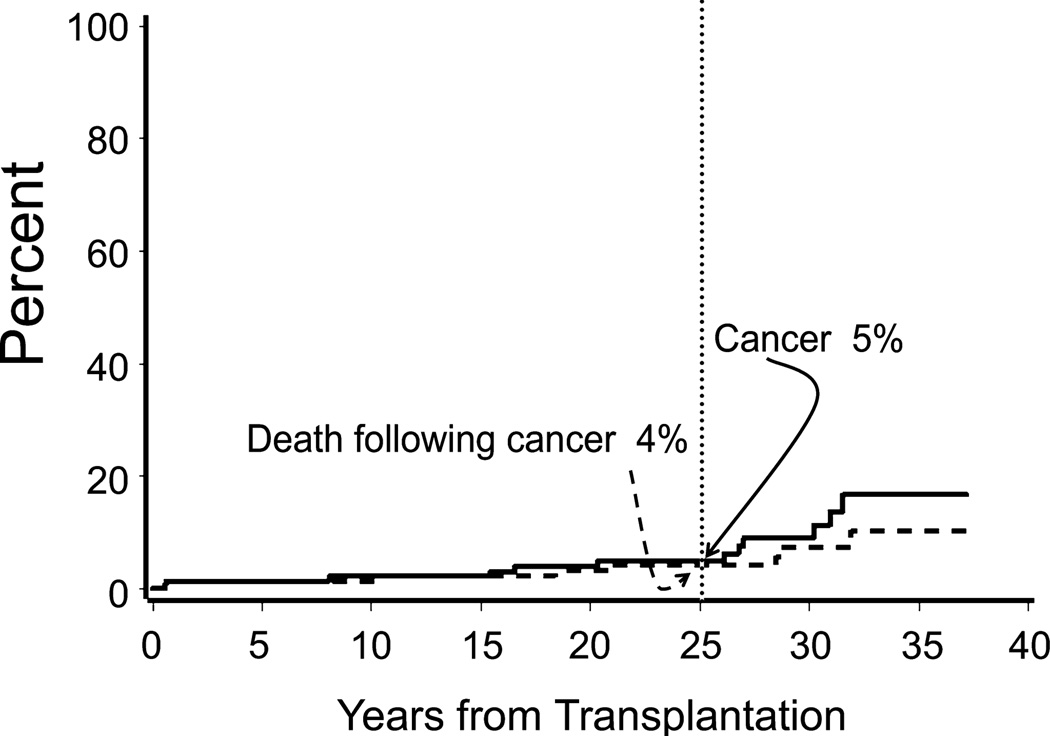
Twelve of the 148 patients (8%) developed cancer after marrow transplantation and all were in Group 1 (Fig 4). Specifically, 5 patients developed skin cancer (squamous cell (n=4), squamous cell and basal cell (n=1) carcinoma) at 8, 15, 20, 26, and 31 years after marrow transplantation. All 5 of the patients who developed skin cancer had a history of chronic skin GVHD. In addition, 2 patients developed leukaemia of host origin [acute lymphoblastic leukaemia (ALL; n=1), acute myeloid leukaemia (AML; n=1)] 6 months after marrow transplantation, and one patient developed myelodysplastic syndrome (MDS) of donor origin 27 years after marrow transplantation. The patient who developed MDS had remission induced after second haematopoietic cell transplantation. Four patients developed solid tumours [cervical (n=1), breast (n=2), hepatitis C-associated hepatocellular carcinoma (n=1)] 17, 27, 30, and 32 years after marrow transplantation.
Fig 4.
Cumulative incidence of post-transplant cancer and cancer-related mortality in 148 children with severe aplastic anaemia.
