Abstract
One year after the revelation by Dr. Furchgott in 1980 that the endothelium was obligatory for acetylcholine to relax isolated arteries, it was clearly shown that the endothelium could also promote contraction. In 1988, Dr. Yanagisawa’s group identified endothelin-1 (ET-1) as the first endothelium-derived contracting factor. The circulating levels of this short (21-amino acid) peptide were quickly determined in humans, and it was reported that, in most cardiovascular diseases, circulating levels of ET-1 were increased, and ET-1 was then tagged as “a bad guy.” The discovery of two receptor subtypes in 1990, ETA and ETB, permitted optimization of the first dual ET-1 receptor antagonist in 1993 by Dr. Clozel’s team, who entered clinical development with bosentan, which was offered to patients with pulmonary arterial hypertension in 2001. The revelation of Dr. Furchgott opened a Pandora’s box with ET-1 as one of the actors. In this brief review, we will discuss the physiological and pathophysiological role of endothelium-derived ET-1 focusing on the regulation of the vascular tone, and as much as possible in humans. The coronary bed will be used as a running example in this review because it is the most susceptible to endothelial dysfunction, but references to the cerebral and renal circulation will also be made. Many of the cardiovascular complications associated with aging and cardiovascular risk factors are initially attributable, at least in part, to endothelial dysfunction, particularly dysregulation of the vascular function associated with an imbalance in the close interdependence of nitric oxide and ET-1.
Keywords: Vascular tone, Inflammation, Cardiovascular diseases
Introduction
Dr. Furchgott rediscovered an extraordinary organ, the endothelium, that protects the arterial wall through the release of nitric oxide (NO) [1, 2] among other factors. Before 1980, the endothelium was merely considered as an anticoagulant sheet of cellophane. The presence of an endothelium-derived constricting factor (EDCF) was perceived 1 year after the revelation of the endothelium-dependent relaxant properties of the endothelium [3, 4]. But, it was 8 years later that Yanagisawa and colleagues identified an EDCF, endothelin-1 (ET-1) [5, 6]. Two receptors for ET-1 were identified 2 years later [7, 8]. Then, shortly after the discovery of ETA and ETB receptors, Martine Clozel’s team presented in 1993 the first orally active endothelin-1 receptor antagonist, Ro 46-2005 [9], and the same team made a structurally modified analog, bosentan (Tracleer), available to patients with pulmonary arterial hypertension at the end of 2001. In less than 15 years, a new factor was identified, its receptors were cloned, and their pharmacology was characterized, a pathology associated with the abnormal function of the ET-1 system and an effective treatment offered to patients in need. It all started from the “so-obvious-today” rediscovery of the endothelial cells by Dr. Furchgott.
In this short review, we will focus on ET-1 and the endothelium and thus, review the role of ET-1 on the vasculature with as much as possible references to the human pathophysiology.
Physiological effects of ET-1 in arteries
Endothelin-1 is one of the most potent endogenous vasoconstrictor identified so far [6]. This peptide induces a long-lasting contraction of isolated porcine coronary arteries with a half maximum effective concentration (EC50) of 0.4± 0.2 nM, which is at least one order of magnitude lower than values reported for other contracting peptides such as angiotensin II, with the exception of urotensin II [10]. In contrast to urotensin II, however, ET-1 induces contraction of almost all arteries and veins tested. There are two additional isoforms of endothelins, ET-2 and ET-3. Among these, ET-1 is the prominent isoform synthesized by the vasculature [6].
ET-1 is released continuously, mostly from endothelial cells (EC), by a constitutive and regulated pathway [11] and contributes to the maintenance of vascular tone [12–15]. NO strongly inhibits the release of ET-1 from the native endothelium [16, 17], which makes ET-1 and NO functionally closely interdependent, with a strong inhibitory effect of ET-1 on NO-mediated dilation, including in human coronary and cerebral arteries, and vice versa [14, 18–21]. The inhibitory effects of ET-1 are not limited to the dilations induced by NO since ET-1, through activation of the protein kinase C pathway, also reduces β-adrenergic receptor-dependent relaxation both in vitro and in vivo [22, 23]. This peptide is therefore a potent “anti-vasodilatory” factor released by the endothelium.
In addition to its genesis by EC, ET-1 is produced by vascular smooth muscle cells (VSMC), cardiomyocytes, leukocytes, macrophages, various neurons, and other cells [24]. ET-1 is not only a potent vasoconstrictor, but at elevated concentrations—pharmacological and pathological—it is also proinflammatory and promotes VSMC proliferation [25–30]. These latter properties of ET-1 are important in pulmonary arterial hypertension and probably other vascular diseases such as atherosclerosis and venous graft occlusion as we will see later. In the physiological environment, ET-1 certainly contributes to cardiovascular homeostasis through several pathways that impact on the regulation of basal vascular tone [12, 24] (Fig. 1).
Fig. 1.
