Abstract
One year after the discovery in 1980 that the endothelium was obligatory for acetylcholine to relax isolated arteries, it was clearly shown that the endothelium could also promote contraction. In 1988, Dr Yanagisawa’s group identified endothelin-1 (ET-1) as the first endothelium-derived contracting factor. The circulating levels of this short (21 amino acids) peptide were quickly determined in humans and it was reported that in most cardiovascular diseases, circulating levels of ET-1 were increased and ET-1 was then recognized as a likely mediator of pathological vasoconstriction in human. The discovery of two receptor subtypes in 1990, ETA and ETB, permitted optimization of bosentan, which entered clinical development in 1993, and was offered to patients with pulmonary arterial hypertension in 2001. In this report, we discuss the physiological and pathophysiological role of endothelium-derived ET-1, the pharmacology of its two receptors, focusing on the regulation of the vascular tone and as much as possible in humans. The coronary bed will be used as a running example, but references to the pulmonary, cerebral, and renal circulation will also be made. Many of the cardiovascular complications associated with aging and cardiovascular risk factors are initially attributable, at least in part, to endothelial dysfunction, particularly dysregulation of the vascular function associated with an imbalance in the close interdependence of NO and ET-1, in which the implication of the ETB receptor may be central.
I. Introduction
The endothelium is an extraordinary organ that protects the arterial wall through the release of nitric oxide (NO) and prostacyclin (PGI2) among other factors (Furchgott & Zawadzki, 1980; Palmer et al., 1987). Before 1980, it was merely considered an inert barrier (Aird, 2007). The presence of an endothelium-derived constricting factor (EDCF) was hypothesized 1 year after the revelation of the relaxant properties of the endothelium (De Mey & Vanhoutte, 1982; Vanhoutte et al., 1986). But it was only 8 years later that Yanagisawa and colleagues identified the peptide endothelin-1 (ET-1) has this long-lasting EDCF (Yanagisawa et al., 1988a, 1988b). Two receptors for ET-1 were identified 2 years later (Arai et al., 1990; Sakurai et al., 1990). Then, shortly after the discovery of ETA and ETB receptors, Martine Clozel and colleagues presented in 1993 the first orally active ET-1 receptor antagonist, Ro 46-2005 (Clozel et al., 1993), and the same team made a structurally modified analog, bosentan (Tracleer), available to patients with pulmonary arterial hypertension (PAH) at the end of 2001. In less than 15 years, a new factor was identified, its receptors were cloned and their pharmacology characterized, a pathology associated with the abnormal function of the ET-1 system, and an effective treatment offered to patients in need. Today, other ET receptor antagonists have been synthesized and are in development, all this being well reviewed recently (Kirkby et al., 2008; Motte et al., 2006). There is still much to be discovered on the role and the mechanisms of action of ET-1. We focus in this chapter on the pharmacology of ET-1 and review the role of ET-1 on the vasculature with as much as possible references to the human pathophysiology.
II. Cardiovascular Physiology of ET-1
ET-1 is one of the most potent vasoconstrictors identified so far (Yanagisawa et al., 1988b) inducing prolonged contraction of isolated canine and nonhuman primate coronary arteries with a half maximal effective concentration (−log[EC50]) of 8. The potency of ET-1 is unequaled, with the exception of urotensin II (−log[EC50] = 9.5; Douglas et al., 2000). ET-1 elicits its effects through two receptors (http://www.iuphar.org/): ETA receptors, located in vascular smooth muscle cells (VSMC) and cardiomyocytes, mediate contraction, whereas ETB receptors, located on vascular endothelial cells (EC), mediate dilation and ET-1 uptake, and regulate ET-1 production (Arai et al., 1990; Barton & Yanagisawa, 2008; Brunner et al., 2006; Callera et al., 2007; Dupuis et al., 1997; Farhat et al., 2008; Komukai et al., 2010; Rubanyi & Polokoff, 1994; Sakurai et al., 1990; Sanchez et al., 2002). Additionally, ETB receptors can also be expressed on VSMC and elicit contractions (Sanchez et al., 2002; Teerlink et al., 1994). It is a known fact that ET-1 is released continuously, mostly from EC, by a constitutive pathway and contributes to the regulation of the vascular tone in general (Brunner et al., 2006; Callera et al., 2007; Rubanyi & Polokoff, 1994). NO, however, strongly inhibits the release of ET-1 from the native endothelium (Boulanger & Luscher, 1990; Luscher et al., 1990); for this reason, it has been suggested that NO and ET-1 regulate each other through an autocrine feedback loop (Alonso & Radomski, 2003; Luscher et al., 1990). In addition to EC, ET-1 is also produced by VSMC, cardiomyocytes, leukocytes, macrophages, various neurons, and other cells (Kedzierski & Yanagisawa, 2001). This peptide is also proinflammatory and promotes VSMC proliferation (Anggrahini et al., 2009; Dashwood et al., 1998a; Davenport & Maguire, 2001; Ihling et al., 2001; Ivey et al., 2008; Ruschitzka et al., 2000). Thus, ET-1 contributes to the cardiovascular homeostasis by regulating basal vascular tone and remodeling (Brunner et al., 2006; Kedzierski & Yanagisawa, 2001).
A. The ET-1 System
1. Endothelins and Their Receptors
There are three isoforms of endothelin produced in humans, ET-1, ET-2, and ET-3 (Inoue et al., 1989a; Saida et al., 1989). They are encoded on chromosomes 6, 1, and 20, respectively (Inoue et al., 1989a). ET-1 binds ETA and ETB receptors with equal affinity, while ETA receptors have approximately 100-fold less affinity for ET-3 than ETB receptors (Davenport, 2002). In addition, snake venom toxins called “sarafotoxins” have been identified by homology. Sarafotoxin 6c (S6c) is a selective ETB receptor agonist (Rubanyi & Polokoff, 1994). ETA and ETB receptors belong to the 7-transmembrane domain (7-TM) family and are encoded by distinct genes on chromosomes 4 and 13, respectively (Sakurai et al., 1990).
ET-1 is produced within the cell in two proteolytic steps from the pre-proET-1, a large precursor peptide of approximately 200 amino acid residues. First, a furin-like neutral endopeptidase cleaves the preproET-1 to bigET-1, an inactive peptide of 41 amino acid residues (Denault et al., 1995; Laporte et al., 1993). Second, bigET-1 is cleaved by the endothelin-converting enzymes (ECE-1 and ECE-2) to the biologically active ET-1, a 21-amino acid residue peptide enclosed by disulfur bonds (Takahashi et al., 1993) mainly by EC (Inoue et al., 1989b). Some alternative pathways to ECE for the synthesis of ET-1 have been reported: a chymostatin-sensitive enzyme, such as chymase, and the matrix metalloproteinase 2 are able to convert bigET-1 to mature ET-1 in human blood vessels (Maguire & Davenport, 2004; Maguire et al., 2001).
The clearance of ET-1 from the circulation after an intravenous injection of radiolabeled ET-1 in rats is rapid (half-life of 40 s; Sirvio et al., 1990), while its pressor effect is long lasting (≈ 1 h at the doses administered) in man (Sirvio et al., 1990; Vierhapper et al., 1990). The majority of ET-1 is retained by the lungs and cleared from the circulation via binding to ETB receptors (Dupuis et al., 1996a, 1996b).
2. Endothelin Receptor Ligands and Pharmacology: Emerging Concepts
There is no selective agonist for the ETA receptor. ET-1 [1–31] has been shown to have more selectivity for ETA compared to ETB receptors (Rossi et al., 2002), but the 31-amino acid peptide has no direct pharmacological effects if not converted via a neutral endopeptidase-dependent mechanism to ET-1 [1–21] (Fecteau et al., 2005). Selective antagonists for the ETA receptor include ZD4054, atrasentan, darusentan, macitentan, ambrisentan, and sitaxsentan (Motte et al., 2006).
In contrast to ETA receptors, selective ETB receptor agonists are available such as S6c and IRL-1620. Selective antagonists of the ETB receptors include BQ788, A192621, RES7011, and IRL2500 (Alexander et al., 2009).
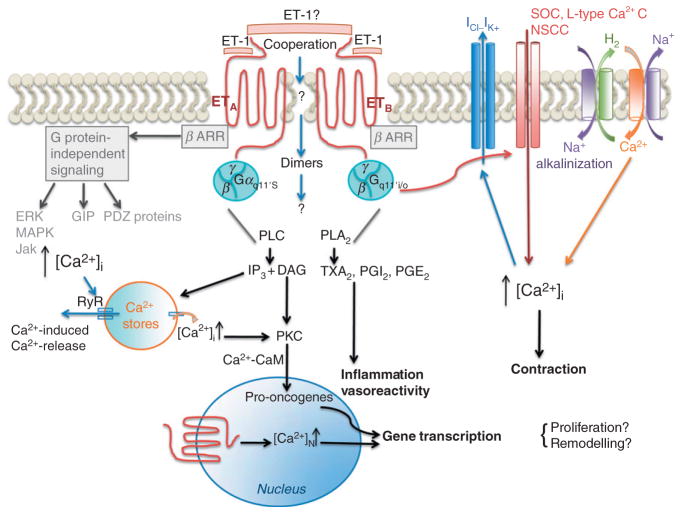
Because ET receptors are 7-TM receptors, their signal transduction (Fig. 1) was first interpreted as a sequential series of events initiated by the binding of the agonist on its receptor. This simplistic view had to be revised with the evidence that activation of a 7-TM receptor can activate simultaneously multiple pathways (Watts, 2010) including some G-protein-independent pathways (Galandrin et al., 2007; Kenakin, 2007; Violin & Lefkowitz, 2007). One well-known example is the activation of the angiotensin II (ANG II) receptor (AT1): this receptor activates both G-protein-dependent path-ways (PLC, PKC, channels, etc.) and β-arrestin-dependent pathways (independent of G proteins, i.e., the src/extracellular signal-regulated kinase/mitogen-activated protein kinase pathway). ANG II activates both signaling pathways; however, the substituted ANG II peptide Sar1, Ile4, Ile8-ANG II (SII) almost exclusively activates the β-arrestin-dependent pathways (Violin & Lefkowitz, 2007). SII is called a “biased agonist” since it activates a preferential signal transduction pathway. Other biased ligands to several 7-TM receptors have been discovered (Gesty-Palmer et al., 2009; Rajagopal et al., 2010; Violin & Lefkowitz, 2007) including the β2-adrenergic receptor: carvedilol, a β-adrenergic receptor antagonist, is able to stabilize a receptor conformation, which, although uncoupled from Gs, is nonetheless able to stimulate β-arrestin-mediated signaling (Drake et al., 2008; Wisler et al., 2007). Through the activation of ETA receptors, ET-1 stimulates both G-protein-dependent and independent pathways (Rosano et al., 2009; Spinella et al., 2009). It is therefore almost obvious that ET-1 acts as a biased ligand. This is also true for ETB receptors, since ET-1 induces internalization of ETB receptors and G-protein-dependent pathways (Farhat et al., 2008; Spinella et al., 2009). Are there specific conditions necessary to reveal the biased activation of ETA by ET-1? What would be the conditions for ET-1 to act like carvedilol does on the β-adrenergic receptor, that is, solely activate the β-arrestin-mediated signaling? Are there pathological conditions that may affect ligand binding and signal transduction? This is an extremely important question because disease states or even aging alone could be responsible for changes in the microdomain such as lipid composition, influencing ligand binding, receptor dimerization (see later), and the subsequent signal transduction. It is known, for example, that oxidized low-density protein can change the microviscosity of the endothelium plasma membrane and alters signal transduction (Hamilton et al., 1994; Thorin et al., 1995). Much, however, needs to be understood from vascular primary cell cultures, isolated vessels, and in vivo preparations.
FIGURE 1. The pleiotropic nature of ET-1 receptor signaling.
Schematic representation of the multiple pathways activated by 7-transmembrane ETA and ETB receptors either directly dependent on G protein activation or independent of G protein activation such as through direct interaction with β-arrestin or PDZ-domain-containing proteins that can act as scaffolds. In addition, ET-1 may act as a bivalent ligand leading to both ETA and ETB receptor activation and possibly dimerization, although this remains to be demonstrated. The signaling pathways activated by either a bivalent ET-1 or a dimerization remain unexplored. Gαs/i/o/q/11, heterotrimeric G protein α subunits of different classes; β arr, β-arrestin; PKC, protein kinase C; Jak, Janus kinase; GIP, other GPCR interacting proteins; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase.
Another recent change in the pharmacological concepts of receptor signal transduction has been introduced by the evidence that ET receptors can form heterodimers, forcing us to change the way we interpreted pharmacological signals (Dai & Galligan, 2006; Evans & Walker, 2008a, 2008b; Gregan et al., 2004; Sauvageau et al., 2006). This observation, however, remains to be translated into physiological significance. So far, heterodimer formation has been reported in heterologous cell preparations expressing ETA and ETB receptors. To the best of our knowledge, we reported the only evidence of potential heterodimers expressed in rat pulmonary arteries (Sauvageau et al., 2006); in these vessels, the pharmacology is complex and difficult to interpret using the classical pharmacological concept of sequential events since for the least, cooperation between the two receptor subtypes exists. In addition, the pharmacology of ET receptors changes in pathological conditions such as experimental pulmonary hypertension in rats (Sauvageau et al., 2009). Although heterodimer formation of ET receptors is likely, the challenge will be to characterize their functions. Another level of complexity has been recently reached with the report that ETB and dopamine D3 receptor heterodimerization (Yu et al., 2009; Zeng et al., 2008): the authors reported aberrant interactions between these two receptors in cultured renal proximal tubule cells with basal D3/ETB receptor coimmunoprecipitation three times greater in Wistar Kyoto rats (WKY) than in spontaneously hypertensive rats (SHR). In vivo, the D3 receptor agonist PD128907 caused natriuresis in WKY, which was partially blocked by ETB receptor antagonism. In contrast, PD128907 blunted sodium excretion in SHR. The authors therefore speculated that there was interaction between the two receptors and that these heterodimers could be partly responsible for hypertension in SHR. If these results can be confirmed in other settings, it will only confirm the complexity of the ET-1 system and offer alternative explanations to unexplained effects of ET-1 in the various systems tested.
When trying to conceive a biological path linking two receptors as dimers, one cannot exclude the possibility that ET-1 is a bivalent ligand, capable of stimulating both receptors simultaneously and thus promoting dimerization of the receptors. This possibility was first proposed by Himeno and collaborators (Himeno et al., 1998), and it was based on the following observation: selective ETB receptor ligands such as S6c, IRL1620, and BQ-788 competitively inhibited 125I-ET-1 binding only when BQ-123 (selective ETA receptor antagonist) was present in the incubation buffer. This therefore suggests that the ETB receptor is capable of binding ET-1 when the ETA receptor is being occupied by BQ-123. A collaboration mechanism between the ETA and the ETB receptors may function in the recognition of ET-1, which is the qualification of a typical “bivalent” ligand. This could be at the basis of the formation of heterodimers at the surface of VSMC (Harada et al., 2002). Unfortunately, no other studies are available that could support this possibility.
Finally, ET-1 can bind with high affinity a newly identified atypical rat receptor, the dual ET-1/ANG II receptor (DEAR) and induces a rise in intra-cellular Ca2+ as efficiently as does ANG II (Ruiz-Opazo et al., 1998). The Dear gene maps to rat chromosome 2 and cosegregates with blood pressure in female F2(normotensive × hypertensive and salt-sensitive [R × S]) intercross rats with highly significant linkage (LOD 3.61) accounting for 14% of blood pressure variance. In Dear−/− mice, angiogenesis is impaired, the neuroepithelial development dysregulated, and is lethal by embryonic day 12.5 (Herrera et al., 2005). Interestingly, mouse DEAR does not bind ANG II as the rat DEAR does, but binds ET-1 and the vascular endothelial growth factor (VEGF) signal peptide (VEGFsp) with equal affinities (Herrera et al., 2005). The hypertension susceptibility in female F2(R × S) intercross rats was validated in humans, in a cohort from northern Sardinia (Glorioso et al., 2007). In the latter, the α1N,K-ATPase (ATP1A1) polymorphism was also tested and concordant with the rat data, and associated with Dear gene polymorphism, albeit in men (and not women). It is interesting that ATP1A1 and Dear are coexpressed in both renal tubular cells and vascular endothelium: it strongly suggests a role in the regulation of blood pressure for these two genes. Altered ATP1A1 and Dear functions in the endothelium could contribute, in combination, to endothelial dysfunction through a putative imbalance of endothelial repair to turnover, because ATP1A1 is implicated in cell proliferation and Dear in angiogenesis. Likewise, ATP1A1 and Dear in renal tubular epithelial cells could affect sodium homeostasis, because ET-1 decreases renal Na, K-ATPase activity. Based on this observation, a net decrease in ET-1/Dear activation could result in greater renal Na+, K+ ATPase activity and increased Na+ reabsorption given the same sodium load, hence salt sensitivity. All these data come from one group of scientists, and the physiology and pharmacology of DEAR has not been studied in depth. We therefore do not know if ET-1 binding site is sensitive to the classical small molecule ET receptor antagonists.
Altogether, these data demonstrate that ET-1 effects are more complex than predicted so far: ETA and ETB receptor cooperation, heterodimerization, the newly discovered DEAR, and a possible bivalent ligand (Fig. 1) are possibilities that have not been fully explored. Based on our data in rat resistance pulmonary arteries (Sauvageau et al., 2006, 2007), we propose that ETA and ETB receptor heterodimerization is an important component in the pharmacological effects of ET-1, although no technique is yet available to evaluate the type of interactions that take place between the two receptors in vivo.
B. Cardiovascular Effects of ET-1
In the systemic and pulmonary circulation, ETA receptors are expressed in the VSMC (Hosoda et al., 1991), while both ETA and ETB receptors are expressed on the surface of VSMC (Ogawa et al., 1991) and EC (Davenport et al., 1993). Both receptors in VSMC induce contraction and cell proliferation in the presence of ET-1 (Clozel et al., 1992; Docherty & MacLean, 1998; LaDouceur et al., 1993; MacLean et al., 1994; Shetty et al., 1993; Sumner et al., 1992). In EC, activation of ETB receptors activates the release of vasodilators and antiproliferative factors such as NO and PGI2 (Clozel et al., 1992; de Nucci et al., 1988; Haynes & Webb, 1993; Muramatsu et al., 1999; Sato et al., 1995). The highest density of ETA/B receptors is found in the lungs and the heart (Simonson & Dunn, 1990).
ET-1 rapidly increases intracellular Ca2+ via activation of the phospholipase C that hydrolyzes phosphatidyl inositol trisphosphate (IP3) and the neutral diacylglycerol (DAG) (Resink et al., 1988). This is followed by a sustained phase of Ca2+ influx associated with activation of secondary multiple intracellular events at the basis of ET-1-induced contraction, relaxation, and secretion (Fig. 1). The rise in IP3 induces a fast and transient increase in [Ca2+]i released from the reticulum, which is at the basis of the activation of membrane-bound channels leading to a sustained increase in [Ca2+]i (Chen & Wagoner, 1991). This leads to numerous signals associated with Ca2+-dependent pathways and Ca2+/calmodulin-dependent pathways (Fig. 1): this includes activation of chloride channels (Haynes & Webb, 1993), the Na+/H+ exchanger resulting in cellular alkalinization and Ca2+ influx through the Na+/Ca2+ exchanger (Grinstein & Rothstein, 1986; Koh et al., 1990), activates Ca2+-induced Ca2+ release from the reticulum via ryanodine receptors and Ca2+-activated K+ channels (Bialecki et al., 1989; Nelson et al., 1995; Simpson & Ashley, 1989). In addition, DAG activates PKC, which leads to numerous intracellular events (Fig. 1) including membrane translocation and activation of phosphokinases (Newton & Keranen, 1994), and damping of the Ca2+ signal (Clerk et al., 1994).
Endothelin receptors are also expressed on the nuclear membrane of VSMC and cardiomyocytes, increasing nuclear Ca2+ concentration, and endogenous nuclear protein kinase activities (Bkaily et al., 2000; Boivin et al., 2003).
