Abstract
The effect of a bacteriophage cocktail (EcoShield™) that is specific against Escherichia coli O157:H7 was evaluated against a nalidixic acid-resistant enterohemorrhagic E. coli O157:H7 RM4407 (EHEC) strain on leafy greens stored under either (1) ambient air or (2) modified atmosphere (MA; 5% O2/35% CO2/60% N2). Pieces (~2 × 2 cm2) of leafy greens (lettuce and spinach) inoculated with 4.5 log CFU/cm2 EHEC were sprayed with EcoShield™ (6.5 log PFU/cm2). Samples were stored at 4 or 10°C for up to 15 d. On spinach, the level of EHEC declined by 2.38 and 2.49 log CFU/cm2 at 4 and 10°C, respectively, 30 min after phage application (p ≤ 0.05). EcoShield™ was also effective in reducing EHEC on the surface of green leaf lettuce stored at 4°C by 2.49 and 3.28 log units in 30 min and 2 h, respectively (p ≤ 0.05).
At 4°C under atmospheric air, the phage cocktail significantly (p ≤ 0.05) lowered the EHEC counts in one day by 1.19, 3.21 and 3.25 log CFU/cm2 on spinach, green leaf and romaine lettuce, respectively compared with control (no bacteriophage) treatments. When stored under MA at 4°C, phages reduced (p ≤ 0.05) EHEC populations by 2.18, 3.50 and 3.13 log CFU/cm2, on spinach, green leaf and romaine lettuce. At 10°C, EHEC reductions under atmospheric air storage were 1.99, 3.90 and 3.99 log CFU/cm2 (p ≤ 0.05), while population reductions under MA were 3.08, 3.89 and 4.34 logs on spinach, green leaf and romaine lettuce, respectively, compared with controls (p ≤ 0.05). The results of this study showed that bacteriophages were effective in reducing the levels of E. coli O157:H7 on fresh leafy produce, and that the reduction was further improved when produce was stored under the MA conditions.
Keywords: E. coli O157:H7, bacteriophage, modified atmosphere packaging, MAP, leafy green
Introduction
Two multi-state enterohemorrhagic E. coli outbreaks linked to bagged baby spinach and shredded lettuce claimed 3 lives and sickened 254 people and raised concerns about the safety of fresh-cut leafy green vegetables.1,2 Though caused by another serotype, E. coli O104:H4, the most recent E. coli outbreak in Europe linked to contaminated fenugreek sprouts resulted in over 50 deaths and over 4,000 hospitalizations in 16 countries.3 After contamination with enteric bacterial pathogens like enterohemorrhagic E. coli or Salmonella spp, leafy greens such as spinach and lettuce will support the survival of enteric bacterial pathogens because currently there is no completely effective killing step during the harvesting, processing and packaging steps. It is estimated foodborne illness costs the United States $152 billion per year, with produce related cases of foodborne illness causing $39 billion per year.4 Enterohemorrhagic E. coli infections in the US incur about $1 billion in costs each year as well.4
Hypochlorite solutions are the most widely used treatment to wash fresh and fresh-cut produce to prevent contamination by enteric bacterial pathogens.5 However, the concentrations at which hypochlorite solutions are used are targeted to prevent cross contamination of produce with enteric bacteria through the washwater between batches of produce.6 The levels of hypochlorite currently used are not sufficient to kill 100% of the pathogenic bacterial cells which could be attached to leafy green or other produce surfaces. The oxidative effect of the free chlorine in hypochlorite solutions is inactivated by the organic substances released from the damaged or cut surfaces of the produce, potentially limiting the effectiveness of the free chlorine in the hypochlorite formulations.7 Therefore, novel, environmentally-friendly and effective intervention approaches are urgently needed to help reduce enteric bacterial populations on fresh produce.