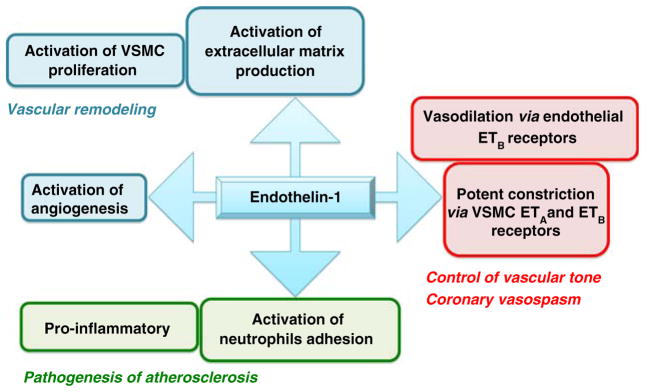
Multiple vascular properties of ET-1
The production of ET-1 is regulated at the gene level [31]: ET-1 messenger RNA (mRNA) is upregulated by inflammatory factors such as transforming growth factor beta, tumor necrosis factor alpha, interleukins, insulin, and angiotensin II, and downregulated by NO, PGI2, hypoxia, and shear stress [12, 24]. It is synthesized as a large protein, the pre-proET-1 or Big ET-1 that is cleaved first to pro-ET-1 and second to ET-1 by ET-converting enzymes (ECE-1 and ECE-2), but additional peptidases can generate ET-1 as demonstrated by experiments in ECE1/2 knockout mice [32]. The biological effects of ET-1 are mediated through activation of the two known ET-1 receptors, ETA and ETB [7, 8]. Although both receptors are important for normal function, the debate in the literature is whether or not both receptors should be blocked to provide most clinical benefit [33, 34]. The ETA has been classified as the bad one, while the ETB is considered as the good one; this distinction is based on the role of ETB in the clearance of circulating ET-1 and on the observation that activation of endothelial ETB induces dilatation by stimulating the release of endothelial prostacyclin and NO. Inhibition of ETB increases circulating ET-1 levels and blood pressure in healthy subjects [35] demonstrating that ETB stimulation is overall vasodilatory. This debate will not be closed unless we learn more on the pathophysiology of ET-1.
Vascular effects of ET-1
In this section, we will mostly, but not exclusively, report data collected in the coronary arteries since excellent reviews have been recently published on the pulmonary vascular bed [36, 37]. The coronary vascular bed is unique because in this vascular territory shear stress, the tangential force per unit area created by flow of blood over the luminal surface of the endothelium is the most unstable. The vascular endothelium-dependent dilation to shear stress permits fine tuning of nutrient delivery to accommodate metabolic demand [38, 39], and the stability of shear stress, rather than its level, contributes to the maintenance of a healthy endothelium [39]. In coronary arteries, blood flow varies throughout the cardiac cycle [40]: blood flows in the coronary arteries during diastole and reverses during systole as the contracting myocardium squeezes the subendocardial coronary arteries. Mechanical stress is nowhere else more pronounced in the circulation. In turn, the coronary circulation is the prime site for endothelial dysfunction. Because of these unique physiological hemodynamic features, coronary arteries display an unusual gene pattern when compared to the aorta: a fivefold lower endothelial NO synthase and a 2.5-fold higher ET-1 mRNA expressions [41]. Based on our current knowledge, this pattern would predispose to endothelial dysfunction and atherosclerosis and is directly in line with the functional interaction existing between NO and ET-1, in favor of ET-1.
Systemic exogenous administration of ET-1 induces a biphasic response, an initial and transient reduction in blood pressure followed by a sustained hypertensive phase. The depressor phase involves endothelial ETB receptors whereas the hypertensive phase is mediated by ETA receptors [42]. Similarly, isolated rat hearts perfused with exogenous ET-1 display a biphasic response: a drop in coronary perfusion pressure associated with coronary dilation was observed at low concentrations of ET-1, while a rise in coronary perfusion pressure associated with a coronary constriction was observed at high concentrations of ET-1 [12]. In addition, the effect of exogenous ET-1 on the coronary circulation may vary according to the mode of administration (bolus, infusions) and the experimental conditions (conscious versus anesthetized) [43]. It has been reported that, in coronary arteries, ETA are far more dominant in VSMC than ETB, and thus that ET-1 acts mainly as a vasoconstrictor [44].
The contribution of endogenously generated ET-1 to the coronary vessel tone in humans was assessed in patients undergoing routine diagnostic cardiac catheterization for suspected coronary artery disease (CAD), by intracoronary infusion of BQ123 (100 nmol/min, for 60 min), a selective antagonist of ETA receptors [45]. Inhibition of ETA receptors caused a coronary dilation in proximal and distal segments, an increase in coronary blood flow, and a decrease in coronary resistance. This result was confirmed by Ganz’s group showing that BQ123 (40 nmol/min, for 60 min) increased coronary artery diameter by 7% compared to 21% with nitroglycerine [46]. In this study, ET-1 was estimated to contribute to 39% of total vasomotor tone in healthy subjects. Similarly, MacCarthy et al. reported that intracoronary infusion of BQ123 (40 nmol/min, for 15 min) in patients with angiographically normal coronary arteries induced a reversible dilation of proximal, mid, and distal vessels, an increase in coronary blood flow, but no changes in systemic hemodynamic [47]. The vasodilatory effect of BQ123 appeared greater in distal vessels, likely explicable by the distribution of ETA in human coronary arteries. BQ123 highlights the importance in ETA-mediated contraction in coronary arteries, but also unmasks the role of endothelial ETB-dependent dilation mediated by NO and prostacyclin release [13, 15].
ET-1 not only exerts direct constrictor effects, but is also able, at low concentrations, to potentiate contractile responses to other vasoconstrictor substances such as norepinephrine and serotonin; on the other hand, the presence of small concentrations of vasoconstrictor substances can amplify the response to ET-1 [15, 48]. In isolated human cerebral arteries, endothelium-derived ET-1 augmented significantly serotonin-induced contraction, and BQ-123 prevented the rise in tone induced by inhibition of NO production [49]. Thus, even subthreshold concentrations of ET-1 may regulate vascular tone and reactivity. This is likely the physiological function of ET-1.
The role of ETB beside clearance of ET-1 and its endothelium-dependent dilatory activity is poorly understood. This is complicated by the possible change in expression of ET-1 receptors during the development of pathologies as evidenced in pulmonary hypertension [50] and renal diseases [51, 52] or by chronically doubling the circulating levels of ET-1 [53]. For example, doubling the circulating levels of ET-1 in dogs induces, likely under the control of ETB receptors, a reduction in cardiac output and heart rate combined with an increased systemic and renal vascular resistance and an antinatriuretic effect [54]. In addition, it has been reported that ETB receptors may play a role in the potentiation of the contraction induced by neurohormones such as angiotensin II and norepinephrine by low doses of ET-1 [55].
