Abstract
The role of lytic bacteriophages in preventing cross contamination of produce has not been evaluated. A cocktail of three lytic phages specific for E. coli O157:H7 (EcoShield™) or a control (phosphate buffered saline, PBS) was applied to lettuce by either; (1) immersion of lettuce in 500 ml of EcoShield™ 8.3 log PFU/ml or 9.8 log PFU/ml for up to 2 min before inoculation with E. coli O157:H7; (2) spray-application of EcoShield™ (9.3 log PFU/ml) to lettuce after inoculation with E. coli O157:H7 (4.10 CFU/cm2) following exposure to 50 μg/ml chlorine for 30 sec. After immersion studies, lettuce was spot-inoculated with E. coli O157:H7 (2.38 CFU/cm2). Phage-treated, inoculated lettuce pieces were stored at 4°C for and analyzed for E. coli O157:H7 populations for up to 7 d. Immersion of lettuce in 9.8 log PFU/ml EcoShield™ for 2 min significantly (p < 0.05) reduced E. coli O157:H7 populations after 24 h when stored at 4°C compared with controls. Immersion of lettuce in suspensions containing high concentrations of EcoShield™ (9.8 log PFU/ml) resulted in the deposition of high concentrations (7.8 log log PFU/cm2) of bacteriophages on the surface of fresh cut lettuce, potentially contributing to the efficacy of the lytic phages on lettuce. Spraying phages on to inoculated fresh cut lettuce after being washed in hypochlorite solution was significantly more effective in reducing E. coli O157:H7 populations (2.22 log CFU/cm2) on day 0 compared with control treatments (4.10 log CFU/cm2). Both immersion and spray treatments provided protection from E. coli O157:H7 contamination on lettuce, but spray application of lytic bacteriophages to lettuce was more effective in immediately reducing E. coli O157:H7 populations fresh cut lettuce.
Keywords: bacteriophages, E. coli O157:H7, lettuce, processing
Introduction
Two outbreaks of Escherichia coli O157:H7 infections in 2006 associated with the consumption of bagged baby spinach and bagged shredded lettuce were collectively responsible for sickening 254 people and causing 3 deaths.1,2 Romaine lettuce contaminated with E. coli O157:H7, grown in California, was responsible for causing a multi-state outbreak of 60 infections in 2011.3 A previous multi-state outbreak associated with E. coli O145 and contaminated Romaine lettuce grown in Arizona resulted in 34 cases of foodborne illness.4 Bagged spinach and shredded lettuce are especially vulnerable to bacterial contamination due to the lack of a kill-step during their harvesting, processing and packaging. Concentrations of chemical disinfectants like sodium hypochlorite used during the processing of fresh cut leafy green commodities may not be sufficient to kill foodborne pathogens attached to intact or cut surfaces of leafy greens, and are primarily used to prevent cross contamination of leafy greens through water.5,6
Outbreaks of foodborne illness associated with the consumption of leafy greens emphasize the need for an effective natural antimicrobial which can be directly applied to fresh cut leafy green to kill enteric pathogens. Bacteriophages are viruses that which can attach to, infiltrate, lyse and kill bacterial cells..7 Phages are the most abundant organisms on earth, and they are highly specific for bacterial species with a lack of ability to cause deleterious effects on human health.8 Thus, they may provide a safe, natural, and effective alternative to chemical sanitizers. Lytic bacteriophages can also use a phenomenon termed “Lysis from without” (LO) to kill bacterial cells, where cell lysis is not mediated through the infective cycle but through a large number of phages adsorbing to the surface of a cell, resulting in cell wall damage, physiological stress and eventual cell lysis.7 The LO mechanism is the most likely route of biocontrol of enteric bacterial pathogens used on refrigerated produce commodities, where the storage temperature prohibits the completion of the full lytic cycle.9 A cocktail of phages specific for Listeria monocytogenes (ListshieldTM) has been approved by the Food and Drug Administration (FDA) for use on ready-to-eat foods Recently, a mixture of E. coli O157:H7-specific bacteriophages (EcoShield™, Intralytix) has been approved through Food Contact notices by FDA and have been granted a temporary exemption by the FSIS for use on meats and on food contact surfaces.10-12 Phages have been found to be naturally present in a variety of food commodities including chicken, pork sausage, deli meats, and also mushrooms, lettuce, refrigerated biscuit dough, and frozen chicken pot pies.13-16 Lytic phage activity has also been assessed on meats, and a cocktail of three O157-specific bacteriophages was effective in reducing populations by 2 log CFU E. coli O157:H7 on the surface of beefsteaks when applied at an multiplicity of infection (MOI) value of 106.17