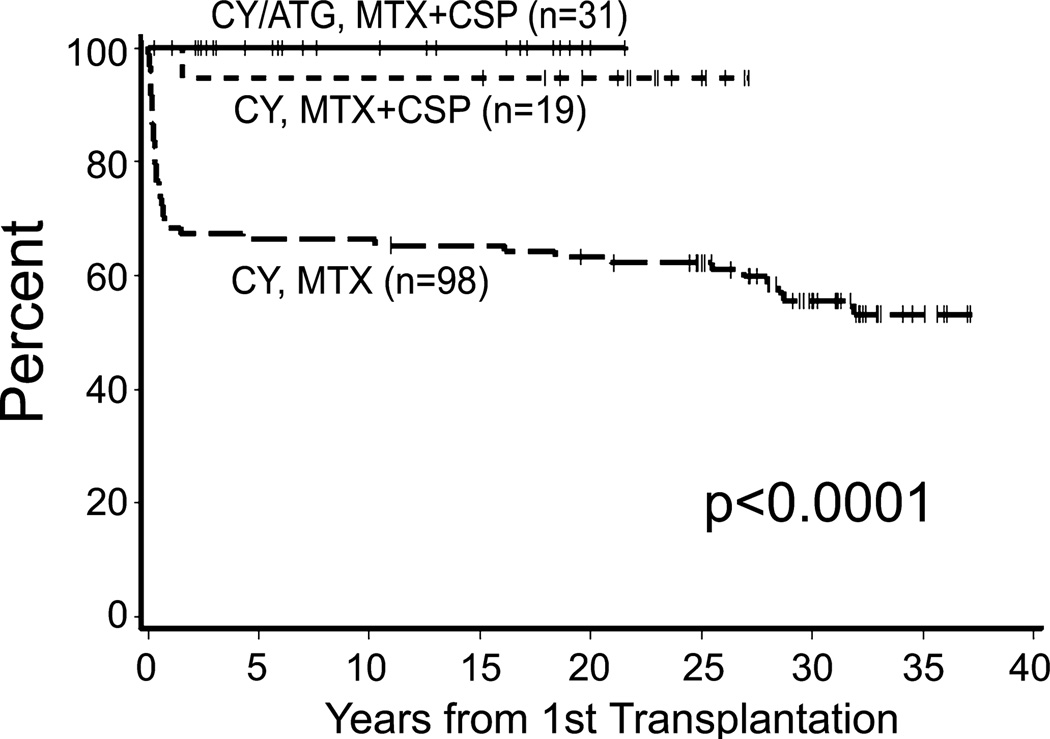
Overall survival
With a median follow up of 25.3 (range, 0.3–37.2) years for living patients, the 5-year survival estimates were 66% for Group 1, 95% for Group 2, and 100% for Group 3 (overall P < 0.0001; Fig 5). The primary causes of death were graft rejection (n=18; 12%) and infections with and without GVHD (n=17; 11%). Importantly, the 5-year survival after graft rejection has improved from 24% to 100% following second marrow transplantation (overall P = 0.0001; Fig 1B). In addition, death due to infections with or without GVHD has decreased over the past 40 years with all of the deaths due to infections with or without GVHD occurring in Group 1. Five patients (3%) died of cancer [(ALL (n=1), metastatic squamous cell carcinoma (n=1), metastatic cervical cancer (n=1), metastatic breast cancer (n=1), and hepatitis C associated hepatocellular carcinoma (n=1)]. Four other patients died of human immunodeficiency virus (HIV)/hepatic failure (n=1), idiopathic interstitial pneumonitis (n=1), suicide (n=1), and an unknown cause (n=1). One patient rejected the graft and developed acute myeloid leukaemia 6 months after graft rejection. This patient was counted as a death due to graft rejection.
Fig 5.
Overall survival according to the conditioning regimen and graft-versus-host disease prophylaxis used in 148 children with severe aplastic anaemia.
ATG=anti-thymocyte globulin; CSP=ciclosporin; CY = cyclophosphamide; MTX=methotrexate.
Twenty-three patients acquired hepatitis C, presumably related to blood product transfusions before or after marrow transplantation [Group 1 (n=15), Group 2 (n=7), and Group 3 (n=1)] (Strasser et al, 1999). In addition, 1 patient had hepatitis B (Group 1) and one patient had HIV (Group 1). Two of the 25 patients with viral infections died as a result [Hepatitis C (n=1), HIV n=1)]. All patients with hepatitis B, C, or HIV were transplanted before 1990.
