Abstract
Background and purpose
The endothelin (ET) system is activated in pulmonary arterial hypertension (PAH). The therapeutic value of pharmacological blockade of ET receptors has been demonstrated in various animal models and led to the current approval and continued development of these drugs for the therapy of human PAH. However, we currently incompletely comprehend what local modifications of this system occur as a consequence of PAH, particularly in small resistance arteries, and how this could affect the pharmacological response to ET receptor antagonists with various selectivities for the receptor subtypes. Therefore, the purposes of this study were to evaluate potential modifications of the pharmacology of the ET system in rat pulmonary resistance arteries from monocrotaline (MCT)-induced pulmonary arterial hypertension.
Experimental approach
ET-1 levels were quantified by ELISA. PreproET-1, ETA and ETB receptor mRNA expressions were quantified in pulmonary resistance arteries using Q-PCR, while protein expression was evaluated by Western blots. Reactivity to ET-1 of isolated pulmonary resistance arteries was measured in the presence of ETA (A-147627), ETB (A-192621) and dual ETA/B (bosentan) receptor antagonists.
Key results
In rats with PAH, plasma ET-1 increased (p < 0.001) while pulmonary levels were reduced (p < 0.05). In PAH arteries, preproET-1 (p < 0.05) and ETB receptor (p < 0.001) gene expressions were reduced, as were ETB receptor protein levels (p < 0.05). ET-1 induced similar vasoconstrictions in both groups. In arteries from sham animals, neither bosentan nor the ETA or the ETB receptor antagonists modified the response. In arteries from PAH rats, however, bosentan and the ETA receptor antagonist potently reduced the maximal contraction, while bosentan also reduced sensitivity (p < 0.01).
Conclusions and implications
The effectiveness of both selective ETA and dual ETA/B receptor antagonists is markedly increased in PAH. Down-regulation of pulmonary resistance arteries ETB receptor may contribute to this finding.
Keywords: Endothelin, Endothelin receptor antagonists, Lung, Pathophysiology, Pharmacology, Pulmonary hypertension, Receptors
1. Introduction
The endothelin (ET) system is activated in pulmonary arterial hypertension (PAH) [1]. Increased plasma levels of ET-1 were detected in patients with various forms of PAH [1] and in various experimental models [2,3]. The chief action of ET-1 is its ability to modulate pulmonary vascular reactivity. Although ET-1 is a strong and potent pulmonary vasoconstrictor, this peptide also has the ability to cause mild vasodilation. This complex biology derives from the existence of two different ET receptor subtypes [4]. The ETA receptor was characterized from bovine lungs [5] and the ETB receptor was cloned from rat lungs [6] where the proportion of these receptors is ~60% ETA and ~40% ETB [3]. The ETA receptor demonstrates higher affinity for ET-1 and ET-2 than ET-3, while the ETB receptor associates equally with all 3 isoforms. Both the ETA and ETB receptors are present on vascular smooth muscle cells and induce direct vasoconstriction and proliferation when stimulated [7,8]. On the other hand, endothelial ETB receptors have been demonstrated to play a dual role. They can induce vasodilation through the release of nitric oxide and prostacyclin [9], but can also cause the release of the potent pulmonary vasoconstrictor thromboxane A2 [10].
The therapeutic value of pharmacological blockade of ET receptors has been demonstrated in various animal models and led to the current approval and continued development of these drugs for the therapy of human PAH [11]. Although the ET system contributes to PAH, we currently incompletely comprehend what local modifications of this system occur as a consequence of PAH, particularly in small resistance arteries. Indeed, previous studies that have evaluated the modified pharmacology of the ET system in PAH were performed on either whole lung homogenate or large pulmonary arteries. However, the increased pulmonary vascular resistance occurs in small pulmonary resistance arteries. Therefore, the purposes of this study were to re-evaluate potential modifica-tions of the pharmacology of the ET system in rat pulmonary resistance arteries from monocrotaline (MCT)-induced pulmonary arterial hypertension, as these results might contribute to the optimization of treatments of PAH. Our results reveal marked changes in pulmonary vasculature sensitivity to ET receptor antagonism in PAH that may be related to a reduction in ETB receptor expression.
2. Methods
This study was approved by the animal research committee of the Montreal Heart Institute and conducted according to the guidelines from the Canadian Council of Animal Care.
2.1. Drugs
MCT was purchased from Sigma Chemical Co. ET-1 was purchased from American Peptide. The ETA receptor antagonist A-147627 and the ETB receptor antagonist A-192621 were kindly provided by Abbott Laboratories whereas bosentan was kindly provided by Actelion.
2.2. Monocrotaline induced PAH
Male Wistar rats (325 ± 10 g) received a single intra-peritoneal injection of either 0.5 ml 0.9% saline (n = 58) or 0.5 ml 60 mg kg−1 MCT (n = 74) [3,12–14]. Five weeks later, rats were anesthetized for hemodynamic measurements as previously described in detail [15]. The pulmonary and cerebral resistance arteries were harvested for vascular reactivity. In addition, pulmonary arteries were snap frozen for RNA and protein extraction.