Bacteriophages are increasingly being evaluated for their effectiveness in killing the surface-borne bacteria on leafy greens and other produce commodities, while avoiding damage to leafy or edible portions of produce desired for their unblemished appearance and fresh consumption. In previous work, phages specific for Salmonella Montevideo and Typhimurium reduced counts of these two serotypes and S. Enteritidis on broccoli seeds by 1.50 log CFU/sample compared with untreated controls.18 In another study, S. Enteritidis populations were reduced by 3.5 log CFU/ wound on fresh cut honeydew melons when stored at 5°C.19 Phages specific for L. monocytogenes which were sprayed on to fresh cut honeydew melons reduced populations by 2 – 4.6 log CFU per sample when stored at 10°C.20 Lytic bacteriophages specific for Salmonella spp were able to reduce S. Javiana in the rhizosphere of growing tomato plants by 5.2 log CFU/g over 14 d.21 A cocktail of five lytic bacteriophages specific for Salmonella spp were applied to blossoms of tomato plants at a level of 6 log PFU/ml, and also treated with 6 log CFU/ml of S. Javiana.21 Resulting tomato fruits had a significantly lower incidence of internalized S. Javiana compared with fruits which received S. Javiana only.21 Other workers have shown that the application lytic phages specific for E. coli O157:H7 to inoculated cut tomatoes reduced E. coli O157:H7 populations by 99%, 94%, and 96% after 24, 120 and 168 h of storage at 10°C.22
Previous work evaluating the effectiveness of lytic bacteriophages against foodborne pathogens has shown that phages can be effective when applied to produce after the introduction of the pathogen; however, the role of lytic bacteriophages in preventing cross-contamination—applying phages before the introduction of the pathogen to the produce surface—has not been evaluated. The effectiveness of using bacteriophages (EcoShieldTM) to prevent contamination of lettuce with E. coli O157:H7 introduced through cross-contamination was investigated.
Results and Discussion
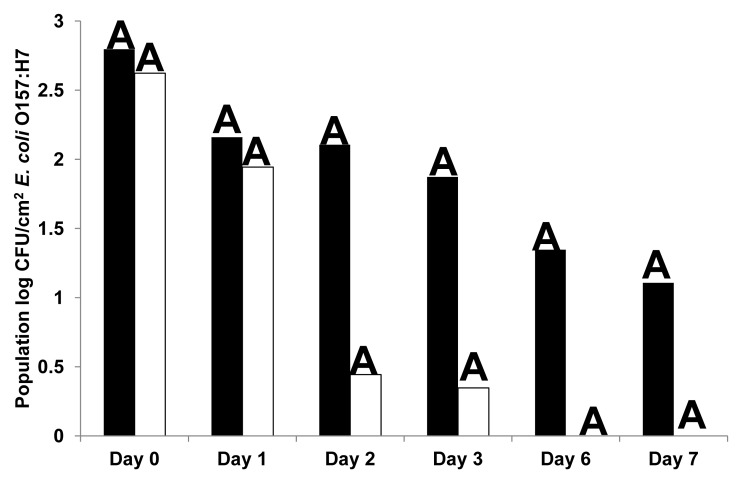
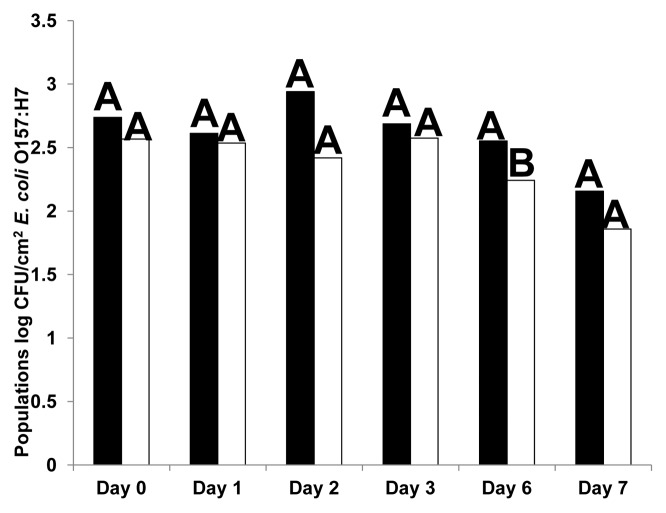
Immersion of cut lettuce pieces in EcoShield™ suspension (9.8 log PFU/ml) for 2 min significantly (p < 0.05) reduced populations of E. coli O157:H7 when immediately applied to fresh cut lettuce stored at 4°C for 1, 3 and 7 d after inoculation, compared with lettuce treated with control (phosphate buffered saline, PBS) (Fig. 1). By days 6 and 7, E. coli O157:H7 populations were reduced to undetectable levels in EcoShield™-treated lettuce samples. However, immersion of lettuce pieces in EcoShield™ suspension for 2 min at a lower titer (8.3 log PFU/ml), and followed by inoculation of E. coli O157:H7, was not as effective as immersion in higher (9.8 log PFU/ml) EcoShield™ suspensions to reduce E. coli O157:H7 populations (Fig. 2). Although significant statistical differences in E. coli O157:H7 populations between control and bacteriophage-treated lettuce were not observed at the lower concentration (8.3 log PFU/ml), populations did decline after 48 h of storage at 4°C, and remained low until day 7. When lettuce pieces were immersed for shorter durations (30 sec) at 8.3 log PFU/ml (Fig. 3), no significant reductions were observed between bacteriophage treatments and control. it was also not as effective as the 2 min immersion. For example, immersion of lettuce in EcoShield™ suspensions at 8.3 log PFU/ml for 30 sec resulted in 4.5 log PFU/cm2 on fresh cut lettuce pieces; but immersion of lettuce in EcoShield™ at higher concentrations (9.8 log PFU/ml) for 2 min resulted in 7.8 log PFU/cm2 on the lettuce surface. MOI values at the lower concentration of EcoShield™ (8.3 log PFU/ml) were < 100; however, MOI at the higher concentration (9.8 log PFU/ml) were > 10000. The longer contact time between the lettuce and bacteriophages (2 min) allowed more bacteriophage particles to adsorb to the surface of the lettuce, leading to the rapid and more effective reduction of E. coli O157:H7.
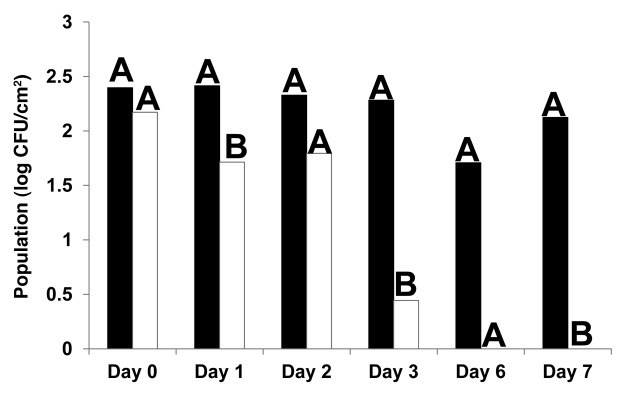
Figure 1. Populations (log CFU/cm2) of E. coli O157:H7 on fresh cut lettuce treated with Control (black) or 9.8 log PFU/ml bacteriophage EcoShield™ (white) for 2 min and stored at 4°C for 7 d. Different capital letters at the top of each bar indicate significant (p < 0.05) differences between control (PBS) and lytic bacteriophages treatment (EcoShield™) within each day.
Figure 2. Populations (log CFU/cm2) of E. coli O157:H7 on fresh cut lettuce treated with Control (black) or 8.3 log PFU/ml bacteriophage EcoShield™ (white) for 2 min and stored at 4°C for 7 d. Different capital letters at the top of each bar indicate significant (p < 0.05) differences between control (PBS) and lytic bacteriophages treatment (EcoShield™) within each day.
Figure 3. Populations (log CFU/cm2) of E. coli O157:H7 on fresh cut lettuce treated with Control (black) or 8.3 log PFU/ml bacteriophage EcoShield™ (white) for 30 sec and stored at 4°C for 7 d. Different capital letters at the top of each bar indicate significant (p < 0.05) differences between control (PBS) and lytic bacteriophages treatment (EcoShield™) within each day.
Currently, fresh cut leafy greens are washed in chlorinated water before being de-watered and packaged into bags before shipment to retail outlets. These washes are not intended to kill bacteria on the surface of fresh cut leafy greens but rather to reduce the likelihood of cross-contamination through water on to fresh cut leafy greens. Lytic bacteriophages applied to lettuce either during a washing step or after dewatering step may provide a more direct mechanism to directly reduce E. coli O157:H7 on fresh cut lettuce. Unlike our previous work, our current study introduces bacteriophages to fresh cut lettuce before the introduction of E. coli O157:H7 on to the surface of lettuce to determine if a pre-treatment of lytic bacteriophages can protect against cross-contamination.22,23 Our results show that application of phages by this method does not cause an immediate reduction of E. coli O157:H7 on the surface of fresh cut lettuce, but reductions do occur during several days of storage at 4°C (Figs. 1 and 2).
Previous work evaluating the effectiveness on leafy greens had utilized spraying of lytic bacteriophages on the commodity after the introduction of 4 log CFU E. coli O157:H7. Abuladze et al. (2008) sprayed ECP-100 (now EcoShield™), a mixture of three lytic bacteriophages specific for E. coli O157:H7, on to spinach, and reduced E. coli O157:H7 populations by 100% and 99% after 24 and 168 h, respectively.22 Similarly, a spray treatment of ECP-100 on fresh cut lettuce following the introduction of E. coli O157:H7, reduced bacterial populations by 1.82 log CFU/cm2 on day 0.23 With both of these experiments, the effect of spray application was rapid and substantial in reducing E. coli O157:H7 populations on lettuce surfaces. The effectiveness of spray application was attributed to increased phage particle dispersion on lettuce leaves. Since the E. coli O157:H7 cells were pre-established on the leaf surface and the phage-treated lettuce was stored at 4°C, the phages are assumed to cause rapid E. coli O157:H7 reductions through the lysis from without (LO) mechanism, mediated by the attachment of multiple bacteriophages to a single cell, lysing the cell membrane without undergoing the entire infective cycle of the bacteriophage.