One such novel approach is the use of bacteriophages (phages) to control bacterial pathogens on produce. Phages are viruses that specifically interact with and infect their respective host bacterial cells. They are extremely diverse and abundant; thus, phages present a promising mechanism to control pathogenic bacteria on produce. In nature, phages are responsible for killing almost half of the bacterial population generated daily.8 Considering the short generation time of the bacteria, phages represent a natural mechanism to keep bacterial populations under control. In the last decade, scientists have researched new phage-based solutions to control pathogenic bacteria in or on foods. Currently, there are four commercial bacteriophage products (AgriPhage™, Listex™ P100, ListShield™, EcoShield™) on the market that received FDA and/or EPA regulatory approvals for use on food items or to decontaminate food processing facilities. In several studies, EHEC-specific phage preparations were reported to significantly reduce the EHEC levels on various foods and hard surfaces.9-11 In another study, EcoShield™ (formerly ECP-100) phage cocktail treatment was able to reduce EHEC counts on fresh-cut lettuce by 1.6 log CFU/cm2 compared with untreated samples after 1 and 2 d of storage at 4°C.12 Separation of the phage particles from lettuce homogenates that contain both E. coli O157:H7 and phage cocktail before plating on agar plates to calculate the surviving E. coli O157:H7 counts showed that the antimicrobial effect of the phage cocktail was seen quickly, almost immediately after the start of treatment and the absence or presence of the unattached, free phage particles did not change the surviving E. coli O157:H7 counts.12 Additionally, fresh cut cantaloupes stored for seven days and treated with EcoShield™ had E. coli O157:H7 populations which were 3.1 log CFU/ml lower than untreated controls.12 Another EHEC-specific phage preparation, BEC8, reduced the experimentally inoculated E. coli O157:H7 levels (4 log CFU) on whole baby spinach and baby romaine lettuce leaves to undetectable levels after 24 h incubation at room temperature (20°C) or higher.13 When combined with trans-cinnamaldehyde (CT), BEC8 was found to be more effective against E. coli O157:H7 and resulted in complete inhibition of the pathogen in all samples at all temperatures.13 In a recent study, EcoShield™ phage preparation was used on beef and lettuce experimentally contaminated with E. coli O157:H7 and found to reduce the bacterial count by 1–2 logs after only 5 min exposure. The reduced levels of the E. coli O157:H7 were maintained for the duration of the study (one week) when foods were stored at 4°C.14
Under current distribution channels, fresh and fresh-cut produce may travel long distances to their eventual retail destination. To preserve the quality attributes of fresh and fresh-cut produce, modified atmosphere packaging (MAP) offers an indispensable advantage. During the transportation of produce, storage temperature and environmental gas concentrations are controlled to reduce the physiological deterioration to prolong shelf life. However, it is unknown how effectively lytic phages can inhibit the growth of bacterial pathogens under the MAP conditions (low oxygen/high carbon dioxide) commonly used in the industry. Thus, the objective of this study was to test and compare the effectiveness of the phage cocktail against EHEC on fresh-cut green leafy vegetables packaged under atmospheric air and modified atmosphere (low O2/high CO2) conditions.
Results and Discussion
Efficacy of the bacteriophage cocktail against E. coli O157:H7 RM4407 NalR (EHEC) on spinach and lettuce
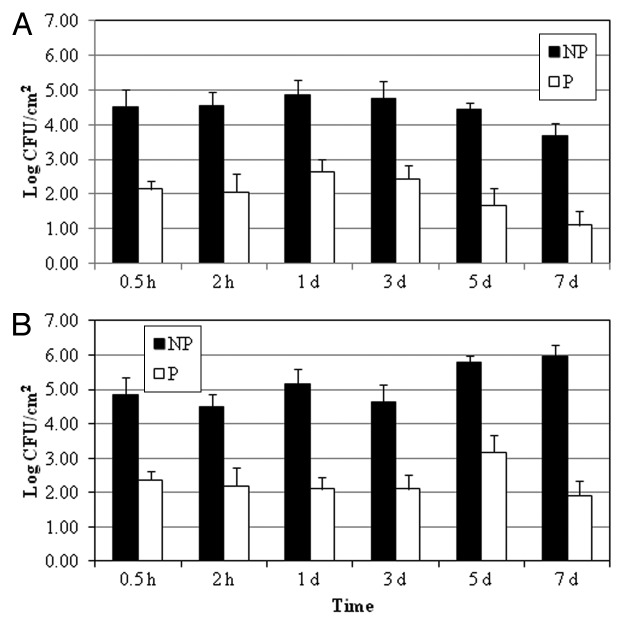
The effectiveness of the EcoShield™ phage cocktail against E. coli O157:H7 RM4407 NalR strain (EHEC) was tested on spinach (Fig. 1) and green leaf lettuce (Fig. 2). Application of EcoShield™ to spinach was able to significantly (p ≤ 0.05) reduce EHEC populations by 2.38 and 2.49 log CFU/cm2 at 4 and 10°C, respectively, after 30 min of storage, compared with control treatments. This significant reduction was maintained over the 7-d storage period at 4°C and 10°C (Fig. 1). In our experimental design, the shortest phage treatment was 30 min and the effect of the phage treatment was observed as early as 30 min after the phage cocktail application on spinach surface. These results are in agreement with the findings of Abuladze et al.,10 who reported a significant decrease in E. coli O157:H7 numbers recovered from the glass coverslips and gypsum boards after 5 min of EcoShield™ treatment. Also, the same study determined that the phage cocktail reduced the E. coli O157:H7 counts on spinach surface from 2.80 log CFU/cm2 to undetectable levels after storage at 10°C for 24 h.10 Our results show that the same phage cocktail is also highly effective against E. coli O157:H7 attached to spinach surfaces.
Figure 1. Effect of EcoShield™ on EHEC on spinach surface. (A) 4°C; (B) 10°C. Data shown as mean ± SD. NP: no phage; P: Phage treatment.
