Abstract
The autoimmune disease systemic lupus erythematosus (SLE) results from an inability of the immune system to discriminate between certain self-antigens and foreign ones. The most common treatment of SLE involves the use of immunosuppressive drugs to reduce inflammation, but these therapies have serious side effects. Three recent papers in Science Translational Medicine redirect focus on neutrophils, platelets, and interferon-α in the pathogenesis of SLE and reinforce the notion that researchers should seek to discover and devise combination therapies that target these processes.
Systemic lupus erythematosus (SLE, or lupus) is an autoimmune disease characterized by multiorgan inflammation. The hallmark of SLE is the ubiquitous presence of serum antibodies directed against nuclear structures, including chromatin and ribonucleoproteins (RNPs) necessary for RNA processing, reflecting an underlying inability of the immune system to discriminate between self- and foreign antigens (1). This fundamental loss of self-tolerance is secondary to predisposing genetic factors (2) in the setting of environmental triggers and stochastic events. SLE currently is treated with broadly acting immunosuppressive drugs to systemically quell inflammation, but these therapies have serious side effects. Three recent papers in Science Translational Medicine suggest potential strategies for the development of combination therapies that target specific aspects of SLE pathogenesis (3–5). In this Perspective, I describe these findings in the context of what is known about how SLE wreaks havoc on the human immune system.
THE SLE PARADIGM
Autoreactive B lymphocytes—those that react to self-antigens—produce autoantibodies to double-stranded (ds) DNA and RNPs released from dying cells, and these autoantibodies play a key pathogenic role in SLE (1). The deposition of immune complexes of these autoantibodies with their respective autoantigens in target organs, such as the kidney, leads to activation of the complement system—a branch of the innate immune system that normally aids the adaptive immune system in clearing pathogens from the organism—and binding of the autoantibodies to Fc receptors (FcRs) on immune cells (6), with subsequent activation of tissue-infiltrating macrophages that promote the inflammatory response and resultant tissue injury (7). Similar mechanisms presumably account for inflammation in other SLE target organs, such as the skin and joints. dsDNA and RNPs also induce autoreactive B cell proliferation and autoantibody production through engagement of their cognate B cell immunoglobulin receptors and Toll-like receptors TLR9 and TLR7, respectively (8). Members of the TLR family of innate immune proteins are found on the surfaces and in the interiors of a variety of immune cells and activate immune responses. These findings indicate that autoantibodies are involved in inflammation and tissue injury as well as in perpetuation of their production by autoreactive B cells. Autoantibodies may also directly injure target cells; for example, anti-dsDNA antibodies that cross-react with N-methyl-d-aspartate (NMDA) receptors on neurons promote excitotoxic cell death in the hippocampus (9), with entry into the central nervous system mediated by breaches in the blood-brain barrier that are promoted by inflammatory cytokines. Target organ injury in SLE may also result from recruitment of inflammatory cells in an antibody-independent manner (10). The immune response in SLE is associated with the production of type I interferon-α (IFN-α) (11–14), with increases in this cytokine paralleling disease activity and severity (11, 15, 16). Such increases lead to enhanced transcription of interferon-responsive genes in immune and other cells (15, 16), thus promoting the inflammatory response in SLE. IFN-α also enhances monocyte maturation to dendritic cells (17), enabling autoreactive T cell activation along with B cell maturation and autoantibody production by plasma cells (18). Type I interferons are primarily produced by innate immune plasmacytoid dendritic cells (pDCs) in response to a viral or bacterial infection. Under these typical circumstances, engagement of TLRs and other innate immune receptors by pathogen-associated nucleic acids triggers a signaling cascade that results in the production of type I interferons (19–22). In the context of SLE, however, this normal physiology is betrayed; pathological activation of pDCs and release of IFN-α (17, 23) occur after FcR-mediated uptake of immune complexes of autoantibodies to chromatin and RNPs with engagement of TLR7 and TLR9 by self-RNAs and -DNA (24), respectively, in a manner analogous to that in B cells. Buttressing this hypothesis is the observation that autoantibodies to RNPs in particular are associated with the robust interferon response in SLE (25).
