Abstract
Background
Histopathology has initially been and is still used to diagnose infectious, degenerative or neoplastic diseases in humans or animals. In addition to qualitative diagnoses semiquantitative scoring of a lesion`s magnitude on an ordinal scale is a commonly demanded task for histopathologists. Multiparametric, semiquantitative scoring systems for mouse models histopathology are a common approach to handle these questions and to include histopathologic information in biomedical research.
Results
Inclusion criteria for scoring systems were a first description of a multiparametric, semiquantiative scoring systems which comprehensibly describe an approach to evaluate morphologic lesion. A comprehensive literature search using these criteria identified 153 originally designed semiquantitative scoring systems for the analysis of morphologic changes in mouse models covering almost all organs systems and a wide variety of disease models. Of these, colitis, experimental autoimmune encephalitis, lupus nephritis and collagen induced osteoarthritis colitis were the disease models with the largest number of different scoring systems. Closer analysis of the identified scoring systems revealed a lack of a rationale for the selection of the scoring parameters or a correlation between scoring parameter value and the magnitude of the clinical symptoms in most studies.
Conclusion
Although a decision for a particular scoring system is clearly dependent on the respective scientific question this review gives an overview on currently available systems and may therefore allow for a better choice for the respective project.
Keywords: Score, Semiquantitative, Histopathology, Colitis, Nephritis, Hepatitis, Encephalitis
Background
Histopathology has initially been and is still used today to diagnose infectious, degenerative or neoplastic diseases in humans or animals. These qualitative diagnoses are based on a sum of observable changes in the morphology of the analyzed tissue. The cognition of these changes is based on the pattern recognition of the observer and the comparison of these patterns with the known physiologic variation in tissue morphology in the respective species. Decades of experience in veterinary pathology show that this approach allows for reproducible qualitative diagnoses by the observer but can also be used for semiquantitative scoring of the lesions magnitude, i.e. on an ordinal scale for instance with a low, medium or high grade trichotomy which correlates with the clinical relevance of the lesions.
Absolute quantification of the lesions extent and severity is however difficult since two main problems hamper absolute quantification, i.e. on a rational scale with absolute values of 1, 2, 3 etc., using standard, non-automated histopathology. First, the detection method is not reliable enough. Despite intensive training and attempts to standardize nomenclature and the definition of lesions there are still unresolved issues in terms of interobserver variation which may be acceptable for qualitative and semiquantitative evaluation but not for absolute quantitation [1]. Second, in most circumstances it is impossible to objectively justify the interval between two values, thus a read out of histopathologic scoring on a rational scale is impossible.
Image analysis by automated calculation of the tissue area affected or cells present per area have been introduced to overcome this problem. These approaches aim at a reliable and reproducible histopathology read out in a rational scale to allow proper statistical processing and at an exclusion of an observer bias [2]. Image analysis approaches usually use one or only few two dimensional planar sections of the tissue of interest to measure three-dimensional objects. This two-dimensional approach thus may also lead to biased results. Stereology, which is based on systematic random sampling and estimates third dimensional information, has been developed to avoid this bias [3]. It can therefore be seen as the most sophisticated method for the quantification of histologic information. It is however by comparison a laborious and complex method which is established in only few laboratories.
Semiquantitative scoring systems are therefore still the most widely used methods to include histopathologic information in biomedical research. These scoring systems usually include multiple parameters which are separately quantified on an ordinal scale and finally combined in a total score. Average scores of the different experimental groups can then be compared by non-parametric statistical tests. The selection of the parameters should be based on the scientific hypothesis or question together with the current knowledge on the morphologic outcome of the investigated disease model. It may therefore be useful to design individual scoring system for each study which in the best possible way answers the particular scientific question. Standard scoring systems for specific disease models on the other hand allow for the comparison of the results of different studies.
Several standard scoring systems for different mouse models have been introduced or emerged in the past 20 years. Histopathologists are therefore repeatedly requested by cooperating scientists to evaluate the outcome of animal studies using standard scoring systems or to elaborate project specific scoring systems. The present review is intended to give a comprehensive overview on the currently most commonly used multiparametric, semiquantitative scoring systems for mouse model histopathology.
Results
Scoring systems for murine intestinal disease models
Eighteen original scoring systems for colitis models could be identified. Most of these scoring systems were designed for dextran sodium sulfate (DSS)-induced colitis models but 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis and several models of immunopathologic colitis were also used to establish scoring systems (Table 1, Figure 1). Eight scoring systems for DSS-induced colitis fulfilled all required parameters and were included in this review. Generally, all of the paper with original DSS colitis scoring systems had a high citation rate but the scoring system described by Cooper is one of the earliest system with the highest citation number up to date and can therefore be seen as a prototype for DSS-colitis scoring [4]. It separates the colon into three segments which are then scored by the parameters of crypt loss, inflammation and affected area. Although later studies refined and increased the number of histopathologic parameters the value of this initial study is the separation of the colon in three segments and the sophisticated approach to the establishment of the scoring system. Remarkably, parameters in this study were chosen and tested according to their correlation with the clinical symptoms of the mice. This is contrast to the vast majority of scoring systems presented in this review, which only rarely stated the rational for choosing the included parameters and did not perform a correlation with the clinical symptoms.
Table 1.
Semiquantitative scoring systems for murine intestinal disease models
| Disease model | Scoring system: parameters (scale width) | Citations |
|---|---|---|
|
Colitis | ||
| DSS-induced [4] |
Proximal + middle + distal colon: crypt loss (0–4), inflammation (0–3) both quantified by the affected area (0–4) |
718 |
| DSS-induced [5] |
Extent, inflammation, necrosis, regeneration (each 3–0) |
321 |
| DSS-induced [6] |
Crypt damage, area involved, regeneration (each 0–4), extent (0–3), inflammation (0–3), |
255 |
| DSS-induced [7] |
Crypt loss (0–4), crypt distortion (0–4), epithelial hyperplasia (0–4), inflammation (0–3) each multiplied by percentage of involved area (0–4) |
185 |
| DSS-induced [8] |
Hyperplasia (0–3), severity (0–3), ulceration (0,1), area involved (0–4) |
147 |
| DSS-induced [9] |
Severity of inflammation, thickness of inflammation, epithelial damage character, extent of epithelial damage (each 0–3) |
130 |
| DSS-induced [10] |
Epithelium (goblet cell/crypt loss), infiltration (each 0–4) |
103 |
| DSS-induced [11] |
Infiltration, tissue damage (each 0–3) |
89 |
| TNBS-induced [12] |
Inflammation, loss of goblet cells, vascular density, transmural infiltration, thickening of the colon wall (together 0–4) |
788 |
| TNBS-induced [13] |
Crypt distortion, goblet cell loss, acute inflammation, chronic inflammation (each 0–2) |
126 |
| TNBS-induced [14] |
Loss of mucosal architecture, cellular infiltration, muscle thickening (each 0–3), crypt abscesses (0,1), goblet cell depletion (0,1) |
97 |
| Acetic acid-induced [15] |
Inflammation, bleeding, ulcer size, deepness, perforation (together 0–6) |
132 |
| TNBS-induced [16] |
Percentage of area, crypt loss (both 0–4) number of follicles, edema, erosion/ulceration, infiltration (each 0–3) |
57 |
| HLA-B27 transgenic mice [17] |
Inflammation, goblet cell loss, mucosal thickening, submucosal infiltration, architecture loss (each 0–4), ulcer, crypt abscess (each 0,1) |
423 |
| IL10-deficient mice [18] |
Mucosal ulceration, epithelial hyperplasia (both 0–3), Lamina propria mononuclear infiltrate, Lamina propria neutrophil infiltrate (each 0–2) |
423 |
| IL10-deficient mice [19] |
Inflammation/epithelial erosion/ulcers/hyperplasia/crypt abscesses/goblet cell depletion (together 0–4) multiplied by no. of affected colon segments (1–5) |
555 |
| MHC missmatch [20] |
Active inflammation (0.5-3), chronic inflammation (0.5-3), villous architecture (1–3) multiplied by the affected area (0.5-4) |
83 |
| Amebic colitis [21] |
Number of amoeba (0–5), ulceration (0-100%), inflammation (0–5) |
58 |
|
Small intestinal diseases | ||
| Clostridial toxicosis [22] |
Epithelial damage, hyperemia/edema, neutrophils (each 0–3) |
202 |
| Intestinal ischemia [23] |
Normal mucosa (0), villous edema (1), subepithelial edema (2), epithelium loss at villi sides (3), denuded villi (4), loss of villous tissue (5), crypt infarction (6), transmucosal infarct (7), transmural infarct (8) |
270 |
| Intestinal ischemia [24] |
Mucosal damage, inflammation, hyperemia/hemorrhage (each 0–5) |
72 |
| Jejunitis [25] |
Villous length, villous tips, epithelium, inflammation, crypt loss (each 0–2), crypt abscesses, hemorrhage (each 0,1) |
15 |
| Bacterial ileitis [26] |
Hemorrhage, villous atrophy/necrosis, edema, congestion, neutrophils, epithelial necrosis (each 0–3) |
1 |
|
Gastric diseases | ||
| Helicobacter-induced [27] | Five areas multiplied by inflammation (0–3) | 49 |
DSS, Dextran sodium sulfate; TNBS, 2,4,6-trinitrobenzene sulfonic acid, MHC, Major histocompatibility complex; HLA-B27, Human Leukocyte Antigen-B27, IL10, Interleukin 10.
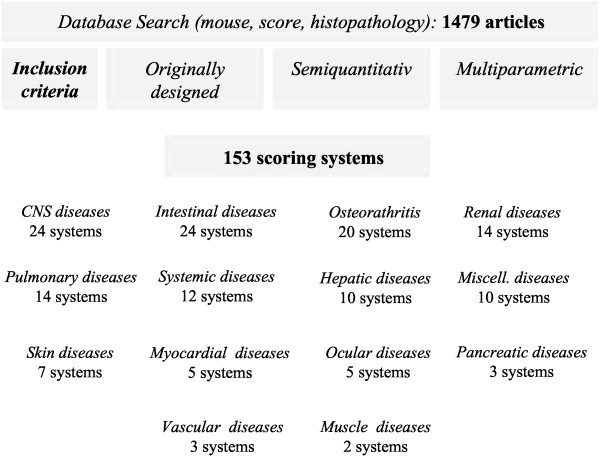
Figure 1.
Flow chart visualizing the approach to identify 153 original, multiparametric, semiquantiative scoring systems by Pubmed data search.