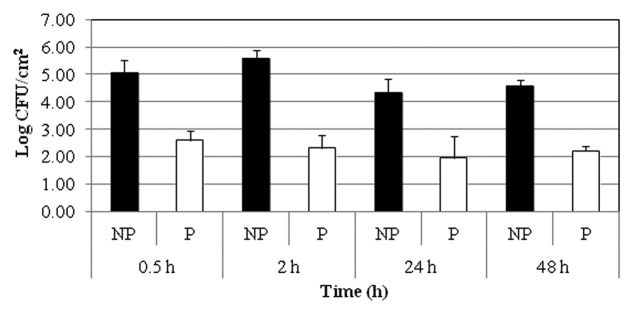
Figure 2. Effect of EcoShield™ on EHEC on green leaf lettuce at 4°C. Experiment was repeated twice in duplicates. NP: No phage; P: Phage treatment.
EcoShield™ significantly (p ≤ 0.05) reduced EHEC populations on green leaf lettuce surface (Fig. 2). These results were similar to those observed with EcoShield™ applied to spinach. The lytic phage activity could be observed as early as in 30 min (the shortest time-point examined) (Fig. 2). EcoShield™ was able to significantly reduce EHEC populations on spinach by 2.49 and 3.28 log units after 30 min and 2 h at 4°C, respectively, compared with control treatments. EHEC populations on the phage-treated lettuce surfaces remained low throughout the entire 48 h incubation period (2.38 log CFU/cm2 reduction).
In a previous study,12 EcoShield™ was reported to reduce E. coli O157:H7 populations on fresh-cut iceberg lettuce compared with control samples after 1 and 2 d of storage at 4°C. The researchers treated 3.76 log CFU/cm2 of E. coli O157:H7 with 5.98 log PFU/cm2 of the phage cocktail on fresh-cut iceberg lettuce surface and obtained a 1.6 log CFU/cm2 decline in bacterial populations. In our current study, we were able to achieve between a 2.49–3.28 log CFU/cm2 reductions in EHEC on day 0 on phage-treated, inoculated green leaf lettuce. The MOI value was 100 in this study, which is equal to what Sharma et al.12 used previously. The differences in observed reductions of E. coli O157:H7 populations could be due to the use of different EHEC strains, inoculation levels, recovery media and lettuce types. Other authors have shown that the surface of lettuce leaves can provide a barrier to the interactions between the bacteriophages and their potential bacterial targets, decreasing the efficacy as compared with broth studies.15 However, the immediate and significant reduction of E. coli O157:H7 was observed in both the current study described here and by Sharma et al.12 indicating the rapidly acting nature of lytic phages on leafy green surfaces. Another recent comparable study found that EcoShield™ significantly (p ≤ 0.05) reduced the counts of target E. coli O157:H7 in ground beef samples by ≥ 94% and in lettuce by 87% only after a 5 min phage treatment.14 The reduced levels of E. coli O157:H7 in treated ground beef samples were maintained during one week of storage at 4°C.14
The stability of the EcoShield™ phage cocktail on the surface of fresh-cut spinach leaves was tested at 4°C in the absence of target EHEC. The phage particles on the produce surface did not change significantly (p > 0.05) during the 17-d storage period at 4°C (Fig. 3).
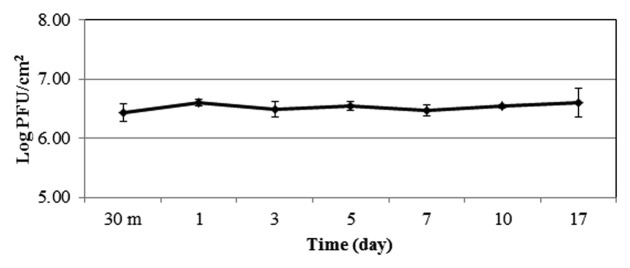
Figure 3. Bacteriophage stability on fresh produce stored at 4°C. Results are the mean of 3 replicates ± SD.
Bacteriophage cocktail vs. EHEC on the surfaces of spinach, romaine and green leaf lettuce stored under MAP
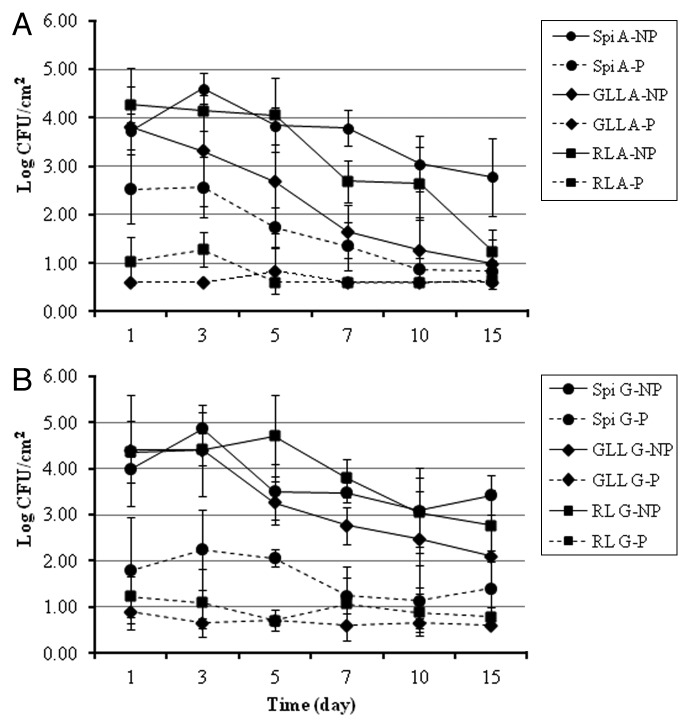
As noted earlier, application of the phage cocktail to leafy green commodities significantly (p ≤ 0.05) lowered the populations of EHEC after 1 d by 1.19 log CFU/cm2 on spinach, 3.21 log CFU/cm2 on green leaf lettuce, and 3.25 log CFU/cm2 on romaine lettuce stored at 4°C when compared with untreated inoculated control leafy green samples under atmospheric air (Fig. 4A). The reduction was further improved when leafy greens were packaged under MA (high CO2/low O2) and stored at 4°C. Specifically, EcoShield™ reduced EHEC populations by 2.18, 3.50 and 3.13 log CFU/cm2 on spinach, green leaf lettuce and romaine lettuce, respectively, stored under the MA conditions, compared with untreated controls (Fig. 4B). The reduction in EHEC population due to the phage treatment under MA storage was not significantly different from that under atmospheric air storage (p > 0.05).
Figure 4. Recovered EHEC counts on green leafy produce contaminated with EHEC, treated with bacteriophage cocktail, and stored at 4°C under; (A) MAP with atmospheric air; (B) MAP with 5% O2, 35% CO2, 60% N2 gas. Experiment was repeated twice in duplicates for each produce. Spi: Spinach; GLL: Green leaf lettuce; RL: Romaine lettuce; A: Air package; G: Gas package; NP: No phage; P: Phage treatment.
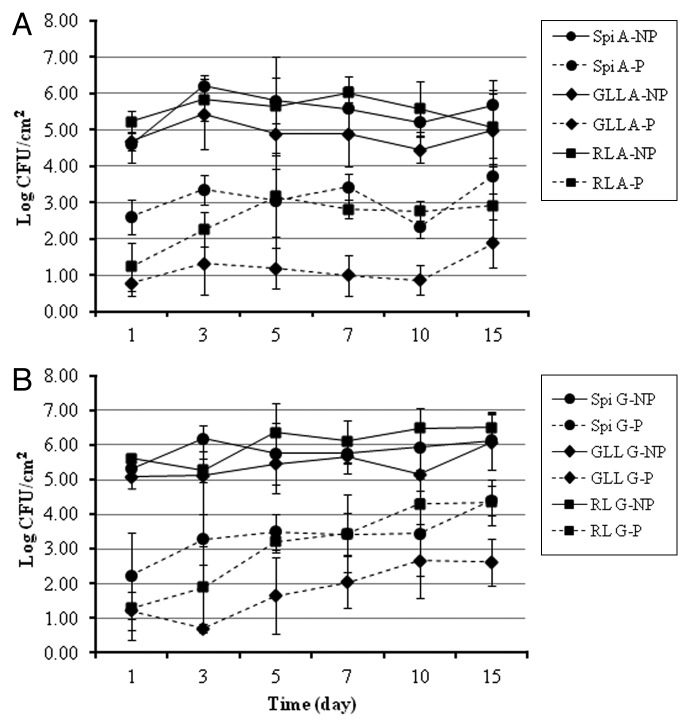
Similar results were obtained when leafy greens were stored at 10°C. As noted earlier, phage-treated leafy greens stored at 10°C under atmospheric air had significantly (p ≤ 0.05) lower EHEC counts than untreated leafy greens. Populations of EHEC decreased after 1 d by 1.99 log CFU/cm2 on spinach, 3.90 log CFU/cm2 on green leaf lettuce, and 3.99 log CFU/cm2 on romaine lettuce (Fig. 5A). When inoculated leafy green samples were stored for 15 d under MA gas condition at 10°C, based on multiple regression analysis the EHEC populations on phage treated spinach, green leaf and romaine lettuce decreased (p ≤ 0.05) on average by 3.08, 3.89 and 4.34 log units, respectively, compared with untreated controls (Fig. 5B).
Figure 5. Recovered EHEC counts on green leafy produce contaminated with EHEC, treated with bacteriophage cocktail, and stored at 10°C under; (A) MAP with atmospheric air; (B) MAP with 5% O2, 35% CO2, 60% N2 gas. Experiment was repeated twice in duplicates. Spi: Spinach; GLL: Green leaf lettuce; RL: Romaine lettuce; A: Air package; G: Gas package; NP: No phage; P: Phage treatment.