1. The Endothelium-Dependent Responses to ET-1
Although ET-1 is known as a potent vasoconstrictor, in healthy animals, in which low levels of blood ET-1 are measured, intracoronary injections of low doses of ET-1 induce a decrease in vascular resistance (Fig. 2): in anesthetized dogs, for example, an intracoronary bolus injection of S6c, a selective ETB receptor agonist, induces a decrease in coronary resistance for doses lower than 1 μg (Teerlink et al., 1994). Likewise, the injection of ET-1 in isolated rat hearts leads to a drop in coronary perfusion pressure at low concentrations of ET-1 (Brunner et al., 2006). In coronary arterial rings isolated from young and healthy pigs, the activation of endothelial ETB receptors induces a significant relaxation (Climent et al., 2005) through the release of NO and PGI2 (Callera et al., 2007; Rubanyi & Polokoff, 1994). In addition, we know that in the human forearm circulation, the increase in blood flow induced by ETA receptor blockade is blunted by ETB receptor antagonism and NOS inhibition (Verhaar et al., 1998), suggesting that endogenous ET-1 exerts a dilatory tone by stimulating endothelial ETB receptors. Nonetheless, the dilatory role of ETB receptors is not significant in isolated human coronary arterial rings (Pierre & Davenport, 1998). However, such ex vivo studies are performed in human coronary vessels isolated from explanted hearts not of healthy subjects, but of patients undergoing cardiac transplantation for ischemic heart disease, vessels in which the expression of endothelial ETB receptors is limited, except in the neovascularization of the atherosclerotic plaque (Bacon et al., 1996), and with a pronounced endothelial dysfunction (Thorin, 2001). This suggests that in pathology the vasodilating effect of endothelial ETB receptor stimulation may be lost. Further data even suggest that in pathology, stimulation of endothelial ETB receptors might be detrimental by inducing effects such as cell adhesion or contraction (Bergdahl et al., 2001; Schneider et al., 2007; Sen et al., 2009).
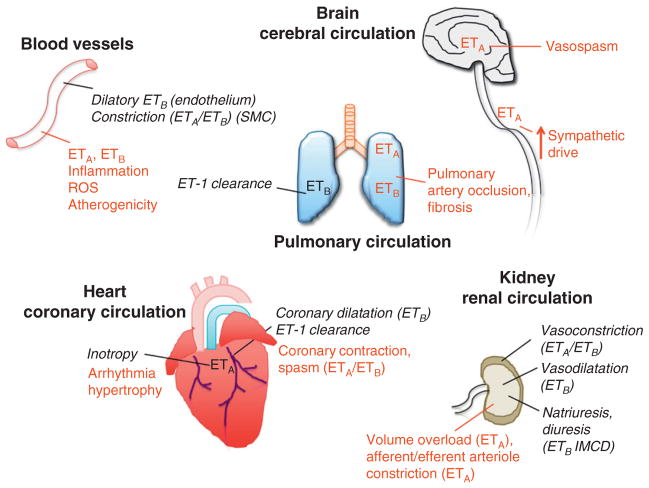
FIGURE 2. Multiple effects of ET-1 in the cardiovascular system.
Physiological responses are presented in italic and black while the responses associated to pathological conditions are in light grey (and in red in the online version).
One final argument supporting a dilatory effect of ET-1 on normal coronary arteries is that the basal production of ET-1 is five times greater toward the lumen than in the interstitial space (Brunner, 1995), which would favor ETB receptor stimulation on the endothelium, although other studies suggest, a polarized secretion of ET-1 toward the underlying VSMCs (Haynes & Webb, 1994; Unoki et al., 1999). Data from the study of Brunner demonstrated that the concentration of free ET-1 in the cardiac interstitial fluid never goes higher than 1 pg/ml (0.4 pM) in healthy animals, which is below the coronary constricting tone, supporting a vasodilatory tone associated with the stimulation of the endothelial ETB autoreceptors in the heart, in physiological conditions.
One should also not underestimate the importance of the concentration of ET-1 in determining the dilatory versus constricting coronary response, because of the heterogeneous distribution of ET-1 receptors as illustrated in cerebral versus pulmonary arteries (Sauvageau et al., 2009). Saturation experiments using iodinated ligands, competition experiments, and reactivity studies using ET-1 receptor antagonists and autoradiography revealed that the expression of ETA receptors is dominant compared to that of ETB receptors in the coronaries of explanted ischemic heart (Bacon & Davenport, 1996; Pierre & Davenport, 1998). The overall effects of ET-1 on vascular tone in vivo are the clear result of the balance between the contraction mediated by VSMC ETA and ETB receptors and the dilation mediated by endothelial ETB receptors (Callera et al., 2007; Thorin et al., 1999). This may explain that dual ET receptor antagonists such as bosentan or macitentan cause no vasodilation in healthy subjects but become vasodilators in pathological vascular beds.
2. Smooth Muscle Contraction, Inflammation, and Vascular Diseases
In rats, injection of bosentan, a dual ETA/B receptor antagonist, does not reduce blood pressure; after blockade of NO production, however, bosentan reduces blood pressure (Richard et al., 1995). This therefore suggests that NO inhibits ET-1-dependent activity in vivo. Bosentan and BQ123 dilate isolated and pressurized rabbit mesenteric arteries in no-flow conditions whether or not NO synthase is blocked (Nguyen et al., 1999). In these latter conditions, however, one might expect a lower influence of NO on the regulation of the vascular diameter since shear stress is nil, therefore favoring the effects of endogenous constrictors such as ET-1. In rat isolated pulmonary arteries, the contraction induced by ET-1 is both ETA and ETB receptor dependent, while in rat cerebral arteries, it is mostly ETA receptor mediated, in agreement with the receptor expression profile in these arteries (Sauvageau et al., 2007, 2009). Coronary vessels, because of the ET-1/NO interdependence and due to their unique hemodynamic features (see later), are very susceptible to higher circulating and locally produced ET-1. The coronary endothelium is prone to dysfunction and highly sensitive to damage, which, with time, accumulates faster in the coronary than in other vascular beds. In addition, coronary endothelial dysfunction is associated with a decline in the contribution of NO in favor of a growing influence of ET-1 (Alonso & Radomski, 2003). Is the increased influence of ET-1 with time in the coronary bed only secondary to the loss of NO or is it due to a change in the ratio of ETA and ETB receptor expression? In any case, this supports the proinflammatory and proconstricting role of ET-1 via its predominant activation on smooth muscle receptors (Griendling et al., 2000; Marsden & Brenner, 1992; Sprague & Khalil, 2009), and could become the basis for the use of ET receptor antagonists to treat coronary artery diseases (CAD).
In cultured cells, it has been shown that ET-1 mRNA is upregulated by inflammatory factors such as TGF-β, TNF-α, interleukins, insulin, and ANG II, and downregulated by NO, PGI2, and shear stress (Boulanger & Luscher, 1990; Brunner et al., 1995; Kohno et al., 1992; Kourembanas et al., 1993; Maemura et al., 1992; Prins et al., 1994). When considering these regulatory mechanisms within the coronary circulation, shear stress is a key element and NO is the key effector (Liu & Gutterman, 2009). In contrast to other vascular beds, wall shear stress in coronary arteries is uneven during the cardiac cycle (Heusch, 2008) and mechanical stress is therefore greatest in the coronary circulation (Thorin & Thorin-Trescases, 2009). In turn, it is not surprising that the coronary circulation is the prime site for endothelial dysfunction. It has been reported that because of these unique physiological hemodynamic features, coronary arteries display an unusual gene pattern when compared to the aorta: a fivefold lower eNOS and a 2.5-fold higher ET-1 mRNA expression (Dancu & Tarbell, 2007). Such a pattern predisposes coronary arteries to endothelial dysfunction and atherosclerosis. Therefore, based only on its physiological characteristics, the coronary circulation should be prone to an increased influence of ET-1 with age: the accumulation of age-related damages would favor ET-1 expression in contrast to that of eNOS and exacerbate endothelial dysfunction (Fig. 1).
Patients with atherosclerosis have elevated plasma levels of ET-1, and an upregulation of ET-1 and its receptors has been described in atherosclerotic arteries and plaques (Barton & Yanagisawa, 2008; Dagassan et al., 1996; Fan et al., 2000; Lerman et al., 1991). BigET-1 and ET-1 immunoreactivity has been found in atherosclerotic regions (Dashwood et al., 1998b; Hasdai et al., 1997). These observations have led to the hypothesis that ET-1 may be associated with the pathogenesis of atherosclerosis (Dashwood & Tsui, 2002; Ivey et al., 2008). In 1998, an important preclinical study (Barton et al., 1998) demonstrated that chronic ETA receptor inhibition improved aortic endothelial dysfunction and reduced the development of atherosclerosis in ApoE knockout mice. Several studies have subsequently demonstrated the beneficial effects of acute intracoronary infusion of the ETA receptor antagonist BQ123 on coronary diameter and coronary flow in patients with CAD. When narrowing the analysis of the dilatory effects of BQ123 to angiographically normal vessels, vessels with plaques and at stenosis, a higher dilation was observed after intracoronary infusion of BQ123 (40 nmol/min, for 60 min) in patients with CAD (Kinlay et al., 2001); in this study, compared with the dilation to nitroglycerin, ET-1 contributed to 39% of coronary vasomotor tone in healthy and angiographically clean vessels, 74% of tone in CAD arteries, and 106% of tone at stenosis. The contribution of NO was, however, not determined: one would assume that the more severe the disease condition, the less NO would be produced, and the more ET-1 would contribute to tone. This hypothesis was tested in 44 patients with CAD in a study published that same year: Halcox et al. provided the first evidence that ET-1, via ETA receptors, contributed to the reduction of endothelial dilatory function (Halcox et al., 2001). The greatest improvement associated with the intracoronary infusion of the ETA receptor antagonist was observed in patients with the greatest endothelial dysfunction as determined in the presence of acetylcholine (Halcox et al., 2001), suggesting that ET-1 contributes to the acute inactivation of NO. However, the tachyphylaxis of NO-dependent dilation occurring following systemic injections of ET-1 could also explain the apparent inactivation of NO by ET-1 (Le Monnier de Gouville et al., 1990); alternatively, we observed that chronic infusion of LU-135252 increased VSMC-sensitivity to NO, suggesting that ET-1 may regulate negatively the sensitivity of the soluble guanylate cyclase (Thorin et al., 2000). Both the selective ETA receptor (BQ123) antagonist and the combination of selective ETA (BQ123) and ETB receptor (BQ788) antagonists improved endothelium-dependent dilation in the coronary arteries of patients with CAD (Bohm et al., 2008). In agreement with these data, using isolated human coronary arteries from idiopathic and atherosclerotic cardiomyopathic hearts, we demonstrated that ET-1-dependent constrictions became more pronounced when the endothelial function was altered (Thorin et al., 1999). Recently, a work by Dr Lerman’s group (Reriani et al., 2010) showed that a chronic (6 months) treatment of patients with premature atherosclerosis with the ETA receptor antagonist atrasentan (10 mg/day) improves coronary endothelial function. Taken together, these data strongly suggest that ET-1 contributes to inactivate the dilatory function of the endothelium in the coronary arterial bed. Hence, the functional contribution of ET-1 is precocious and appears to rise with the severity of CAD.