When comparing all original colitis models it becomes obvious that a wide variety of appellation for the most common parameters inflammation, crypt and surface epithelial damage were used (Table 1). These differences in the nomenclature make it however difficult to directly compare the different scoring systems. Less often used parameters in the colitis scoring systems were goblet cell loss, regeneration, muscular and epithelial hyperplasia, edema and the separation between acute and chronic inflammation. In some cases these singularities of the respective scoring system seem to be dependent on the objectives of the study while in most cases the rationale for selection of the parameters was not given.
The number of identified scoring systems for small intestinal disease was significantly lower than for colitis models and had on average lesser citations (Table 1). Two independent scoring systems were identified for intestinal ischemia which include the comprehensible parameters of hyperemia and hemorrhage as well as inflammation and epithelial damage and in the case of the higher cited publication by Park et al. several other more sophisticated parameters [23,24]. Only two scoring systems for small intestinal enteritis were detected which both included villous morphology, epithelial damage and inflammation as the main features (Table 1). Surprisingly, only one semiquantitative scoring system for gastritis was identified. Wang et al. scored the severity of Helicobacter-induced gastritis in a uniparametric scoring of five gastric areas. For the sake of completeness this studies was included in this review although it did not fulfill the criterion of multiple parameters [27].
Scoring systems for murine osteoarthritis models
Seventeen original semiquantitative, multiparametric scoring systems were identified for murine osteoarthritis models (Table 2). Three of these, designated as osteoarthritis in Table 2, are scoring systems for human idiopathic arthritis and were transferred to the murine model to allow for comparisons of the model with the human disease. Of these the the score developed by Mankin et al. has by far the highest citation number which is most probably influenced by its common application in the description of human osteoarthritic lesion [28]. The majority of mouse specific systems were established in collagen-induced models while models of instability-induced or bacterial arthritis models were only rarely used. Of these, the scoring system by William et al. had the highest citation number [29]. Twelve of the thirteen osteoarthritis scoring systems include an evaluation of cartilage damage, nine included an infiltration parameter, seven included changes in perichondral bone structure and six studies evaluated the extend of synovial infiltration (Table 2). Other parameters like tidemark integrity, proteoglycan content of the cartilage, pannus formation or synovial hyperplasia were used only in the minority or single scoring systems. Again, in most of these descriptions of original scoring systems no rational for inclusion of the respective parameters is given but their usefulness can be comprehended by reflecting the general pathogenesis of osteoarthritis.
Table 2.
Semiquantitative scoring systems for murine osteoarthritis models
| Osteoarthritis model | Scoring system: parameters (scale width) | Citations |
|---|---|---|
| Arthritis [28] |
Cartilage structure (0–6), cells (0–3), Safranin-O-stain (0–4), tidemark integrity (0–1) |
1322 |
| Arthritis [30] |
Cartilage destruction (0–6), optional subgrading (subdivision in 2 subgrades) |
197 |
| Arthritis [31] |
Synovial lining, resident cell density, inflammation (each 0–3) |
49 |
| Collagen-induced [29] |
Extent of synovitis, cartilage loss, bone erosions (together 0–3) |
713 |
| Collagen-induced [32] |
Inflammation, cartilage destruction, bone erosion (each 0–3) |
273 |
| Collagen-induced [33] |
Infiltration in the exudate, infiltration of the synovial membrane, cartilage destruction, bone erosion (each 0–3) |
198 |
| Collagen-induced [34] |
Joint exudate (0–5), proteoglycan depletion (0–3) |
139 |
| Collagen-induced [35] |
Bone resorption, inflammation, cartilage damage (each 0–5) |
95 |
|
Mycobacterium butyricum-induced [36] |
Synovial thickening, infiltration, pannus formation (each 0–3) |
95 |
| Adjuvant-induced [37] |
Percentage of affected area, synovial hyperplasia, cartilage destruction, bone erosion (each 0–3) |
64 |
| IL1-induced arthritis [38] |
Synovial infiltration, proteoglycan depletion, cartilage damage (each 0–3) |
41 |
| Instability-induced [39] |
Matrix structure, matrix staining, cellularity, subchondral bone (each 0–8) |
85 |
| Instability-induced [40] |
Cartilage destruction (0–4), osteophyte formation (0–3) |
85 |
| Hereditary arthritis [41] |
Articular cartilage structure (0–8), Toluidine blue-staining (0–6) |
52 |
| Bacterial arthritis [42] |
Infiltration (0–4), pannus formation, cartilage destruction, extra-articular manifestations, tail lesion (each 0,1) |
145 |
| Cartilage repair [43] |
Cell morphology (0–4), matrix staining (0–3), surface regularity (0–3), thickness (0–2), integration of donor cartilage (0–2) |
819 |
| Cartilage repair [44] |
Cellular morphology (0,2,4), Safranin-O stain (0–3), structural regularity (0–3), structural integrity (0–2), thickness (0–2), bonding (0–2), hypocellularity (0–3), chondrocyte clustering (0–2), adjacent degeneration (0–3) |
297 |
| Cartilage repair [45] |
Relative defect area (0–4), integration of repair tissue (0–3), matrix staining (0–4), cellular morphology (0–5), defect architecture (0–4), surface architecture, percentage of new bone (0–4), tidemark formation (0–4) |
222 |
| Cartilage repair [46] |
Filled depth (0–4), integration (0–2), surface architecture (0–3), cell morphology (0–3), cellularity (0–2), tidemark formation (0–4), Toloudin blue stain (0–2) |
130 |
| Cartilage repair [47] | Defect filling (0–4), osteochondral reconstruction (0–2), matrix staining (0–4), cell morphology (0–4) | 96 |
IL1, Interleukin 1.
Scoring systems for murine renal disease models
Fourteen original multiparametric, semiquantitative scoring systems for murine models of renal diseases fulfilled the required criteria for inclusion in this review (Table 3). Scoring systems for murine Lupus erythematous models were the dominant model in the category of renal disease models with four appearances. Austin et al. published the lupus nephritis score with the highest citation number [48]. It uses a complex scoring system with 10 parameters and a scale width of four and five respectively and was thus more sophisticated than the other scoring systems which used only four different parameters.
Table 3.
Semiquantitative scoring systems for murine renal disease models
| Renal disease model | Scoring system: parameters (scale width) | Citation |
|---|---|---|
| Lupus nephritis [48] |
Activity index (glomerular/tubulointerstitial abnormalities (6-tier, each 0–4)); chronicity index (4-tier, each 0–3) |
358 |
| Lupus nephritis [49] |
Mesangial thickening, extent of changes (together 0–4) |
44 |
| Lupus nephritis [50] |
Glomerular cell proliferation, lobulation, hyaline droplets, macrophage infiltration (together 0–3) |
25 |
| Lupus nephritis [51] |
Glomerular inflammation, proliferation, crescent formation, necrosis (each 0–3) |
1 |
| Toxic nephropathy [52] |
Glomerular cellularity, hypertrophy, thrombosis, dilation (together 0–5) |
4 |
| Toxic nephropathy [53] |
Glomerular injury, tubular cysts/casts, podocyte hyperplasia, interstitial inflammation (each 0–4) |
33 |
| Hypertension nephropathy [54] |
Mesangial matrix, percentage of glomerular affection (each 0–4) |
524 |
| Diabetic-/Hypertension-induced glomerulosclerosis [55] |
Arteriole hyalinization, glomerular sclerosis (0–4), interstitial volume (%) |
152 |
| Diabetic nephropathy [56] |
Glomerulosclerosis, interstitial fibrosis (each 0–4) |
15 |
| Crescentic glomerulonephritis[57] |
Fibrin deposition, immunoglobulin deposition, tubular damage, glomerular crescents (each 0–3) |
8 |
| MRL/MPJ mouse glomerulonephritis [58] |
Glomerular infiltration, crescents, necrosis, tubular casts, interstitial infiltrates (each 0–4) |
244 |
| Age-associated changes [59] |
Mesangial proliferation, sclerosis, hyalinization (together 0–5) |
27 |
| HIV-nephropathy [60] |
Tubuloepithelial degeneration/regeneration, tubular casts, dilation, interstitial infiltration, glomerular sclerosis, collapse, podocyte hyperplasia (each 0–3) |
47 |
| Obstructive nephropathy [61] | Tubular dilation/atrophy, interstitial fibrosis (each 0–3) | 4 |
HIV, Human immunodeficiency virus; MRL, Murphy Roths Large.
Glomerular cellularity and proliferation were the terms most commonly used in all renal scoring systems except one scoring system for obstructive nephropathy model [61]. In addition, one half of the systems included tubulointerstitial infiltration and fibrosis in the scoring system.
Scoring systems for murine models of neurologic disease
Twenty-two original scoring systems for murine models of central nervous system (CNS) disease were identified (Table 4). Scoring systems for experimental autoimmune encephalomyelitis (EAE) and stroke clearly dominated results. Due to the wide variety of diseases covered by the system the selection of parameters to be analyzed also had a wide variation and was clearly dependent on the pathophysiology of the disease. But again, the rationale for inclusion of parameters was not consistently given.
Table 4.
Semiquantitative scoring systems for murine central nervous disease models
| Disease model | Scoring system: parameters (scale width) | Citations |
|---|---|---|
|
Stroke | ||
| Focal ischemia [62] |
Ischemic neuronal damage (0–3) |
2176 |
| Focal Ischemia[63] |
18 areas x neuronal injury (0–5) |
23 |
| Global ischemia [64] |
Infarcts in 3 cerebral regions (0–4), hippocampus infarction (0–4) |
100 |
| Global ischemia [65] |
Eight regions x neuronal cell los/gliosis/iron deposition/gliosis (0–3) |
95 |
| Peripheral nerve ischemia [66] |
Edema, fiber regeneration (each 0–4) |
21 |
|
Multiple sclerosis models | ||
| EAE [67] |
Inflammation, neuronal degeneration (each 0–4) |
160 |
| EAE [68] |
Inflammation (cells/cuff), axonal injury, axonal loss (each 0–4) |
81 |
| EAE[69] |
Lesion severity, myelin loss/tissue injury, acute inflammation, chronic inflammation (each 0–5) |
79 |
| Theiler's murine encephalomyelitis virus [70] |
Neuropil inflammation, demyelination, necrosis, meningeal inflammation (each 0–4) |
77 |
| EAE [71] |
Infiltration, demyelination (together 0,1) in 16 regions |
64 |
| EAE [72] |
Inflammation, necrosis (each 0–3) |
53 |
| EAE [73] |
Inflammation (cuffs/100 m2), demyelination (lesions/mm2) |
52 |
| EAE [74] |
Meningitis (0–2), perivascular cuffing (0–5), demyelination (0–3) |
43 |
| EAE [75] |
Spinal cord demyelination (0–3), inflammatory cells (No./mm2) |
22 |
|
Spinal cord trauma (SCT) | ||
| SCT [76] |
Gray matter degeneration/infarction (0–4) in 5 μm serial sections |
33 |
| SCT [77] |
150 μm intervals x Area affected by neuronal degeneration, malacia (0–4) |
23 |
|
Encephalitis/Meningitis |
|
|
| Streptococcus meningitis [78] |
Meningeal inflammation (0–3) x four regions |
46 |
|
Trypanosoma encephalitis [79] |
Meningitis, perivascular cuffing, neuropil infiltration (each 0–4) |
41 |
|
Miscellaneous CNS diseases | ||
| Oxidative damage [80] |
7 areas x necrosis (0–3) |
27 |
| Senescence [81] |
Spongiosis (0–3), lipofuscin positive cells (%) |
23 |
| Spinocerebellar ataxia [82] | Molecular layer thickness, Purkinje cell loss (each 0–3) | 10 |
EAE, Experimental autoimmune encephalomyelitis, SCT, Spinal cord trauma, CNS, Central nervous system.
A striking feature of CNS disease scoring systems was the relatively low number of ordinal scales for parameter´s magnitudes and the common inclusion of multiple anatomical sites into the scoring system (Table 4). This discrepancy is not addressed in the respective publications but may be based on the anatomical diversity of the CNS. Furthermore, the inclusion of absolute values like lesions/mm2 occurred significantly more often in CNS disease scoring than for other organs although this is not comprehensible in each case. Only one scoring system occurred for a peripheral nerve system disease which has been developed to evaluate peripheral nerve ischemia in a relatively simple two-tier system with a zero to four-scale [66].
Scoring systems for murine models of pulmonary diseases
Fourteen original semiquantitative, multiparametric scoring systems were identified for pulmonary diseases (Table 5). Of these pulmonary fibrosis and pulmonary inflammation were diseases with the highest number scoring systems and citations. For instance, the scoring system developed by Ashcroft, which is a relatively simple multiparametric but single scaled system, is commonly used for the evaluation of lung fibrosis [83]. Three scoring systems were developed for models of general acute lung inflammation (Table 5). They used the parameters of edema and anatomical site specific inflammation as parameters to evaluate the relative amount of inflammatory response. Similar parameters in a wide variety of combinations were used to develop scoring systems for diverse infectious pneumonia models. This variation is again in most cases not based on reasonable argument for the inclusion of a certain parameter in a certain model and therefore not in all cases clearly associated with the supposed pathogen-associated pathogenesis of the respective pneumonia.
Table 5.
Semiquantitative scoring systems for murine models of pulmonary diseases
| Pulmonary disease models | Scoring system: parameters (scale width) | Citations |
|---|---|---|
| Lung fibrosis [83] |
Alveolar/bronchial wall thickening, structure distortion, fibrosis (together 0–8) |
273 |
| Lung fibrosis [84] |
Fibroblastic foci, established fibrosis, intraalveolar macrophages (each 0–6) |
116 |
| Cystic fibrosis [85] |
Lymphoid infiltrate (0–5), goblet cell hyperplasia (0–2), mucus retention (0–3), bronchiolitis (0–5), pneumonia (0–3), edema (0–2) |
101 |
| Ventilator-induced lung injury [86] |
Alveolar congestion, hemorrhage, neutrophils in airspaces/vessel walls, alveolar wall thickness, hyaline membranes (each 0–4) |
78 |
| Pulmonary ischemia [87] |
Edema, inflammatory cell infiltration, vascular congestion, alveolar hemorrhage (each 0–3) |
5 |
| Smoke-induced pneumopathy [88] |
Alveolar emphysema, atelectasis, infiltration, hemorrhage, alveolar wall thickness, perivascular/peribronchiolar edema (each 0–3) |
4 |
| Lung inflammation [89] |
Perivascular edema (0–3), perivascular/-bronchiolar inflammation (0–3), goblet cell metaplasia (0–2) |
32 |
| Lung inflammation [90] |
Alvolear wall inflammation, perivenous regions, periarterial/peribronchial regions, venous/arterial endothelial lesion (each 0–3) |
30 |
| Acute lung inflammation [91] |
Alveolar necrosis, vascular congestion, infiltration by neutrophils/ macrophages (each 0–4) |
6 |
| RSV pneumonia [92] |
Peribronchiolitis, alveolitis, perivasculitis, hypertrophy of mucus-producing glands, eosinophilia (each 0–5). |
26 |
| Mycoplasma pneumonia [93] |
Quantity/quality of (peri-)bronchial infiltrates, bronchial luminal exudate, perivascular infiltrate, parenchymal pneumonia (0–3) |
56 |
|
Pneumocystis carinii infection [94] |
Cyst number (0–4), inflammation (0–5) |
87 |
| Streptococcus pneumonia [95] | Bronchitis, edema, interstitial inflammation, intraalveolar inflammation, pleuritis, endothelialitis (each 0–4) | 0 |
Scoring systems for myocardial, vascular and muscular disease models
Three original scoring systems for the evaluation of viral myocarditis were identified [96-98]. All of them included the evaluation of the parameters of myocardial necrosis and inflammation (Table 6). In addition, two of them also included calcification as a parameter while fibrosis and Evans blue-staining as a marker of myofiber damage were used as a parameter of myocardial disease only once.
Table 6.
Semiquantitative scoring systems for cardiovascular and muscle disease models
| Disease model | Scoring system: parameters (scale width) | Citations |
|---|---|---|
|
Myocardial diseases | ||
| EMCV-induced myocarditis [96] |
Myocardial necrosis, infiltration, calcification, fibrosis (together 0–4) |
50 |
| Coxsackievirus-induced myocarditis [97] |
Myocardial necrosis, infiltration, calcification (together 0–4) |
33 |
| Coxsackievirus-induced myocarditis [98] |
Necrosis, inflammation, Evans blue-stain (each 0–4) |
17 |
| Dilated cardiomyopathy [99] |
Myocardial necrosis, fibrosis (together 0–4) |
20 |
| Chronic cardiotoxicity [100] |
Qualitative/quantitative myocardial degeneration score (each 0–4) |
72 |
|
Vascular diseases | ||
| Aneurysm [101] |
Extent of medial, adventitial disruption/size of lesion (together 0–6) |
21 |
| Atherosclerosis [102] |
Medial erosion, foam cells, buried fibrous caps, chondrocyte-like cells, lateral xanthomas (each 0,1) |
2 |
| Vasculitis [103] |
Infiltration, elastic lamina destruction, intimal thickening (together 0–3) |
8 |
|
Muscle diseases | ||
| Ischemic necrosis [104] |
Infiltration, necrosis, hemorrhage (together 0–10) |
14 |
| Trypanosoma myositis [105] | Number of parasites, eosinophilic infiltration (each 0–3) | 0 |
EMCV, Encephalomyocarditis virus.
Three semiquantitative scoring systems for the most important human vascular diseases were identified: atherosclerosis, aneurysms and vasculitis (Table 6) [101-103]. They all cover several aspects of the pathogenesis and pathophysiology of the diseases but have been generally rarely cited yet. The aneurysm scoring system grades the severity of the disease by the extent of medial and adventitial lesion together with the general size of the lesion [102]. The atherosclerosis scoring systems uses a 5-tier system with a 0–1 scale width [102], whereas the vasculitis score uses the parameters infiltration, elastic lamina destruction and intimal thickening, thus indicating that the system may only be useful for evaluation of larger vessel types [103].
Two scoring systems for muscular disease models were identified (Table 6). One recently published scoring system evaluated the extent of Trypanosoma-induced myositis [104] while the other scoring system was developed to quantify the extent of ischemia-induced muscle necrosis by the parameters necrosis, infiltration and hemorrhage [105].
Scoring systems for hepatic and pancreatic diseases
Ten original scoring systems for chronic hepatitis have been developed or used for the quantification chronic hepatic disease (Table 7). The scoring systems by Ishak and Knodell are both highly cited scoring systems and cover almost all possible histomorphologic changes in chronically inflamed livers [106,107]. The two identified scoring systems for acute hepatitis quantify lesions by grading the extent of inflammation and necrosis, similar to the Ishak system for chronic hepatitis [108,109].
Table 7.
Semiquantitative scoring systems for murine hepatic and pancreatic disease models
| Disease model | Scoring system: parameters (scale width) | Citations |
|---|---|---|
|
Hepatic disease | ||
| Chronic active hepatitis [107] |
Periportal bridging (0–10), intralobular necrosis, portal inflammation, fibrosis (each 0–4) |
2,609 |
| Chronic hepatitis [106] |
Periportal/septal inflammation (0–4), confluent necrosis (0–6), focal necrosis/apoptosis/inflammation (0–6), portal inflammation (0–4), fibrosis (0–6) |
2,001 |
| Chronic hepatitis [110] |
Mitotic activity, portal inflammation, ductular proliferation, councilman bodies, fibrosis (each 0–3) |
107 |
| Hepatic fibrosis [108] |
Centrolobular vein/perisinusoidal space fibrosis (each 0–2), portal tract fibrosis (0–3), septa number (0–3), septa width (0–5) |
135 |
| Acute hepatitis [111] |
Steatosis (0–4), necrosis, inflammation (each 0–2), |
218 |
| Acute hepatitis [109] |
Portal/lobular inflammation (each 0–3) |
41 |
| Nutritional hepatopathy [112] |
Hepatocyte degeneration, portal inflammation, portal fibrosis (each 0–3) |
25 |
| Alcohol-induced hepatopathy [113] |
Steatosis (%), inflammation/necrosis/fibrosis (each 0–2) |
8 |
| Non-alcoholic Steatohepatitis [114] |
Steatosis (0–3), hepatocellular ballooning (0–2), lobular inflammation (0–2) |
1,149 |
| Hepatic ischemia [115] |
Location, necrosis (together 0–4) |
12 |
|
Pancreatic disease | ||
| Acute pancreatits [116] |
Edema, necrosis, inflammation, hemorrhage, fat necrosis (each 0–4) |
307 |
| Acute pancreatitis [117] |
Edema, necrosis, inflammation, vacuolization (each 0–4) |
144 |
| Acute pancreatitis [118] |
Edema, necrosis, inflammation, hemorrhage (each 0–4) |
89 |
| Acute pancreatitis [119] |
Acinar-cell ghosts (%), acinar cells vacuolization/swelling (%) |
42 |
| Ischemia-induced acute pancreatitis [120] |
Edema, necrosis, infiltration, hemorrhage, vacuolization (each 0–3) |
14 |
| Autoimmune pancreatitis [121] |
Infiltration, necrosis, lipomatosis (0–4) |
42 |
| Insulitis [122] | Islet infiltration, destruction, atrophy (0–5) | 49 |
Five scoring systems for the evaluation of acute pancreatitis have been identified (Table 7). The first and most commonly cited scoring system was published by Schmidt et al. [116]. It uses the five parameters edema, necrosis, inflammation, hemorrhage and fat necrosis to score the extent of pancreatic lesions. All four later developed scoring systems only marginally modified the parameters by omitting a single parameter or including vacuolization as an additional marker (Table 7). In addition, one multiparametric but several uniparametric (data not shown) scoring systems were identified for the quantification of insulitis in mice models. The scoring system by Papaccio et al. uses islet infiltration, atrophy and destruction as parameters for the evaluation of Isle of Langerhans inflammation [122].