Thus, a disease paradigm in lupus has emerged in which excessive autoantibody production by B cells promotes interferon release by pDCs, with the latter then promoting inflammation while driving autoreactive B cell maturation. But how is this inflammatory response initiated and why is it maintained? Genome-wide association studies provide critical clues, identifying in SLE patients gene variants that lead to deregulation of potentially autoreactive T and B cells with tolerance loss and subsequent autoantibody production; disrupted clearance of the DNA- and RNA-containing autoantigens that are necessary for activation of autoreactive B cells and for formation of pathogenic immune complexes; and altered responsiveness of intracellular signaling pathways that promote IFN-α release (2). Genetic polymorphisms in the major histocompatibility complex and in FcR and complement protein genes that promote aberrant handling of immune complexes and regulation of B cell tolerance also appear to contribute to disease pathogenesis (2). In the setting of this genetic background, SLE is then initiated by environmental triggers (2), with a likely contribution from the random activation of autoreactive cells (26), a randomness inherent in the immune system, as it needs to anticipate responses to a vast range of potential pathogens. Although considerable insight has been made in understanding the pathogenic steps that lead to tissue injury in SLE, gaps in the disease paradigm remain. There is an incomplete understanding of the cellular sources and characteristics of self-antigens that are required for the aberrant immune response in SLE, as well as the precise steps by which complexes of autoantibodies and autoantigens elicit the injurious inflammatory response. Careful clinical observations have also identified unexplained findings in SLE patients with active disease who have heightened interferon responses. For example, the blood of such patients contains a large number of immature neutrophils, which reflects enhanced bone marrow release; this event is likely a consequence of the death of mature neutrophils upon activation by cytokines, including type I interferons, that are elevated in active SLE. While mature dying neutrophils are found at sites of organ injury in SLE and apparently contribute to tissue injury, their precise role in disease promotion is uncertain.
UNRIDDLING THE NEUTROPHIL
Let us consider three questions: What are the steps that lead to neutrophil activation in SLE, what are the consequences of this activation, and how do these physiological consequences further inform us about self-antigen–driven autoreactivity in SLE? Two papers in this issue of Science Translational Medicine provide answers to these questions, while placing the aberrant activation of neutrophils in SLE squarely within the current disease paradigm in which autoantibody production with immune-complex formation triggers aberrant interferon release by pDCs. Lande et al. (3) and Garcia-Romo et al. (4) found that neutrophils from patients with SLE are activated by IFN-α, with release of neutrophil extracellular traps (NETs) that contain unwound DNA, and antimicrobial peptides. Under physiological circumstances, NET release (NETosis) by neutrophils leads to entrapment and killing of pathogenic bacteria, fungi, and parasites (27). However, in SLE, NETs drive IFN-α production by pDCs after engagement of TLR9, thus promoting cytokine-driven disease progression (Fig. 1). Necessary contributors to this process include autoantibodies and their cognate autoantigens. Garcia-Romo et al. demonstrated that immune complexes containing anti-RNP autoantibodies bound to Fc gamma receptor IIA (FcγRIIa) on neutrophils with TLR7 engagement and generation of reactive oxygen species (ROS). TLR7 expression in neutrophils is enhanced by pDC-secreted IFN-α. It is the FcγRIIa-mediated anti-RNP binding to neutrophils that promotes their death by NETosis, as this binding drives the generation of ROS, which are necessary for activation of neutrophil enzymes and their movement to the cell’s nucleus to initiate the DNA unwinding critical for NET generation (28).
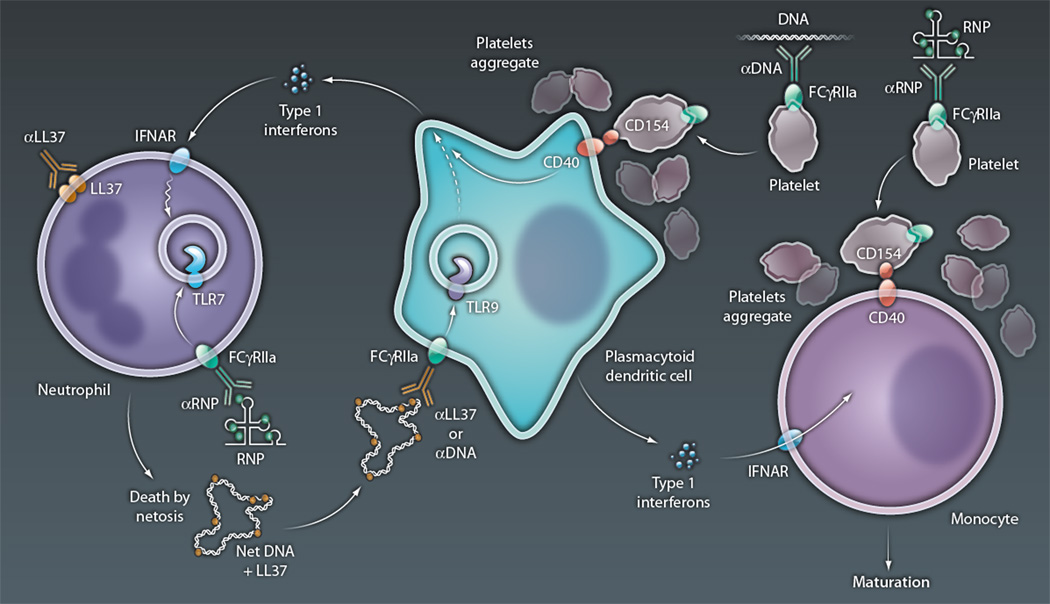
Figure 1. Immune cell medley in SLE.