DISCUSSION
SAA is a life-threatening bone marrow failure disorder characterized by a hypocellular marrow and pancytopenia. Allogeneic marrow transplantation using HLA-matched related donors provides curative therapy for patients with SAA and is the preferred therapy for paediatric patients (Kobayashi et al, 2006; Locasciulli et al, 2007). Long-term survivals of approximately 80–90% following marrow transplantation have been reported in several studies that included children (Schrezenmeier et al, 2007; Locasciulli et al, 2007; Kennedy-Nasser et al, 2006; Pulsipher et al, 2011). Here we report a single centre series of 148 children with SAA who received HLA-matched related marrow grafts over the past 4 decades.
In the current paediatric cohort, 5-year survival has significantly improved, from 66% to 100%, during the past 4 decades. One important factor contributing to the improvement in survival was the understanding and abrogation of marrow graft rejection. Patients in Group 1 had the lowest survival rate due to a high incidence of graft rejection and an inability to deal with rejections effectively. Preclinical canine studies and clinical observations suggested that rejections were largely due to transfusion-induced sensitization to minor histocompatibility antigens (Storb et al, 1970b; Storb et al, 1977). Rejection rates were lower in patients who had not received transfusions before marrow transplantation (Storb et al, 1980). Subsequent experimental studies showed that the risk of sensitization to minor antigens was reduced by leucocyte depletion and by in vitro irradiation (2000 cGy) of the transfusion products (Bean et al, 1991). These methods have now become standard clinical practice in the treatment of newly diagnosed SAA and probably contributed to the lower rejection risk in more recently transplanted patients. At the same time, preclinical studies led to the inclusion of ATG in the conditioning regimen, as a means to eliminate T cells in the recipient responsible for graft rejection. ATG plus CY was first shown to facilitate second marrow grafts after initial graft rejection (Storb et al, 1987). As a consequence, the overall mortality associated with graft rejection has been eliminated over the past 40 years due to the success of second marrow transplantation. This is highlighted by the fact that there have been no patient deaths due to graft rejection since 1987.
The encouraging results with second marrow transplantation prompted the introduction of CY/ATG as a conditioning regimen for initial marrow transplantation beginning in 1988 (Group 3) (Storb et al, 1994; Storb et al, 2001). With this change, rejection declined from 32% to 7% (p=0.05). It is likely; however, that increasing use of leuco-depleted irradiated blood products before marrow transplantation contributed to the decreased rejection rate. A randomized study comparing CY to CY/ATG conditioning in 134 paediatric and adult patients, conducted at 29 centres during a 7-year period did not show outcome differences between the two study arms and was prematurely closed because of slow accrual. Rejection rates in both arms were 18% and 16%, and 5-year survivals were 74% and 80%, respectively (Champlin et al, 2007).
Prevention of acute GVHD is a second area of progress. In the early 1970s, “long” MTX (102 days) was the only regimen used for GHVD prevention based on preclinical canine studies. CSP monotherapy was then introduced in the late 1970s but was shown convincingly in prospective randomized trials to be no better than MTX (Storb et al, 1988). However, canine studies showed that a short course of MTX combined with CSP was superior to either drug alone (Deeg et al, 1982), and these preclinical results were confirmed in subsequent clinical trials (Storb et al, 1986a; Storb et al, 1986b). Accordingly, the rate of grades III–IV acute GVHD declined from 15% with MTX to 3% grade III (no grade IV) with MTX/CSP. These observations have been confirmed by others (Locatelli et al, 2000). The increase in grade II acute GVHD among patients in Group 3 was primarily due to an increased diagnostic sensitivity for GVHD of the upper intestinal tract, reflecting the aggressive use of endoscopy for evaluation of gut symptoms at our centre, as previously reported (Martin et al, 2004). Our study supports this finding. Of the 31 patients in Group 3, 11 were diagnosed with grade II acute GVHD. Of these 11 patients, 7 had grade II GVHD that was endoscopy-proven stage 1 gut GVHD. In the remaining 4 patients, the incidence of grade II acute GVHD was 13% compared to 6% in Group 1 and 11% in Group 2, which was not significantly different. Therefore, it is possible that the incidence of grade II acute GVHD was underestimated in patients transplanted in Groups 1 and 2.