ET-1, at low concentrations, potentiates coronary contractile responses to other vasoconstrictor substances such as norepinephrine and serotonin (Garcia-Villalon et al., 2008; Rubanyi & Polokoff, 1994; Thorin et al., 1998). In human cerebral arteries, a reduction in endothelium-derived ET-1 accounts for the dilatory effects of endothelial α2-adrenergic receptor stimulation (Thorin et al., 1998). Consequently, even subthreshold concentrations of ET-1 may regulate vascular tone and reactivity in conditions where NO production is reduced, that is, with age and in patients presenting with risk factors for cardiovascular diseases.
The potential physiological roles of ETB receptors, in addition to acting as clearance receptors for ET-1 and stimulating NO release, remain poorly understood. This is likely due to the limited final effects of the stimulation of endothelial ETB receptors on the in vitro vascular function and the possible change in the expression of ET-1 receptors during the development of pathologies as evidenced in pulmonary hypertension (Sauvageau et al., 2009). A change in receptor expression is likely to change the pharmacology of the system. The consequences of receptor inhibition in young and healthy subjects or in old and diseased patients should obviously be different. Since most clinical data have been collected in an elderly population most likely showing some degree of endothelial dysfunction, it is quite possible that our understanding of ET-1 function as a proconstrictor and proinflammatory factor is only a reflection of these data and experimental environment, and thus may not illustrate the effects of ET-1 in young and healthy subjects. In support of this statement, the induction of endothelial damage eliminates ETB-receptor-dependent relaxation in pig coronary arteries (Climent et al., 2005). The seminal demonstration that acetylcholine induces a contraction of coronary arteries in patients with CAD, but a dilation otherwise (Ludmer et al., 1986), is a good example of such a case.
Therefore, based on the literature reviewed so far, one can infer that at physiological and low concentrations, ET-1 predominantly induces dilations, while at pharmacological concentrations it induces contractions. The impact of the inevitable endothelial dysfunction when using isolated arteries from explanted human hearts may have led researchers to underestimate the endothelial dilatory component of ET-1. The production of NO may be reduced, but an alternative explanation may be the loss of coupling between the ETB receptor and the NO pathway without affecting the ability of NO to clear ET-1 from the circulation. For example, acetylcholine induces a contraction of coronary vessels isolated from patients with ischemic heart disease, but substance-P still produces near-maximal relaxation by stimulating NO production (Thorin, 2001). A change in the expression or coupling of the endothelial ETB receptor cannot be excluded in an elderly population (> 65 years of age) and in patients with CAD.
3. Pulmonary Circulation
The pulmonary circulation is highly susceptible to elevated levels of ET-1 which have been associated with PAH (Stewart et al., 1991). Circulating levels of ET-1 are a good marker of disease severity (pulmonary vascular resistance, right atrial pressure, and pulmonary artery oxygen saturation) and predict poor prognosis (Stewart et al., 1991). Upregulation of ET-1 production by the lungs and changes in ET receptor expression could be at the basis of the dysregulation and PAH (Sauvageau et al., 2009; Takahashi et al., 2001). In animal models of PAH, both dual antagonists of ETA and ETB receptors (bosentan) and selective ETA receptor antagonists (sitaxsentan, atrasentan, TBC-3711) are effective in reducing pulmonary artery resistance and inhibiting vascular remodeling. In humans, both types of antagonists are used (Kirkby et al., 2008; Motte et al., 2006).
Bosentan (Tracleer) was approved for the treatment of PAH in 2001 based on two clinical trials, “Study 351” with 32 class III patients with idiopathic PAH or associated with systemic sclerosis (Channick et al., 2001) and the important BREATHE-1 study that included 150 patients with idiopathic PAH, 47 with systemic sclerosis–associated PAH, and 16 with systemic lupus erythematosus–associated PAH (Rubin et al., 2002). In the latter study, bosentan improved exercise capacity, the functional class, and increased the time to clinical worsening. Sitaxsentan was approved for treatment of PAH in 2006 based on the STRIDE-1 results (Barst et al., 2004). Recently, ambrisentan has been approved for the treatment of PAH (Galie et al., 2008). No clinical advantages have been demonstrated between dual ET receptor antagonist and selective ETA receptor antagonists.
4. Cardiac Myocyte Function and Heart Failure
ET-1 has positive cardiac inotropic effects in healthy animals and humans (Kang & Walker, 2006; Katoh et al., 1998; Kelly et al., 1990; Li et al., 1991; Pieske et al., 1999), but not in failing human hearts (MacCarthy et al., 2000; Pieske et al., 1999). ET-1 enhances myocyte contractility by activating ETA receptor-phospholipase Cβ-PKCε signaling complexes preferentially localized in cardiac T-tubules (Robu et al., 2003). It has been shown that ET-1 is devoid of any significant effects on basal L-type Ca2+ channel activity, but exerts a potent inhibitory effect against isoprenaline-enhanced L-type Ca2+ channel current (He et al., 2000; Watanabe & Endoh, 2000). This effect is mediated through ETA receptors coupled to pertussis toxin–sensitive G proteins (He et al., 2000; Thomas et al., 1997).
As mentioned earlier, ET-1 has growth-promoting effects (Inada et al., 1999). Because there is a correlation between ventricular mass and ET-1 concentration in the blood, it is possible that ET-1 contributes to the ventricular hypertrophy in patients with ischemic heart failure and dilated cardiomyopathy (Tsutamoto et al., 2000) and in rats following coronary artery ligation (Loennechen et al., 2001). ET-1 also promotes sympathetic tone, especially in heart failure as demonstrated in rabbits (Liu et al., 2001) and dogs (McConnell et al., 2000). This may partly explain the proarrhythmic effects of ET-1 (Burrell et al., 2000; Yorikane & Koike, 1990; Yorikane et al., 1990), while circulating levels of ET-1 have been associated with arrhythmia in patients with decompensated heart failure (Aronson & Burger, 2003a, 2003b; Aronson et al., 2001).
5. Renal Effects of ET-1
The effects of ET-1 on the kidney are complex. Exogenous administration of ET-1 induces a vasoconstriction in the renal cortex and a vasodilatation in the medulla (Rubinstein et al., 1995). The latter is mediated by ETB receptors while the former is dependent on both ETA and ETB receptor activation (Dhaun et al., 2006). Acutely, selective ETA receptor antagonism with BQ123 reduced blood pressure, proteinuria, and pulse wave velocity on top of standard treatment in patients with nondiabetic chronic kidney diseases (Dhaun et al., 2009). In diabetic patients with chronic kidney disease, however, chronic ETA receptor antagonism with avosentan is deleterious due to fluid overload and congestive heart failure (Mann et al., 2010). In the inner-medullary-collecting duct of mice, ET-1 induces an autocrine natriuretic and diuretic effect which seems mediated by ETB receptors, since specific inner-medullary-collecting duct deletion of ETB but not ETA receptors leads to salt-sensitive hypertension (Bagnall et al., 2006; Ge et al., 2006). However, the renal effects of ETB receptor antagonism or deletion are inhibited by ETA receptor antagonism, showing that it is the reactive increase in ET-1 acting on ETA receptors, not the deletion of ETB receptors per se, which is responsible for hypertension and tissue injury (Matsumura et al., 2000). Dual ET antagonists seem to give very low rates of fluid retention and edema in the clinical setting. In PAH clinical trials with Tracleer, combined adverse events of fluid retention or edema were reported in 1.7% (placebo-corrected) of patients (Tracleer US package insert, 2009). In a Phase II study in hypertensive patients, the novel dual ET antagonist macitentan did not cause peripheral edema (Press release Actelion Dec 2006). It is therefore possible that ET receptor antagonists can be used safely in patients with renal diseases, but this remains to be validated in a proper clinical trial.
III. Conclusion
Numerous clinical developments are ongoing with ET receptor antagonists (Aubert & Juillerat-Jeanneret, 2009). Our understanding of receptor pharmacology in general is changing with the appearance of new concepts including dimerization and G-protein-independent signaling. These changes apply to ET-1 and its receptors. One major weakness, however, which applies to many other pharmacological systems, is our lack of knowledge of the evolution of ET-1 and its receptors in the aging human and how this influences cardiovascular function in combination with risk factors for cardiovascular diseases. The critical role for ET-1 in controlling cardiovascular function is evident by the fact that its clinical importance was established within a few years after its discovery. It is likely that work in this area will continue to yield novel therapies for the treatment of cardiovascular disease.
Acknowledgments
The laboratory of Dr. Eric Thorin is supported by the Canadian Institutes for Health Research (MOP 14496 and 89733), the Heart and Stroke Foundation of Quebec, and the Foundation of the Montreal Heart Institute.
Abbreviations
- 7-TM
7-transmembrane domain
- ANG II
angiotensin II
- EDCF
endothelium-derived constricting factor
- ET-1
endothelin-1
- GPCR
G protein-coupled receptor
- PAH
pulmonary arterial hypertension
- PGI2
prostacyclin
- S6c
sarafotoxin 6c
Footnotes
Conflict of Interest: Dr. Martine Clozel is Chief Scientific Officer at Actelion Pharmaceuticals.