Type I interferons, released from pDCs in SLE, prime neutrophils for death by NETosis, with up-regulation of TLR7 in endosomes and translocation of microbial peptides (such as LL37) to the cell surface. NETosis is then initiated by FcR-mediated uptake of anti-RNP and RNP immune complexes, with the RNA component of the latter engaging TLR7 in conjunction with release of ROS required for NET formation, and by the binding of surface-associated LL37 by anti-LL37 autoantibodies, presumably also leading to ROS release. NETs released from dying neutrophils are then taken up by pDCs, directly or as an immune complex of NET-associated LL37 and DNA, along with anti-LL37 or anti-DNA autoantibodies. In either case, the DNA component of the NETs engages TLR9 in endosomes, with the activation of genes that lead to interferon release and thus further neutrophil priming. Immune complexes of anti-DNA and DNA, or anti-RNP and RNPs, also activate platelets via an FcR-mediated pathway, with up-regulation of surface CD154 and aggregation. Aggregated platelets form complexes with pDCs and monocytes, enabling CD40-mediated activation after its engagement by platelet-expressed CD154. CD40 activation of pDCs leads to interferon release, whereas that of monocytes promotes their maturation to antigen-presenting dendritic cells, a process also enabled by interferons released by pDCs. The latter APCs serve as efficient activators of autoreactive T cells in lupus, which can promote release of autoantibodies (anti-LL37, anti-RNPs, and anti-DNA) by autoreactive B cells (not shown).
CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE
Both Lande et al. (3) and Garcia-Romo et al. (4) show that NETs in SLE contain LL37, a prominent DNA-binding antimicrobial peptide that facilitates NET-mediated pathogen killing and, in autoimmunity, enables self-DNA and -RNA to engage TLR9 and TLR7, respectively, in pDCs, with subsequent enhancement of IFN-α release (29, 30). Lande et al. dissected the mechanism behind this effect, showing that LL37 protects NET DNA from extracellular degradation while concomitantly facilitating the DNA’s uptake by and activation of pDCs via TLR9 engagement with subsequent IFN-α production. Indeed, internalization of DNA complexes via FcγRIIas on pDCs was increased in vitro by anti-LL37 and anti-DNA autoantibodies, which the authors detected in significant amounts in sera samples from SLE patients but not in those from scleroderma patients or healthy subjects. Lande et al. also suggest that complexes of self-DNA and antimicrobial peptides contribute to the activation of autoreactive B cells in SLE, with the resulting generation of anti-LL37 autoantibodies. This conclusion was based upon the correlation of these autoantibodies with those directed against DNA, an autoantigen known to trigger autoreactive B cell activation. Intriguingly, the authors also showed that neutrophils exposed to IFN-α in vitro displayed increased amounts of antimicrobial peptides on their membranes where they were bound by antimicrobial peptide antibodies to stimulate NET release. Thus, like those of Garcia-Romo et al. (4), the findings by Lande et al. revealed that IFN-α primes neutrophils for death by NETosis. These two studies push type I interferons front and center in the pathogenesis of SLE, demonstrating that release of NETs by neutrophils and their uptake and activation of pDCs drive the chronic IFN-α production seen in many patients with SLE. They also demonstrate a novel pathogenic function of autoantibodies in the disease, beyond their established role in immune complex–mediated renal disease, by directly tying the autoantibodies to NETosis of neutrophils. What other insights into SLE pathogenesis can we take from these papers? Most importantly, perhaps, they illustrate the feed-forward nature of the disease: Interferons promote neutrophil activation, which, in the setting of autoantibody engagement, begets NET formation with subsequent pDC activation and interferon production, events that ultimately promote further immune system activation. The central importance of IFN-α to the disease process provides additional support for interferon blockade as a therapeutic strategy in SLE. While such a strategy is theoretically fraught with hazard given the role of this cytokine in host defenses, blockade of other critical cytokines, such as TNF-α, interleukin (IL)-1, and IL-6, has proven to be beneficial in the treatment of other autoimmune diseases—rheumatoid arthritis, Crohn’s disease, and systemic onset juvenile arthritis—with acceptable toxicity profiles.
The work of Lande et al. (3) and Garcia-Romo et al. (4) also underscores the importance of TLR engagement in SLE pathogenesis. Blockade of TLR signaling is efficacious in animal models of autoimmunity and offers a second approach to disease intervention (31). TLR blockade is also therapeutically appealing in that it enhances the effect of corticosteroids on suppression of IFN-α production by pDCs (32), thus offering the potential to avoid use of high doses of these toxic agents in SLE patients.