2.3. ET-1 levels
Plasma and whole lung tissue homogenate samples were passed on Sep-Pak C18 columns (Waters, Milford, MA) before determination of ET-1 levels by ELISA according to the manufacturer’s instructions (Biomedica, Medicorp, Montreal, Quebec, Canada) [15].
2.4. Quantification of gene expression of preproET-1, ETA and ETB receptors by quantitative polymerase chain reaction (Q-PCR)
Total RNA was extracted from pulmonary resistance arteries isolated from the pulmonary right inferior lobe using an RNeasy mini-kit (Qiagen Inc.). The reverse transcriptase reaction contained 5 ng per μl total RNA (each sample), M-MLV reverse transcriptase (800 U, Invitrogen), RNAseOUT (40 U, Invitrogen), reverse primer (4 pmol l−1, Invitrogen), dNTPs (0.5 mmol l−1, MBI Fermentas), and supplied optimal buffers. PCR was performed with 1 ng of cDNA template containing the appropriate primer concentration; pre-proET-1 (150 nmol l−1); ETA receptor (150 nmol l−1); ETB receptor (200 nmol l−1) and SYBR Green PCR master mix (Applied Bio-Systems). The primers were as follow:
| Rat preproET-1 | Forward | 5′-CTGGAGACCCCGCAGGTCCAA-3′ |
| Reverse | 5′-GTGGGAAGTAAGTCTTTCAAGGATCGC-3′ | |
| Rat ETA receptor | Forward | 5′-TTCCCTCTTCACTTAAGC CGAA-3′ |
| Reverse | 5′-GACAACAGCAACAGAGGC ATGA-3′ | |
| Rat ETB receptor | Forward | 5′-CTAGCCATCACTGCGATCTT-3′ |
| Reverse | 5′-CAG AAT CCT GCT GAG GTG AA-3′ | |
| Rat cyclophiline A | Forward | 5′-AGGTCCTGGCATCTTGTC-3′ |
| Reverse | 5′-TGATCTTCTTGCTGGTCT-3′ |
PCR products were purified, sequenced and confirmed to be the genes of interest. Cyclophiline A was chosen as the housekeeping gene as the expression did not change with MCT treatment.
2.5. Quantification of protein expression of the ETA and ETB receptors by Western blot
Small pulmonary resistance arteries from the pulmonary right inferior lobe were obtained and pooled for each experimental condition. Standard protein extraction was assessed followed by protein quantification using the Bradford technique. Proteins (50 μg per lane) were separated on 10% acrylamide SDS-PAGE. Following SDS-PAGE, samples were transferred onto either nitro-cellulose (ETA receptor) or PVDF (ETB receptor) membranes. Membranes were incubated overnight with the primary antibody for the ETA receptor (Abcam, anti-rabbit, 1:1000) or for the ETB receptor (Alomone, anti-rabbit, 1:500). Equal protein loading was verified by Red Ponceau.
2.6. Vascular reactivity studies
Experiments were conducted on isolated pulmonary arteries and on the basilar artery using a wire myograph as previously described [16,17]. The general contractile capacities of pulmonary arteries from both groups were evaluated with: a high potassium solution (127 mmol l−1), a concentration–response curve to ET-1 (0.1 nmol l−1 to 0.3 μmol l−1) in both absolute values (mg of tension) and as percentage of the maximal vasoconstriction (127 mmol l−1 KCl). Preparations of pulmonary arteries were subjected to ET-1 (0.1 nmol l−1 to 0.3 μmol l−1) either in the presence of the ETA receptor antagonist (A-147627, 10 nmol l−1, ETA:ETB ~1800:1), the ETB receptor antagonist (A-192621, 1 μmol l−1, ETA:ETB ~1:1400) or the dual antagonist bosentan (10 μmol l−1, ETA:ETB ~40:1). In order to test potential improvement in efficacy of the ET receptor antagonists in PAH, the concentrations of the antagonists were chosen based on concentration–response curves for normal pulmonary resistance arteries [17,18] as these concentrations caused no or minimal effects on the normal response. The basilar arteries were subjected to ET-1-induced vasoconstriction in the absence and presence of the dual antagonist bosentan.
2.7. Immunofluorescence of ET receptors in pulmonary and basilar arteries
The lungs (n = 3) and the basilar artery (n = 3) were oriented to cross-section the arteries of interest and 8 μmol l−1 cryocuts were performed. The ETB receptor antibody (Alomone, rabbit 1:200) was incubated with α-smooth muscle actin antibody (Sigma, mouse, 1:200). Anti-rabbit Alexa 555 and anti-mouse Alexa 647 (Molecular Probes, donkey, 1:800) antibodies were diluted in their respective antibody diluents and applied. Each experimental condition was performed in triplicates. Confocal imaging and deconvolution were performed as previously described in detail [15].