References
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circulation Research. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. British Journal of Pharmacology. 2009;158(Suppl 1):S42–S43. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso D, Radomski MW. The nitric oxide-endothelin-1 connection. Heart Failure Reviews. 2003;8:107–115. doi: 10.1023/a:1022155206928. [DOI] [PubMed] [Google Scholar]
- Anggrahini DW, Emoto N, Nakayama K, Widyantoro B, Adiarto S, Iwasa N, Nonaka H, Rikitake Y, Kisanuki YY, Yanagisawa M, Hirata K. Vascular endothelial cell-derived endothelin-1 mediates vascular inflammation and neointima formation following blood flow cessation. Cardiovascular Research. 2009;82:143–151. doi: 10.1093/cvr/cvp026. [DOI] [PubMed] [Google Scholar]
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Aronson D, Burger AJ. Neurohormonal prediction of mortality following admission for decompensated heart failure. The American Journal of Cardiology. 2003a;91:245–248. doi: 10.1016/s0002-9149(02)03119-3. [DOI] [PubMed] [Google Scholar]
- Aronson D, Burger AJ. Neurohumoral activation and ventricular arrhythmias in patients with decompensated congestive heart failure: Role of endothelin. Pacing and Clinical Electrophysiology. 2003b;26:703–710. doi: 10.1046/j.1460-9592.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- Aronson D, Mittleman MA, Burger AJ. Role of endothelin in modulation of heart rate variability in patients with decompensated heart failure. Pacing and Clinical Electrophysiology. 2001;24:1607–1615. doi: 10.1046/j.1460-9592.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Aubert JD, Juillerat-Jeanneret L. Therapeutic potential of endothelin receptor modulators: Lessons from human clinical trials. Expert Opinion on Therapeutic Targets. 2009;13:1069–1084. doi: 10.1517/14728220903074570. [DOI] [PubMed] [Google Scholar]
- Bacon CR, Cary NR, Davenport AP. Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circulation Research. 1996;79:794–801. doi: 10.1161/01.res.79.4.794. [DOI] [PubMed] [Google Scholar]
- Bacon CR, Davenport AP. Endothelin receptors in human coronary artery and aorta. British Journal of Pharmacology. 1996;117:986–992. doi: 10.1111/j.1476-5381.1996.tb15292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, Webb DJ, Kotelevtsev YV. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48:286–293. doi: 10.1161/01.HYP.0000229907.58470.4c. [DOI] [PubMed] [Google Scholar]
- Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, Zwicke D, Duncan B, Dixon RA, Frumkin LR. Sitaxsentan therapy for pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- Barton M, Haudenschild CC, d’Uscio LV, Shaw S, Munter K, Luscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Canadian Journal of Physiology and Pharmacology. 2008;86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Valdemarsson S, Adner M, Sun XY, Hedner T, Edvinsson L. Enhanced endothelin-1-induced contractions in mesenteric arteries from rats with congestive heart failure: Role of ET(B) receptors. European Journal of Heart Failure. 2001;3:293–299. doi: 10.1016/s1388-9842(01)00138-6. [DOI] [PubMed] [Google Scholar]
- Bialecki RA, Izzo NJ, Jr, Colucci WS. Endothelin-1 increases intracellular calcium mobilization but not calcium uptake in rabbit vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1989;164:474–479. doi: 10.1016/0006-291x(89)91744-0. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Choufani S, Hassan G, El-Bizri N, Jacques D, D’Orleans-Juste P. Presence of functional endothelin-1 receptors in nuclear membranes of human aortic vascular smooth muscle cells. Journal of Cardiovascular Pharmacology. 2000;36:S414–S417. doi: 10.1097/00005344-200036051-00121. [DOI] [PubMed] [Google Scholar]
- Bohm F, Jensen J, Svane B, Settergren M, Pernow J. Intracoronary endothelin receptor blockade improves endothelial function in patients with coronary artery disease. Canadian Journal of Physiology and Pharmacology. 2008;86:745–751. doi: 10.1139/Y08-081. [DOI] [PubMed] [Google Scholar]
- Boivin B, Chevalier D, Villeneuve LR, Rousseau E, Allen BG. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. The Journal of Biological Chemistry. 2003;278:29153–29163. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. The Journal of Clinical Investigation. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner F. Tissue endothelin-1 levels in perfused rat heart following stimulation with agonists and in ischaemia and reperfusion. Journal of Molecular and Cellular Cardiology. 1995;27:1953–1963. doi: 10.1016/0022-2828(95)90017-9. [DOI] [PubMed] [Google Scholar]
- Brunner F, Bras-Silva C, Cerdeira AS, Leite-Moreira AF. Cardiovascular endothelins: Essential regulators of cardiovascular homeostasis. Pharmacology & Therapeutics. 2006;111:508–531. doi: 10.1016/j.pharmthera.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Brunner F, Stessel H, Kukovetz WR. Novel guanylyl cyclase inhibitor, ODQ reveals role of nitric oxide, but not of cyclic GMP in endothelin-1 secretion. FEBS Letters. 1995;376:262–266. doi: 10.1016/0014-5793(95)01297-x. [DOI] [PubMed] [Google Scholar]
- Burrell KM, Molenaar P, Dawson PJ, Kaumann AJ. Contractile and arrhythmic effects of endothelin receptor agonists in human heart in vitro: Blockade with SB 209670. The Journal of Pharmacology and Experimental Therapeutics. 2000;292:449–459. [PubMed] [Google Scholar]
- Callera G, Tostes R, Savoia C, Muscara MN, Touyz RM. Vasoactive peptides in cardiovascular (patho)physiology. Expert Review of Cardiovascular Therapy. 2007;5:531–552. doi: 10.1586/14779072.5.3.531. [DOI] [PubMed] [Google Scholar]
- Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: A randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- Chen C, Wagoner PK. Endothelin induces a nonselective cation current in vascular smooth muscle cells. Circulation Research. 1991;69:447–454. doi: 10.1161/01.res.69.2.447. [DOI] [PubMed] [Google Scholar]
- Clerk A, Bogoyevitch MA, Anderson MB, Sugden PH. Differential activation of protein kinase C isoforms by endothelin-1 and phenylephrine and subsequent stimulation of p42 and p44 mitogen-activated protein kinases in ventricular myocytes cultured from neonatal rat hearts. The Journal of Biological Chemistry. 1994;269:32848–32857. [PubMed] [Google Scholar]
- Climent B, Fernandez N, Sanz E, Sanchez A, Monge L, Garcia-Villalon AL, Dieguez G. Enhanced response of pig coronary arteries to endothelin-1 after ischemia-reperfusion. Role of endothelin receptors, nitric oxide and prostanoids. European Journal of Pharmacology. 2005;524:102–110. doi: 10.1016/j.ejphar.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Clozel M, Breu V, Burri K, Cassal JM, Fischli W, Gray GA, Hirth G, Loffler BM, Muller M, Neidhart W, et al. Pathophysiological role of endothelin revealed by the first orally active endothelin receptor antagonist. Nature. 1993;365:759–761. doi: 10.1038/365759a0. [DOI] [PubMed] [Google Scholar]
- Clozel M, Gray GA, Breu V, Loffler BM, Osterwalder R. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochemical and Biophysical Research Communications. 1992;186:867–873. doi: 10.1016/0006-291x(92)90826-7. [DOI] [PubMed] [Google Scholar]
- Dagassan PH, Breu V, Clozel M, Kunzli A, Vogt P, Turina M, Kiowski W, Clozel JP. Up-regulation of endothelin-B receptors in atherosclerotic human coronary arteries. Journal of Cardiovascular Pharmacology. 1996;27:147–153. doi: 10.1097/00005344-199601000-00023. [DOI] [PubMed] [Google Scholar]
- Dai X, Galligan JJ. Differential trafficking and desensitization of human ET(A) and ET(B) receptors expressed in HEK 293 cells. Experimental Biology and Medicine (Maywood) 2006;231:746–751. [PubMed] [Google Scholar]
- Dancu MB, Tarbell JM. Coronary endothelium expresses a pathologic gene pattern compared to aortic endothelium: Correlation of asynchronous hemodynamics and pathology in vivo. Atherosclerosis. 2007;192:9–14. doi: 10.1016/j.atherosclerosis.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Mehta D, Izzat MB, Timm M, Bryan AJ, Angelini GD, Jeremy JY. Distribution of endothelin-1 (ET) receptors (ET(A) and ET(B)) and immunoreactive ET-1 in porcine saphenous vein-carotid artery interposition grafts. Atherosclerosis. 1998a;137:233–242. doi: 10.1016/s0021-9150(97)00249-9. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Timm M, Muddle JR, Ong AC, Tippins JR, Parker R, McManus D, Murday AJ, Madden BP, Kaski JC. Regional variations in endothelin-1 and its receptor subtypes in human coronary vasculature: Pathophysiological implications in coronary disease. Endothelium. 1998b;6:61–70. doi: 10.3109/10623329809053405. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Tsui JC. Endothelin-1 and atherosclerosis: Potential complications associated with endothelin-receptor blockade. Atherosclerosis. 2002;160:297–304. doi: 10.1016/s0021-9150(01)00586-x. [DOI] [PubMed] [Google Scholar]
- Davenport AP. International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacological Reviews. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Maguire JJ. The endothelin system in human saphenous vein graft disease. Current Opinion in Pharmacology. 2001;1:176–182. doi: 10.1016/s1471-4892(01)00026-1. [DOI] [PubMed] [Google Scholar]
- Davenport AP, O’Reilly G, Molenaar P, Maguire JJ, Kuc RE, Sharkey A, Bacon CR, Ferro A. Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: Evidence for expression of ETB receptors in human vascular smooth muscle. Journal of Cardiovascular Pharmacology. 1993;22(Suppl 8):S22–S25. doi: 10.1097/00005344-199322008-00008. [DOI] [PubMed] [Google Scholar]
- De Mey JG, Vanhoutte PM. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circulation Research. 1982;51:439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- de Nucci G, Thomas R, D’Orleans-Juste P, Antunes E, Walder C, Warner TD, Vane JR. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denault JB, Claing A, D’Orleans-Juste P, Sawamura T, Kido T, Masaki T, Leduc R. Processing of proendothelin-1 by human furin convertase. FEBS Letters. 1995;362:276–280. doi: 10.1016/0014-5793(95)00249-9. [DOI] [PubMed] [Google Scholar]