In summary, the endogenous ET-1-dependent control of tone in normal arteries depends on the balance between ETA- and ETB-mediated effects and on factors such as receptor distribution and endothelial integrity.
Physiological control of vascular remodeling
It has been reported that ET-1 modulates the expression of extracellular matrix (ECM) and matrix metalloproteinases, the main enzymes that degrade ECM molecules [56]. This confers to ET-1 a role in vascular remodeling. Vessel remodeling, however, should be considered as an adaptive response and is usually associated with endothelial dysfunction and vascular disease. ET-1, via ETA, mediates intimal hyperplasia in human saphenous vein graft [26, 27]. Similarly, when compared to control healthy internal mammary artery segments, segments isolated from patients with CAD displayed increased in situ immunostaining to ET-1 and ETA/ETB in VSMC, increased total and type 1 collagen, and a higher rate of VSMC proliferation, suggesting that ET-1 plays a role in arterial remodeling associated with CAD [57]. Recently, it has been reported that, in vascular endothelial cell ET-1 knockout mice, neointima formation induced by arterial (carotid) ligation was reduced, SMC proliferation decreased, and expression of endothelial adhesion molecules was inhibited [25]. This is a direct evidence for the role of endothelial ET-1 in mediating neointima formation following vascular injury. Hence, beyond its known vasoconstrictor effects, ET-1 is an important mediator of vascular remodeling. The links between ET-1-induced signaling events and the vascular remodeling are largely unknown, since the role of ET-1 in remodeling has been evidenced under stress conditions only.
ET-1 stimulates angiogenesis
Another mean by which ET-1 may be beneficial to tissue perfusion is through its ability to stimulate angiogenesis. Angiogenesis is the final common pathway in ischemia-induced neovascularization as well as formation of collateral vessels in cardiovascular diseases. Both in vitro experiments using isolated endothelial cells and in vivo models showed that ET-1 induces angiogenesis via ETB [58, 59]. Tissue hypoxia is a physiological stimulus for angiogenesis, and ET-1 production is also enhanced by hypoxia. In cardiomyocytes, ET-1 triggers connective tissue growth factor, a fibrotic mediator that regulates cell proliferation, migration, and ECM accumulation and thus plays a role in angiogenesis and tissue repair [60]. The angiogenic property of ET-1 could potentially be involved in the neovascularization described in atherosclerotic human coronary arteries.
In summary, the physiological roles of ET-1 involve maintenance of normal blood vessel tone, cellular proliferation, tissue development and repair, and angiogenesis.
Pathophysiological vascular effects of ET-1
The rise in circulating ET-1 levels
Under normal physiological conditions, the plasmatic concentration of ET-1 is around 1 pM, but it has been reported that the local concentration of ET-1 within the vascular wall is 100-fold higher than that of plasma levels [24]. The changes in plasmatic concentration of ET-1 may therefore not directly reflect changes in ET-1 release/clearance/breakdown associated with pathological conditions. Nonetheless, it has been shown that doubling circulating ET-1 levels acutely in dogs decreased heart rate and cardiac output without affecting blood pressure, and therefore increasing systemic vascular resistance [54]. This experimental increase in ET-1 was also associated with a rise in renal vascular resistance in association with a decreased glomerular filtration rate, without affecting coronary flow and pulmonary wedge pressure. On the other hand, doubling circulating levels of ET-1 chronically (7 days) in rats increased systolic blood pressure [53]. Futhermore, α2-adrenergic receptor-dependent contraction of isolated mesenteric arteries was increased, blunted by dual ETA/B inhibition but not by selective ETA inhibition with BQ123. Therefore, chronic elevation of ET-1 leads to changes in vascular reactivity possibly by affecting ETB-dependent responses.
Circulating levels of ET-1 have been reported to be elevated in patients with CAD and atherosclerosis, pulmonary hypertension, diabetes [29, 30, 34, 61–64], in patients with hypertension associated with renal failure [24], and in patients with heart failure [13, 65–68]. Many of the cardiovascular complications associated with aging and cardiovascular risk factors are attributable, at least in part, to endothelial dysfunction, particularly dysregulation of the vascular tone induced by an imbalance between NO and ET-1. Therefore, the known reduction of NO bioavailability in association with risk factors for cardiovascular diseases and disease states could also explain the rise in ET-1. Subsequently, ET-1 may feed forward by stimulating the production of damaging reactive oxygen species (ROS) [69] and hasten the decline in endothelial function [39]; on the one hand, prevention of NO production increases ET-1 release [16], while on the other hand, blockade of ET-1 receptors improves NO-dependent, flow-mediated dilation in patients with CAD [61]. The chronic rise in ET-1 may then lead to a molecular remodeling of the endothelin system (receptors, enzyme, associated proteins, etc.) and affect the cardiovascular system. By opposition, chronic blockade of ETA (4 weeks) in rats with congestive heart failure partially restored guanylate cyclase sensitivity and the loss of endothelial cell/smooth muscle cell communication [70]. Therefore, changes in ET-1 levels or blockade of its receptors have functional consequences involving proteins and pathways outside of the endothelin system itself.