Scoring systems for skin and ocular diseases and miscellaneous disease models
Murine psoriasis models are the only skin disease model with more than one identified scoring system (Table 8). Both scoring systems offer a wide variety of parameters for the evaluation of epidermal and dermal changes in models of this relevant human disease [123,124]. In addition, scoring systems for dermal sclerosis, burn scars, atopic dermatitis and epithelial irritation were identified (Table 8).
Table 8.
Semiquantitative scoring systems for murine models of eye, skin and miscellaneous diseases
| Disease model | Scoring system: parameters (scale width) | Citations |
|---|---|---|
|
Skin diseases | ||
| Burn scars [125] |
Epidermal hyperplasia/hyperkeratosis, hair follicles, apocrine glands, smooth muscles, fibroplasia, vascular proliferation (each 0,1), collagen orientation (0–3) |
26 |
| Systemic sclerosis [126] |
Dermal inflammation, thickened collagen bundles, dermal thickness (each 0–3) |
18 |
| Atopic dermatitis [127] |
Epidermal hypertrophy, hyperkeratosis, parakeratosis, erosion, inflammation, edema, ulcer (each 0–4) |
8 |
| UV radiation-induced skin damage [128] |
Epidermal thickness (0–3), dermal cellularity (0–3), dermal cyst changes (0–5) |
7 |
| Epithelial irritation [129] |
Leukocyte infiltration (0–5), epithelial reaction (0,1) |
1 |
| Psoriasis [123] |
Munro abscesses (1.5), hyperkeratosis (0.5), parakeratosis (1), length of rete ridges (0.5–1.5), lack of granular layer (1), acanthosis (1), dermis lymphocytic infiltrate (0.5–1.5), papillary papillae congestion (1), thinning above papillae (0.5) |
18 |
| Psoriasis [124] |
Epidermal thickness, Stratum corneum thinning, extent of Stratum granulosum/parakeratosis/inflammation, microabscesses (each 0–3) |
5 |
|
Ocular diseases | ||
| Diabetic retinopathy [130] |
Inflammation (leukocytes/100 μm retina length), leukostasis (leukocytes per vessel lumen) |
25 |
| Oxygen induced retinopathy [131] |
Blood vessel growth, tufts, tortuosity, extraretinal neovascularization, vasoconstriction (each 0–3), hemorrhage (0,1) |
48 |
| Bacterial endophthalmitis [132] |
Inflammation, retinal architecture (each 0–4), |
18 |
| Autoimmune uveoretinitis [133] |
Infiltration (0–4) |
285 |
| Endotoxin uveitis [134] |
Inflammation (0–3) |
47 |
|
Miscellaneous diseases | ||
| Abdominal adhesion [135] |
Inflammation, fibrosis, necrosis/abscess, granulomas (each 0–3) |
100 |
| Abdominal adhesion [136] |
Vessel number, neutrophil infiltration, neutrophils at site (each 0–3) |
4 |
| Abdominal adhesion [137] |
Fibrotic matrix, collagen fibers, fibroblast proliferation (together 1–5) |
n.r. |
| Embryonic development [138] |
Grading (0–5) of 17 parameters |
429 |
| Wound healing [139] |
Infiltration, granulation tissue, fibroblasts, collagen deposition (together 0–12) |
369 |
| Thyroiditis [140] |
Number of inflammatory foci, parenchymal destruction (together 0–4) |
77 |
| Vaginitis [141] |
Epithelial disruption, leucocyte infiltration, edema, vascular injection (each 0–4) |
74 |
| Esophagitis [142] |
Epithelial damage/hemorrhage (0–4), inflammation (0–3) |
6 |
| Lymph node [143] |
Heterophils, apoptotic histiocytes, sinus histiocytosis, follicular hyperplasia (together 0–5) |
1 |
| Spermatogenic activity [144] | Presence of spermatozoa/spermatides/germ cells/sertoli cells (together 0–10) | n.r. |
n.r., not reported; UV, ultraviolet light.
Five original scoring systems for the evaluation of ocular diseases were identified. Two of these systems use only one parameter for the evaluation of autoimmune and endotoxin uveitis. For the sake of completeness these scoring systems are also displayed in Table 8, although they do not fulfill requirements for inclusion [133,134]. Furthermore, the identified scoring system for diabetic retinopathy uses two parameters evaluated in absolute numbers of leukocytes per area [130].
Three scoring systems for the evaluation of abdominal adhesions after traumatic or toxic irritation of the peritoneum could be identified. Interestingly, not all scoring systems for abdominal adhesion use fibrosis as a parameter for the grading of the adhesions [135-137]. Finally, very helpful scoring systems for the evaluation of embryonic development and wound healing could be identified (Table 8).
Scoring systems for systemic diseases and transplant rejection
Three original scoring systems analyzing lesions associated with graft-versus-host disease (GvHD) are available (Table 9). The most cited scoring system by Hill et al. exclusively covers intestinal lesions associated with GvHD [145]. This system allows a very thorough analysis of intestinal lesion using a wide variety of parameters in the small and large intestine. The other two systems also cover intestinal lesions but provide additional parameters for the analysis of hepatic [146] or hepatic and skin lesions [147].
Table 9.
Semiquantitative scoring systems for systemic disease models
| Disease model | Scoring system: parameters (scale width) | Citations |
|---|---|---|
|
Graft-versus-host disease (GvHD) | ||
| Intestinal acute GvHD [145] |
Small intestine: villous blunting, crypt regeneration/apoptosis/loss, enterocyte loss, infiltration (each 0–4); Colon: crypt regeneration, colonocyte vacuolization, crypt apoptosis/destruction, infiltration (each 0–4) |
389 |
| Acute GvHD [146] |
Small/large bowel: villous blunting, crypt regeneration/apoptosis/loss, luminal sloughing, infiltration, mucosal ulceration, epithelial/vacuolization (each 0–4), liver: portal infiltration, bile duct apoptosis/sloughing, parenchymal apoptosis/abscesses/mitoses, steatosis, cholestasis (each 0–4) |
170 |
| Acute GvHD [147] |
Skin: epidermal damage, dermal collagen density, dermal infiltration, subcutaneous fat loss, hair follicle loss (each 0–2), intestine: crypt apoptosis, inflammation(each 0–4), liver: bile duct injury (0–4) |
50 |
|
Hemorrhagic shock | ||
| Lung lesion [148] |
Alveolar membrane thickening, congestion, edema, intraalveolar hemorrhage, interstitial, intraalveolar infiltration (each 0–3) |
36 |
| Intestinal, pulmonary lesions [149] |
Intestine: % injury (number of edematous villi x 0,5 + number of villi with epithelial damage x 1), lung: number of neutrophils in 10 fields |
2 |
| Pulmonary, intestinal, renal, hepatic lesions [150] |
Lung: atelectasis, hemorrhage, edema, congestion, inflammation, hyp-eraeration (each 0–3); ileal mucosal damage, (0–5), liver: congestion, necrosis, vacuolization (each 0–3), Kidney: epithelial swelling, tubular dilation, necrosis, edema, microthrombosis (each 0–3) |
2 |
|
Immunotoxicity | ||
| [151] |
Complete assessment of all lymphoid organs (each 0–4) |
50 |
|
Transplant rejection | ||
| Banff classification for renal rejection [152] |
Tubulitis, arteritis, mononuclear cell interstitial infiltrates, glomerulitis, interstitial fibrosis, tubular atrophy, glomerulopathy, mesangial matrix increase, vascular fibrous intimal thickening, arteriolar hyaline thickening (each 0–3) |
1.727 |
| Heart rejection 1990 [153] |
Infiltration, myocyte damage (0–4) |
1401 |
| Heart rejection 2005 [154] |
Infiltration, myocyte damage (0–3) |
365 |
| Lung rejection [155] |
Inflammation (0–4), lymphocyte infiltration (0–1), bronchiolitis obliterans (0–4), Vascular rejection (0–1), vasculitis (0–1) |
362 |
| Skin rejection [156] | Acanthosis, ulceration, necrosis, inflammation, granulation tissue (0–5) | 1 |
GvHD, Graft versus host disease.
Three original scoring systems have been developed for the analysis lesions associated with hemorrhagic shock (Table 9). The scoring system with the highest citation number only focusses on the pulmonary lesion and offers a variety of parameters for the evaluation of shock-induced lesions in the lung [148]. The other two scoring system also include parameters for lung evaluation but both offer additional parameters for the quantification of intestinal changes [149,150] or in one case offer scoring systems for renal and hepatic lesions [150] associated with hemorrhagic shock.
The identified scoring system for immunotoxicity of toxins more or less demands the analysis of all immune organs but gives a good guideline in terms of the nomenclature of morphologic changes associated with the toxin application (Table 9) [151].
Finally, the three scoring systems which have been used for murine transplant rejection models have consistently been developed for the evaluation of tissues from human patients (Table 9). They are all used in mouse models unmodified to allow for better conclusions from the mouse models for the situation in the human patient.
Discussion
Extensive research of the literature identified 146 originally designed semiquantitative, multiparametric scoring systems for the histopathology of mouse models. These scoring systems cover almost all organs systems and a wide variety of disease models. Colitis and especially ulcerative colitis was the disease model with the largest number of different scoring system closely followed by experimental autoimmune encephalitis (EAE), lupus nephritis and collagen induced osteoarthritis.