- Dhaun N, Goddard J, Webb DJ. The endothelin system and its antagonism in chronic kidney disease. Journal of the American Society of Nephrology. 2006;17:943–955. doi: 10.1681/ASN.2005121256. [DOI] [PubMed] [Google Scholar]
- Dhaun N, Macintyre IM, Melville V, Lilitkarntakul P, Johnston NR, Goddard J, Webb DJ. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension. 2009;54:113–119. doi: 10.1161/HYPERTENSIONAHA.109.132670. [DOI] [PubMed] [Google Scholar]
- Docherty CC, MacLean MR. EndothelinB receptors in rabbit pulmonary resistance arteries: Effect of left ventricular dysfunction. The Journal of Pharmacology and Experimental Therapeutics. 1998;284:895–903. [PubMed] [Google Scholar]
- Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, Ohlstein EH, Willette RN. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. British Journal of Pharmacology. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. beta-arrestin-biased agonism at the beta2-adrenergic receptor. The Journal of Biological Chemistry. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: Exclusive role of ETB receptors. Journal of Applied Physiology. 1996a;81:1510–1515. doi: 10.1152/jappl.1996.81.4.1510. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Goresky CA, Rose CP, Stewart DJ, Cernacek P, Schwab AJ, Simard A. Endothelin-1 myocardial clearance, production, and effect on capillary permeability in vivo. The American Journal of Physiology. 1997;273:H1239–H1245. doi: 10.1152/ajpheart.1997.273.3.H1239. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Stewart DJ, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation. 1996b;94:1578–1584. doi: 10.1161/01.cir.94.7.1578. [DOI] [PubMed] [Google Scholar]
- Evans NJ, Walker JW. Endothelin receptor dimers evaluated by FRET, ligand binding, and calcium mobilization. Biophysical Journal. 2008a;95:483–492. doi: 10.1529/biophysj.107.119206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans NJ, Walker JW. Sustained Ca2+ signaling and delayed internalization associated with endothelin receptor heterodimers linked through a PDZ finger. Canadian Journal of Physiology and Pharmacology. 2008b;86:526–535. doi: 10.1139/Y08-050. [DOI] [PubMed] [Google Scholar]
- Fan J, Unoki H, Iwasa S, Watanabe T. Role of endothelin-1 in atherosclerosis. Annals of New York Academy of Sciences. 2000;902:84–93. doi: 10.1111/j.1749-6632.2000.tb06303.x. discussion 93–94. [DOI] [PubMed] [Google Scholar]
- Farhat N, Matouk CC, Mamarbachi AM, Marsden PA, Allen BG, Thorin E. Activation of ETB receptors regulates the abundance of ET-1 mRNA in vascular endothelial cells. British Journal of Pharmacology. 2008;153:1420–1431. doi: 10.1038/bjp.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau MH, Honore JC, Plante M, Labonte J, Rae GA, D’Orleans-Juste P. Endothelin-1 (1-31) is an intermediate in the production of endothelin-1 after big endothelin-1 administration in vivo. Hypertension. 2005;46:87–92. doi: 10.1161/01.HYP.0000170460.24604.23. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: Implications for drug discovery. Trends in Pharmacological Sciences. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ. Ambrisentan for the treatment of pulmonary arterial hypertension: Results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- Garcia-Villalon AL, Amezquita YM, Monge L, Fernandez N, Salcedo A, Dieguez G. Endothelin-1 potentiation of coronary artery contraction after ischemia-reperfusion. Vascular Pharmacology. 2008;48:109–114. doi: 10.1016/j.vph.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. The American Journal of Physiology—Renal Physiology. 2006;291:F1274–F1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Science Translational Medicine. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso N, Herrera VL, Bagamasbad P, Filigheddu F, Troffa C, Argiolas G, Bulla E, Decano JL, Ruiz-Opazo N. Association of ATP1A1 and dear single-nucleotide polymorphism haplotypes with essential hypertension: Sex-specific and haplotype-specific effects. Circulation Research. 2007;100:1522–1529. doi: 10.1161/01.RES.0000267716.96196.60. [DOI] [PubMed] [Google Scholar]
- Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. The Journal of Biological Chemistry. 2004;279:27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. The Journal of Membrane Biology. 1986;90:1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Nour KR, Zalos G, Quyyumi AA. Coronary vasodilation and improvement in endothelial dysfunction with endothelin ET(A) receptor blockade. Circulation Research. 2001;89:969–976. doi: 10.1161/hh2301.100980. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Thorin E, McCulloch J, Dominiczak MH, Reid JL. Chronic exposure of bovine aortic endothelial cells to native and oxidized LDL modifies phosphatidylinositol metabolism. Atherosclerosis. 1994;107:55–63. doi: 10.1016/0021-9150(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Harada N, Himeno A, Shigematsu K, Sumikawa K, Niwa M. Endothelin-1 binding to endothelin receptors in the rat anterior pituitary gland: Possible formation of an ETA-ETB receptor heterodimer. Cellular and Molecular Neurobiology. 2002;22:207–226. doi: 10.1023/a:1019822107048. [DOI] [PubMed] [Google Scholar]
- Hasdai D, Mathew V, Schwartz RS, Smith LA, Holmes DR, Jr, Katusic ZS, Lerman A. Enhanced endothelin-B-receptor-mediated vasoconstriction of small porcine coronary arteries in diet-induced hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17:2737–2743. doi: 10.1161/01.atv.17.11.2737. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Endothelium-dependent modulation of responses to endothelin-I in human veins. Clinical Science (London) 1993;84:427–433. doi: 10.1042/cs0840427. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- He JQ, Pi Y, Walker JW, Kamp TJ. Endothelin-1 and photoreleased diacylglycerol increase L-type Ca2+ current by activation of protein kinase C in rat ventricular myocytes. Journal de Physiologie. 2000;524(Pt 3):807–820. doi: 10.1111/j.1469-7793.2000.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera VL, Ponce LR, Bagamasbad PD, VanPelt BD, Didishvili T, Ruiz-Opazo N. Embryonic lethality in Dear gene-deficient mice: New player in angiogenesis. Physiological Genomics. 2005;23:257–268. doi: 10.1152/physiolgenomics.00144.2005. [DOI] [PubMed] [Google Scholar]
- Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: Benefit from selective bradycardic agents. British Journal of Pharmacology. 2008;153:1589–1601. doi: 10.1038/sj.bjp.0707673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno A, Shigematsu K, Taguchi T, Niwa M. Endothelin-1 binding to endothelin receptors in the rat anterior pituitary gland: Interaction in the recognition of endothelin-1 between ETA and ETB receptors. Cellular and Molecular Neurobiology. 1998;18:447–452. doi: 10.1023/a:1022557717481. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Nakao K, Hiroshi A, Suga S, Ogawa Y, Mukoyama M, Shirakami G, Saito Y, Nakanishi S, Imura H. Cloning and expression of human endothelin-1 receptor cDNA. FEBS Letters. 1991;287:23–26. doi: 10.1016/0014-5793(91)80007-p. [DOI] [PubMed] [Google Scholar]
- Ihling C, Szombathy T, Bohrmann B, Brockhaus M, Schaefer HE, Loeffler BM. Coexpression of endothelin-converting enzyme-1 and endothelin-1 in different stages of human atherosclerosis. Circulation. 2001;104:864–869. doi: 10.1161/hc3301.094742. [DOI] [PubMed] [Google Scholar]
- Inada T, Fujiwara H, Hasegawa K, Araki M, Yamauchi-Kohno R, Yabana H, Fujiwara T, Tanaka M, Sasayama S. Upregulated expression of cardiac endothelin-1 participates in myocardial cell growth in Bio14.6 Syrian cardiomyopathic hamsters. Journal of the American College of Cardiology. 1999;33:565–571. doi: 10.1016/s0735-1097(98)00564-6. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proceedings of the National Academy of Sciences of the United States of America. 1989a;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. The Journal of Biological Chemistry. 1989b;264:14954–14959. [PubMed] [Google Scholar]
- Ivey ME, Osman N, Little PJ. Endothelin-1 signalling in vascular smooth muscle: Pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis. 2008;199:237–247. doi: 10.1016/j.atherosclerosis.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kang M, Walker JW. Endothelin-1 and PKC induce positive inotropy without affecting pHi in ventricular myocytes. Experimental Biology and Medicine (Maywood) 2006;231:865–870. [PubMed] [Google Scholar]
- Katoh H, Terada H, Iimuro M, Sugiyama S, Qing K, Satoh H, Hayashi H. Heterogeneity and underlying mechanism for inotropic action of endothelin-1 in rat ventricular myocytes. British Journal of Pharmacology. 1998;123:1343–1350. doi: 10.1038/sj.bjp.0701743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierski RM, Yanagisawa M. Endothelin system: The double-edged sword in health and disease. Annual Review of Pharmacology and Toxicology. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- Kelly RA, Eid H, Kramer BK, O’Neill M, Liang BT, Reers M, Smith TW. Endothelin enhances the contractile responsiveness of adult rat ventricular myocytes to calcium by a pertussis toxin-sensitive pathway. The Journal of Clinical Investigation. 1990;86:1164–1171. doi: 10.1172/JCI114822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Collateral efficacy in drug discovery: Taking advantage of the good (allosteric) nature of 7TM receptors. Trends in Pharmacological Sciences. 2007;28:407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Behrendt D, Wainstein M, Beltrame J, Fang JC, Creager MA, Selwyn AP, Ganz P. Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation. 2001;104:1114–1118. doi: 10.1161/hc3501.095707. [DOI] [PubMed] [Google Scholar]
- Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: Great expectations or bleak house? British Journal of Pharmacology. 2008;153:1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh E, Morimoto S, Kim S, Nabata T, Miyashita Y, Ogihara T. Endothelin stimulates Na+/H+ exchange in vascular smooth muscle cells. Biochemistry International. 1990;20:375–380. [PubMed] [Google Scholar]
- Kohno M, Horio T, Yokokawa K, Kurihara N, Takeda T. C-type natriuretic peptide inhibits thrombin- and angiotensin II-stimulated endothelin release via cyclic guanosine 3′,5′-monophosphate. Hypertension. 1992;19:320–325. doi: 10.1161/01.hyp.19.4.320. [DOI] [PubMed] [Google Scholar]
- Komukai K, Jin OU, Morimoto S, Kawai M, Hongo K, Yoshimura M, Kurihara S. Role of Ca2+/calmodulin-dependent protein kinase II in the regulation of the cardiac L-type Ca2+ current during endothelin-1 stimulation. American Journal of Physiology Heart and Circulatory Physiology. 2010;298:H1902–H1907. doi: 10.1152/ajpheart.01141.2009. [DOI] [PubMed] [Google Scholar]
- Kourembanas S, McQuillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. The Journal of Clinical Investigation. 1993;92:99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDouceur DM, Flynn MA, Keiser JA, Reynolds E, Haleen SJ. ETA and ETB receptors coexist on rabbit pulmonary artery vascular smooth muscle mediating contraction. Biochemical and Biophysical Research Communications. 1993;196:209–215. doi: 10.1006/bbrc.1993.2236. [DOI] [PubMed] [Google Scholar]
- Laporte S, Denault JB, D’Orleans-Juste P, Leduc R. Presence of furin mRNA in cultured bovine endothelial cells and possible involvement of furin in the processing of the endothelin precursor. Journal of Cardiovascular Pharmacology. 1993;22(Suppl 8):S7–S10. doi: 10.1097/00005344-199322008-00004. [DOI] [PubMed] [Google Scholar]
- Le Monnier de Gouville AC, Lippton H, Cohen G, Cavero I, Hyman A. Vasodilator activity of endothelin-1 and endothelin-3: Rapid development of cross-tachyphylaxis and dependence on the rate of endothelin administration. The Journal of Pharmacology and Experimental Therapeutics. 1990;254:1024–1028. [PubMed] [Google Scholar]
- Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. The New England Journal of Medicine. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Li K, Stewart DJ, Rouleau JL. Myocardial contractile actions of endothelin-1 in rat and rabbit papillary muscles. Role of endocardial endothelium. Circulation Research. 1991;69:301–312. doi: 10.1161/01.res.69.2.301. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gutterman DD. Vascular control in humans: Focus on the coronary microcirculation. Basic Research in Cardiology. 2009;104:211–227. doi: 10.1007/s00395-009-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Pliquett RU, Brewer E, Cornish KG, Shen YT, Zucker IH. Chronic endothelin-1 blockade reduces sympathetic nerve activity in rabbits with heart failure. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2001;280:R1906–R1913. doi: 10.1152/ajpregu.2001.280.6.R1906. [DOI] [PubMed] [Google Scholar]
- Loennechen JP, Stoylen A, Beisvag V, Wisloff U, Ellingsen O. Regional expression of endothelin-1, ANP, IGF-1, and LV wall stress in the infarcted rat heart. American Journal of Physiology. Heart and Circulatory Physiology. 2001;280:H2902–H2910. doi: 10.1152/ajpheart.2001.280.6.H2902. [DOI] [PubMed] [Google Scholar]
- Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. The New England Journal of Medicine. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Yang Z, Tschudi M, von Segesser L, Stulz P, Boulanger C, Siebenmann R, Turina M, Buhler FR. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circulation Research. 1990;66:1088–1094. doi: 10.1161/01.res.66.4.1088. [DOI] [PubMed] [Google Scholar]
- MacCarthy PA, Grocott-Mason R, Prendergast BD, Shah AM. Contrasting inotropic effects of endogenous endothelin in the normal and failing human heart: Studies with an intracoronary ET(A) receptor antagonist. Circulation. 2000;101:142–147. doi: 10.1161/01.cir.101.2.142. [DOI] [PubMed] [Google Scholar]
- MacLean MR, McCulloch KM, Baird M. Endothelin ETA- and ETB-receptor-mediated vasoconstriction in rat pulmonary arteries and arterioles. Journal of Cardiovascular Pharmacology. 1994;23:838–845. doi: 10.1097/00005344-199405000-00022. [DOI] [PubMed] [Google Scholar]
- Maemura K, Kurihara H, Morita T, Oh-hashi Y, Yazaki Y. Production of endothelin-1 in vascular endothelial cells is regulated by factors associated with vascular injury. Gerontology. 1992;38(Suppl 1):29–35. doi: 10.1159/000213360. [DOI] [PubMed] [Google Scholar]
- Maguire J, Davenport AP. Alternative pathway to endothelin-converting enzyme for the synthesis of endothelin in human blood vessels. Journal of Cardiovascular Pharmacology. 2004;44(Suppl 1):S27–S29. doi: 10.1097/01.fjc.0000166219.65593.af. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Kuc RE, Davenport AP. Vasoconstrictor activity of novel endothelin peptide, ET-1(1–31), in human mammary and coronary arteries in vitro. British Journal of Pharmacology. 2001;134:1360–1366. doi: 10.1038/sj.bjp.0704384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G. Avosentan for overt diabetic nephropathy. Journal of American Society of Nephrology. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. The American Journal of Physiology. 1992;262:C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Kuro T, Kobayashi Y, Konishi F, Takaoka M, Wessale JL, Opgenorth TJ, Gariepy CE, Yanagisawa M. Exaggerated vascular and renal pathology in endothelin-B receptor-deficient rats with deoxycorticosterone acetate-salt hypertension. Circulation. 2000;102:2765–2773. doi: 10.1161/01.cir.102.22.2765. [DOI] [PubMed] [Google Scholar]
- McConnell PI, Olson CE, Patel KP, Blank DU, Olivari MT, Gallagher KP, Quenby-Brown E, Zucker IH. Chronic endothelin blockade in dogs with pacing-induced heart failure: Possible modulation of sympathoexcitation. Journal of Cardiac Failure. 2000;6:56–65. doi: 10.1016/s1071-9164(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Motte S, McEntee K, Naeije R. Endothelin receptor antagonists. Pharmacology & Therapeutics. 2006;110:386–414. doi: 10.1016/j.pharmthera.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Oka M, Morio Y, Soma S, Takahashi H, Fukuchi Y. Chronic hypoxia augments endothelin-B receptor-mediated vasodilation in isolated perfused rat lungs. The American Journal of Physiology. 1999;276:L358–L364. doi: 10.1152/ajplung.1999.276.2.L358. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Newton AC, Keranen LM. Phosphatidyl-L-serine is necessary for protein kinase C′s high-affinity interaction with diacylglycerol-containing membranes. Biochemistry. 1994;33:6651–6658. doi: 10.1021/bi00187a035. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Vequaud P, Thorin E. Effects of endothelin receptor antagonists and nitric oxide on myogenic tone and alpha-adrenergic-dependent contractions of rabbit resistance arteries. Cardiovascular Research. 1999;43:755–761. doi: 10.1016/s0008-6363(99)00170-4. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Nakao K, Arai H, Nakagawa O, Hosoda K, Suga S, Nakanishi S, Imura H. Molecular cloning of a non-isopeptide-selective human endothelin receptor. Biochemical and Biophysical Research Communications. 1991;178:248–255. doi: 10.1016/0006-291x(91)91806-n. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pierre LN, Davenport AP. Endothelin receptor subtypes and their functional relevance in human small coronary arteries. British Journal of Pharmacology. 1998;124:499–506. doi: 10.1038/sj.bjp.0701865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieske B, Beyermann B, Breu V, Loffler BM, Schlotthauer K, Maier LS, Schmidt-Schweda S, Just H, Hasenfuss G. Functional effects of endothelin and regulation of endothelin receptors in isolated human nonfailing and failing myocardium. Circulation. 1999;99:1802–1809. doi: 10.1161/01.cir.99.14.1802. [DOI] [PubMed] [Google Scholar]
- Prins BA, Hu RM, Nazario B, Pedram A, Frank HJ, Weber MA, Levin ER. Prostaglandin E2 and prostacyclin inhibit the production and secretion of endothelin from cultured endothelial cells. The Journal of Biological Chemistry. 1994;269:11938–11944. [PubMed] [Google Scholar]
- Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reriani M, Raichlin E, Prasad A, Mathew V, Pumper GM, Nelson RE, Lennon R, Rihal C, Lerman LO, Lerman A. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010;122:958–966. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink TJ, Scott-Burden T, Buhler FR. Endothelin stimulates phospholipase C in cultured vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1988;157:1360–1368. doi: 10.1016/s0006-291x(88)81025-8. [DOI] [PubMed] [Google Scholar]
- Richard V, Hogie M, Clozel M, Loffler BM, Thuillez C. In vivo evidence of an endothelin-induced vasopressor tone after inhibition of nitric oxide synthesis in rats. Circulation. 1995;91:771–775. doi: 10.1161/01.cir.91.3.771. [DOI] [PubMed] [Google Scholar]
- Robu VG, Pfeiffer ES, Robia SL, Balijepalli RC, Pi Y, Kamp TJ, Walker JW. Localization of functional endothelin receptor signaling complexes in cardiac transverse tubules. The Journal of Biological Chemistry. 2003;278:48154–48161. doi: 10.1074/jbc.M304396200. [DOI] [PubMed] [Google Scholar]
- Rosano L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi GP, Andreis PG, Colonna S, Albertin G, Aragona F, Belloni AS, Nussdorfer GG. Endothelin-1[1-31]: A novel autocrine-paracrine regulator of human adrenal cortex secretion and growth. The Journal of Clinical Endocrinology and Metabolism. 2002;87:322–328. doi: 10.1210/jcem.87.1.8134. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA. Endothelins: Molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacological Reviews. 1994;46:325–415. [PubMed] [Google Scholar]
- Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. The New England Journal of Medicine. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Gurbanov K, Hoffman A, Better OS, Winaver J. Differential effect of endothelin-1 on renal regional blood flow: Role of nitric oxide. Journal of Cardiovascular Pharmacology. 1995;26(Suppl 3):S208–S210. [PubMed] [Google Scholar]