Multiple regression analysis of the entire data that includes all three leafy greens under both atmospheric air and MA at 4 and 10°C, showed that the phage treatment significantly (p ≤ 0.05) reduced the EHEC populations by 2.64 log CFU/cm2 on average compared with untreated contaminated samples. The EHEC populations in all samples stored at 4°C started to decline steadily after day 3; however, EHEC populations on the phage-treated samples were consistently lower than the untreated controls at each time point throughout the study. Storage at 10°C significantly (p ≤ 0.05) increased the EHEC populations recovered from the control (untreated by bacteriophage) leafy green leaves by 1.84 log CFU/cm2, on average, compared with control leafy green leaves stored at 4°C. The populations of EHEC on control leaf samples did not decline during the 15 d storage period at 10°C (Fig. 5A and B). Storage under MA resulted in an average of 0.40 log CFU/cm2 increase (p ≤ 0.05) in EHEC counts compared with the storage under atmospheric air. High CO2 concentrations in MAP might have blocked the growth of spoilage bacteria, which in turn minimizes the bacterial competition against EHEC resulting in slightly higher EHEC counts than atmospheric air storage.
EHEC populations on the untreated control leaves sprayed with peptone water (PW) and stored under atmospheric air conditions at 4°C decreased during the 15-d study by 0.94 log CFU/cm2 on spinach, 2.82 log CFU/cm2 on green leaf lettuce, and 3.04 log CFU/cm2 on romaine lettuce. After 15 d of storage at 4°C under MA, reductions were 0.55, 2.30, and 1.60 log CFU/cm2 on spinach, green leaf and romaine lettuces, respectively. These observed reductions in EHEC populations are in general agreement with the results of previous studies on shredded lettuce.16,17 One study found that E. coli O157:H7 counts on shredded lettuce decreased by 1 log CFU/cm2 after 10 d incubation at 5°C.16 In another study with shredded iceberg lettuce stored at 4°C for 10 d, E. coli O157:H7 population declined by 1.70 log inside gas permeable package with perforations, 0.85 log inside gas permeable package with an initial N2 flush, and 1.10 log inside gas-impermeable package filled with high CO2/low O2.17 Our results differ from those reported in a previous study,18 which reported only 0.43 log decrease of the E. coli O157:H7 counts on romaine lettuce stored at 4°C for 9 d. However, in that study the lettuce samples were not incubated under MAP conditions. The particular EHEC strain used, storage atmosphere and temperature conditions, specific constituents and the concentrations of the indigenous microflora, and the initial bacterial load might be responsible for the discrepancies between the current report and the previously published literature.19