2.8. Statistical analysis
All values are expressed as mean ± SEM. The concentration–response curves were fitted using a 5-parameter logistic fit to determine the maximal responses as well as the EC50 values. When maximal constriction could not be fitted with the 5-parameter logistic, we used the experimental data maximal response (Emax) to perform our statistics. At the end of the protocol, the maximal vasoconstriction was determined by changing the PSS with a high potassium solution (127 mmol l−1 KCl). ET-1-induced vasoconstrictions are expressed as a percentage of the maximal response. The differences between groups were evaluated with unpaired two-tailed Student’s t tests. Statistical significance was assumed when p < 0.05.
3. Results
3.1. Effect of MCT treatment on hemodynamics parameters
Five weeks following the injection of MCT, the animals developed severe PAH as evidenced by higher right ventricular systolic pressure (77 ± 3 mm Hg, n = 58, p < 0.001) when compared to sham rats (26 ± 1 mm Hg, n = 74).
3.2. ET-1 levels
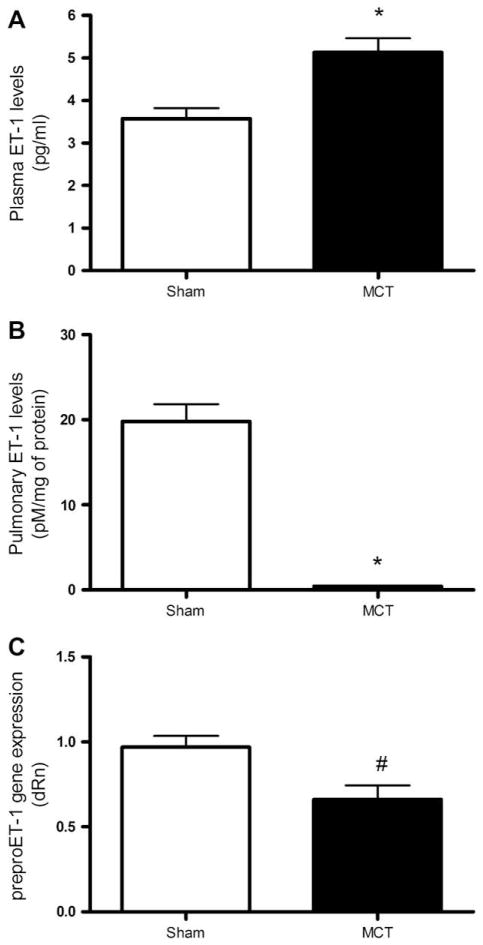
As shown in Fig. 1A, arterial ET-1 levels were significantly higher in MCT-treated rats (p < 0.001) compared to sham-treated rats. ET-1 pulmonary tissue levels were, however, strongly reduced in MCT (p < 0.001) compared to sham rats (Fig. 1B). This was associated with a reduction in preproET-1 mRNA levels in pulmonary arteries of MCT rats (p < 0.05, Fig. 1C).
Fig. 1.
ET-1 levels and gene expression. (A) Arterial plasma ET-1 levels in sham (n = 17) and MCT rats (n = 18). (B) Pulmonary ET-1 levels in sham (n = 16) and MCT rats (n = 19). (C) PreproET-1 gene expression in pulmonary resistance arteries normalized with cyclophiline A. Values are expressed as relative quantity (dRn). *p < 0.001, #p < 0.05.
3.3. Quantification of gene expression of ETA and ETB receptors in pulmonary resistance arteries
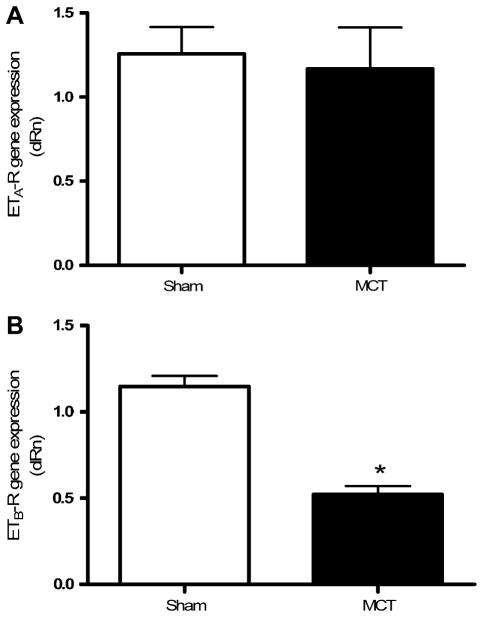
ETB receptor mRNA levels were reduced in PAH (p < 0.001) compared to normotensive rats (Fig. 2B). In contrast, MCT treatment did not significantly modify the genomic expression of the ETA receptor (Fig. 2A).
Fig. 2.
ET receptor gene expression. Gene expression of the ETA (A) and ETB (B) receptors in pulmonary resistance arteries of sham (n = 6) and MCT-treated rats (n = 4) normalized with cyclophiline A. Values are expressed as relative quantity (dRn). *p < 0.001.
3.4. Quantification of protein expression of the ETA and ETB receptors
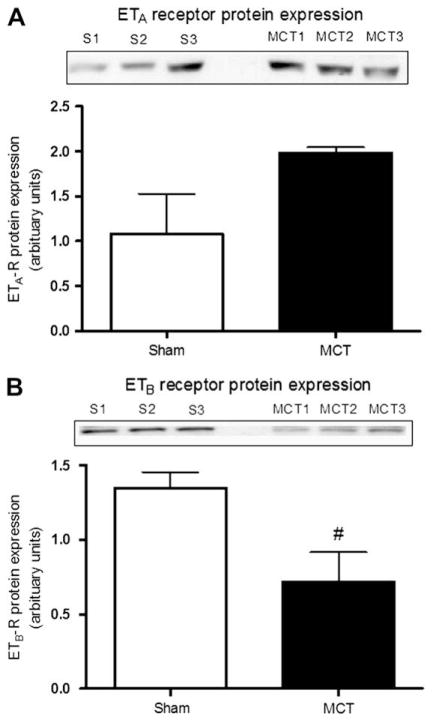
ETB receptor protein expression was also reduced in MCT-treated rats (p < 0.05, Fig. 3B) while that of the ETA receptor tended to increase but was however non-significant (Fig. 3A).
Fig. 3.
ET receptor protein expression. Protein expression of the ETA (A) and ETB (B) receptors in pulmonary resistance arteries of both sham (n = 3) and MCT-treated rats (n = 3). Blots illustrated represent 3 experiments and the bar graph represents the summarized results of the 3 experiments in which blot intensities were normalized with Ponceau red. #p < 0.05.
3.5. Vascular reactivity studies
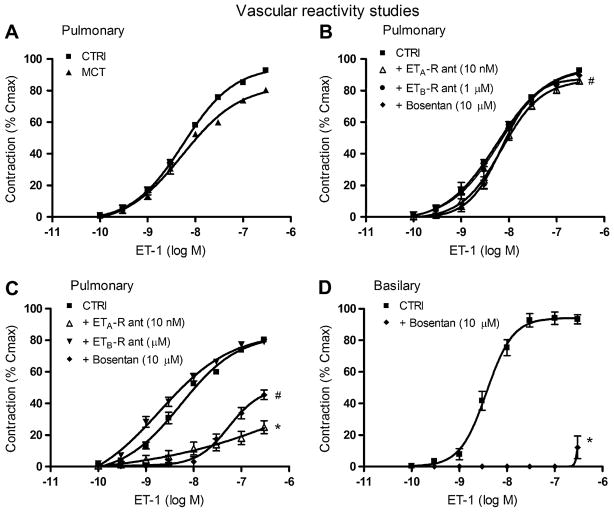
The maximal contraction induced by a high external K+ solution (127 mmol l−1) was significantly reduced in pulmonary arteries isolated from MCT rats (279 ± 16 mg, p < 0.01) when compared to sham-treated rats (377 ± 20 mg). Similarly, the maximal response induced by ET-1 in absolute values (mg of tension) was also significantly reduced in isolated vessels from MCT (350 ± 10 mg, p < 0.001) when compared to sham (560 ± 8 mg) rats. When expressed as a % of the maximal contraction induced by high external K+ solution, however, the vasoconstriction induced by ET-1 was preserved in arteries isolated from MCT-treated rats when compared to those from sham-treated rats (Fig. 4A), without change in vascular sensitivity (pEC50 8.2 ± 0.2 and pEC50 8.4 ± 0.3, respectively). In pulmonary arteries isolated from sham animals, none of the antagonists diminished the contractile response induced by ET-1 (Fig. 4B) or the vascular sensitivity to ET-1 at the concentrations used (pEC50; ETA receptor antagonist: 8.2 ± 0.2, ETB receptor antagonist: 8.3 ± 0.2, bosentan: 8.2 ± 0.1). In PAH and when used at the same concentrations, the selective ETA receptor antagonist and the dual ETA/B receptor antagonist strongly limited ET-1-induced contraction (p < 0.001). Only bosentan, however, reduced the vascular sensitivity to ET-1 (7.2 ± 0.2, p < 0.01) (Fig. 4C). The ETB receptor antagonist A-192621 alone had no significant effect on the contraction induced by ET-1 in arteries isolated from either sham-treated or MCT animals. In isolated cerebral arteries from control animals, ET-1-induced vasoconstriction (Emax 94 ± 3%, pEC50 8.4 ± 0.1) was completely abolished by bosentan at the same concentration (10 μmol l−1) used in pulmonary arteries (Emax 40 ± 3%, p < 0.001, pEC50 not measurable) (Fig. 4D), revealing the uniqueness of pulmonary artery sensitivity to ET-1 and antagonism.
Fig. 4.
Vascular reactivity. Endothelin-1-induced pulmonary vasoconstriction in sham- and MCT-treated rats (A). Endothelin-1-induced pulmonary vasoconstriction in the presence of an ETA-R antagonist (open triangle, 10 nmol 1−1), an ETB-R antagonist (filled circles, 1 μmol l−1) and the dual antagonist Bosentan (filled diamond, 10 μmol l−1) in both sham (B) and MCT rats (C). n = 6–8/group. (D) Endothelin-1-induced vasoconstriction of basilar arteries in the absence and presence of bosentan (filled diamond, 10 μmol l−1). n = 6–8/group. Values are mean ± SEM. *p < 0.001, #p < 0.05.