The number of citations for the publication including the scoring system varied between few citations and up to 2176. The citation number clearly reflects the value of the scientific work shown in the papers and thus also indirectly reflects the quality of the included scoring systems. In some cases there is even clear evidence that the high citation number is directly based on the “gold” standard character of the scoring system and its regular use in mouse models or human tissues, for instance the score from Mankin et al. for osteoarthritis evaluation and the scores developed by Knodell and Ishak et al. for chronic hepatitis or the score by Cooper et al. for DSS-colitis [4,28,106,107]. Nevertheless, after careful analysis of the publication it also became obvious that scoring systems in publications with a small number of citation also proofed to be of expedience for certain scientific question.
Assuming that the main function of scoring systems is the analysis of the influences of experimental factors on the microscopical tissue morphology the selected parameters should be consciously chosen to be able to reflect the potential changes. The lack of a rationale for the selection of the parameters was therefore an emerging and surprising finding during the literature search for this study. Although the selection of parameters in most scoring systems is comprehensibly based on the common knowledge on the pathogenesis of the disease modeled in the mouse, there is only rarely a clear statement or a line of argument for choosing a parameter. Even less often the correlation between scoring parameter value and the magnitude of the clinical symptoms or the differences in the extent of the experimental factor is given as for instance in the excellent study of Cooper et al. [4]. This lack is most probably due to the time-consuming work involved, but it may however tremendously increase the value and the scientific merit of the scoring system.
Conclusion
In summary, a final judgment of the quality and the usefulness of the scoring systems presented was not an aim of this study and is after all most probably not possible since the value of a scoring system clearly depends on the scientific question, the underlying hypothesis, the model characteristics and the pathogenesis of the disease. This review may however give an overview on currently available scoring systems and may therefore allow for a better choice for the respective project.
Methods
Selection of scoring systems
The systematic review was prepared according to the PRISMA guidelines [157]. All items were considered and can be viewed in Additional file 1. Scoring systems were identified by a comprehensive Pubmed search (http://www.ncbi.nlm.nih.gov/pubmed/) using a combination of the search terms “mouse”, “score”, “histopathology”. This led to the identification of 1479 publication by October 30, 2012 (Figure 1). Full text versions of all publications were obtained and analyzed for the description of multiparametric, semiquantitative, scoring systems for the histopathology of mouse models. Inclusion of a mouse scoring system in this overview was based on the fulfillment of six parameters.
First, the scoring system had to be based on the semiquantitative evaluation of histopathologic changes in murine tissues. Thus, approaches using digital image analysis for absolute quantification of lesion area, cell number or immunohistochemical signals or scoring systems with dominance of immunohistochemical markers as evaluation parameters were not included.
Second, only scoring systems evaluating more than one histomorphologic parameter were included in the review. Nevertheless, scoring systems with high citation numbers which combined several parameters in a uniparametric score were also included. For instance, if a highly cited scoring system integrated the presence and extent of crypt abscesses, epithelial sloughing and submucosal infiltration into a single score of 0 to 4 the study was also included.
Third, the scoring approach had to be comprehensibly described to allow for reproduction by the reader.
Fourth, the scoring system had to be originally designed for the presented study without citation of former publications. If former publications were cited as the source of the scoring system, the string of citations was followed back to the study originally describing the scoring systems. If scorings systems were not referenced to older studies but similar approaches were detected in earlier publication, only the older study was included in this review.
Fifth, the scoring systems were generally grouped by the organ affected and analyzed. Systemic diseases and transplantation models were included in separate groups. If the number of identified scoring systems for a specific disease model exceeded ten, only the then most cited scoring systems were included in this review.
Citation number was obtained using Thomson Reuters Web Science© (http://apps.webofknowledge.com).
Competing interests
The author declares that he has no competing interests.
Author’s contributions
RK had the idea of the project, performed the internet search, the data analysis and wrote the abstract.
Supplementary Material
PRISMA 2009 Checklist.
Acknowledgments
This study was supported by the FCT grant BD/43731/2008 and the DFG grant KL 2240/1-1. Literature search was tremenduously supported by Camillo Krawczyk, Freie Universität Berlin.
References
- Renshaw AA, Gould EW. Measuring errors in surgical pathology in real-life practice - Defining what does and does not matter. Am J Clin Pathol. 2007;127(1):144–152. doi: 10.1309/5KF89P63F4F6EUHB. [DOI] [PubMed] [Google Scholar]
- Riber-Hansen R, Vainer B, Steiniche T. Digital image analysis: a review of reproducibility, stability and basic requirements for optimal results. APMIS. 2012;120(4):276–289. doi: 10.1111/j.1600-0463.2011.02854.x. [DOI] [PubMed] [Google Scholar]
- Boyce RW, Dorph-Petersen KA, Lyck L, Gundersen HJG. Design-based Stereology: Introduction to Basic Concepts and Practical Approaches for Estimation of Cell Number. Toxicol Pathol. 2010;38(7):1011–1025. doi: 10.1177/0192623310385140. [DOI] [PubMed] [Google Scholar]
- Cooper HS, Murthy SNS, Shah RS, Sedergran DJ. Clinicopathological Study of Dextran Sulfate Sodium Experimental Murine Colitis. Lab Invest. 1993;69(2):238–249. [PubMed] [Google Scholar]
- Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran Sulfate Sodium-Induced Colitis Occurs in Severe Combined Immunodeficient Mice. Gastroenterology. 1994;107(6):1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Palmen MJHJ, Akol H, Bloemena E, Pena AS, Meuwissen SGM, van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114(3):385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SNS, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of Dextran Sulfate Sodium-Induced Murine Colitis by Intracolonic Cyclosporine. Digest Dis Sci. 1993;38(9):1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- Mahler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, Sundberg JP. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol-Gastr L. 1998;274(3):G544–G551. doi: 10.1152/ajpgi.1998.274.3.G544. [DOI] [PubMed] [Google Scholar]
- Hudert CA, Weylandt KH, Lu Y, Wang JD, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. P Natl Acad Sci USA. 2006;103(30):11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116(2):238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G, Bidlingmaier C, Siegmund B, Albrich S, Schulze J, Tschoep K, Eigler A, Lehr HA, Endres S. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J Pharmacol Exp Ther. 2000;292(1):22–30. [PubMed] [Google Scholar]
- Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to Interleukin-12 Abrogate Established Experimental Colitis in Mice. J Exp Med. 1995;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T, Fujihashi K, Rennert PD, Iwatani K, Kiyono H, McGhee JR. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J Exp Med. 1999;189(8):1169–1179. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard CB, Wallace JL. Reactivation of Hapten-Induced Colitis and Its Prevention by Antiinflammatory Drugs. Am J Physiol-Gastr L. 1995;269(1):G119–G125. doi: 10.1152/ajpgi.1995.269.1.G119. [DOI] [PubMed] [Google Scholar]
- Macpherson BR, Pfeiffer CJ. Experimental Production of Diffuse Colitis in Rats. Digestion. 1978;17(2):135–150. doi: 10.1159/000198104. [DOI] [PubMed] [Google Scholar]
- ten Hove T, van den Blink B, Pronk I, Drillenburg P, Peppelenbosch MP, van Deventer SJH. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50(4):507–512. doi: 10.1136/gut.50.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta(2) microglobulin transgenic rats. J Clin Invest. 1996;98(4):945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116(5):1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, ThompsonSnipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98(4):1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology. 2001;121(6):1428–1436. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- Houpt ER, Glembocki DJ, Obrig TG, Moskaluk CA, Lockhart LA, Wright RL, Seaner RM, Keepers TF, Wilkins TD, Petri WA. The mouse model of amebic colitis reveals mouse strain susceptibility to infection and exacerbation of disease by CD4(+) T cells. J Immunol. 2002;169(8):4496–4503. doi: 10.4049/jimmunol.169.8.4496. [DOI] [PubMed] [Google Scholar]
- Pothoulakis C, Castagliuolo I, Lamont JT, Jaffer A, Okeane JC, Snider RM, Leeman SE. Cp-96,345, a Substance-P Antagonist, Inhibits Rat Intestinal Responses to Clostridium-Difficile Toxin-a but Not Cholera-Toxin. P Natl Acad Sci USA. 1994;91(3):947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PO, Haglund U, Bulkley GB, Falt K. The Sequence of Development of Intestinal Tissue-Injury after Strangulation Ischemia and Reperfusion. Surgery. 1990;107(5):574–580. [PubMed] [Google Scholar]
- Lane JS, Todd KE, Lewis MPN, Gloor B, Ashley SW, Reber HA, McFadden DW, Chandler CF. Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery. 1997;122(2):288–294. doi: 10.1016/s0039-6060(97)90020-9. [DOI] [PubMed] [Google Scholar]