Coronary arterial vasospasm
The evidence that ET-1 plasma levels are elevated in the coronary circulation of patients during angina, that ET-1 induces a long-lasting contraction in coronary arteries, and that subthreshold concentrations of ET-1 potentiate the coronary constrictor effects of other vasoconstrictors make ET-1 an ideal candidate for the initiation and the maintenance of coronary arterial spasm [15]. This hypothesis has been validated recently in a case report [71]. In this report, a 46-year-old patient with severe and treatment-resistant coronary vaso-spasm was treated successfully with the endothelin receptor antagonist bosentan. In acute coronary syndrome, it was reported that the concentration of ET-1 in the thrombus exceeded 280 times that of angiotensin II, norepinephrine, and serotonin [72]. Importantly, thrombus homogenates exerted vasoconstrictions of isolated porcine coronary artery rings that were inhibited by the dual ET-1 receptor antagonist tezosentan. The recent demonstration that following coronary artery endothelial dysfunction ET-1 is essential for a ROS-dependent coronary vasospasm [69], a mechanism that could also be involved in coronary spasm postcardiac transplantation [73], provides a strong rationale for testing ETA/B antagonists in spastic angina.
Endothelial dysfunction and atherosclerosis
In 1995, it was shown that treatment of cholesterol-fed hamsters with the selective ETA receptor antagonist BMS-182874 decreased the area of the fatty streak by reducing the number and size of macrophage foam cells [74], strongly suggesting a proinflammatory role of ETA activation. Lüscher’s group demonstrated that ETA inhibition improved endothelial dysfunction and reduced atherosclerosis development in ApoE knockout mice [75]. In pigs fed with a high-cholesterol diet, both selective ETA and dual ETA/B chronic treatment selectively increased coronary blood flow induced by intracoronary infusion of acetylcholine [76], while following myocardial infarction in rats, treatment with both the selective ETA antagonist LU 135252 and the dual ETA/B antagonist bosentan improved acetylcholine-induced relaxation [77]. Several studies have reported the effects of intracoronary infusion of the ETA antagonist BQ123 on coronary diameter and coronary flow in patients with CAD. As in healthy patients, Dr. Webb’s group showed that BQ123 (100 nmol/min, for 60 min) increased diameter and coronary flow [45]. In contrast, Ganz’s group reported that BQ123 (40 nmol/min, for 60 min) increased more coronary artery diameter in patients with CAD than without [46]. In this latter study, compared with the dilation to nitroglycerin, ET-1 contributed to 39% of coronary vasomotor tone in healthy subjects, 74% of tone in CAD arteries, and 106% of tone at stenoses. The contribution of NO, that is the endothelial dilatory function, was, however, not determined: one would assume that the more severe the diseases state the less NO would be produced (if any), and the more ET-1 would contribute to tone. This hypothesis was tested in 44 patients with CAD in a study published that same year in 2001: Halcox et al. provided the first evidence that ET-1, via ETA, contributed to reduce the endothelial dilatory function [63]. Intracoronary infusion of BQ123 (200 nmol/min, for 1 h) in patients with atherosclerosis resulted in coronary artery dilation and an improvement of acetylcholine-induced endothelium-dependent dilation. The patients with the greatest endothelial dysfunction benefited the most from the intracoronary infusion of the ETA antagonist [63]. In pig coronary arteries subjected to ischemia reperfusion, another model of endothelial dysfunction, the vasoreactivity to exogenous ET-1 was increased and associated with a reduction in endothelial ETB-mediated dilation and an increase in vascular smooth muscle ETB-dependent contraction [78]. Bohm et al. reported that both selective ETA (BQ123) and the combination of selective ETA (BQ123) and ETB (BQ788) antagonists improved endothelial-dependent dilation in coronary arteries from patients with CAD [61]. Again, the dilation was higher in severely stenotic segments, demonstrating that the importance of ET-1 in the control of vascular tone is increased in atherosclerosis. In agreement with these data, using isolated human coronary arteries from idiopathic and atherosclerotic cardiomyopathic hearts, we demonstrated that ET-1-dependent constriction became more important when endothelial function was altered [21]. Taken together, these data strongly suggest that ET-1 is an important contributor to endothelial dysfunction in the coronary arterial bed. The role of the ETB needs also to be considered because its expression increases in experimental hypercholesterolemia, promoting contraction of isolated pig coronary arterial rings at low concentrations (0.1 nM) of ET-1 and sarafotoxin-6c, a selective ETB agonist [79]. Hence, the functional contribution of ET-1 appears to rise with the severity of the disease. Raised plasma levels of ET-1 have been reported in patients with atherosclerosis, and upregulation of ET-1 and its receptors has been described in atherosclerotic arteries and plaques [64, 67, 80, 81]. Big ET-1 and ET-1 immunoreactivity has been found in regions of atherosclerosis [79, 82]. The constrictor, proinflammatory, chemoattractant, and mitogenic properties of ET-1 also clearly support a role for ET-1 in the pathogenesis of atherosclerosis [29, 83].
Additional studies brought another perspective on the potential clinical development of ET-1 receptor antagonists based on the interactions between ET-1 and angiotensin II. ET-1 receptor inhibition combined with angiotensin-converting enzyme (ACE) inhibitors has been shown to improve renal hemodynamics and sodium excretion in patients with chronic kidney diseases [84, 85]. Most interestingly, dual ETA/B antagonism improved endothelium-dependent dilation in the forearm in atherosclerotic patients treated with an ACE inhibitor [86]. Similarly, ETA antagonism and ACE inhibition were efficient at preventing diabetic renal lesions induced by diabetes in rats [51], and a recent study in treatment resistant hypertension [87] shows efficacy on blood pressure and proteinuria in patients already treated with renin–angiotensin system blockers. Since endothelin antagonists do not produce hyperkalemia, and there is tremendous unmet need in the field of chronic proteinuric nephropathy, this seems to be a very attractive area for further clinical development. This dual therapeutic approach may also be efficient in several other cardiovascular diseases including CAD, peripheral vascular diseases, and diabetes.