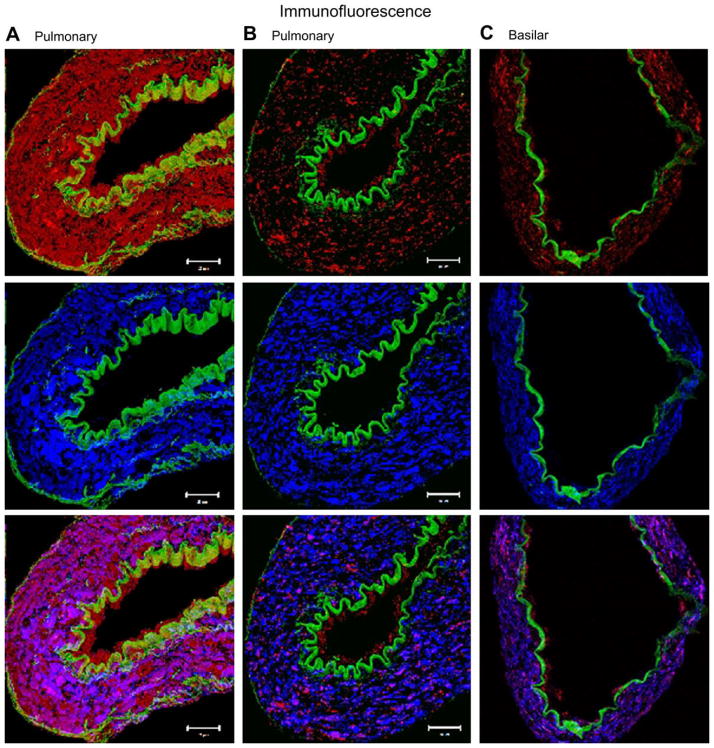
3.6. Immunohistology of ET receptors in pulmonary and basilar arteries
Examples of composite Z-stack images obtained with ETB receptor and α-smooth muscle actin antibodies in pulmonary arteries isolated from sham- and MCT-treated rats, and in the basilar artery from control rats, are presented in Fig. 5. Auto-fluorescence of the internal elastic lamina and external elastic lamina (in green) enables easy delineation of the endothelium. We can notice a significant reduction in the fluorescence intensity of both the endothelial and smooth muscle ETB receptors in the MCT-treated rats when compared to controls. Moreover, the fluorescence intensity of the ETB receptor in the basilar artery is comparable to the fluorescence intensity of this receptor in pulmonary arteries isolated from MCT-treated rats.
Fig. 5.
Immunofluorescence and confocal imaging. Confocal imaging representing the distribution of the ETB receptor in transverse 8 μmol l−1 thick sections of small pulmonary arteries of both sham (A) and MCT rats (B) and in the basilar artery of control rats (C). The first row of figures (from left to right) displays the fluorescence of the ETB (in red) and both the internal elastic lamina (IEL) and external elastic lamina (EEL) (in green), which enable easy demarcation of the endothelium from the media. The second row displays the fluorescence of smooth muscle actin (in blue), which is limited to the media. The third row represents the co-localisation of the ETB and smooth muscle actin.
4. Discussion and conclusions
This study was designed to evaluate the local modifications of the ET system in pulmonary resistance arteries from rats with MCT-induced PAH and assess how these modifications could affect the response to ET receptor antagonist with various selectivities. Our main findings in the MCT model of PAH are that: (1) although plasma levels of ET-1 are increased, there is reduced local pulmonary production of this peptide; (2) there is a significant down-regulation of the ETB receptor in the pulmonary resistance arteries from PAH rats; (3) the relative pulmonary vasoconstriction to ET-1 is not modified by PAH; and finally (4) the inhibitory effect of the selective ETA receptor antagonist and the dual ETA/B receptor antagonist bosentan on the ET-1 response is greatly increased in isolated pulmonary resistance arteries from PAH rats. These results reveal pathophysiological events that could be potentially responsible for the effectiveness of ET receptor antagonists in the treatment of PAH.
This study confirms that, in the MCT-induced model of PAH; there is an increase in ET-1 plasma levels and a decrease in pulmonary tissue level of this peptide. Hills et al. [24] have suggested that regional variations in lung ET-1 production decrease whole lung homogenate concentrations but increase local concentration, such as those adjacent to the vascular smooth muscle. We found a reduction in preproET-1 mRNA expression in pulmonary resistance arteries, which therefore excludes the possible regional variation in lung ET-1 production, but confirms that in this particular model pulmonary production of ET-1 is reduced. Hence, despite lower pulmonary levels of the peptide, there is nevertheless proven benefit in blockade of this system in PAH. The increased plasma levels could therefore come from other tissues such as the kidney and the heart, where an increased expression of preproET-1 mRNA has been observed in this model [2]. Importantly, a reduced pulmonary clearance likely contributes to the observed increase in plasma ET-1, the pulmonary circulation playing a major role in the removal of circulating ET-1, and a reduced pulmonary clearance of this peptide contributes to the hyperendothelinemia observed in both human and animal model of PAH [24–27]. Our group has previously reported a reduction in ETB-receptor-dependent clearance in the MCT model of PAH [28]. Reduced pulmonary clearance is likely explained by the marked down-regulation of the ETB receptor in this pathological model.