The partial oxygen and carbon dioxide concentrations were measured for the entire duration of the MAP studies (Fig. 6). When all leafy green samples are considered, the oxygen level in the packages filled with atmospheric air dropped on average by 2.2% and 5.9%; whereas, CO2 levels increased by 1.1% and 3.7% at 4 and 10°C, respectively (Fig. 6A). Under MA storage at 4°C, O2 level increased by 1.8%, while CO2 level decreased by 18.1% in 15 d period. When the MA samples were stored at 10°C, both O2 and CO2 levels declined by 1.4% and 14.1%, respectively, over the same time period (Fig. 6B). The different O2 and CO2 concentrations used in this study did not significantly (p > 0.05) alter the efficiency of the phage cocktail in killing EHEC cells on leafy green leaves. Several previous studies have also exhibited similar results. For example, one study reported no effect on E. coli O157:H7 populations by the storage atmosphere that is composed of 3% O2 and 97% N2.20 Another study concluded that E. coli growth on shredded iceberg lettuce was not affected by any of the 4 different MAP conditions examined at 13 and 22°C.21 Lastly, a recent study noted that the various O2 and CO2 levels developed inside the different packaging film materials during 10 d of incubation at 5 and 25°C did not change the survival or the growth of EHEC on shredded romaine lettuce.16
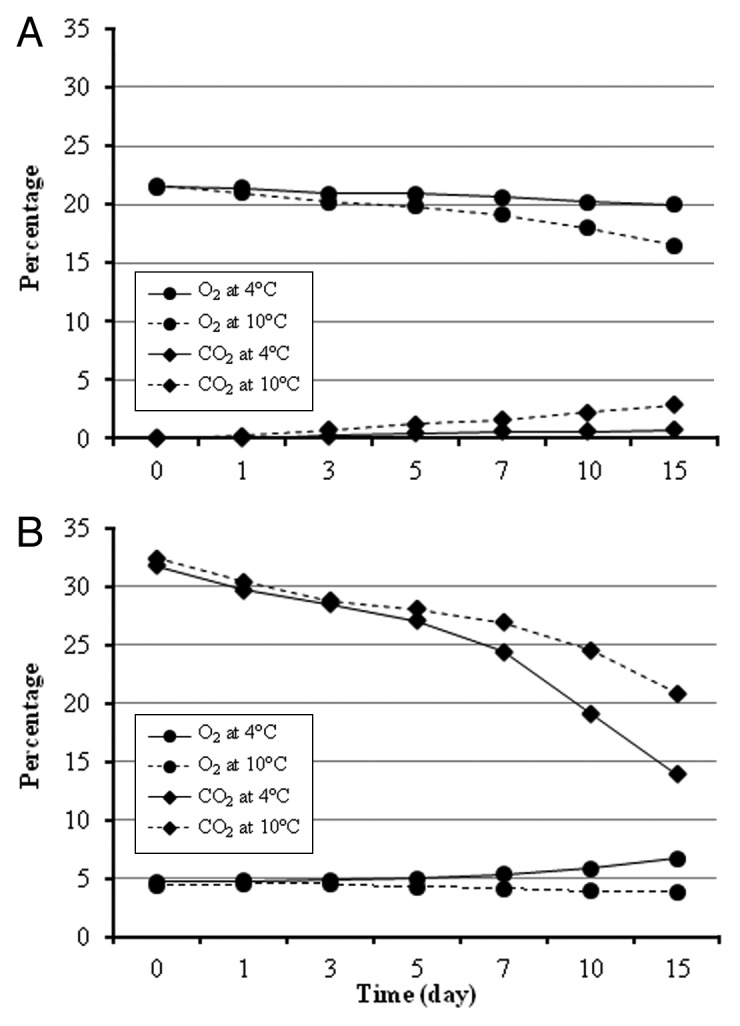
Figure 6. Partial percentages of O2 and CO2 during MAP experiment. (A) MAP with atmospheric air; (B) MAP with 5% O2, 35% CO2, 60% N2 gas.
Conclusions
In this study, the effect of an EHEC-specific phage cocktail (EcoShield™) against a nalidixic acid-resistant EHEC strain on fresh-cut leafy greens stored under different atmospheric conditions at 4 and 10°C was investigated. EcoShield™ was effective in significantly (p ≤ 0.05) reducing EHEC populations on spinach, green leaf lettuce and romaine lettuce under both atmospheric and MA conditions at both 4 and 10°C. These results indicate that the application of lytic bacteriophage mixtures specific for EHEC can be effective in reducing EHEC populations on fresh cut leafy greens under conditions (temperature and atmospheric composition) mimicking those commonly encountered during industrial produce processing, storage and transportation.
Materials and Methods
Bacterial strain and bacteriophage cocktail
Nalidixic acid resistant (up to 50 µg/ml) strain of E. coli O157:H7 RM4407 (EHEC) was obtained from USDA ARS, Environmental Microbial and Food Safety Laboratory, Beltsville, MD. This strain was previously associated with leafy green outbreak. It was cultured on Tryptic Soy Agar (Becton Dickinson) supplemented with 50 µg/ml nalidixic acid (Sigma-Aldrich) (TSAN) at 37°C. A single colony was inoculated into Tryptic Soy Broth (Becton Dickinson) supplemented with 50 µg/ml nalidixic acid (TSBN) and incubated at 37°C.
EcoShield™ bacteriophage cocktail (1010 PFU/ml in PBS, pH 7.4), specific against E. coli O157:H7, was provided by Intralytix, Inc., Baltimore, MD. The cocktail consists of three E. coli O157:H7-specific lytic phages (ECML-4, ECML-117 and ECML-134) in the Myoviridae family isolated from the environment. Phage cocktail was serially diluted to yield a final concentration of 7 log PFU/ml in sterile 1.5% peptone water (PW) (w/v) (Becton Dickinson) immediately before treatment. PW was chosen as the salt residues from phosphate buffered saline (PBS) caused dehydration of the leaf pieces during long incubation periods.