The incidence rates of chronic GVHD have remained low throughout the three time periods except for a period during the late 1970s and early 1980s when transfused patients received combined marrow and buffy coat grafts (Storb et al, 1982). Combined grafts were administered to overcome the problem of graft rejection. While the manoeuvre was effective in decreasing graft rejection, it was associated with higher incidences of chronic GVHD (Storb et al, 1983). Introduction of the CY/ATG regimen permitted the return to marrow as the sole source of stem cells, and the cumulative incidence of chronic GVHD since 1988 has decreased to 10%. A recent combined analysis of adult and paediatric patients with SAA at our centre suggested that limiting the graft to 2.0 to 2.5 × 108 total nucleated cells (corrected for donor’s peripheral blood leucocyte count)/kg of recipient’s actual weight could reduce the incidence of chronic GVHD further without increasing the rejection risk (Kahl et al, 2005). A recent retrospective analysis of combined European Group for Blood and Marrow Transplantation (EBMT)/ Center for International Blood & Marrow Transplant Research (CIBMTR) data emphasized that outcomes were worse and the risk of chronic GVHD was higher with peripheral blood progenitor grafts compared to marrow, especially in patients who were younger than 20 years (Schrezenmeier et al, 2007).
A third area of major progress has been advances in the management of infectious diseases after transplantation (Goodman et al, 1992; Boeckh et al, 1996; Limaye et al, 2001). In particular, measures to prevent infection included the use of trimethoprim-sulfamethoxazole or other prophylaxis regimens for prevention of Pneumocystis jiroveci pneumonia, prophylactic fluconazole for prevention of yeast infections, and acyclovir for prevention of herpes simplex virus and varicella reactivation. In addition, preemptive treatment with ganciclovir or foscarnet was used if cytomegalovirus was detected.
Several studies have evaluated the effectiveness of non-transplant alternative therapies for patients with SAA with immune suppression alone and reported overall survivals of 60–80% (Scheinberg et al, 2008; Bacigalupo et al, 2000; Rosenfeld et al, 1995; Pulsipher et al, 2011). CSP and ATG are most commonly used and are currently the treatment of choice for patients who lack HLA-matched related donors. Initial response rates to immunosuppressive therapy range from 60 to 75%, but 10–35% will fail to respond or relapse (Scheinberg et al, 2008; Pulsipher et al, 2011). Attempts to improve initial response rates by increasing immune suppression with the addition of mycophenolate mofetil or sirolimus to ATG/CSP have not had a major impact (Scheinberg et al, 2006). A recent EBMT study compared the outcomes of 2479 patients with SAA who received first-line treatment with marrow transplantation (n=1567) or immunosuppressive therapy (n=912) (Locasciulli et al, 2007). Survival was significantly better in patients who received marrow transplantation and has improved over time, similar to findings at our centre. Survival for patients receiving immunosuppressive therapy has also improved over the past 2 decades, albeit not to the same extent. Of note, however, recent studies indicate that determination of pre-treatment telomere length and reticulocyte counts identifies patients who are less likely to respond to immunosuppressive therapy and, therefore, should be considered for transplantation early in the disease course (Scheinberg et al, 2010; Scheinberg et al, 2009a). Immunosuppressive therapy also carries the risk of clonal evolution to PNH, MDS, or AML (Scheinberg et al, 2008; Scheinberg et al, 2009b). In our cohort of 148 children, median follow-up 25 years, 12 patients (8%) developed malignancies following transplantation. Four of the 12 patients had solid tumours (breast cancer (n=2), cervical cancer, or hepatitis C-associated hepatocellular carcinoma), which may or may not have been late effects from the transplantation procedure. In addition, in the patient who developed AML, retrospective analysis revealed abnormal cytogenetics on the patient’s pre-transplant marrow identical to those identified at the time of AML diagnosis, suggesting that this patient presumably had MDS rather than acquired SAA at the time of transplantation (Appelbaum et al, 1984). A detailed discussion of late effects and quality of life in patients with SAA was reviewed by Sanders et al, (2011). In general, patients with SAA have normal growth, development, and quality of life.
In summary, this study demonstrated significant improvement in survival over the past 40 years among children with SAA who received HLA-matched related marrow grafts. The reasons for this improvement include decreased incidences of graft rejection and improved survivals after second marrow transplantation for graft rejection, decreased rates of grades III–IV acute GVHD, a low incidence of chronic GVHD with marrow grafts, and improved supportive care. This study confirms the efficacy of allogeneic marrow transplantation as primary therapy for children with SAA who have HLA-matched related donors.
Acknowledgments
The authors would like to thank the transplant and long-term follow-up teams, the referring physicians, and the database managers, particularly Paul Hoffmeister and Gary Schoch, for their invaluable help with the study, and Bonnie Larson, Helen Crawford, and Sue Carbonneau for help with manuscript preparation.
Funding: The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD grants P01HL036444, P30CA015704, and K23HL085288. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
Footnotes
AUTHORSHIP AND DISCLOSURES
L.M.B designed the study, researched and analysed data, and wrote the manuscript. A.E.W, H.J.D., M.E.D.F., P.J.M., P.A.C., K.D., F.R.A. and R.S. provided interpretation and analysis of data, edited the manuscript. B.E.S. performed statistical analyses and edited the manuscript. R.S. was the principal investigator, providing support, and takes primary responsibility for the paper.
REFERENCES
- Appelbaum FR, Storb R, Ramberg RE, Shulman HM, Buckner CD, Clift RA, Deeg HJ, Fefer A, Sanders J, Stewart P, Sullivan K, Witherspoon R, Thomas ED. Allogeneic marrow transplantation in the treatment of preleukemia. Annals of Internal Medicine. 1984;100:689–693. doi: 10.7326/0003-4819-100-5-689. [DOI] [PubMed] [Google Scholar]
- Bacigalupo A, Bruno B, Saracco P, Di Bona E, Locasciulli A, Locatelli F, Gabbas A, Dufour C, Arcese W, Testi G, Broccia G, Carotenuto M, Coser P, Barbui T, Leoni P, Ferster A. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO) Blood. 2000;95:1931–1934. [PubMed] [Google Scholar]
- Bean MA, Storb R, Graham T, Raff R, Sale GE, Schuening F, Appelbaum FR. Prevention of transfusion-induced sensitization to minor histocompatibility antigens on DLA-identical canine marrow grafts by gamma irradiation of marrow donor blood. Transplantation. 1991;52:956–960. doi: 10.1097/00007890-199112000-00004. [DOI] [PubMed] [Google Scholar]
- Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–4071. [PubMed] [Google Scholar]
- Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, Bredeson CN, Eapen M, Horowitz MM. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg HJ, Storb R, Weiden PL, Raff RF, Sale GE, Atkinson K, Graham TC, Thomas ED. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft-versus-host disease, and induction of tolerance. Transplantation. 1982;34:30–35. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- Doney K, Leisenring W, Storb R, Appelbaum FR for the Seattle Bone Marrow Transplant Team. Primary treatment of acquired aplastic anemia: outcomes with bone marrow transplantation and immunosuppressive therapy. Annals of Internal Medicine. 1997;126:107–115. doi: 10.7326/0003-4819-126-2-199701150-00003. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Herman R, Rolland-Grinton M, Jones-Wallace D, Blanchette V, Calderwood S, Doyle J, Halperin D, Leaker M, Saunders EF, Zipursky A, Freedman MH. Improved survival in severe acquired aplastic anemia of childhood. Bone Marrow Transplantation. 2000;26:1149–1156. doi: 10.1038/sj.bmt.1702699. [DOI] [PubMed] [Google Scholar]
- Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, Shadduck RK, Shea TC, Stiff P, Friedman DJ, Powderly WG, Silber JL, Horowitz H, Lichtin A, Wolff SN, Mangan KF, Silver SM, Weisdorf D, Ho WG, Gilbert G, Buell D. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. New England Journal of Medicine. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- Kahl C, Leisenring W, Deeg HJ, Chauncey TR, Flowers MED, Martin PJ, Sanders JE, Storb R. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long-term follow-up. British Journal of Haematology. 2005;130:747–751. doi: 10.1111/j.1365-2141.2005.05667.x. [DOI] [PubMed] [Google Scholar]
- Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons; 1980. [Google Scholar]
- Kennedy-Nasser AA, Leung KS, Mahajan A, Weiss HL, Arce JA, Gottschalk S, Carrum G, Khan SP, Heslop HE, Brenner MK, Bollard CM, Krance RA. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biology of Blood and Marrow Transplantation. 2006;12:1277–1284. doi: 10.1016/j.bbmt.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Yabe H, Hara J, Morimoto A, Tsuchida M, Mugishima H, Ohara A, Tsukimoto I, Kato K, Kigasawa H, Tabuchi K, Nakahata T, Ohga S, Kojima S. Preceding immunosuppressive therapy with antithymocyte globulin and ciclosporin increases the incidence of graft rejection in children with aplastic anaemia who underwent allogeneic bone marrow transplantation from HLA-identical siblings. British Journal of Haematology. 2006;135:693–696. doi: 10.1111/j.1365-2141.2006.06352.x. [DOI] [PubMed] [Google Scholar]
- Koc S, Leisenring W, Flowers MED, Anasetti C, Deeg HJ, Nash RA, Sanders JE, Witherspoon RP, Storb R, Appelbaum FR, Martin PJ. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- Limaye AP, Huang M-L, Leisenring W, Stensland L, Corey L, Boeckh M. Cytomegalovirus (CMV) DNA load in plasma for the diagnosis of CMV disease before engraftment in hematopoietic stem-cell transplant recipients. Journal of Infectious Diseases. 2001;183:377–382. doi: 10.1086/318089. [DOI] [PubMed] [Google Scholar]
- Locasciulli A, Oneto R, Bacigalupo A, Socie G, Korthof E, Bekassy A, Schrezenmeier H, Passweg J, Fuhrer M. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2007;92:11–18. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Bruno B, Zecca M, Van-Lint MT, McCann S, Arcese W, Dallorso S, Di Bartolomeo P, Fagioli F, Locasciulli A, Lawler M, Bacigalupo A. Cyclosporin A and short-term methotrexate versus cyclosporin A as graft versus host disease prophylaxis in patients with severe aplastic anemia given allogeneic bone marrow transplantation from an HLA-identical sibling: results of a GITMO/EBMT randomized trial. Blood. 2000;96:1690–1697. [PubMed] [Google Scholar]
- Martin PJ. Documentation of engraftment and characterization of chimerism following hematopoietic cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplantation. Oxford, UK: Wiley-Blackwell; 2009. p. 365. [Google Scholar]
- Martin PJ, McDonald GB, Sanders JE, Anasetti C, Appelbaum FR, Deeg HJ, Nash RA, Petersdorf EW, Hansen JA, Storb R. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2004;10:320–327. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Longton G, Thornquist M. A qualifier Q for the survival function to describe the prevalence of a transient condition. Statistics in Medicine. 1991;10:413–421. doi: 10.1002/sim.4780100313. [DOI] [PubMed] [Google Scholar]
- Przepiorka D, Weisdorf D, Martin P, Klingemann H-G, Beatty P, Hows J, Thomas ED. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- Pulsipher MA, Young NS, Tolar J, Risitano AM, Deeg HJ, Anderlini P, Calado R, Kojima S, Eapen M, Harris R, Scheinberg P, Savage SE, Maciejewski JP, Tiu RV, Difronzo N, Horowitz MM, Antin JH. Optimization of therapy for severe aplastic anemia based on clinical, biological, and treatment response parameters: conclusions of an international working group on severe aplastic anemia convened by the Blood and Marrow Transplant Clinical Trials Network, March 2010. Biology of Blood and Marrow Transplantation. 2011;17:291–299. doi: 10.1016/j.bbmt.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–3065. [PubMed] [Google Scholar]
- Sanders JE, Woolfrey AE, Carpenter PA, Storer BE, Hoffmeister PA, Deeg HJ, Flowers MED, Storb RF. Late effects among pediatric patients followed for nearly 4 decades after transplantation for severe aplastic anemia. Blood. 2011;118:1421–1428. doi: 10.1182/blood-2011-02-334953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos GW, Owens AH., Jr Allogeneic marrow transplants in cyclophosphamide treated mice. Transplantation Proceedings. 1969;1:44–46. [PubMed] [Google Scholar]
- Scheinberg P, Nunez O, Wu C, Young NS. Treatment of severe aplastic anaemia with combined immunosuppression: anti-thymocyte globulin, ciclosporin and mycophenolate mofetil. British Journal of Haematology. 2006;133:606–611. doi: 10.1111/j.1365-2141.2006.06085.x. [DOI] [PubMed] [Google Scholar]
- Scheinberg P, Wu CO, Nunez O, Young NS. Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine. Journal of Pediatrics. 2008;153:814–819. doi: 10.1016/j.jpeds.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. British Journal of Haematology. 2009a;144:206–216. doi: 10.1111/j.1365-2141.2008.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg P, Wu CO, Nunez O, Scheinberg P, Boss C, Sloand EM, Young NS. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica. 2009b;94:348–354. doi: 10.3324/haematol.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia [Erratum appears in JAMA. 2010 Nov 3;304(17):1901] Journal of the American Medical Association. 2010;304:1358–1364. doi: 10.1001/jama.2010.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrezenmeier H, Passweg JR, Marsh JC, Bacigalupo A, Bredeson CN, Bullorsky E, Camitta BM, Champlin RE, Gale RP, Fuhrer M, Klein JP, Locasciulli A, Oneto R, Schattenberg AV, Socie G, Eapen M. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007;110:1397–1400. doi: 10.1182/blood-2007-03-081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb R, Blume KG, O'Donnell MR, Chauncey T, Forman SJ, Deeg HJ, Hu WW, Appelbaum FR, Doney K, Flowers MED, Sanders J, Leisenring W. Cyclophosphamide and antithymocyte globulin to condition patients with aplastic anemia for allogeneic marrow transplantations: the experience in four centers. Biology of Blood and Marrow Transplantation. 2001;7:39–44. doi: 10.1053/bbmt.2001.v7.pm11215697. [DOI] [PubMed] [Google Scholar]
- Storb R, Epstein RB, Rudolph RH, Thomas ED. Allogeneic canine bone marrow transplantation following cyclophosphamide. Transplantation. 1969;7:378–386. doi: 10.1097/00007890-196905000-00007. [DOI] [PubMed] [Google Scholar]
- Storb R, Buckner CD, Dillingham LA, Thomas ED. Cyclophosphamide regimens in rhesus monkeys with and without marrow infusion. Cancer Research. 1970a;30:2195–2203. [PubMed] [Google Scholar]
- Storb R, Epstein RB, Rudolph RH, Thomas ED. The effect of prior transfusion on marrow grafts between histocompatible canine siblings. Journal of Immunology. 1970b;105:627–633. [PubMed] [Google Scholar]
- Storb R, Prentice RL, Thomas ED. Marrow transplantation for treatment of aplastic anemia. An analysis of factors associated with graft rejection. New England Journal of Medicine. 1977;296:61–66. doi: 10.1056/NEJM197701132960201. [DOI] [PubMed] [Google Scholar]
- Storb R, Thomas ED, Buckner CD, Clift RA, Deeg HJ, Fefer A, Goodell BW, Sale GE, Sanders JE, Singer J, Stewart P, Weiden PL. Marrow transplantation in thirty"untransfused" patients with severe aplastic anemia. Annals of Internal Medicine. 1980;92:30–36. doi: 10.7326/0003-4819-92-1-30. [DOI] [PubMed] [Google Scholar]
- Storb R, Doney KC, Thomas ED, Appelbaum F, Buckner CD, Clift RA, Deeg HJ, Goodell BW, Hackman R, Hansen JA, Sanders J, Sullivan K, Weiden PL, Witherspoon RP. Marrow transplantation with or without donor buffy coat cells for 65 transfused aplastic anemia patients. Blood. 1982;59:236–246. [PubMed] [Google Scholar]