Other workers who used a spot inoculation technique, whereby bacteriophages specific for E. coli O157:H7 were applied to the same spot as E. coli O157:H7 was applied on a baby spinach leaf, also observed increased bacterial reductions after 24 h compared with 10 min or 1 h at all tested storage temperatures of 4, 8 and 23°C.24 These findings, coupled with the results presented here, support that immersion or spot application can cause reductions of E. coli O157:H7 populations after 24 or 48 h periods at 4°C, but not as rapidly as when bacteriophages are spray-applied to lettuce.
Unlike previous studies with a spray application after the introduction of E. coli O157:H7 to the lettuce surface, no immersion treatment in our current study resulted in the immediate reduction of E. coli O157:H7 on day 0 as was previously observed.23 The immersion of pieces in the EcoShield™ solution may have impeded the mobility of the bacteriophage on the surface of the lettuce pieces compared with the spray application. The dispersion of bacteriophage particles on the surface of lettuce pieces achieved through spraying may compensate for the limited mobility of bacteriophages on cut lettuce,25 whereas immersion may not be as effective a method for optimal distribution of the phages on the lettuce surface. Recent work using model E. coli and coliphages with a bioluminescent reporting system to measure bacterial viability reported that lytic phages reduced the number of bacterial cells on the surface of the lettuce compared with controls (non-phage treated) lettuce within 5 h, as measured through reduced bioluminescence on the lettuce surface stored at room temperature after 24 h.25 In our immersion studies of lettuce using bacteriophage concentrations of 8.3 or 9.8 log PFU/ml for 2 min, the lack of an immediate bacteriophage-mediated decline in E. coli O157:H7 populations may be attributable to the more localized and limited dispersion of the phage particles on the lettuce surface. Spraying lettuce surface tissue with bacteriophages, as previous studies have shown, may have allowed the bacteriophages to be more active on the surface of the tissue and not penetrate deeply into the tissue, thus facilitating the rapid interaction between E. coli O157:H7 cells and the introduced bacteriophages. Furthermore, the storage of the lettuce at 4°C, with the refrigeration temperature limiting metabolic activity, may have also retarded the interactions between phage particles and bacterial cells. In an effort to simulate and evaluate the lytic bacteriophages in a fresh cut leafy green processing/washing regime, phages were sprayed on to lettuce after treatment with 50 μg/ml sodium hypochlorite (NaOCl) for 30 sec and then de-watered. On day 0 (immediately after treatment), lettuce treated with NaOCl only (3.00 log CFU/cm2), NaOCl and EcoShield™ (2.22 log CFU/cm2), or EcoShield™ only (3.28 log CFU/cm2) all had significantly lower E. coli O157:H7 populations than control treatment (4.10 log CFU/cm2 (Table 1). The control treatment was phosphate buffer, with no EcoShield™ or hypochlorite treatment. However, there were no significant statistical differences between E. coli O157:H7 populations on lettuce receiving hypochlorite or EcoShield™ treatments. After 24 h of storage at 4°C, the same trends were observed as on Day 1—lettuce which received no chemical or EcoShield™ treatments had significantly higher E. coli O157:H7 populations (4.86 log CFU/cm2) than those treated with NaOCl (3.48 log CFU/cm2), NaOCl and EcoShield™ (3.32 log CFU/cm2), or EcoShield™ alone (3.54 log CFU/cm2). On both day 0 and day 1, lettuce treated with sodium hypochlorite and EcoShield™ bacteriophages had lower E. coli O157:H7 populations than lettuce treated with either NaOCl or EcoShield™ alone (although differences between treatments other than control were not statistically significant). This finding suggests that lytic bacteriophages could be used synergistically with chemical sanitizers to reduce E. coli O157:H7 populations, and are more effective than employing solely a chemical or biological agent. Furthermore, bacteriophage titers on the surface of lettuce pieces sprayed with EcoShield™ were 7.2 and 6.4 log PFU/cm2 on day 0 and 1, respectively. The effective MOI was > 1000. These titers are similar to those observed on lettuce pieces treated with 50 μg/ml hypochlorite for 30 sec followed by spraying with EcoShield™ (6.8 and 6.7 log PFU/cm2) on days 0 and 1, respectively. Apparently the residual chlorine on lettuce leaves did not inactivate the bacteriophage particle or reduce the lytic potential of bacteriophages applied against E. coli O157:H7 introduced to the lettuce surface. The MOI of the spray study was 10-fold lower (> 1000) than observed in the immersion study at 9.8 log PFU/ml (> 10,000), and therefore is unlikely responsible for the increased effectiveness of the spray treatment compared with the immersion treatment. These results are similar to those observed using lytic bacteriophages specific for Listeria monocytogenes in a suspension with a quaternary ammonium compound-based sanitizer, QUATAL.26 Those workers observed that a suspension containing three listeriaphages and QUATAL in combination was significantly more effective in reducing L. monocytogenes populations on stainless steel and polypropylene cylinders than separate treatments containing either listeriaphages or QUATAL. Listeriaphage concentrations were not reduced in the presence of QUATAL utilized at 50 ug/ml concentration.26
Table 1. Populations (log CFU/cm2) of E. coli O157:H7 on fresh cut lettuce treated with: control (no treatment), sodium hypochlorite for 30 sec (NaOCl), NaOCl and then EcoShield™ (NaOCl+ EcoShield™), or EcoShield™ only.
| E. coli O157:H7 population (log CFU/cm2) on fresh cut lettuce | ||||
|---|---|---|---|---|
| |
Control |
NaOCl |
NaOCl + EcoShield™ |
EcoShield™ |
| Day 0 |
4.10 A |
3.00 B |
2.22 B |
3.28 B |
| Day 1 | 4.86 A | 3.48 B | 3.32 B | 3.54 B |
Within each row (day), different letters following log value indicates significant (p < 0.05) differences between treatments. Populations were determined immediately after treatment or after 24 h storage at 4°C.
The preliminary findings presented here indicate that an immersion treatment of lytic bacteriophages for fresh cut lettuce or a combination of a hypochlorite wash and E. coli O157:H7-specific bacteriophages have the potential to be applied to leafy greens in a processing facility and/or a field setting. The incorporation of lytic bacteriophages into washwater used to process fresh cut lettuce, or a subsequent treatment before dewatering, may not affect an immediate reduction in E. coli O157:H7 populations, but populations do decline after 24 h or 48 h at 4°C in our current study. Although the impact of the modified atmosphere packaging (MAP) used to preserve leafy green appearance and quality on the efficacy of lytic bacteriophages was not assessed in our work, other workers have shown that EcoShield™ was effective on fresh cut spinach stored under MAP conditions currently used for fresh cut leafy greens.27 Current harvesting and processing of iceberg lettuce intended for bagged salads or shredded lettuce packages begins with a head of iceberg lettuce being cut at the stalk in the field, trimmed of damaged leaves, and then cored before being placed on a conveyor belt and deposited into a bin to be transported to the processing facility.28 Lettuce, as a member of family Asteraceae is a latex producer, especially when its phloem cells are damaged, releasing lactucin-laden latex.29 The wounding generated by coring produces latex has been shown to predispose lettuce to opportunistic growth of E. coli O157:H7 and exacerbate contamination.30 Cored areas of lettuce heads are sprayed with 50–150 ppm chlorine (as hypochlorite) wash on the coveyor belt in the field to primarily remove latex, but such concentrations are insufficient to kill 100% of an enteric bacterial pathogen present.31 The demonstrated synergistic effect of the hypochlorite and lytic bacteriophages as studied under our experimental conditions provide an opportunity to test direct antimicrobial mitigation against potential E. coli O157:H7 contamination of fresh cut lettuce in a field processing setting.
In summary, the mode of application (immersion and spraying) affected the efficacy of lytic bacteriophage treatments to reduce E. coli O157:H7 on fresh cut lettuce. Although both methods provided a degree of protection from introduction of E. coli O157:H7 to fresh cut lettuce, spray application of lytic bacteriophages specific for E. coli O157:H7 to lettuce surfaces was more effective in immediately reducing E. coli O157:H7 populations on lettuce surfaces compared with immersion of lettuce in EcoShield™ solutions. The presence of E. coli O157:H7 on the surface of the leaf before spraying may have promoted more rapid and frequent interactions between phage particles and E. coli O157:H7 compared with the addition of E. coli O157:H7 to lettuce leaves prior to immersion in a lytic bacteriophage solution. A synergistic effect between lytic bacteriophage treatment and sodium hypochlorite wash was also observed, indicating the suitability of using lytic bacteriophages in the presence of such sanitizers. Immersion of fresh cut lettuce into a lytic bacteriophage solution reduced E. coli O157:H7 counts only after 24 to 48 h after of when stored at 4°C, indicating that bacteriophages required extended periods of time to reduce E. coli O157:H7 populations during refrigerated storage. The use of lytic bacteriophages can reduce E. coli O157:H7 on fresh cut lettuce. More research is needed to optimize the application of E. coli O157:H7-specific bacteriophages against E. coli O157:H7 on leafy greens under field- and facility-processing conditions.
Materials and Methods
Strains and bacteriophage preparations used
Escherichia coli O157:H7 gfp86, which expresses the green fluorescent protein, was obtained from the USDA-ARS Microbial Food Safety Laboratory (Fratamico et al., 1997).32 The isolate was streaked from frozen stock on to tryptic soy agar (TSA)(Becton Dickinson) supplemented with 100 μg/ml ampicillin (TSAA) (Sigma-Aldrich) and incubated at 37°C for 24 h. An isolated colony was then inoculated into tryptic soy broth (Becton Dickinson) supplemented with ampicillin (Sigma Aldrich) (TSBA) and incubated with agitation (175 rpm) for 24 h at 37°C. Cultures were then serially diluted in 0.1% peptone water for either dilution into sterile water or direct application to fresh cut lettuce. EcoShield™ consists of three E. coli O157:H7-specific lytic bacteriophages (ECML-4, ECML-117 and ECML-134) of the Myoviridae family isolated from fresh and salt water environments, and was obtained from Intralytix, Inc.21 EcoShield™ was diluted in phosphate buffered saline (PBS) (Sigma Aldrich) to various concentrations for immersion and spray applications.
Treatment of fresh cut lettuce by immersion in lytic bacteriophage suspension
Iceberg lettuce (Lactuca sativa) was purchased at a grocery store in Beltsville, MD. The plastic packaging of heads of iceberg lettuce (Lactuca sativa) was rinsed with 70% ethanol before removal. Outer leaves of the lettuce were removed and inner leaves were cut with a sterile stainless steel scalpel. A sterile metal coupon was then used to cut 3 cm × 3 cm pieces of lettuce on a sterile metal tray. For immersion studies, 24 pieces of lettuce were immersed in 500 mL of sterile water containing EcoShield™ in a 1 L sterile glass beaker with a frequently spinning stir-bar to simulate flume water in fresh cut lettuce processing. Pieces of lettuce were immersed in 8.3 log PFU/ml for 30 sec or 2 mins 9.8 PFU/ml EcoShield™ for 2 min. Twenty-four pieces of lettuce were also washed with phosphate buffer (PBS) as a control for the same periods of time. Excess EcoShield™ suspension or PBS was then removed from lettuce pieces by using a household salad spinner (Oxoid). Pieces of lettuce were allowed to dry for 10 min before each piece was spot inoculated with 50 μl of 5.1 log CFU / ml. E. coli O157:H7. The inoculum was applied using a 100 μl pipet, dispensing 8–10 droplets on each piece of lettuce. Lettuce was allowed to dry for 30 min in a biosafety cabinet at 25°C. Pieces of lettuce were stored on a piece of wetted filter paper and sealed (with parafilm) in a plastic Petri dish for up to 7 d at 4°C.
Spray treatment of inoculated cut lettuce in combination with EcoShield™ and sodium hypochlorite solution
Pieces of lettuce (9 cm2) were spot inoculated with E. coli O157:H7 with 50 ul of 6.9 log CFU/ml and allowed to dry for 90 min. Pieces of lettuce were either placed in 500 ml of a 50 μg/ml sodium hypochlorite (NaOCl) solution (Sigma Aldrich), made up in 0.05 M potassium phosphate buffer, or 500 ml PBS. NaOCl solutions were prepared in sterile chlorine-saturated 1 L glass beaker. Free chlorine concentrations were measured using a DR 720 colorimeter (Hach Inc.) and appropriate chlorine testing methodology. Inoculated lettuce was washed in NaOCl solution for 30 sec. Pieces of lettuce were then either immediately placed in in 15 ml Dey Engley (Becton Dickson) to neutralize the NaOCl in a 50-ml disposable conical tube (Fisher Scientific), or in a salad spinner to removed excess moisture and ‘spun’ for 15 sec. Subsequently the pieces of lettuce were then sprayed immediately with EcoShield™ (9.3 log PFU/ml) or PBS. Pieces of lettuce were treated with either PBS or EcoShield™ administered using a hand-held air-brush (Model 200, Badger Air-Brush Co.) attached to an air compressor (Badger Air Brush Co.) The air brush was filled with 20 ml of EcoShield™ (9.3 PFU/ml), and calibrated to deliver 2.4 ml over a 500 cm2 surface area,20 which resulted in 5.98 log PFU EcoShield™/cm2 concentration in the manner described above. After lettuce pieces were sprayed with EcoShield™ suspension, individual pieces were either placed in 15 ml of PBS for microbial analysis or stored at 4°C for 24 h as described above.
Microbiological analysis of E. coli O157:H7 populations on cut lettuce
After appropriate duration, one piece of lettuce inoculated with E. coli O157:H7 treated with EcoShield™ or PBS and were added to 15 ml of PBS in a 50-ml conical tube and homogenized with a Polytron Pt 2100 laboratory blender (Kinematica AG) for 30 sec. Two pieces of lettuce from each treatment were analyzed on each day of analysis. The blender was rinsed with 70% ethanol and sterile deionized water to prevent cross-contamination between each sample. A volume of 1 ml of the lettuce homogenate was surfaced-plated (0.25 ml on four plates) on sorbitol MacConkey agar (SMAC) (Becton Dickinson) supplemented with ampicillin (SMACA), or homogenates were serially diluted in 0.1% peptone water and then enumerated (0.1 ml, in duplicate) on SMACA. Plates were incubated at 37°C for 24 h before enumeration, and fluorescent colonies of E. coli O157:H7 gfp86,from lettuce samples were counted under UV light (395 nm). Colony counts were converted to log CFU/cm2 for statistical analysis. The limit of detection was 0.22 log CFU/cm2. Previous work has indicated that separating bacteriophages by centrifugation does not affect the bacteriophage titers; therefore this was not performed in this experiment.23
Bacteriophage enumeration
Bacteriophage concentrations were enumerated from lettuce or solutions containting EcoShield™ by soft agar overlay methods. Letttuce pieces treated with bacteriophages by either immersion or spray but not inoculated with E. coli O157:H7 were homogenized as described above either on Day 0 or after appropriate storage duration at 4°C. Homogenates were then serially diluted and mixed with 0.1 ml of stationary phage cultures of E. coli O157:H7 gfp86. Diluted homogeanates were then gently mixed with 4 ml 0.7% soft agar (held at 50°C), and poured onto a TSAA plate. After soft agar had solidified, plates were incubated at 37°C for 24 h. Plaques resulting from bacteriophage lysis of the E. coli O157:H7 lawn were counted and titers were coverted to log PFU/ml or log PFU/g.