Leafy green vegetable preparation
Commercially packaged, pre-washed, ready-to-eat fresh spinach, conventional green leaf and romaine lettuce were purchased from a local grocery store (Greensboro, NC), brought to the laboratory, and stored immediately at 4°C for up to 1 d until use. All produce were handled with 70% ethanol-treated nitrile gloves. Outer leaves of the lettuces, damaged spinach and lettuce leaves, and leaves that did not look healthy or edible were discarded. Lettuce leaves were washed gently for about 10 sec under running tap water. Using a sterile knife and surface-disinfected glass cutting board, spinach and lettuce leaves were cut into 2 × 2 cm2 pieces. The pieces of the leaves were immediately placed into a sterile stomacher bag to reduce wilting due to water loss and kept at room temperature during preparation steps. The leaves were then placed as a grid on aluminum foil sheet for bacteria and phage inoculation. EHEC cultures were diluted in peptone water to adjust the population to ca. 7 log CFU/ml. Ten microliters from the adjusted EHEC culture was inoculated gently on top side of the 2 × 2 cm2 of leaf pieces. Extra care was given to distribute the total volume evenly in 15–20 small droplets on the entire surface by gently touching the surface of the leaf pieces without disturbing or damaging the surface. The inoculated droplets did not contact the cut edges of the leaves. Contaminated leaf pieces were air-dried for 20 min to allow for bacterial attachment and then sprayed with 7 log PFU/ml EcoShield™ phage cocktail or peptone water (control). A small fingertip sprayer (Bottle Crew) that delivers 100 µl in a single spray volume was used to deliver the phage cocktail at a concentration of ~6.5 log PFU/cm2 of leaf. The phage was sprayed from 30 cm above the surface, with one stroke (100 μl) per leaf plus one extra stroke for the leaves around the edges of the grid. Phage concentrations recovered from the leaf pieces that were not inoculated with EHEC were obtained to verify the repeatability of the method. The leaf pieces were incubated up to 7 d at 4 and 10°C in sterile Petri dishes humidified with sterile, wet filter paper. To determine the surviving EHEC counts, the leaf pieces were placed in glass tubes with 9 ml peptone water using sterile forceps, homogenized with a handheld laboratory blender (Kinematica AG, Polytron PT 1200 E). Between the leaf samples, the blender was rinsed in a 3 step process; tap water, 100% denatured ethanol and sterile water to prevent cross-contamination of EHEC. The homogenized leaves were diluted in 1.5% peptone water and plated on MacConkey agar (Becton Dickinson) containing 25 µg/ml Nalidixic acid (MACN). After the overnight incubation at 37°C, violet EHEC colonies were enumerated and recovered EHEC populations were expressed as log CFU/cm2.
Phage titration on fresh produce
To determine the active phage counts on fresh produce stored at 4°C for up to 17 d, uninoculated spinach leaf samples were treated with EcoShield™ as described above and were homogenized and serially diluted in peptone water before being used in the soft agar overlay assay22 with some modifications. Briefly, cultures of EHEC were serially diluted in 0,1% peptone water, and 100 μl of ca. 7 log CFU/ml EHEC was mixed with 1 ml of diluted, phage-treated spinach homogenates. This mixture was then added to 3.5 ml of warm (50°C) soft agar made from Lysogeny Broth (Fisher Scientific) containing 0.75% agar (w/v) (Fisher Scientific) and gently vortexed. The content of the tube was then quickly poured onto LB plate. Once the top agar layer solidified, the plates were incubated at 37°C. Clear plaques formed by the phages were counted the next day to enumerate bacteriophages, and phage titers were then converted to log PFU/cm2.
Effect of the phage cocktail against E. coli O157:H7 on leafy greens at 4 and 10°C under different modified atmosphere packages (MAP)
Inoculation of the EHEC on the leaf pieces and treatment of phage cocktail were performed in the same manner as explained above. The leaf pieces were incubated up to 15 d at 4 and 10°C inside sterile Petri dishes humidified with sterile, wet filter papers under two different atmospheric conditions, atmospheric air (A) and modified gas mixture (G). Modified gas mixture contained 5% O2, 35% CO2 and 60% N2. Leaf samples inside the Petri dishes were placed in vacuum pouches (Prime Source Vacuum Pouches), and filled with the desired gas mixture by using a table-top vacuum packaging machine (Supervac). After the incubation, the surviving EHEC populations were determined the same way as explained above.
Statistical analysis
All experiments were repeated twice with at least two replicates in each trial. Populations of EHEC recovered from spinach and lettuce homogenates treated with either control or EcoShield™ phage cocktail were log transformed and subjected to analysis of variance (ANOVA), multiple regression analysis, and Fisher’s least significant difference test using SAS, version 9.2 (Cary, NC). The level of statistical significance was p ≤ 0.05.
Acknowledgments
The authors would like to thank Ms. Bonita Hardy for her help in executing the experiments. This study was supported by the USDA-NIFA (project #NCX-2007-03435).
Glossary
Abbreviations:
- EHEC
nalidixic acid-resistant enterohemorrhagic E. coli
Disclosure of Potential Conflicts of Interest
A.S. holds an equity stake in Intralytix, Inc., a Maryland corporation involved with the development of phage preparations (including EcoShield™) for various practical applications.