- Storb R, Prentice RL, Sullivan KM, Shulman HM, Deeg HJ, Doney KC, Buckner CD, Clift RA, Witherspoon RP, Appelbaum FR, Sanders JE, Stewart PS, Thomas ED. Predictive factors in chronic graft-versus-host disease in patients with aplastic anemia treated by marrow transplantation from HLA-identical siblings. Annals of Internal Medicine. 1983;98:461–466. doi: 10.7326/0003-4819-98-4-461. [DOI] [PubMed] [Google Scholar]
- Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Hansen J, Hill R, Longton G, Lum L, Martin P, McGuffin R, Sanders J, Singer J, Stewart P, Sullivan K, Witherspoon R, Thomas ED. Marrow transplantation for severe aplastic anemia: Methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986a;68:119–125. [PubMed] [Google Scholar]
- Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Doney K, Farewell V, Hansen J, Hill R, Lum L, Martin P, McGuffin R, Sanders J, Stewart P, Sullivan K, Witherspoon R, Yee G, Thomas ED. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. New England Journal of Medicine. 1986b;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- Storb R, Weiden PL, Sullivan KM, Appelbaum FR, Beatty P, Buckner CD, Clift RA, Doney KC, Hansen J, Martin PJ, Sanders JE, Stewart P, Witherspoon RP, Thomas ED. Second marrow transplants in patients with aplastic anemia rejecting the first graft: Use of a conditioning regimen including cyclophosphamide and antithymocyte globulin. Blood. 1987;70:116–121. [PubMed] [Google Scholar]
- Storb R, Deeg HJ, Fisher LD, Appelbaum F, Buckner CD, Bensinger W, Clift R, Doney K, Irle C, McGuffin R, Martin P, Sanders J, Schoch G, Singer J, Stewart P, Sullivan K, Witherspoon R, Thomas ED. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: Long-term follow-up of three controlled trials. Blood. 1988;71:293–298. [PubMed] [Google Scholar]
- Storb R, Deeg HJ, Pepe M, Doney K, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Hansen J, Hill R, Longton G, Anasetti C, Martin P, Loughran TP, Sanders J, Singer J, Stewart P, Sullivan KM, Witherspoon R, Thomas ED. Graft-versus-host disease prevention by methotrexate combined with cyclosporin compared to methotrexate alone in patients given marrow grafts for severe aplastic anaemia: Long-term follow-up of a controlled trial. British Journal of Haematology. 1989;72:567–572. doi: 10.1111/j.1365-2141.1989.tb04325.x. [DOI] [PubMed] [Google Scholar]
- Storb R, Etzioni R, Anasetti C, Appelbaum FR, Buckner CD, Bensinger W, Bryant E, Clift R, Deeg HJ, Doney K, Flowers M, Hansen J, Martin P, Pepe M, Sale G, Sanders J, Singer J, Sullivan KM, Thomas ED, Witherspoon RP. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood. 1994;84:941–949. [PubMed] [Google Scholar]
- Strasser SI, Myerson D, Spurgeon CL, Sullivan KM, Storer B, Schoch HG, Kim S, Flowers MED, McDonald GB. Hepatitis C virus infection and bone marrow transplantation: a cohort study with 10-year follow-up. Hepatology. 1999;29:1893–1899. doi: 10.1002/hep.510290609. [DOI] [PubMed] [Google Scholar]
- Stucki A, Leisenring W, Sandmaier BM, Sanders J, Anasetti C, Storb R. Decreased rejection and improved survival of first and second marrow transplants for severe aplastic anemia (a 26-year old retrospective analysis) Blood. 1998;92:2742–2749. [PubMed] [Google Scholar]
- Sullivan KM, Agura E, Anasetti C, Appelbaum FR, Badger C, Bearman S, Erickson K, Flowers M, Hansen JA, Loughran T, Martin P, Matthews D, Petersdorf E, Radich J, Riddell S, Rovira D, Sanders J, Schuening F, Siadak M, Storb R, Witherspoon RP. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Seminars in Hematology. 1991;28:250–259. [PubMed] [Google Scholar]
- Young NS. Aplastic anaemia [Review] Lancet. 1995;346:228–232. doi: 10.1016/s0140-6736(95)91273-8. [DOI] [PubMed] [Google Scholar]