- Ruiz-Opazo N, Hirayama K, Akimoto K, Herrera VL. Molecular characterization of a dual endothelin-1/Angiotensin II receptor. Molecular Medicine. 1998;4:96–108. [PMC free article] [PubMed] [Google Scholar]
- Ruschitzka F, Moehrlen U, Quaschning T, Lachat M, Noll G, Shaw S, Yang Z, Teupser D, Subkowski T, Turina MI, Luscher TF. Tissue endothelin-converting enzyme activity correlates with cardiovascular risk factors in coronary artery disease. Circulation. 2000;102:1086–1092. doi: 10.1161/01.cir.102.10.1086. [DOI] [PubMed] [Google Scholar]
- Saida K, Mitsui Y, Ishida N. A novel peptide, vasoactive intestinal contractor, of a new (endothelin) peptide family. Molecular cloning, expression, and biological activity. The Journal of Biological Chemistry. 1989;264:14613–14616. [PubMed] [Google Scholar]
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Sanchez R, MacKenzie A, Farhat N, Nguyen TD, Stewart DJ, Mercier I, Calderone A, Thorin E. Endothelin B receptor-mediated regulation of endothelin-1 content and release in cultured porcine aorta endothelial cell. Journal of Cardiovascular Pharmacology. 2002;39:652–659. doi: 10.1097/00005344-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Sato K, Oka M, Hasunuma K, Ohnishi M, Kira S. Effects of separate and combined ETA and ETB blockade on ET-1-induced constriction in perfused rat lungs. The American Journal of Physiology. 1995;269:L668–L672. doi: 10.1152/ajplung.1995.269.5.L668. [DOI] [PubMed] [Google Scholar]
- Sauvageau S, Thorin E, Caron A, Dupuis J. Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Experimental Biology and Medicine (Maywood) 2006;231:840–846. [PubMed] [Google Scholar]
- Sauvageau S, Thorin E, Caron A, Dupuis J. Endothelin-1-induced pulmonary vasoreactivity is regulated by ET(A) and ET(B) receptor interactions. Journal of Vascular Research. 2007;44:375–381. doi: 10.1159/000102534. [DOI] [PubMed] [Google Scholar]
- Sauvageau S, Thorin E, Villeneuve L, Dupuis J. Change in pharmacological effect of endothelin receptor antagonists in rats with pulmonary hypertension: Role of ETB-receptor expression levels. Pulmonary Pharmacology & Therapeutics. 2009;22:311–317. doi: 10.1016/j.pupt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ET (A) and ET(B) receptors in cardiovascular disease. Annual Review of Pharmacology and Toxicology. 2007;47:731–759. doi: 10.1146/annurev.pharmtox.47.120505.105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen U, Tyagi N, Patibandla PK, Dean WL, Tyagi SC, Roberts AM, Lominadze D. Fibrinogen-induced endothelin-1 production from endothelial cells. American Journal of Physiology. Cell Physiology. 2009;296:C840–C847. doi: 10.1152/ajpcell.00515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty SS, Okada T, Webb RL, DelGrande D, Lappe RW. Functionally distinct endothelin B receptors in vascular endothelium and smooth muscle. Biochemical and Biophysical Research Communications. 1993;191:459–464. doi: 10.1006/bbrc.1993.1240. [DOI] [PubMed] [Google Scholar]
- Simonson MS, Dunn MJ. Cellular signaling by peptides of the endothelin gene family. The FASEB Journal. 1990;4:2989–3000. doi: 10.1096/fasebj.4.12.2168326. [DOI] [PubMed] [Google Scholar]
- Simpson AW, Ashley CC. Endothelin evoked Ca2+ transients and oscillations in A10 vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1989;163:1223–1229. doi: 10.1016/0006-291x(89)91108-x. [DOI] [PubMed] [Google Scholar]
- Sirvio ML, Metsarinne K, Saijonmaa O, Fyhrquist F. Tissue distribution and half-life of 125I-endothelin in the rat: Importance of pulmonary clearance. Biochemical and Biophysical Research Communications. 1990;167:1191–1195. doi: 10.1016/0006-291x(90)90649-8. [DOI] [PubMed] [Google Scholar]
- Spinella F, Garrafa E, Di Castro V, Rosano L, Nicotra MR, Caruso A, Natali PG, Bagnato A. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Research. 2009;69:2669–2676. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochemical Pharmacology. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: Marker or mediator of disease? Annals of Internal Medicine. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- Sumner MJ, Cannon TR, Mundin JW, White DG, Watts IS. Endothelin ETA and ETB receptors mediate vascular smooth muscle contraction. British Journal of Pharmacology. 1992;107:858–860. doi: 10.1111/j.1476-5381.1992.tb14537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Matsushita Y, Iijima Y, Tanzawa K. Purification and characterization of endothelin-converting enzyme from rat lung. The Journal of Biological Chemistry. 1993;268:21394–21398. [PubMed] [Google Scholar]
- Takahashi H, Soma S, Muramatsu M, Oka M, Ienaga H, Fukuchi Y. Discrepant distribution of big endothelin (ET)-1 and ET receptors in the pulmonary artery. The European Respiratory Journal. 2001;18:5–14. doi: 10.1183/09031936.01.00075501. [DOI] [PubMed] [Google Scholar]
- Teerlink JR, Breu V, Sprecher U, Clozel M, Clozel JP. Potent vasoconstriction mediated by endothelin ETB receptors in canine coronary arteries. Circulation Research. 1994;74:105–114. doi: 10.1161/01.res.74.1.105. [DOI] [PubMed] [Google Scholar]
- Thomas GP, Sims SM, Karmazyn M. Differential effects of endothelin-1 on basal and isoprenaline-enhanced Ca2+ current in guinea-pig ventricular myocytes. Journal de Physiologie. 1997;503(Pt. 1):55–65. doi: 10.1111/j.1469-7793.1997.055bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorin E. Different contribution of endothelial nitric oxide in the relaxation of human coronary arteries of ischemic and dilated cardiomyopathic hearts. Journal of Cardiovascular Pharmacology. 2001;37:227–232. doi: 10.1097/00005344-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Thorin E, Hamilton C, Dominiczak AF, Dominiczak MH, Reid JL. Oxidized-LDL induced changes in membrane physico-chemical properties and [Ca2+]i of bovine aortic endothelial cells. Influence of vitamin E. Atherosclerosis. 1995;114:185–195. doi: 10.1016/0021-9150(94)05482-x. [DOI] [PubMed] [Google Scholar]
- Thorin E, Lucas M, Cernacek P, Dupuis J. Role of ET(A) receptors in the regulation of vascular reactivity in rats with congestive heart failure. American Journal of Physiology. Heart and Circulatory Physiology. 2000;279:H844–H851. doi: 10.1152/ajpheart.2000.279.2.H844. [DOI] [PubMed] [Google Scholar]
- Thorin E, Nguyen TD, Bouthillier A. Control of vascular tone by endogenous endothelin-1 in human pial arteries. Stroke. 1998;29:175–180. doi: 10.1161/01.str.29.1.175. [DOI] [PubMed] [Google Scholar]
- Thorin E, Parent R, Ming Z, Lavallee M. Contribution of endogenous endothelin to large epicardial coronary artery tone in dogs and humans. The American Journal of Physiology. 1999;277:H524–H532. doi: 10.1152/ajpheart.1999.277.2.H524. [DOI] [PubMed] [Google Scholar]
- Thorin E, Thorin-Trescases N. Vascular endothelial ageing, heartbeat after heartbeat. Cardiovascular Research. 2009;84:24–32. doi: 10.1093/cvr/cvp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T, Horie H, Sugimoto Y, Kinoshita M. Transcardiac extraction of circulating endothelin-1 across the failing heart. The American Journal of Cardiology. 2000;86:524–528. doi: 10.1016/s0002-9149(00)01006-7. [DOI] [PubMed] [Google Scholar]
- Unoki H, Fan J, Watanabe T. Low-density lipoproteins modulate endothelial cells to secrete endothelin-1 in a polarized pattern: A study using a culture model system simulating arterial intima. Cell and Tissue Research. 1999;295:89–99. doi: 10.1007/s004410051215. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Rubanyi GM, Miller VM, Houston DS. Modulation of vascular smooth muscle contraction by the endothelium. Annual Review of Physiology. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Verhaar MC, Strachan FE, Newby DE, Cruden NL, Koomans HA, Rabelink TJ, Webb DJ. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–756. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- Vierhapper H, Wagner O, Nowotny P, Waldhausl W. Effect of endothelin-1 in man. Circulation. 1990;81:1415–1418. doi: 10.1161/01.cir.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends in Pharmacological Sciences. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Endoh M. Antiadrenergic effects of endothelin-1 on the L-type Ca2+ current in dog ventricular myocytes. Journal of Cardiovascular Pharmacology. 2000;36:344–350. doi: 10.1097/00005344-200009000-00010. [DOI] [PubMed] [Google Scholar]
- Watts SW. Endothelin receptors: What’s new and what do we need to know? American Journal of Physiology—Regulatory, Integrative and Comparative Physiology. 2010;298:R254–R260. doi: 10.1152/ajpregu.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: Carvedilol stimulates beta-arrestin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Inoue A, Ishikawa T, Kasuya Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S, Goto K, et al. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proceedings of the National Academy of Sciences of the United States of America. 1988a;85:6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988b;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yorikane R, Koike H. The arrhythmogenic action of endothelin in rats. Japanese Journal of Pharmacology. 1990;53:259–263. doi: 10.1254/jjp.53.259. [DOI] [PubMed] [Google Scholar]
- Yorikane R, Shiga H, Miyake S, Koike H. Evidence for direct arrhythmogenic action of endothelin. Biochemical and Biophysical Research Communications. 1990;173:457–462. doi: 10.1016/s0006-291x(05)81080-0. [DOI] [PubMed] [Google Scholar]
- Yu C, Yang Z, Ren H, Zhang Y, Han Y, He D, Lu Q, Wang X, Yang C, Asico LD, Hopfer U, Eisner GM, Jose PA, Zeng C. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. American Journal of Hypertension. 2009;22:877–883. doi: 10.1038/ajh.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney International. 2008;74:750–759. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]