Footnotes
Previously published online: www.landesbioscience.com/journals/bacteriophage/article/24620
References
- 1.Centers for Disease Control and Prevention (CDC) Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach - United States, September 2006. MMWR Morb Mortal Wkly Rep. 2006;55:1–2. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Multistate Outbreak of E. coli O157 Infections, November-December 2006. 2006 [Google Scholar]
- 3.WHO. EHEC outbreak: Update 5 (03-06-11). 2011 [Google Scholar]
- 4.Scharff RL. Health-related costs from foodborne illness in the United States. Produce Safety Project 2010:1-28 [Google Scholar]
- 5.Takeuchi K, Frank JF. Quantitative determination of the role of lettuce leaf structures in protecting Escherichia coli O157:H7 from chlorine disinfection. J Food Prot. 2001;64:147–51. doi: 10.4315/0362-028x-64.2.147. [DOI] [PubMed] [Google Scholar]
- 6.Behrsing J, Winkler S, Franz P, Premier R. Efficacy of chlorine for inactivation of Escherichia coli on vegetables. Postharvest Biol Technol. 2000;19:187–92. doi: 10.1016/S0925-5214(00)00092-2. [DOI] [Google Scholar]
- 7.Marriott N. Principles of Food Sanitation. Gaithersburg, MD: Aspen Publishers, Inc., 1999. [Google Scholar]
- 8.Suttle CA. Viruses in the sea. Nature. 2005;437:356–61. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- 9.Viazis S, Akhtar M, Feirtag J, Diez-Gonzalez F. Reduction of Escherichia coli O157:H7 viability on hard surfaces by treatment with a bacteriophage mixture. Int J Food Microbiol. 2011;145:37–42. doi: 10.1016/j.ijfoodmicro.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Abuladze T, Li M, Menetrez MY, Dean T, Senecal A, Sulakvelidze A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl Environ Microbiol. 2008;74:6230–8. doi: 10.1128/AEM.01465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma M, Ryu JH, Beuchat LR. Inactivation of Escherichia coli O157:H7 in biofilm on stainless steel by treatment with an alkaline cleaner and a bacteriophage. J Appl Microbiol. 2005;99:449–59. doi: 10.1111/j.1365-2672.2005.02659.x. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M, Patel JR, Conway WS, Ferguson S, Sulakvelidze A. Effectiveness of bacteriophages in reducing Escherichia coli O157:H7 on fresh-cut cantaloupes and lettuce. J Food Prot. 2009;72:1481–5. doi: 10.4315/0362-028x-72.7.1481. [DOI] [PubMed] [Google Scholar]
- 13.Viazis S, Akhtar M, Feirtag J, Diez-Gonzalez F. Reduction of Escherichia coli O157:H7 viability on leafy green vegetables by treatment with a bacteriophage mixture and trans-cinnamaldehyde. Food Microbiol. 2011;28:149–57. doi: 10.1016/j.fm.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Carter CD, Parks A, Abuladze T, Li M, Woolston J, Magnone J, et al. Bacteriophage cocktail significantly reduces Escherichia coli O157: H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage. 2012;2:178–85. doi: 10.4161/bact.22825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Z, Simmons CW, VanderGheynst JS, Nitin N. Quantitative real time measurements of bacteria–bacteriophages interactions in fresh lettuce leaves. J Food Eng. 2012;111:176–85. doi: 10.1016/j.jfoodeng.2011.12.025. [DOI] [Google Scholar]
- 16.Oliveira M, Usall J, Solsona C, Alegre I, Viñas I, Abadias M. Effects of packaging type and storage temperature on the growth of foodborne pathogens on shredded ‘Romaine’ lettuce. Food Microbiol. 2010;27:375–80. doi: 10.1016/j.fm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Sharma M, Lakshman S, Ferguson S, Ingram DT, Luo Y, Patel J. Effect of modified atmosphere packaging on the persistence and expression of virulence factors of Escherichia coli O157:H7 on shredded iceberg lettuce. J Food Prot. 2011;74:718–26. doi: 10.4315/0362-028X.JFP-10-427. [DOI] [PubMed] [Google Scholar]
- 18.Carey CM, Kostrzynska M, Thompson S. Escherichia coli O157:H7 stress and virulence gene expression on Romaine lettuce using comparative real-time PCR. J Microbiol Methods. 2009;77:235–42. doi: 10.1016/j.mimet.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Velasco G, Davis M, Boyer RR, Williams RC, Ponder MA. Alterations of the phylloepiphytic bacterial community associated with interactions of Escherichia coli O157:H7 during storage of packaged spinach at refrigeration temperatures. Food Microbiol. 2010;27:476–86. doi: 10.1016/j.fm.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Raouf UM, Beuchat LR, Ammar MS. Survival and growth of Escherichia coli O157:H7 on salad vegetables. Appl Environ Microbiol. 1993;59:1999–2006. doi: 10.1128/aem.59.7.1999-2006.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz C, Hotchkiss JH. Comparative growth of Escherichia coli O157:H7, spoilage organisms and shelf-life of shredded iceberg lettuce stored under modified atmospheres. J Sci Food Agric. 1996;70:433–8. doi: 10.1002/(SICI)1097-0010(199604)70:4<433::AID-JSFA518>3.0.CO;2-Q. [DOI] [Google Scholar]
- 22.Leverentz B, Conway WS, Janisiewicz W, Camp MJ. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J Food Prot. 2004;67:1682–6. doi: 10.4315/0362-028x-67.8.1682. [DOI] [PubMed] [Google Scholar]