- de Koning BAE, van Dieren JM, Lindenbergh-Kortleve DJ, van der Sluis M, Matsumoto T, Yamaguchi K, Einerhand AW, Samsom JN, Pieters R, Nieuwenhuis EES. Contributions of mucosal immune cells to methotrexate-induced mucositis. Int Immunol. 2006;18(6):941–949. doi: 10.1093/intimm/dxl030. [DOI] [PubMed] [Google Scholar]
- Stewart-Tull DES, Coote JG, Thompson DH, Candlish D, Wardlaw AC, Candlish A. Virulence spectra of typed strains of Campylobacter jejuni from different sources: a blinded in vivo study. J Med Microbiol. 2009;58(5):546–553. doi: 10.1099/jmm.0.005611-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Sturegard E, Rupar R, Nilsson HO, Aleljung PA, Carlen B, Willen R, Wadstrom T. Infection of BALB/c a mice by spiral and coccoid forms of Helicobacter pylori. J Med Microbiol. 1997;46(8):657–663. doi: 10.1099/00222615-46-8-657. [DOI] [PubMed] [Google Scholar]
- Mankin HJ, Dorfman H, Lippiell L, Zarins A. Biochemical and Metabolic Abnormalities in Articular Cartilage from Osteo-Arthritic Human Hips .2. Correlation of Morphology with Biochemical and Metabolic Data. J Bone Joint Surg Am. 1971;53(3):523. [PubMed] [Google Scholar]
- Williams RO, Feldmann M, Maini RN. Antitumor Necrosis Factor Ameliorates Joint Disease in Murine Collagen-Induced Arthritis. P Natl Acad Sci USA. 1992;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, Haupl T. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49(4):358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- Joosten LAB, Helsen MMA, Saxne T, van de Loo FAJ, Heinegard D, van den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163(9):5049–5055. [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Laceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7(5):563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Adkison AM, Raptis SZ, Kelley DG, Pham CTN. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109(3):363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendele A, McComb J, Gould T, McAbee T, Sennello G, Chlipala E, Guy M. Animal models of arthritis: Relevance to human disease. Toxicol Pathol. 1999;27(1):134–142. doi: 10.1177/019262339902700125. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Kohsaka H, Inoue N, Terada Y, Ito H, Hirokawa K, Miyasaka N. Induction of the p16(INK4a) senescence gene as a new therapeutic strategy for the treatment of rheumatoid arthritis. Nat Med. 1999;5(7):760–767. doi: 10.1038/10480. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Szabo A, Nemeth J, Jakab B, Pinter E, Banvolgyi A, Kereskai L, Keri G, Szolcsanyi J. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund's adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50(5):1677–1685. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- Alten R, Gram H, Joosten LA, van den Berg WB, Sieper J, Wassenberg S, Burmester G, van Riel P, Diaz-Lorente M, Bruin GJ. et al. The human anti-IL-1 beta monoclonal antibody ACZ885 is effective in joint inflammation models in mice and in a proof-of-concept study in patients with rheumatoid arthritis. Arthritis Res Ther. 2008;10(3):R67. doi: 10.1186/ar2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolphi K, Gerwin N, Verzijl N, van der Kraan P, van den Berg W. Pralnacasan, an inhibitor of interleukin-1 beta converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthr Cartilage. 2003;11(10):738–746. doi: 10.1016/s1063-4584(03)00153-5. [DOI] [PubMed] [Google Scholar]
- Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, Uchida M, Ogata N, Seichi A, Nakamura K. et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartilage. 2005;13(7):632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Huebner JL, Hanest MA, Beekman B, TeKoppele JM, Kraus VB. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthr Cartilage. 2002;10(10):758–767. doi: 10.1053/joca.2002.0821. [DOI] [PubMed] [Google Scholar]
- Bremell T, Abdelnour A, Tarkowski A. Histopathological and Serological Progression of Experimental Staphylococcus-Aureus Arthritis. Infect Immun. 1992;60(7):2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76(4):579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- O'Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68(7):1017–1035. [PubMed] [Google Scholar]
- Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1997;79(10):1452–1463. doi: 10.2106/00004623-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84(2):276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat (Basel) 1992;143(4):335–340. doi: 10.1159/000147272. [DOI] [PubMed] [Google Scholar]
- Austin HA, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, Decker JL, Balow JE. Prognostic Factors in Lupus Nephritis - Contribution of Renal Histologic Data. Am J Med. 1983;75(3):382–391. doi: 10.1016/0002-9343(83)90338-8. [DOI] [PubMed] [Google Scholar]
- Wang BY, Yamamoto Y, El-Badri NS, Good RA. Effective treatment of autoimmune disease and progressive renal disease by mixed bone-marrow transplantation that establishes a stable mixed chimerism in BXSB recipient mice. P Natl Acad Sci USA. 1999;96(6):3012–3016. doi: 10.1073/pnas.96.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Ono M, Qu WM, Zhang MC, Mori S, Nakatsuru S, Nakamura Y, Sawasaki T, Endo Y, Nose M. Implication of allelic polymorphism of osteopontin in the development of lupus nephritis in MRL/lpr mice. Eur J Immunol. 2005;35(5):1510–1520. doi: 10.1002/eji.200425672. [DOI] [PubMed] [Google Scholar]
- Keil DE, Peden-Adams MM, Wallace S, Ruiz P, Gilkeson GS. Assessment of trichloroethylene (TCE) exposure in murine strains genetically-prone and non-prone to develop autoimmune disease. J Environ Sci Heal A. 2009;44(5):443–453. doi: 10.1080/10934520902719738. [DOI] [PubMed] [Google Scholar]
- Chen SM, Mukoyama T, Sato N, Yamagata SI, Arai Y, Satoh N, Ueda S. Induction of nephrotoxic serum nephritis in inbred mice and suppressive effect of colchicine on the development of this nephritis. Pharmacol Res. 2002;45(4):319–324. doi: 10.1006/phrs.2002.0948. [DOI] [PubMed] [Google Scholar]
- Zheng ZY, Schmidt-Ott KM, Chua S, Foster KA, Frankel RZ, Pavlidis P, Barasch J, D'Agati VD, Gharavi AG. A Mendelian locus on chromosome 16 determines susceptibility to doxorubicin nephropathy in the mouse. P Natl Acad Sci USA. 2005;102(7):2502–2507. doi: 10.1073/pnas.0409786102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij L, Azar S, Keane W. Mesangial Immune Injury, Hypertension, and Progressive Glomerular Damage in Dahl Rats. Kidney Int. 1984;26(2):137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- Bader R, Bader H, Grund KE, Mackensenhaen S, Christ H, Bohle A. Structure and Function of the Kidney in Diabetic Glomerulosclerosis Correlations between Morphological and Functional Parameters. Pathol Res Pract. 1980;167(2–4):204–216. doi: 10.1016/S0344-0338(80)80051-3. [DOI] [PubMed] [Google Scholar]
- Yabuki A, Tahara T, Taniguchi K, Matsumoto M, Suzuki S. Neuronal nitric oxide synthase and cyclooxygenase-2 in diabetic nephropathy of type 2 diabetic OLETF rats. Exp Anim Tokyo. 2006;55(1):17–25. doi: 10.1538/expanim.55.17. [DOI] [PubMed] [Google Scholar]
- Lai PC, Smith J, Bhangal G, Chaudhry KA, Chaudhry AN, Keith JC, Tam FWK, Pusey CD, Cook HT. Interleukin-11 reduces renal injury and glomerular NF-Kappa B activity in murine experimental glomerulonephritis. Nephron Exp Nephrol. 2005;101(4):E146–E154. doi: 10.1159/000087938. [DOI] [PubMed] [Google Scholar]
- Watson ML, Rao JK, Gilkeson GS, Ruiz P, Eicher EM, Pisetsky DS, Matsuzawa A, Rochelle JM, Seldin MF. Genetic-Analysis of Mrl-Lpr Mice - Relationship of the Fas Apoptosis Gene to Disease Manifestations and Renal Disease-Modifying Loci. J Exp Med. 1992;176(6):1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumura W, Sugino N, Nagasawa R, Kubo S, Hirokawa K, Maruyama N. Age-Associated Changes in Renal Glomeruli of Mice. Exp Gerontol. 1989;24(3):237–249. doi: 10.1016/0531-5565(89)90015-6. [DOI] [PubMed] [Google Scholar]
- Gharavi AG, Ahmad T, Wong RD, Hooshyar R, Vaughn J, Oller S, Frankel RZ, Bruggeman LA, D'Agati VD, Klotman PE. et al. Mapping a locus for susceptibility to HIV-1-associated nephropathy to mouse chromosome 3. P Natl Acad Sci USA. 2004;101(8):2488–2493. doi: 10.1073/pnas.0308649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, Yasuda K, Ratliff B, Stoessel A, Sharkovska Y, Yamamoto I, Jasmin JF, Bachmann S, Lisanti MP, Chander P. et al. Postobstructive regeneration of kidney is derailed when surge in renal stem cells during course of unilateral ureteral obstruction is halted. Am J Physiol-Renal. 2010;298(2):F357–F364. doi: 10.1152/ajprenal.00542.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli WA, Levy DE, Duffy TE. Regional Cerebral Blood-Flow and Glucose-Metabolism Following Transient Forebrain Ischemia. Ann Neurol. 1982;11(5):499–509. doi: 10.1002/ana.410110510. [DOI] [PubMed] [Google Scholar]
- Barber PA, Hoyte L, Colbourne F, Buchan AM. Temperature-regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke. 2004;35(7):1720–1725. doi: 10.1161/01.STR.0000129653.22241.d7. [DOI] [PubMed] [Google Scholar]
- Thoresen M, Bagenholm R, Loberg EM, Apricena F, Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child. 1996;74(1):F3–F9. doi: 10.1136/fn.74.1.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810(1–2):114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- Iida H, Schmelzer JD, Schmeichel AM, Wang Y, Low PA. Peripheral nerve ischemia: reperfusion injury and fiber regeneration. Exp Neurol. 2003;184(2):997–1002. doi: 10.1016/S0014-4886(03)00385-6. [DOI] [PubMed] [Google Scholar]
- Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160(6):2940–2946. [PubMed] [Google Scholar]
- Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, Slavin S, Abramsky O, Karussis D. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol-Chicago. 2008;65(6):753–761. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Teuscher C. Identification of genetic loci controlling the characteristics and severity of brain and spinal cord lesions in experimental allergic encephalomyelitis. Am J Pathol. 2000;157(2):637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PD, McGavern DB, Lin XQ, Njenga MK, Leibowitz J, Pease LR, Rodriguez M. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J Neurosci. 1998;18(18):7306–7314. doi: 10.1523/JNEUROSCI.18-18-07306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JJ, Du CG, Coon M, Sriram S, Klaus SJ. Prevention of experimental allergic encephalomyelitis via inhibition IL-12 signaling and IL-12-mediated Th1 differentiation: An effect of the novel anti-inflammatory drug lisofylline. J Immunol. 1998;161(12):7015–7022. [PubMed] [Google Scholar]
- Soilu-Hanninen M, Roytta M, Salmi A, Salonen R. Therapy with antibody against leukocyte integrin VLA-4 (CD49d) is effective and safe in virus-facilitated experimental allergic encephalomyelitis. J Neuroimmunol. 1997;72(1):95–105. doi: 10.1016/s0165-5728(96)00158-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Cui YS, Chen JL, Lu M, Elias SB, Chopp M. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034(1–2):34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Kuang LQ, Theil DJ, Fujinami RS. Antibody association with a novel model for primary progressive multiple sclerosis: Induction of relapsing-remitting and progressive forms of EAE in H2(s) mouse strains. Brain Pathol. 2000;10(3):402–418. doi: 10.1111/j.1750-3639.2000.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZQ, Hu XQ, Zhu CS, Wang DJ, Zheng XP, Liu QT. Overexpression of CNTF in Mesenchymal Stem Cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. J Neuroimmunol. 2009;206(1–2):58–69. doi: 10.1016/j.jneuroim.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Sirin BH, Ortac R, Cerrahoglu M, Saribulbul O, Baltalarli A, Celebisoy N, Iskesen I, Rendeci O. Ischaemic preconditioning reduces spinal cord injury in transient ischaemia. Acta Cardiol. 2002;57(4):279–285. doi: 10.2143/AC.57.4.2005427. [DOI] [PubMed] [Google Scholar]
- Sheng HX, Spasojevic I, Warner DS, Batinic-Haberle I. Mouse spinal cord compression injury is ameliorated by intrathecal cationic manganese (III) porphyrin catalytic antioxidant therapy. Neurosci Lett. 2004;366(2):220–225. doi: 10.1016/j.neulet.2004.05.050. [DOI] [PubMed] [Google Scholar]
- Gerber J, Raivich G, Wellmer A, Noeske C, Kunst T, Werner A, Bruck W, Nau R. A mouse model of Streptococcus pneumoniae meningitis mimicking several features of human disease. Acta Neuropathol. 2001;101(5):499–508. doi: 10.1007/s004010000326. [DOI] [PubMed] [Google Scholar]
- Kennedy PGE, Rodgers J, Jennings FW, Murray M, Leeman SE, Burke JM. A substance P antagonist, RP-67,580, ameliorates a mouse meningoencephalitic response to Trypanosoma brucei brucei. P Natl Acad Sci USA. 1997;94(8):4167–4170. doi: 10.1073/pnas.94.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RA, Jiang XN, Francisco C, Christen S, Vexler ZS, Tauber MG, Ferriero DM. Manipulation of antioxidant pathways in neonatal murine brain. Pediatr Res. 2004;56(4):656–662. doi: 10.1203/01.PDR.0000139413.27864.50. [DOI] [PubMed] [Google Scholar]
- Chan YC, Hosoda K, Tsai CJ, Yamamoto S, Wang MF. Favorable effects of tea on reducing the cognitive deficits and brain morphological changes in senescence-accelerated mice. J Nutr Sci Vitaminol (Tokyo) 2006;52(4):266–273. doi: 10.3177/jnsv.52.266. [DOI] [PubMed] [Google Scholar]
- Oz G, Nelson CD, Koski DM, Henry PG, Marjanska M, Deelchand DK, Shanley R, Eberly LE, Orr HT, Clark HB. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2010;30(10):3831–3838. doi: 10.1523/JNEUROSCI.5612-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft T, Simpson JM, Timbrell V. Simple Method of Estimating Severity of Pulmonary Fibrosis on a Numerical Scale. J Clin Pathol. 1988;41(4):467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AG, Fulford LG, Colby TV, du Bois RM, Hansell DM, Wells AU. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Resp Crit Care. 2002;166(2):173–177. doi: 10.1164/rccm.2109039. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Dorin JR, Mclachlan G, Ranaldi V, Lamb D, Doherty C, Govan J, Porteous DJ. Lung-Disease in the Cystic-Fibrosis Mouse Exposed to Bacterial Pathogens. Nat Genet. 1995;9(4):351–357. doi: 10.1038/ng0495-351. [DOI] [PubMed] [Google Scholar]
- Nishina K, Mikawa K, Takao Y, Shiga M, Maekawa N, Obara H. Intravenous lidocaine attenuates acute lung injury induced by hydrochloric acid aspiration in rabbits. Anesthesiology. 1998;88(5):1300–1309. doi: 10.1097/00000542-199805000-00022. [DOI] [PubMed] [Google Scholar]
- Makiuchi A, Yamaura K, Mizuno S, Matsumoto K, Nakamura T, Amano J, Ito KI. Hepatocyte growth factor prevents pulmonary ischernia-reperfusion injury in mice. J Heart Lung Transpl. 2007;26(9):935–943. doi: 10.1016/j.healun.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Yang YL, Tang GJ, Wu YL, Yien HW, Lee TS, Kou YR. Exacerbation of wood smoke-induced acute lung injury by mechanical ventilation using moderately high tidal volume in mice. Resp Physiol Neurobi. 2008;160(1):99–108. doi: 10.1016/j.resp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Wohlford-Lenane C, Chulada P, Bradbury JA, Scarborough PE, Roggli V, Langenbach R, Schwartz DA. Airway inflammation and responsiveness in prostaglandin H synthase-deficient mice exposed to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2001;25(4):457–465. doi: 10.1165/ajrcmb.25.4.4505. [DOI] [PubMed] [Google Scholar]
- Curtis JL, Byrd PK, Warnock ML, Kaltreider HB. Requirement of Cd4-Positive T-Cells for Cellular Recruitment to the Lungs of Mice in Response to a Particulate Intratracheal Antigen. J Clin Invest. 1991;88(4):1244–1254. doi: 10.1172/JCI115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveillard M, Soltner C, Kempf M, Saint-Andre JP, Lemarie C, Randrianarivelo C, Seifert H, Wolff M, Joly-Guillou ML. The virulence variability of different Acinetobacter baumannii strains in experimental pneumonia. J Infection. 2010;60(2):154–161. doi: 10.1016/j.jinf.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Barends M, van Oosten M, de Rond CGH, Dormans JAMA, Osterhaus ADME, Neijens HJ, Kimman TG. Timing of infection and prior immunization with respiratory syncytial virus (RSV) in RSV-enhanced allergic inflammation. J Infect Dis. 2004;189(10):1866–1872. doi: 10.1086/386341. [DOI] [PubMed] [Google Scholar]
- Cimolai N, Taylor GP, Mah D, Morrison BJ. Definition and Application of a Histopathological Scoring Scheme for an Animal-Model of Acute Mycoplasma-Pneumoniae Pulmonary Infection. Microbiol Immunol. 1992;36(5):465–478. doi: 10.1111/j.1348-0421.1992.tb02045.x. [DOI] [PubMed] [Google Scholar]
- Beck JM, Warnock ML, Curtis JL, Sniezek MJ, Arraj-Peffer SM, Kaltreider HB, Shellito JE. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am J Respir Cell Mol Biol. 1991;5(2):186–197. doi: 10.1165/ajrcmb/5.2.186. [DOI] [PubMed] [Google Scholar]
- Lammers AJ, de Porto AP, de Boer OJ, Florquin S, van der Poll T. The role of TLR2 in the host response to pneumococcal pneumoniain absence of the spleen. BMC Infect Dis. 2012;12(1):139. doi: 10.1186/1471-2334-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R, Liu P, Wee L, Butany J, Sole MJ. Verapamil Ameliorates the Clinical and Pathological Course of Murine Myocarditis. J Clin Invest. 1992;90(5):2022–2030. doi: 10.1172/JCI116082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Kishimoto C, Kurokawa M, Ochiai H, Sasayama S. Effects of Polyethylene-Glycol Conjugated Superoxide-Dismutase on Coxsackievirus B3 Myocarditis in Mice. Cardiovasc Res. 1992;26(10):956–961. doi: 10.1093/cvr/26.10.956. [DOI] [PubMed] [Google Scholar]
- Wang YX, Da Cunha V, Vincelette J, White K, Velichko S, Xu Y, Gross C, Fitch RM, Halks-Miller M, Larsen BR. et al. Antiviral and myocyte protective effects of murine interferon-beta and -alpha 2 in coxsackievirus B3-induced myocarditis and epicarditis in Balb/c mice. Am J Physiol-Heart C. 2007;293(1):H69–H76. doi: 10.1152/ajpheart.00154.2007. [DOI] [PubMed] [Google Scholar]
- Kanda T, Araki M, Nakano M, Imai S, Suzuki T, Murata K, Kobayashi I. Chronic Effect of Losartan in a Murine Model of Dilated Cardiomyopathy - Comparison with Captopril. J Pharmacol Exp Ther. 1995;273(2):955–958. [PubMed] [Google Scholar]
- Rahman A, More N, Schein PS. Doxorubicin-Induced Chronic Cardiotoxicity and Its Protection by Liposomal Administration. Cancer Res. 1982;42(5):1817–1825. [PubMed] [Google Scholar]
- Ghazalpour A, Wang XP, Lusis AJ, Mehrabian M. Complex inheritance of the 5-lipoxygenase locus influencing atherosclerosis in mice. Genetics. 2006;173(2):943–951. doi: 10.1534/genetics.106.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BJ, Wang SS, Wang XP, Wu XH, Lusis AJ. Genetic Regulation of Atherosclerotic Plaque Size and Morphology in the Innominate Artery of Hyperlipidemic Mice. Arterioscl Throm Vas. 2009;29(3):348–A318. doi: 10.1161/ATVBAHA.108.176685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Miyazaki T, Lu LM, Ono M, Ito MR, Terada M, Mori S, Hata K, Nozaki Y, Nakatsuru S. et al. Genetic basis of tissue specificity of vasculitis in MRL/lpr mice. Arthritis Rheum. 2003;48(5):1445–1451. doi: 10.1002/art.10952. [DOI] [PubMed] [Google Scholar]
- Carter WO, Bull C, Bortolon E, Yang L, Jesmok GJ, Gundel RH. A murine skeletal muscle ischemia-reperfusion injury model: differential pathology in BALB/c and DBA/2N mice. J Appl Physiol. 1998;85(5):1676–1683. doi: 10.1152/jappl.1998.85.5.1676. [DOI] [PubMed] [Google Scholar]
- Nascentes GAN, Meira WSF, Lages-Silva E, Ramirez LE. Immunization of Mice with a Trypanosoma cruzi-Like Strain Isolated from a Bat: Predictive Factors for Involvement of Eosinophiles in Tissue Damage. Vector-Borne Zoonot. 2010;10(10):989–997. doi: 10.1089/vbz.2009.0185. [DOI] [PubMed] [Google Scholar]
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and Application of a Numerical Scoring System for Assessing Histological Activity in Asymptomatic Chronic Active Hepatitis. Hepatology. 1981;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- Chevallier M, Guerret S, Chossegros P, Gerard F, Grimaud JA. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology. 1994;20(2):349–355. [PubMed] [Google Scholar]
- Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G. Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol. 2002;32(2):552–560. doi: 10.1002/1521-4141(200202)32:2<552::AID-IMMU552>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- VanNieuwkerk CMJ, Elferink RPJO, Groen AK, Ottenhoff R, Tytgat GNJ, Dingemans KP, Weerman MAVB, Offerhaus GJA. Effects of ursodeoxycholate and cholate feeding on liver disease in FVB mice with a disrupted mdr2 P-glycoprotein gene. Gastroenterology. 1996;111(1):165–171. doi: 10.1053/gast.1996.v111.pm8698195. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Khettry U, Sadrzadeh SMH. Lactobacillus Feeding Reduces Endotoxemia and Severity of Experimental Alcoholic Liver (Disease) P Soc Exp Biol Med. 1994;205(3):243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- Curran TJ, Uzoaru I, Das JB, Ansari G, Raffensperger JG. The Effect of Cholecystokinin-Octapeptide on the Hepatobiliary Dysfunction Caused by Total Parenteral-Nutrition. J Pediatr Surg. 1995;30(2):242–247. doi: 10.1016/0022-3468(95)90568-5. [DOI] [PubMed] [Google Scholar]
- Sancho-Bru P, Bataller R, Fernandez-Varo G, Moreno M, Ramalho LN, Colmenero J, Mari M, Claria J, Jimenez W, Arroyo V. et al. Bradykinin attenuates hepatocellular damage and fibrosis in rats with chronic liver injury. Gastroenterology. 2007;133(6):2019–2028. doi: 10.1053/j.gastro.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Cavalieri B, Perrelli MG, Aragno M, Ramadori P, Poli G, Cutrin JC. Ischaemic preconditioning modulates the activity of Kupffer cells during in vivo reperfusion injury of rat liver. Cell Biochem Funct. 2003;21(4):299–305. doi: 10.1002/cbf.1028. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A Better Model of Acute-Pancreatitis for Evaluating Therapy. Ann Surg. 1992;215(1):44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112(3):960–967. doi: 10.1053/gast.1997.v112.pm9041259. [DOI] [PubMed] [Google Scholar]
- Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120(2):284–289. doi: 10.1016/s0039-6060(96)80299-6. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Centorrino T, Ciccolo A, Van de Loo FAJ, Britti D, Caputi AP, Thiemermann C. Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein. Shock. 2002;17(5):416–422. doi: 10.1097/00024382-200205000-00013. [DOI] [PubMed] [Google Scholar]
- Dembinski A, Warzecha Z, Ceranowicz P, Dembinski M, Cieszkowski J, Pawlik WW, Tomaszewska R, Konturek SJ, Konturek PC. Effect of ischemic preconditioning on pancreatic regeneration and pancreatic expression of vascular endothelial growth factor and platelet-derived growth factor-A in ischemia/reperfusion-induced pancreatitis. J Physiol Pharmacol. 2006;57(1):39–58. [PubMed] [Google Scholar]
- Kanno H, Nose M, Itoh J, Taniguchi Y, Kyogoku M. Spontaneous Development of Pancreatitis in the Mrl/Mp Strain of Mice in Autoimmune Mechanism. Clin Exp Immunol. 1992;89(1):68–73. doi: 10.1111/j.1365-2249.1992.tb06879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaccio G, Nicoletti F, Pisanti FA, Bendtzen K, Galdieri M. Prevention of spontaneous autoimmune diabetes in NOD mice by transferring in vitro antigen-pulsed syngeneic dendritic cells. Endocrinology. 2000;141(4):1500–1505. doi: 10.1210/endo.141.4.7437. [DOI] [PubMed] [Google Scholar]
- Baker BS, Brent L, Valdimarsson H, Powles AV, Alimara L, Walker M, Fry L. Is Epidermal-Cell Proliferation in Psoriatic Skin-Grafts on Nude-Mice Driven by T-Cell Derived Cytokines. Brit J Dermatol. 1992;126(2):105–110. doi: 10.1111/j.1365-2133.1992.tb07805.x. [DOI] [PubMed] [Google Scholar]
- Kvist PH, Svensson L, Hagberg O, Hoffmann V, Kemp K, Ropke MA. Comparison of the effects of vitamin D products in a psoriasis plaque test and a murine psoriasis xenograft model. J Transl Med. 2009;7:107. doi: 10.1186/1479-5876-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Thode HC, McClain SA. Development of a histomorphologic scale to quantify cutaneous scars after burns. Acad Emerg Med. 2000;7(10):1083–1088. doi: 10.1111/j.1553-2712.2000.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Takagawa S, Katayama I, Mizushima Y, Nishioka K. Effect of superoxide dismutase on bleomycin-induced dermal sclerosis: Implications for the treatment of systemic sclerosis. J Invest Dermatol. 1999;113(5):843–847. doi: 10.1046/j.1523-1747.1999.00758.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hamada K, Tategaki A, Kishida H, Tanaka H, Kitano M, Miyamoto T. Oral Administration of Lactic Acid Bacteria Isolated from Traditional South Asian Fermented Milk 'Dahi' Inhibits the Development of Atopic Dermatitis in NC/Nga Mice. J Nutr Sci Vitaminol (Tokyo) 2009;55(3):271–278. doi: 10.3177/jnsv.55.271. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim JS, Song MS, Seo HS, Moon CJ, Kim JC, Jo SK, Jang JS, Kim SH. Photoprotective Effect of Red Ginseng against Ultraviolet Radiation-induced Chronic Skin Damage in the Hairless Mouse. Phytother Res. 2009;23(3):399–403. doi: 10.1002/ptr.2640. [DOI] [PubMed] [Google Scholar]
- Hjortsjo C, Saxegaard E, Young A, Dahl JE. In vivo and in vitro irritation testing of low concentrations of hydrofluoric acid. Acta Odontol Scand. 2009;67(6):360–365. doi: 10.1080/00016350903117118. [DOI] [PubMed] [Google Scholar]
- Gubitosi-Klug RA, Talahalli R, Du YP, Nadler JL, Kern TS. 5-lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes. 2008;57(5):1387–1393. doi: 10.2337/db07-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins RD, Yu K, Sanders RJ, Nandgaonkar BN, Rotschild T, Rifkin DB. Diltiazem reduces retinal neovascularization in a mouse model of oxygen induced retinopathy. Curr Eye Res. 1999;18(1):20–27. doi: 10.1076/ceyr.18.1.20.5390. [DOI] [PubMed] [Google Scholar]
- Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31(11):955–965. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z, Nussenblatt RB. A New Model of Autoimmune-Disease - Experimental Autoimmune Uveoretinitis Induced in Mice with 2 Different Retinal Antigens. J Immunol. 1988;140(5):1490–1495. [PubMed] [Google Scholar]
- Cousins SW, Guss RB, Howes EL, Rosenbaum JT. Endotoxin-Induced Uveitis in the Rat - Observations on Altered Vascular-Permeability, Clinical Findings, and Histology. Exp Eye Res. 1984;39(5):665–676. doi: 10.1016/0014-4835(84)90065-4. [DOI] [PubMed] [Google Scholar]
- Tyrell J, Silberman H, Chandrasoma P, Niland J, Shull J. Absorbable Versus Permanent Mesh in Abdominal Operations. Surg Gynecol Obstet. 1989;168(3):227–232. [PubMed] [Google Scholar]
- Corona R, Verguts J, Schonman R, Binda MM, Mailova K, Koninckx PR. Postoperative inflammation in the abdominal cavity increases adhesion formation in a laparoscopic mouse model. Fertil Steril. 2011;95(4):1224–1228. doi: 10.1016/j.fertnstert.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Suga H, Teraoka S, Ota K, Komemushi S, Furutani S, Yamauchi S, Margolin S. Preventive effect of pirfenidone against experimental sclerosing peritonitis in rats. Exp Toxicol Pathol. 1995;47(4):287–291. doi: 10.1016/s0940-2993(11)80261-7. [DOI] [PubMed] [Google Scholar]
- Brown NA, Fabro S. Quantitation of Rat Embryonic-Development Invitro - a Morphological Scoring System. Teratology. 1981;24(1):65–78. doi: 10.1002/tera.1420240108. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. Pdgf and Fgf Stimulate Wound-Healing in the Genetically Diabetic Mouse. Am J Pathol. 1990;136(6):1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H, Sharp GC, Medling B, Tang HW. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12(3):157–165. doi: 10.1006/jaut.1999.0272. [DOI] [PubMed] [Google Scholar]
- Eckstein P, Jackson MCN, Millman N, Sobrero AJ. Comparison of Vaginal Tolerance Tests of Spermicidal Preparations in Rabbits and Monkeys. J Reprod Fertil. 1969;20(1):85. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- Poplawski C, Sosnowski D, Szaflarska-Poplawska A, Sarosiek J, McCallum R, Bartuzi Z. Role of bile acids, prostaglandins and COX inhibitors in chronic esophagitis in a mouse model. World J Gastroentero. 2006;12(11):1739–1742. doi: 10.3748/wjg.v12.i11.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A, Hewicker-Trautwein M, Erdmann N, Angrisani N, Reifenrath J, Meyer-Lindenberg A. Comparison of morphological changes in efferent lymph nodes after implantation of resorbable and non-resorbable implants in rabbits. Biomed Eng Online. 2011;10:32. doi: 10.1186/1475-925X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen SG. Testicular biopsy score count a method for registration of spermatogenesis in human testes. Normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan LY, Ferrara JLM. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90(8):3204–3213. [PubMed] [Google Scholar]
- Cooke KR, Hill GR, Crawford JM, Bungard D, Brinson YS, Delmonte J, Ferrara JLM. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102(10):1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173(9):5467–5475. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- Murao Y, Loomis W, Wolf P, Hoyt DB, Junger WG. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock. 2003;20(1):29–34. doi: 10.1097/01.shk.0000071060.78689.f1. [DOI] [PubMed] [Google Scholar]
- Ahmadi-Yazdi C, Williams B, Oakes S, Moore FD. Attenuation of the Effects of Rat Hemorrhagic Shock with a Reperfusion Injury-Inhibiting Agent Specific to Mice. Shock. 2009;32(3):295–301. doi: 10.1097/SHK.0b013e3181995e0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mees ST, Gwinner M, Marx K, Faendrich F, Schroeder J, Haier J, Kahlke V. Influence of sex and age on morphological organ damage after hemorrhagic shock. Shock. 2008;29(6):670–674. doi: 10.1097/shk.0b013e31815c3ea0. [DOI] [PubMed] [Google Scholar]
- Kuper CF, Harleman JH, Richter-Reichelm HB, Vos JG. Histopathologic approaches to detect changes indicative of immunotoxicity. Toxicol Pathol. 2000;28(3):454–466. doi: 10.1177/019262330002800317. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB. et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9(6):587–593. [PubMed] [Google Scholar]
- Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM. et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transpl. 2005;24(11):1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Yousem SA, Berry GJ, Brunt EM, Chamberlain D, Hruban RH, Sibley RK, Stewart S, Tazelaar HD. A Working Formulation for the Standardization of Nomenclature in the Diagnosis of Heart and Lung Rejection - Lung Rejection Study-Group. J Heart Transplant. 1990;9(6):593–601. [PubMed] [Google Scholar]
- Gaucher S, Nicco C, Jarraya M, Batteux F. Viability and Efficacy of Coverage of Cryopreserved Human Skin Allografts in Mice. Int J Low Extr Wound. 2010;9(3):132–140. doi: 10.1177/1534734610380024. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Checklist.