PAH could modify the expression profile of ET receptors on pulmonary resistance arteries. Therefore, we evaluated gene and protein expressions of ET receptors in pulmonary resistance arteries. ETA receptor mRNA expression was not modified by PAH. Although the protein expression of the ETA receptor tended to increase in MCT rats, these results were however non-significant. ETB receptor mRNA expression was reduced by approximately 45% in PAH, confirming the results obtained from whole lung homogenates [20–22]. Likewise, ETB receptor protein levels were reduced. The reduced expression of the ETB receptor could modify the ET-1-induced pulmonary vasoconstriction. In fact, it has been previously reported that the main pulmonary artery vascular responsiveness to ET-1 was reduced after 25 days in the MCT model of PAH [2]. Using isolated pulmonary resistance arteries, we established that the relative ET-1 responsiveness is maintained 5 weeks after MCT injury, despite overall reduced smooth muscle contractility. This therefore demonstrates that in small pulmonary resistance arteries, PAH does not specifically target the ET-1-induced smooth muscle contractile potential. More so, this study demonstrates that the reduced expression of the ETB receptor did not modify ET-1-induced vasoconstriction. This suggests that the ETA receptor compensates for the reduction in ETB receptors to maintain normal contractility to ET-1.
The reduced expression of the ETB receptor in pulmonary resistance arteries could also modify the response to endothelin receptor antagonists. To investigate this possibility, we compared the effects of selective and dual ET receptor antagonists on the contractile response in both sham and PAH rats. In pulmonary arteries isolated from sham animals, selective ETA, selective ETB and dual ETA/B (bosentan) receptor antagonists had no significant effect on ET-1-induced response. We have previously demonstrated that at these concentrations, the selective ETA and ETB receptor antagonists had little effect on ET-1-dependent response [17]. It is difficult, however, to reconcile the lack of effect of the dual antagonist bosentan as this antagonist simultaneously blocks both receptors (ETA/ETB) responsible for ET-1-induced pulmonary vasoconstriction. Similar results were reported in a previous study in which, this dual antagonist could reduce ET-1-induced vasoconstriction in both tail and mesenteric arteries isolated from the rat but not in pulmonary vessels [19]. In the present study, we also evaluated the effect of a similar concentration of bosentan in rat cerebral arteries. In the isolated basilar artery, bosentan completely abolished the response induced by ET-1, thus corroborating that the pharmacology observed in the pulmonary vascular bed is unique. More interestingly, bosentan potently inhibited the contraction induced by ET-1 in pulmonary arteries isolated from rats with PAH. Bosentan reduced the maximal response as well as the vascular sensitivity to ET-1. The selective ETA receptor antagonist also greatly reduced the maximal vasoconstriction induced by ET-1 without, however, affecting vascular sensitivity.
In PAH rats, bosentan efficiently inhibits ET-1-induced contraction as in isolated cerebral arteries from control rats. We performed immunofluorescence studies in both types of arteries, which qualitatively confirm that the proportion of the ETB receptor in cerebral artery of control animals is comparable to that in the pulmonary arteries of MCT-treated rats. Consequently, the fact that the basilar arteries exhibit little ETB receptor at basal level likely explains the inhibitory effects of bosentan in these vessels and in pulmonary arteries isolated from PAH rats. The immunofluorescence studies also allow us to appreciate the fact that both the endothelial and the smooth muscle ETB receptors seem to be reduced in pulmonary arteries from PAH rats. It is well known that the endothelial ETB receptor can affect pulmonary vascular reactivity by releasing nitric oxide and prostacycline [10]. Previous investigators such as Ivy et al. have demonstrated that the ETB receptor plays a protective role in rats with PAH induced by chronic hypoxia. Indeed, they have demonstrated that ETB receptor deficient rats display an exaggerated response to hypoxic pulmonary hypertension [31]. Hence, if the endothelial ETB receptor has a protective role and its expression is reduced in PAH, this could have functional consequences on pulmonary vascular reactivity of MCT-treated rats. Indeed, this would imply that the vasodilator role of the ETB receptor is compromised in PAH and therefore could favour increased pulmonary vasoconstriction via the stimulation of the ETB receptor present on vascular smooth muscle cells. However, we have demonstrated, in a previous publication, that the endothelial ETB receptor plays a minor vasodilator role in the ET-1-induced vasoconstriction of rat’s small pulmonary arteries. Indeed, we have demonstrated that removal of the endothelium does not modify the maximal response or the vascular sensitivity to ET-1 or sarafotoxin 6c [17].