Conclusion
Although there are now a number of cardiovascular indications for endothelin receptor antagonists, and a number under consideration, the physiology of ET-1 needs more understanding. ET-1 is essential for normal physiological function, and its well-known interaction with NO should be sufficient to stimulate more research. More data may stimulate clinical development of ET receptors antagonists in atherosclerosis and diabetes among others [33, 34, 88]. The legacy of Dr. Furchgott is not an endothelium with a Janus face but a cellular layer that finely regulates tone and maintains wall integrity with high levels of control. Dysfunctions develop with age or prematurely with exposure to cardiovascular risk factors, and the loss of NO probably unbalances the system favoring ET-1-dependent pathogenesis.
Contributor Information
Eric Thorin, Department of Surgery and Research Center, Institut de Cardiologie de Montréal, Université de Montréal, Montréal, QC, Canada.
David J. Webb, British Heart Foundation Centre of Research Excellence (BHF CoRE), Centre for Cardiovascular Science, University of Edinburgh, Edinburgh, Scotland, UK
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982;51:439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte PM, Rubanyi GM, Miller VM, Houston DS. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa M, Inoue A, Ishikawa T, Kasuya Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S, Goto K, et al. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci USA. 1988;85:6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 7.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 9.Clozel M, Breu V, Burri K, Cassal JM, Fischli W, Gray GA, Hirth G, Loffler BM, Muller M, Neidhart W, et al. Pathophysiological role of endothelin revealed by the first orally active endothelin receptor antagonist. Nature. 1993;365:759–761. doi: 10.1038/365759a0. [DOI] [PubMed] [Google Scholar]
- 10.McDonald J, Batuwangala M, Lambert DG. Role of urotensin II and its receptor in health and disease. J Anesth. 2007;21:378–389. doi: 10.1007/s00540-007-0524-z. [DOI] [PubMed] [Google Scholar]
- 11.Russell FD, Davenport AP. Secretory pathways in endothelin synthesis. Br J Pharmacol. 1999;126:391–398. doi: 10.1038/sj.bjp.0702315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunner F, Bras-Silva C, Cerdeira AS, Leite-Moreira AF. Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther. 2006;111:508–531. doi: 10.1016/j.pharmthera.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Callera G, Tostes R, Savoia C, Muscara MN, Touyz RM. Vasoactive peptides in cardiovascular (patho)physiology. Expert Rev Cardiovasc Ther. 2007;5:531–552. doi: 10.1586/14779072.5.3.531. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TD, Vequaud P, Thorin E. Effects of endothelin receptor antagonists and nitric oxide on myogenic tone and alpha-adrenergic-dependent contractions of rabbit resistance arteries. Cardiovasc Res. 1999;43:755–761. doi: 10.1016/s0008-6363(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 15.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- 16.Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luscher TF, Yang Z, Tschudi M, von Segesser L, Stulz P, Boulanger C, Siebenmann R, Turina M, Buhler FR. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circ Res. 1990;66:1088–1094. doi: 10.1161/01.res.66.4.1088. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert P, Tremblay J, Thorin E. Endothelium-derived endothelin-1 reduces cerebral artery sensitivity to nitric oxide by a protein kinase C-independent pathway. Stroke. 2001;32:2351–2355. doi: 10.1161/hs1001.096007. [DOI] [PubMed] [Google Scholar]
- 19.Ming Z, Parent R, Thorin E, Lavallee M. Endothelin-dependent tone limits acetylcholine-induced dilation of resistance coronary vessels after blockade of NO formation in conscious dogs. Hypertension. 1998;32:844–848. doi: 10.1161/01.hyp.32.5.844. [DOI] [PubMed] [Google Scholar]
- 20.Thorin E. Influence of nitric oxide synthase inhibition and endothelin-1 receptor blockade on acetylcholine-induced coronary artery contraction in vitro in dilated and ischemic cardiomyopathies. J Cardiovasc Pharmacol. 2001;38:90–98. doi: 10.1097/00005344-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Thorin E, Parent R, Ming Z, Lavallee M. Contribution of endogenous endothelin to large epicardial coronary artery tone in dogs and humans. Am J Physiol. 1999;277:H524–H532. doi: 10.1152/ajpheart.1999.277.2.H524. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert P, Thorin E. Endothelin-1 limits vascular smooth muscle beta-adrenergic receptor sensitivity by a PKC-dependent pathway. J Cardiovasc Pharmacol. 2003;42:534–538. doi: 10.1097/00005344-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Okajima M, Parent R, Thorin E, Lavallee M. Pathophysiological plasma ET-1 levels antagonize beta-adrenergic dilation of coronary resistance vessels in conscious dogs. Am J Physiol Heart Circ Physiol. 2004;287:H1476–H1483. doi: 10.1152/ajpheart.00297.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 25.Anggrahini DW, Emoto N, Nakayama K, Widyantoro B, Adiarto S, Iwasa N, Nonaka H, Rikitake Y, Kisanuki YY, Yanagisawa M, Hirata K. Vascular endothelial cell-derived endothelin-1 mediates vascular inflammation and neointima formation following blood flow cessation. Cardiovasc Res. 2009;82:143–151. doi: 10.1093/cvr/cvp026. [DOI] [PubMed] [Google Scholar]
- 26.Dashwood MR, Mehta D, Izzat MB, Timm M, Bryan AJ, Angelini GD, Jeremy JY. Distribution of endothelin-1 (ET) receptors (ET(A) and ET(B)) and immunoreactive ET-1 in porcine saphenous vein-carotid artery interposition grafts. Atherosclerosis. 1998;137:233–242. doi: 10.1016/s0021-9150(97)00249-9. [DOI] [PubMed] [Google Scholar]
- 27.Davenport AP, Maguire JJ. The endothelin system in human saphenous vein graft disease. Curr Opin Pharmacol. 2001;1:176–182. doi: 10.1016/s1471-4892(01)00026-1. [DOI] [PubMed] [Google Scholar]
- 28.Ihling C, Szombathy T, Bohrmann B, Brockhaus M, Schaefer HE, Loeffler BM. Coexpression of endothelin-converting enzyme-1 and endothelin-1 in different stages of human atherosclerosis. Circulation. 2001;104:864–869. doi: 10.1161/hc3301.094742. [DOI] [PubMed] [Google Scholar]
- 29.Ivey ME, Osman N, Little PJ. Endothelin-1 signalling in vascular smooth muscle: pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis. 2008;199:237–247. doi: 10.1016/j.atherosclerosis.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Ruschitzka F, Moehrlen U, Quaschning T, Lachat M, Noll G, Shaw S, Yang Z, Teupser D, Subkowski T, Turina MI, Luscher TF. Tissue endothelin-converting enzyme activity correlates with cardiovascular risk factors in coronary artery disease. Circulation. 2000;102:1086–1092. doi: 10.1161/01.cir.102.10.1086. [DOI] [PubMed] [Google Scholar]
- 31.Farhat N, Matouk CC, Mamarbachi AM, Marsden PA, Allen BG, Thorin E. Activation of ETB receptors regulates the abundance of ET-1 mRNA in vascular endothelial cells. Br J Pharmacol. 2008;153:1420–1431. doi: 10.1038/bjp.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, Clouthier DE, Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105:1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhaun N, Pollock DM, Goddard J, Webb DJ. Selective and mixed endothelin receptor antagonism in cardiovascular disease. Trends Pharmacol Sci. 2007;28:573–579. doi: 10.1016/j.tips.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: great expectations or bleak house? Br J Pharmacol. 2008;153:1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strachan FE, Spratt JC, Wilkinson IB, Johnston NR, Gray GA, Webb DJ. Systemic blockade of the endothelin-B receptor increases peripheral vascular resistance in healthy men. Hypertension. 1999;33:581–585. doi: 10.1161/01.hyp.33.1.581. [DOI] [PubMed] [Google Scholar]
- 36.Davie NJ, Schermuly RT, Weissmann N, Grimminger F, Ghofrani HA. The science of endothelin-1 and endothelin receptor antagonists in the management of pulmonary arterial hypertension: current understanding and future studies. Eur J Clin Invest. 2009;39(Suppl 2):38–49. doi: 10.1111/j.1365-2362.2009.02120.x. [DOI] [PubMed] [Google Scholar]
- 37.Pullamsetti SS, Schermuly RT. Endothelin receptor antagonists in preclinical models of pulmonary hypertension. Eur J Clin Invest. 2009;39(Suppl 2):3–13. doi: 10.1111/j.1365-2362.2009.02115.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Gutterman DD. Vascular control in humans: focus on the coronary microcirculation. Basic Res Cardiol. 2009;104:211–227. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorin E, Thorin-Trescases N. Vascular endothelial ageing, heartbeat after heartbeat. Cardiovasc Res. 2009;84:24–32. doi: 10.1093/cvr/cvp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol. 2008;153:1589–1601. doi: 10.1038/sj.bjp.0707673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dancu MB, Tarbell JM. Coronary endothelium expresses a pathologic gene pattern compared to aortic endothelium: correlation of asynchronous hemodynamics and pathology in vivo. Atherosclerosis. 2007;192:9–14. doi: 10.1016/j.atherosclerosis.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 42.D’Orleans-Juste P, Labonte J, Bkaily G, Choufani S, Plante M, Honore JC. Function of the endothelin(B) receptor in cardiovascular physiology and pathophysiology. Pharmacol Ther. 2002;95:221–238. doi: 10.1016/s0163-7258(02)00235-8. [DOI] [PubMed] [Google Scholar]
- 43.Lavallee M, Thorin E. Role of ET-1 in the regulation of coronary circulation. Can J Physiol Pharmacol. 2003;81:570–577. doi: 10.1139/y03-014. [DOI] [PubMed] [Google Scholar]
- 44.Bacon CR, Davenport AP. Endothelin receptors in human coronary artery and aorta. Br J Pharmacol. 1996;117:986–992. doi: 10.1111/j.1476-5381.1996.tb15292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyriakides ZS, Kremastinos DT, Bofilis E, Tousoulis D, Antoniadis A, Webb DJ. Endogenous endothelin maintains coronary artery tone by endothelin type A receptor stimulation in patients undergoing coronary arteriography. Heart. 2000;84:176–182. doi: 10.1136/heart.84.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinlay S, Behrendt D, Wainstein M, Beltrame J, Fang JC, Creager MA, Selwyn AP, Ganz P. Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation. 2001;104:1114–1118. doi: 10.1161/hc3501.095707. [DOI] [PubMed] [Google Scholar]
- 47.MacCarthy PA, Pegge NC, Prendergast BD, Shah AM, Groves PH. The physiological role of endogenous endothelin in the regulation of human coronary vasomotor tone. J Am Coll Cardiol. 2001;37:137–143. doi: 10.1016/s0735-1097(00)01042-1. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Villalon AL, Amezquita YM, Monge L, Fernandez N, Salcedo A, Dieguez G. Endothelin-1 potentiation of coronary artery contraction after ischemia-reperfusion. Vascul Pharmacol. 2008;48:109–114. doi: 10.1016/j.vph.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Thorin E, Nguyen TD, Bouthillier A. Control of vascular tone by endogenous endothelin-1 in human pial arteries. Stroke. 1998;29:175–180. doi: 10.1161/01.str.29.1.175. [DOI] [PubMed] [Google Scholar]
- 50.Sauvageau S, Thorin E, Villeneuve L, Dupuis J. Change in pharmacological effect of endothelin receptor antagonists in rats with pulmonary hypertension: role of ETB-receptor expression levels. Pulm Pharmacol Ther. 2009;22:311–317. doi: 10.1016/j.pupt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gagliardini E, Corna D, Zoja C, Sangalli F, Carrara F, Rossi M, Conti S, Rottoli D, Longaretti L, Remuzzi A, Remuzzi G, Benigni A. Unlike each drug alone, Lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.00340.2009. [DOI] [PubMed] [Google Scholar]
- 52.Longaretti L, Benigni A. Endothelin receptor selectivity in chronic renal failure. Eur J Clin Invest. 2009;39(Suppl 2):32–37. doi: 10.1111/j.1365-2362.2009.02119.x. [DOI] [PubMed] [Google Scholar]
- 53.Thorin E, Cernacek P, Dupuis J. Endothelin-1 regulates tone of isolated small arteries in the rat: effect of hyperendothelinemia. Hypertension. 1998;31:1035–1041. doi: 10.1161/01.hyp.31.4.1035. [DOI] [PubMed] [Google Scholar]
- 54.Lerman A, Hildebrand FL, Jr, Aarhus LL, Burnett JC., Jr Endothelin has biological actions at pathophysiological concentrations. Circulation. 1991;83:1808–1814. doi: 10.1161/01.cir.83.5.1808. [DOI] [PubMed] [Google Scholar]
- 55.Gossl M, Mitchell A, Lerman A, Opazo Saez A, Schafers RF, Erbel R, Philipp T, Wenzel RR. Endothelin-B-receptor-selective antagonist inhibits endothelin-1 induced potentiation on the vasoconstriction to noradrenaline and angiotensin II. J Hyper-tens. 2004;22:1909–1916. doi: 10.1097/00004872-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Abraham D, Dashwood M. Endothelin—role in vascular disease. Rheumatology (Oxford) 2008;47(Suppl 5):v23–v24. doi: 10.1093/rheumatology/ken282. [DOI] [PubMed] [Google Scholar]
- 57.Sutherland AJ, Nataatmadja MI, Walker PJ, Cuttle L, Garlick RB, West MJ. Vascular remodeling in the internal mammary artery graft and association with in situ endothelin-1 and receptor expression. Circulation. 2006;113:1180–1188. doi: 10.1161/CIRCULATIONAHA.105.582890. [DOI] [PubMed] [Google Scholar]
- 58.Goligorsky MS, Budzikowski AS, Tsukahara H, Noiri E. Co-operation between endothelin and nitric oxide in promoting endothelial cell migration and angiogenesis. Clin Exp Pharmacol Physiol. 1999;26:269–271. doi: 10.1046/j.1440-1681.1999.03029.x. [DOI] [PubMed] [Google Scholar]
- 59.Morbidelli L, Orlando C, Maggi CA, Ledda F, Ziche M. Proliferation and migration of endothelial cells is promoted by endothelins via activation of ETB receptors. Am J Physiol. 1995;269:H686–H695. doi: 10.1152/ajpheart.1995.269.2.H686. [DOI] [PubMed] [Google Scholar]
- 60.Recchia AG, Filice E, Pellegrino D, Dobrina A, Cerra MC, Maggiolini M. Endothelin-1 induces connective tissue growth factor expression in cardiomyocytes. J Mol Cell Cardiol. 2009;46:352–359. doi: 10.1016/j.yjmcc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 61.Bohm F, Jensen J, Svane B, Settergren M, Pernow J. Intracoronary endothelin receptor blockade improves endothelial function in patients with coronary artery disease. Can J Physiol Pharmacol. 2008;86:745–751. doi: 10.1139/Y08-081. [DOI] [PubMed] [Google Scholar]
- 62.Celebi H, Catakoglu AB, Kurtoglu H, Sener M, Hanavdelogullari R, Demiroglu C, Aytekin V, Aytekin S. The relation between coronary flow rate, plasma endothelin-1 concentrations, and clinical characteristics in patients with normal coronary arteries. Cardiovasc Revasc Med. 2008;9:144–148. doi: 10.1016/j.carrev.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Halcox JP, Nour KR, Zalos G, Quyyumi AA. Coronary vasodilation and improvement in endothelial dysfunction with endothelin ET(A) receptor blockade. Circ Res. 2001;89:969–976. doi: 10.1161/hh2301.100980. [DOI] [PubMed] [Google Scholar]
- 64.Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- 65.Alonso D, Radomski MW. The nitric oxide-endothelin-1 connection. Heart Fail Rev. 2003;8:107–115. doi: 10.1023/a:1022155206928. [DOI] [PubMed] [Google Scholar]
- 66.Angerio AD. The role of endothelin in heart failure. Crit Care Nurs Q. 2005;28:355–359. doi: 10.1097/00002727-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- 68.Kelland NF, Webb DJ. Clinical trials of endothelin antagonists in heart failure: a question of dose? Exp Biol Med (Maywood) 2006;231:696–699. [PubMed] [Google Scholar]