Statistical analysis
All experiments were repeated at least three times. Populations of E. coli O157:H7 gfp 86 recovered from lettuce homogenates treated with either control or EcoShield™ were subjected to an analysis of variance and Duncan’s least-significant-difference test. The data were analyzed using Statistical Analysis Software (SAS 9.1.2)
Acknowledgments
This work was funded by the USDA-Agricultural Research Service, “Microbial Ecology and Safety of Fresh on-Farm Organically Grown Produce.” Rishi Banerjee, Mary Theresa Callahan and Ajay Singh contributed to this work. Any reference to a commercial product does not construe an endorsement.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bacteriophage/article/24323
References
- 1.Anonymous, Centers for Disease Control and Prevention (CDC) Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach--United States, September 2006. MMWR Morb Mortal Wkly Rep. 2006;55:1045–6. [PubMed] [Google Scholar]
- 2.Anonymous. Multistate Outbreak of E. coli O157:H7 infections, November-December 2006, daily web update. 2006 available at: http://www.cdc.gov/ecoli/2006/december/121406.htm Accessed: 01-08-2009.
- 3.Anonymous. Investigation announcement: Multistate outbreak of E. coli O157:H7 infections linked to Romaine lettuce. 2012 Available at: http://www.cdc.gov/ecoli/2011/ecoliO157/romainelettuce/120711/index.html Accessed: 10-26-2012.
- 4.Anonymous. Investigation update: Multistate outbreak of human E. coli O145 infections linked to shredded Romaine lettuce from a single processing facility. 2010. Available at: http://www.cdc.gov/ecoli/2010/ecoli_o145/index.html Accessed: 10-26-2012.
- 5.Behrsing J, Jaeger J, Horlock F, Kita N, Franz P, Premier R. Survival of Listeria innocua, Salmonella Salford and Escherichia coli on the surface of fruit with inedible skin. Postharvest Biol Technol. 2003;29:249–56. doi: 10.1016/S0925-5214(03)00004-8. [DOI] [Google Scholar]
- 6.Takeuchi K, Frank JF. Penetration of Escherichia coli O157:H7 into lettuce tissues as affected by inoculum size and temperature and the effect of chlorine treatment on cell viability. J Food Prot. 2000;63:434–40. doi: 10.4315/0362-028x-63.4.434. [DOI] [PubMed] [Google Scholar]
- 7.Abedon ST. Lysis from without. Bacteriophage. 2011;1:46–9. doi: 10.4161/bact.1.1.13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brussow H, Kutter E. 2005. Genomics and evolution of tailed phages. In: Kutter E and Sulakvelidze A. eds. Bacteriophages: biology and applications Washington D.C.: CRC Press, 2005: 91-128. [Google Scholar]
- 9.Sharma M, Sharma GC. Bacteriophages. In: Gomez-Lopez VM ed. Decontamination of fresh and minimally processed produce Oxford, U.K.: Wiley-Blackwell, 2012: 285-295. [Google Scholar]
- 10.Anonymous. Escherichia coli O157:H7 specific bacteriophages; temporary exemption for the requirement of a tolerance. 2011; Fed. Register 76: 71 (April 13, 2011) p. 20542. Available at: http://www.gpo.gov/fdsys/pkg/FR-2011-04-13/pdf/2011-8712.pdf Accessed on: 7-05-2011.