It is intriguing that only bosentan was able to reduce the vascular sensitivity to ET-1 in PAH while the selective ETA receptor antagonist was only able to reduce the maximal vasoconstriction to ET-1. It is tempting to speculate that there is an inter-play between the two ET receptor subtypes that determine the pharmacological response of the antagonists. By blocking both receptor subtypes, bosentan may preserve the inter-play, while selective ETA receptor inhibition unbalances it. Previous investigators such as Mickley [29] and Adner [30] have previously reported the existence of an interaction between the two receptor subtypes in small mesenteric arteries. We have also recently demonstrated the existence of an interaction between the ETA and ETB receptor to induce the ET-1 vasoconstriction in pulmonary resistance arteries [17]. Whether this inter-play occurs post-receptor or takes place at the receptor level, remains to be determined. It is known that ET receptors can from heterodimers in cultured transfected HEK cells [23] as well as in rat pulmonary arteries as we previously reported [16]. The functional significance of the dimerization needs to be investigated but could contribute to our findings.
There is a marked down-regulation of the ETB receptor in pulmonary resistance arteries from rats with MCT-induced PAH. This reduced expression of the ETB receptor explains the inhibitory effects of the selective ETA (A-147627) and a dual ETA/ETB receptor antagonist (bosentan) on ET-1-induced constriction of pulmonary resistance arteries in the MCT model of PAH. Above all, this study provides a functional rational for using ET antagonists in PAH.
Acknowledgments
Dr. Jocelyn Dupuis is a National Researcher from the «Fonds de la recherche en santé du Québec». Stéphanie Sauvageau is supported by a scholarship from the Heart and Stroke Foundation of Canada. Supported by the Canadian Institutes for Health Research.
References
- 1.Michel RP, Langleben D, Dupuis J. The endothelin system in pulmonary hypertension. Can J Physiol Pharmacol. 2003;81:542–54. doi: 10.1139/y03-008. [DOI] [PubMed] [Google Scholar]
- 2.Miyauchi T, Yorikane R, Sakai S, Sakurai T, Okada M, Nishikibe M, et al. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary hypertension. Circ Res. 1993;73:887–97. doi: 10.1161/01.res.73.5.887. [DOI] [PubMed] [Google Scholar]
- 3.Jasmin JF, Cernacek P, Dupuis J. Activation of the right ventricular endothelin (ET) system in the monocrotaline model of pulmonary hypertension: response to chronic ETA receptor blockade. Clin Sci (Lond) 2003;105:647–53. doi: 10.1042/CS20030139. [DOI] [PubMed] [Google Scholar]
- 4.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–2. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–5. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 7.Davie N, Haleen SJ, Upton PD, Yacoub MH, Morrell NW, Wharton J. ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am J Respir Crit Care Med. 2002;165:398–405. doi: 10.1164/ajrccm.165.3.2104059. [DOI] [PubMed] [Google Scholar]
- 8.MacLean MR, McCulloch KM, Baird M. Endothelin ETA- and ETB-receptor-mediated vasoconstriction in rat pulmonary arteries and arterioles. J Cardiovasc Pharmacol. 1994;23:838–45. doi: 10.1097/00005344-199405000-00022. [DOI] [PubMed] [Google Scholar]
- 9.de Nucci G, Thomas R, D’Orleans-Juste P, Antunes E, Walder C, Warner TD, et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;85:9797–800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Orleans-Juste P, Claing A, Telemaque S, Maurice MC, Yano M, Gratton JP. Block of endothelin-1-induced release of thromboxane A2 from the guinea pig lung and nitric oxide from the rabbit kidney by a selective ETB receptor antagonist, BQ-788. Br J Pharmacol. 1994;113:1257–62. doi: 10.1111/j.1476-5381.1994.tb17133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:62S–7S. doi: 10.1016/j.jacc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Dupuis J, Prie S. The ET(A)-receptor antagonist LU 135252 prevents the progression of established pulmonary hypertension induced by monocrotaline in rats. J Cardiovasc Pharmacol Ther. 1999;4:33–9. doi: 10.1177/107424849900400106. [DOI] [PubMed] [Google Scholar]
- 13.Jasmin JF, Lucas M, Cernacek P, Dupuis J. Effectiveness of a nonselective ET(A/B) and a selective ET(A) antagonist in rats with monocrotaline-induced pulmonary hypertension. Circulation. 2001;103:314–8. doi: 10.1161/01.cir.103.2.314. [DOI] [PubMed] [Google Scholar]