- 69.Saitoh S, Matsumoto K, Kamioka M, Ohkawara H, Kaneshiro T, Ishibashi T, Maruyama Y. Novel pathway of endothelin-1 and reactive oxygen species in coronary vasospasm with endothelial dysfunction. Coron Artery Dis. 2009;20:400–408. doi: 10.1097/MCA.0b013e32832e5c8c. [DOI] [PubMed] [Google Scholar]
- 70.Thorin E, Lucas M, Cernacek P, Dupuis J. Role of ET(A) receptors in the regulation of vascular reactivity in rats with congestive heart failure. Am J Physiol Heart Circ Physiol. 2000;279:H844–H851. doi: 10.1152/ajpheart.2000.279.2.H844. [DOI] [PubMed] [Google Scholar]
- 71.Vermeltfoort IA, Raijmakers PG, Kamphuisen PW. Improved myocardial perfusion preceding clinical response on bosentan treatment for coronary vasospasm. Acta Cardiol. 2009;64:415–417. doi: 10.2143/AC.64.3.2038032. [DOI] [PubMed] [Google Scholar]
- 72.Adlbrecht C, Bonderman D, Plass C, Jakowitsch J, Beran G, Sperker W, Siostrzonek P, Glogar D, Maurer G, Lang IM. Active endothelin is an important vasoconstrictor in acute coronary thrombi. Thromb Haemost. 2007;97:642–649. doi: 10.1160/th06-08-0479. [DOI] [PubMed] [Google Scholar]
- 73.Badiwala MV, Ramzy D, Tumiati LC, Tepperman ED, Sheshgiri R, Prodger JL, Feindel CM, Rao V. Donor pretreatment with hypertonic saline attenuates primary allograft dysfunction: a pilot study in a porcine model. Circulation. 2009;120:S206–S214. doi: 10.1161/CIRCULATIONAHA.108.843169. [DOI] [PubMed] [Google Scholar]
- 74.Kowala MC, Rose PM, Stein PD, Goller N, Recce R, Beyer S, Valentine M, Barton D, Durham SK. Selective blockade of the endothelin subtype A receptor decreases early atherosclerosis in hamsters fed cholesterol. Am J Pathol. 1995;146:819–826. [PMC free article] [PubMed] [Google Scholar]
- 75.Barton M, Haudenschild CC, d’Uscio LV, Shaw S, Munter K, Luscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Best PJ, McKenna CJ, Hasdai D, Holmes DR, Jr, Lerman A. Chronic endothelin receptor antagonism preserves coronary endothelial function in experimental hypercholesterolemia. Circulation. 1999;99:1747–1752. doi: 10.1161/01.cir.99.13.1747. [DOI] [PubMed] [Google Scholar]
- 77.Bauersachs J, Fraccarollo D, Galuppo P, Widder J, Ertl G. Endothelin-receptor blockade improves endothelial vasomotor dysfunction in heart failure. Cardiovasc Res. 2000;47:142–149. doi: 10.1016/s0008-6363(00)00083-3. [DOI] [PubMed] [Google Scholar]
- 78.Climent B, Fernandez N, Sanz E, Sanchez A, Monge L, Garcia-Villalon AL, Dieguez G. Enhanced response of pig coronary arteries to endothelin-1 after ischemia-reperfusion. Role of endothelin receptors, nitric oxide and prostanoids. Eur J Pharmacol. 2005;524:102–110. doi: 10.1016/j.ejphar.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Hasdai D, Mathew V, Schwartz RS, Smith LA, Holmes DR, Jr, Katusic ZS, Lerman A. Enhanced endothelin-B-receptor-mediated vasoconstriction of small porcine coronary arteries in diet-induced hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:2737–2743. doi: 10.1161/01.atv.17.11.2737. [DOI] [PubMed] [Google Scholar]
- 80.Dagassan PH, Breu V, Clozel M, Kunzli A, Vogt P, Turina M, Kiowski W, Clozel JP. Up-regulation of endothelin-B receptors in atherosclerotic human coronary arteries. J Cardiovasc Pharmacol. 1996;27:147–153. doi: 10.1097/00005344-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 81.Fan J, Unoki H, Iwasa S, Watanabe T. Role of endothelin-1 in atherosclerosis. Ann NY Acad Sci. 2000;902:84–93. doi: 10.1111/j.1749-6632.2000.tb06303.x. discussion 93-84. [DOI] [PubMed] [Google Scholar]
- 82.Dashwood MR, Timm M, Muddle JR, Ong AC, Tippins JR, Parker R, McManus D, Murday AJ, Madden BP, Kaski JC. Regional variations in endothelin-1 and its receptor subtypes in human coronary vasculature: pathophysiological implications in coronary disease. Endothelium. 1998;6:61–70. doi: 10.3109/10623329809053405. [DOI] [PubMed] [Google Scholar]
- 83.Dashwood MR, Tsui JC. Endothelin-1 and atherosclerosis: potential complications associated with endothelin-receptor blockade. Atherosclerosis. 2002;160:297–304. doi: 10.1016/s0021-9150(01)00586-x. [DOI] [PubMed] [Google Scholar]
- 84.Dhaun N, Ferro CJ, Davenport AP, Haynes WG, Goddard J, Webb DJ. Haemodynamic and renal effects of endothelin receptor antagonism in patients with chronic kidney disease. Nephrol Dial Transplant. 2007;22:3228–3234. doi: 10.1093/ndt/gfm364. [DOI] [PubMed] [Google Scholar]
- 85.Dhaun N, Macintyre IM, Melville V, Lilitkarntakul P, Johnston NR, Goddard J, Webb DJ. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension. 2009;54:113–119. doi: 10.1161/HYPERTENSIONAHA.109.132670. [DOI] [PubMed] [Google Scholar]
- 86.Bohm F, Beltran E, Pernow J. Endothelin receptor blockade improves endothelial function in atherosclerotic patients on angiotensin converting enzyme inhibition. J Intern Med. 2005;257:263–271. doi: 10.1111/j.1365-2796.2005.01448.x. [DOI] [PubMed] [Google Scholar]
- 87.Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, Linseman JV, Wiens BL, Warren MS, Lindholm LH. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet. 2009 doi: 10.1016/S0140-6736(09)61500-2. [DOI] [PubMed] [Google Scholar]
- 88.Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension. 2008;52:452–459. doi: 10.1161/HYPERTENSIONAHA.108.117366. [DOI] [PubMed] [Google Scholar]