- 11.Anonymous Listeria-specific bacteriophage preparation. Food additives permitted for direct addition to food for human consumption. 21 CFR Part 172.785. Fed Regist. 2006;71:47729–32. [Google Scholar]
- 12.Anonymous. US Food and Drug Adminsitration. Food Contact Notice 1018. 2011. available at: http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=fcsListing&id=1018
- 13.Greer GG. Bacteriophage control of foodborne bacteriat. J Food Prot. 2005;68:1102–11. doi: 10.4315/0362-028x-68.5.1102. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy JE, Wei CI, Oblinger JL. Characterization of coliphages recovered from foods according to temperature of infectivity. J Food Prot. 1986;49:952–4. doi: 10.4315/0362-028X-49.12.952. [DOI] [PubMed] [Google Scholar]
- 15.Greer GG, Dilts BD. Control of Brochothrix thermosphacta spoilage of pork adipose tissue using bacteriophages. J Food Prot. 2002;65:861–3. doi: 10.4315/0362-028x-65.5.861. [DOI] [PubMed] [Google Scholar]
- 16.Sulakveldize A. Safety by nature: potential bacteriophage applications. Microbe. 2011;6:122–6. [Google Scholar]
- 17.O’Flynn G, Ross RP, Fitzgerald GF, Coffey A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl Environ Microbiol. 2004;70:3417–24. doi: 10.1128/AEM.70.6.3417-3424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pao S, Randolph SP, Westbrook EW, Shen H. Use of bacteriophages to control Salmonella in experimentally contaminated sprout seeds. J Food Sci. 2004;69:M127–9. doi: 10.1111/j.1365-2621.2004.tb10720.x. [DOI] [Google Scholar]
- 19.Leverentz B, Conway WS, Alavidze Z, Janisiewicz WJ, Fuchs Y, Camp MJ, et al. Examination of bacteriophage as a biocontrol method for salmonella on fresh-cut fruit: a model study. J Food Prot. 2001;64:1116–21. doi: 10.4315/0362-028x-64.8.1116. [DOI] [PubMed] [Google Scholar]
- 20.Leverentz B, Conway WS, Camp MJ, Janisiewicz WJ, Abuladze T, Yang M, et al. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl Environ Microbiol. 2003;69:4519–26. doi: 10.1128/AEM.69.8.4519-4526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye J, Kostrzynska M, Dunfield K, Warriner K. Evaluation of a biocontrol preparation consisting of Enterobacter asburiae JX1 and a lytic bacteriophage cocktail to suppress the growth of Salmonella Javiana associated with tomatoes. J Food Prot. 2009;72:2284–92. doi: 10.4315/0362-028x-72.11.2284. [DOI] [PubMed] [Google Scholar]
- 22.Abuladze T, Li M, Menetrez MY, Dean T, Senecal A, Sulakvelidze A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl Environ Microbiol. 2008;74:6230–8. doi: 10.1128/AEM.01465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma M, Patel JR, Conway WS, Ferguson S, Sulakvelidze A. Effectiveness of bacteriophages in reducing Escherichia coli O157:H7 on fresh-cut cantaloupes and lettucet. J Food Prot. 2009;72:1481–5. doi: 10.4315/0362-028x-72.7.1481. [DOI] [PubMed] [Google Scholar]
- 24.Viazis S, Akhtar M, Feirtag J, Diez-Gonzalez F. Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food Microbiol. 2011;28:149–57. doi: 10.1016/j.fm.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Luo Z, Simmons CW. VanderGheynst, Nitin N. Quantitative real time measurements of bacteria-bacteriophages interactions in fresh lettuce leaves. J Food Eng. 2012;111:176–85. doi: 10.1016/j.jfoodeng.2011.12.025. [DOI] [Google Scholar]
- 26.Roy B, Ackermann H-W, Pandian S, Picard G, Goulet J. Biological inactivation of adhering Listeria monocytogenes by listeriaphages and a quaternary ammonium compound. Appl Environ Microbiol. 1993;59:2914–7. doi: 10.1128/aem.59.9.2914-2917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyacioglu O, Goktepe I, Sharma M, Sulakvelidze A. Inhibition of E. coli O157:H7 on the surface of fresh spinach by bacteriophage ECP-100 and modified atmosphere packaging (Poster). Institute of Food Technologists 2010 Annual Meeting. 2010. [Google Scholar]
- 28.United States Department of Agriculture. The commercial storage of fruits, vegetables and florist and nursery stocks. Agriculture Handbook. 2006; number 66. Available at: www.ba.ars.usda.gov/hb66/contents.html
- 29.Agarwal AA, Konno K. Latex: A model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu Rev Ecol Evol Syst. 2009;40:311–31. doi: 10.1146/annurev.ecolsys.110308.120307. [DOI] [Google Scholar]
- 30.Brandl MT. Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl Environ Microbiol. 2008;74:5285–9. doi: 10.1128/AEM.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suslow TV. Chlorination in the production of and postharvest handling of fresh fruits and vegetables; chap. 6: fruit and vegetable processing. In: McLaren, D. (ed). Use of chlorine-based sanitizers and disinfectants in the food manufacturing industry. Food Processing Center at the University of Nebraska, Lincoln. 2000. p. 2-15. [Google Scholar]
- 32.Fratamico PM, Deng MY, Strobaugh TP, Palumbo SA. Construction and characterization Escherichia coli O157:H7 strains expressing firefly luciferase and green fluorescent protein and their use in survival studies. J Food Prot. 1997;60:1167–73. doi: 10.4315/0362-028X-60.10.1167. [DOI] [PubMed] [Google Scholar]