- 14.Prie S, Stewart DJ, Dupuis J. Endothelin A receptor blockade improves nitric oxide-mediated vasodilation in monocrotaline-induced pulmonary hypertension. Circulation. 1998;97:2169–74. doi: 10.1161/01.cir.97.21.2169. [DOI] [PubMed] [Google Scholar]
- 15.Migneault A, Sauvageau S, Villeneuve L, Thorin E, Fournier A, Leblanc N, et al. Chronically elevated endothelin levels reduce pulmonary vascular reactivity to nitric oxide. Am J Respir Crit Care Med. 2005;171:506–13. doi: 10.1164/rccm.200403-340OC. [DOI] [PubMed] [Google Scholar]
- 16.Sauvageau S, Thorin E, Caron A, Dupuis J. Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Exp Biol Med (Maywood) 2006;231:840–6. [PubMed] [Google Scholar]
- 17.Sauvageau S, Thorin E, Caron A, Dupuis J. Endothelin-1-induced pulmonary vasoreactivity is regulated by ET(A) and ET(B) receptor interactions. J Vasc Res. 2007;44:375–81. doi: 10.1159/000102534. [DOI] [PubMed] [Google Scholar]
- 18.Wessale JL, Adler AL, Novosad EI, Calzadilla SV, Dayton BD, Marsh KC, et al. Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: ex vivo and in vivo studies. Clin Sci. 2002;103(Suppl 48):112S–7S. doi: 10.1042/CS103S112S. [DOI] [PubMed] [Google Scholar]
- 19.Angus J, Wright C. The endothelin system in cardiopulmonary diseases. Allschwil, Switzerland: Friedrich Reinhardt Verlag; 2002. Endothelin and the sympathetic nervous system; pp. 97–125. [Google Scholar]
- 20.Gosselin R, Gutkowska J, Baribeau J, Perreault T. Endothelin receptor changes in hypoxia-induced pulmonary hypertension in the newborn piglet. Am J Physiol. 1997;273:L72–9. doi: 10.1152/ajplung.1997.273.1.L72. [DOI] [PubMed] [Google Scholar]
- 21.Ivy DD, Le Cras TD, Horan MP, Abman SH. Increased lung preproET-1 and decreased ETB-receptor gene expression in fetal pulmonary hypertension. Am J Physiol. 1998;274:L535–41. doi: 10.1152/ajplung.1998.274.4.L535. [DOI] [PubMed] [Google Scholar]
- 22.Yorikane R, Miyauchi T, Sakai S, Sakurai T, Yamaguchi I, Sugishita Y, et al. Altered expression of ETB-receptor mRNA in the lung of rats with pulmonary hypertension. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S336–8. doi: 10.1097/00005344-199322008-00088. [DOI] [PubMed] [Google Scholar]
- 23.Gregan B, Schaefer M, Rosenthal W, Oksche A. Fluorescence resonance energy transfer analysis reveals the existence of endothelin-A and endothelin-B receptor homodimers. J Cardiovasc Pharmacol. 2004;44:S30–3. doi: 10.1097/01.fjc.0000166218.35168.79. [DOI] [PubMed] [Google Scholar]
- 24.Hill NS, Warburton RR, Pietras L, Klinger JR. Nonspecific endothelin-receptor antagonist blunts monocrotaline-induced pulmonary hypertension in rats. J Appl Physiol. 1997;83:1209–15. doi: 10.1152/jappl.1997.83.4.1209. [DOI] [PubMed] [Google Scholar]
- 25.Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endo-thelin-1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol. 1996;81:1510–5. doi: 10.1152/jappl.1996.81.4.1510. [DOI] [PubMed] [Google Scholar]
- 26.Dupuis J, Stewart DJ, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation. 1996;94:1578–84. doi: 10.1161/01.cir.94.7.1578. [DOI] [PubMed] [Google Scholar]
- 27.Langleben D, Dupuis J, Langleben I, Hirsch AM, Baron M, Senécal JL, et al. Etiology-specific endothelin-1 clearance in human precapillary pulmonary hypertension. Chest. 2006;129:689–95. doi: 10.1378/chest.129.3.689. [DOI] [PubMed] [Google Scholar]
- 28.Dupuis J, Jasmin JF, Prie S, Cernacek P. Importance of local production of endothelin-1 and of the ET(B) receptor in the regulation of pulmonary vascular tone. Pulm Pharmacol Ther. 2000;13:135–40. doi: 10.1006/pupt.2000.0242. [DOI] [PubMed] [Google Scholar]
- 29.Mickley EJ, Gray GA, Webb DJ. Activation of endothelin ETA receptors masks the constrictor role of endothelin ETB receptors in rat isolated small mesenteric arteries. Br J Pharmacol. 1997;120:1376–82. doi: 10.1038/sj.bjp.0701036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adner M, Shankley N, Edvinsson L. Evidence that ET-1, but not ET-3 and S6b, ET(A)-receptor mediated contractions in isolated rat mesenteric arteries are modulated by co-activation of ET(B) receptors. Br J Pharmacol. 2001;133:927–35. doi: 10.1038/sj.bjp.0704135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivy DD, Yanagisawa M, Gariepy CE, Gebb SA, Colvin KL, McMurtry IF. Exaggerated hypoxic pulmonary hypertension in endothelin B receptor-deficient rats. Am J Physiol Lung Cell Mol Physiol. 2002;282:L703–12. doi: 10.1152/ajplung.00272.2001. [DOI] [PubMed] [Google Scholar]