PLATELETS AS PLAYERS
A recent publication in Science Translational Medicine adds additional insight into the pathogenic events that trigger IFN-α release in SLE. Duffau et al. (5) found that circulating immune complexes of anti-dsDNA plus dsDNA and anti-RNP plus RNPs activate platelets from SLE patients, as measured by expression of CD154 and selectins, via engagement of FcγRIIa. CD154 is a molecule known to be critical for dendritic cell and B cell activation upon engagement of its receptor CD40. In SLE, the activated platelets form aggregates with monocytes and pDCs, resulting in enhanced interferon expression by the latter that was dependent on CD40 signaling mediated by CD154 binding (Fig. 1). Thus platelets, like neutrophils, are activated by immune complexes of autoantibodies and autoantigens in patients with SLE, and this activation contributes to the release of IFN-α by pDCs and promotion of the inflammatory response. Notably, engagement of CD40 on dendritic cells, in the setting of TLR engagement, helps license these cells to overcome immune tolerance (33). Platelets may therefore contribute to activation of autoreactive T cells by dendritic cells in SLE.
It is also tempting to speculate that the accelerated vascular disease seen in patients with active SLE (34) might be linked to their exuberant platelet activation (35). Importantly, as demonstrated by Duffau et al. (5), depletion of platelets or their inhibition by clopidrogel—an oft-prescribed preventative of thromboembolic disease that down-regulates CD154 expression—improved disease parameters and survival in a mouse model of SLE (which is a model system that accurately reproduces characteristics of the human illness), suggesting that such therapy might be beneficial for the accelerated vascular disease in SLE. Although Duffau et al. did not ask whether clopidrogel affected the interferon response in the mouse model, as might be predicted, this therapeutic approach is nonetheless appealing given that vascular disease is now a leading cause of morbidity and mortality in SLE patients (34). The authors also suggest that statins might be beneficial in the treatment of such patients, given their effectiveness in reduction of platelet activation. Despite this compelling rationale, a recently completed (albeit a relatively short-term 2-year) trial of statin therapy in SLE was ineffective in reduction of clinical parameters associated with vascular disease (36). Paradoxically, statins boost release of extracellular NETs by neutrophils, enabling their antibacterial capability (37), although it is not known whether or how these agents might affect the pathogenic NET formation triggered by autoantibodies and interferons in SLE, as described by Lande et al. (3) and Garcia-Romo et al. (4).
The work of Duffau et al. (5) also reminds us of the appeal of therapeutic blockade of CD154-CD40 interactions. Treatment with an anti-CD154 antibody has proven to be beneficial in SLE patients, yielding a reduction in disease activity (as measured by an SLE disease activity index) and immune-complex–mediated glomerulonephritis—inflammation of blood vessels in the kidney—in the setting of diminished pathogenic autoantibody formation (38). These effects were thought to be a consequence of blocking the pathogenic T cell–B cell interactions that are a hallmark of SLE, as evidenced by interdiction in pathological B cell maturation; however, we now might consider that a reduction in activated platelet–driven interferon activity contributes to the anti-CD154 effect. Although this therapy was complicated by thromboembolic disease (apparently triggered by binding of the antibody to platelet FcRs with engagement of the surface-associated CD154 and platelet aggregation) (39), the work of Duffau et al. adds weight to the idea that other approaches being developed for blockade of CD40 signaling in SLE might be beneficial. The results from all three papers argue that therapeutic intervention at any place in the self-perpetuating disease cycle in SLE might be beneficial. However, clinical trials have not always borne out this concept, with notable therapeutic disappointments, including B cell depletion with anti-CD20 and interruption of T cell–antigen presenting cell interactions with CTLA4Ig. Rather, combination therapy directed at more than one potentially pathological step might be a more suitable alternative. Combinations of immunosuppressive agents such as cyclophosphamide or mycophenolate mofetil and corticosteroids have historically been beneficial in the therapy of some patients with lupus nephritis (40, 41); however, use of these agents, particularly cyclophosphamide and corticosteroids, is limited by significant toxicities. Thus, these three new studies offer additional justification to continue rational targeting of pathogenic steps in disease, perhaps using combination therapies, while providing the knowledge to effectively monitor immune responsiveness to avoid undue toxicity from such interventions.
REFERENCES AND NOTES
- 1.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: Positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 2.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: New insights from fine mapping and genome-wide association studies. Nat. Rev. Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu Y-J, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001180. 73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001201. 73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffau P, Seneschal J, Nicco C, Richez C, Lazaro E, Douchet I, Bordes C, