Abstract
ABT-199 is a new selective small molecule inhibitor of BCL-2 that appears to spare platelets while achieving potent antitumor activity. Assays that can predict the efficacy of ABT-199 in individual tumors will be critical in determining how best to incorporate this promising agent into the armamentarium of cancer therapies.
The B-cell lymphoma/leukemia 2 (BCL-2) family regulates critical life/death decisions of cells via the mitochondrial pathway of apoptosis (Davids and Letai, 2012). BCL-2 inhibits death by binding the BH3 domains of pro-death BCL-2 family proteins and thus prevents mitochondrial outer membrane permeabilization, which can be considered the point of commitment to apoptosis. BCL-2 has several anti-apoptotic cousins, including BCL-XL and MCL-1, each of which possesses a distinct, hydrophobic BH3-binding pocket. Lymphoid malignancies are frequently addicted to BCL-2 for their survival. Since most of these cancers, including chronic lymphocytic leukemia (CLL), remain incurable with conventional therapies, agents that specifically target BCL-2 are under urgent investigation.
Early efforts to target the BCL-2 family were met with disappointment in the clinic. Agents such as the antisense oligonucleotide oblimersen sodium and the small molecule obatoclax showed promise as BCL-2 antagonists in pre-clinical testing, but had little clinical activity. A potential mechanistic shortcoming of these agents is that they were never conclusively shown to specifically engage their purported BCL-2 family targets in patients.
Abbott Laboratories (now AbbVie, Inc.) has developed a series of BH3-mimetic small molecules that bind to the BH3 binding sites of anti-apoptotic proteins like BCL-2. ABT-737, which binds BCL-2, BCL-XL and BCL-w, was the first molecule studied extensively pre-clinically (Oltersdorf et al., 2005). Many subsequent experiments support its killing in an on-target fashion in cell lines, primary human cancer cells, and animal models. ABT-263 (navitoclax) was the first of this series to enter the clinic. Like ABT-737, it bound BCL-2, BCL-XL and BCL-w, but it had the perceived advantage of being orally bioavailable. Clinical activity was observed, particularly in lymphoid cancers (Roberts et al., 2012); however, because navitoclax binds not only to BCL-2 but also to BCL-XL, the drug causes predictable, dose-dependent thrombocytopenia (Figure 1A). This is an on-target effect due to the reliance of platelets on BCL-XL for survival, and it provides pharmacodynamic evidence of the mechanism of action of the drug. Indeed, drugs claiming to inhibit BCL-XL that do not cause significant thrombocytopenia in vivo should be viewed with caution. The modest clinical activity of navitoclax is likely related to the inability to optimize its dose due to this dose-limiting thrombocytopenia.
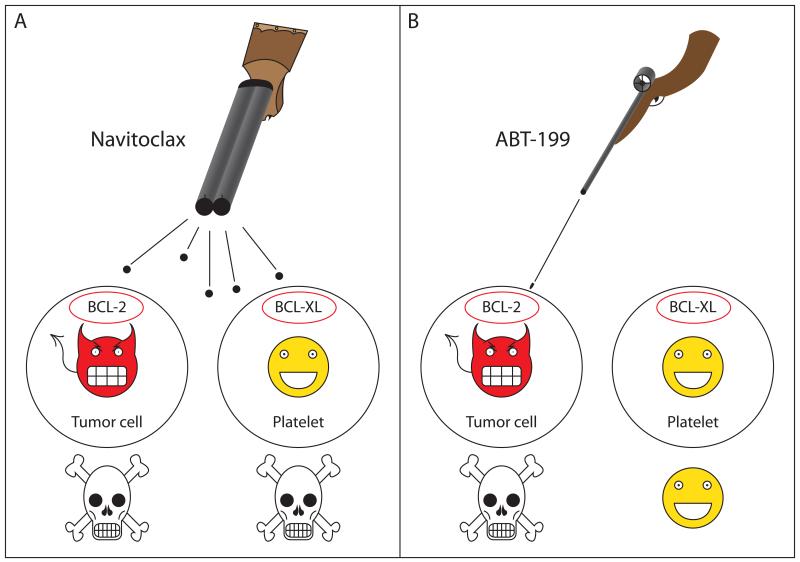
Figure 1. ABT-199 selectively kills BCL-2 dependent tumor cells while sparing platelets.
(A) Navitoclax (ABT-263) binds with high affinity to both BCL-2 and BCL-XL. Since many tumors, particularly lymphoid malignancies, are addicted to BCL-2 for survival, this potently induces tumor cell apoptosis. Platelets depend primarily on BCL-XL for survival, and therefore are also destroyed by navitoclax. (B) ABT-199 is specific for BCL-2 and induces selective death of BCL-2 dependent tumor cells while sparing platelets.
Souers and colleagues recently reported the re-engineering of navitoclax to create ABT-199, a highly potent and selective inhibitor of BCL-2 (Souers et al., 2013). Through an elegant structure-based reverse engineering process, ABT-199 maintains a sub-nanomolar affinity for BCL-2, but binds over three orders of magnitude less avidly to BCL-XL, suggesting the drug may not cause clinically significant thrombocytopenia (Figure 1B). In an in vitro cell culture model, the authors provide convincing evidence that ABT-199 selectively kills BCL-2 dependent, but not BCL-XL dependent cells and that it kills through the mitochondrial pathway of apoptosis. ABT-199 also potently kills a diverse array of non-Hodgkin lymphoma (NHL) and acute myelogenous leukemia cell lines, suggesting that the drug has the potential to be efficacious in a wide variety of hematologic malignancies. In vivo, ABT-199 suppresses tumor growth in several human hematologic tumor xenograft models and shows at least additive efficacy in combination with chemotherapy. Importantly, the authors also show compelling data from both in vitro and in vivo experiments that, as predicted, ABT-199 causes markedly less thrombocytopenia than navitoclax.
The true measure of a drug’s efficacy must come from clinical trials, and the authors provide some very preliminary but promising data in 3 CLL patients. The rapid reduction of absolute lymphocyte count in just 8 hours along with a concomitant reduction in palpable lymphadenopathy in all 3 patients suggests that the drug is more potent in CLL patients than navitoclax. The lack of thrombocytopenia in this limited data set is also a promising sign that the preclinical studies predicting less thrombocytopenia will be validated in the clinic. A similar lack of thrombocytopenia accompanied by significant anti-tumor activity was reported in an early interim analysis of a phase I study of ABT-199 in NHL patients at the 2012 American Society of Hematology Annual Meeting. Also notable in the Souers et al. article is that markedly elevated LDH and phosphate levels were seen during the same early time frame when the anti-tumor response was observed, reflecting significant tumor lysis, a clinical syndrome with potentially serious consequences if not managed aggressively. Whether modifications to the study design for future patients are adequate to reduce the risk of tumor lysis will be critical to the clinical development of ABT-199.
The use of rational biomarkers has become essential to optimizing the development of targeted cancer therapies, and ABT-199 is no exception. A challenge in developing a predictive biomarker for ABT-199 is understanding the molecular biology underlying BCL-2 addiction. The levels of over a dozen BCL-2 family proteins, both pro- and anti-death, fluctuate rapidly, and protein-protein interactions or post-translational modifications can have a major influence on the behavior of the system. Functional biomarkers are one way to probe the physiology of this dynamic system in real time. One such assay is BH3 profiling (Certo et al., 2006), which measures the mitochondrial response to different BH3-only peptides, each with its own selectivity of interaction with anti-apoptotic members. Results can be used to determine the relative dependence of a cell on a particular anti-apoptotic protein such as BCL-2, BCL-XL, or MCL-1. In many examples of primary cancer cells and cancer cell lines, BH3 profiling has correctly predicted sensitivity to ABT-737 (Del Gaizo Moore et al., 2007; Deng et al., 2007; Vo et al., 2012).
Central to the fate of a cancer cell exposed to chemotherapy is its proximity to the threshold of apoptosis, a cellular state also detected through BH3 profiling that is known as “mitochondrial priming”. Mitochondrial priming helps explain why CLL cells are generally quite sensitive to chemotherapy despite having high levels of BCL-2 protein. Most BCL-2 in primed cells is bound by pro-death BCL-2 family members such as BIM, thereby perching these cells precariously near the precipice of apoptosis. Mitochondrial priming also provides insight into the reason why chemotherapy may be effective against malignant cells while sparing hematopoietic stem cells and pretreatment priming levels in patient samples correlate with clinical response to cytotoxic chemotherapy (Davids et al., 2012; Ni Chonghaile et al., 2011; Vo et al., 2012). Agents like ABT-199 afford the exciting possibility of selectively increasing the mitochondrial priming of certain cancer cells, identified by the proper predictive biomarker, which might then be more effectively treated with conventional chemotherapy.
Clinical response to navitoclax was strongly associated with high BIM-MCL-1 or BIM-BCL-2 ratios, providing evidence of the selectivity of the drug for targeting the BCL-2 family in patients (Roberts et al., 2012). Additional correlative studies built into the ongoing clinical trials of ABT-199 include analysis of BCL2 gains, BCL2 amplification, as well as BH3 profiling. These assays have the potential to help predict the toxicities and efficacy of ABT-199, perhaps even one day to guide personalized therapy of cancer patients. Whether ABT-199 will be most effective as a single agent or in combination with other agents will be important to determine. Moreover, the biological insights gained through correlative studies will help inform the rational design of combination trials to determine the optimal role for ABT-199 in the treatment of cancer.
References
- Certo M, Moore Vdel G, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, Wilson WH, Brown JR, Letai A. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012;120:3501–3509. doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol. 2012;30:3127–3135. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. The Journal of clinical investigation. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, Carney DA, He SZ, Huang DC, Xiong H, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013 doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, Deangelo DJ, Frattini, M G, Letai A. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–355. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]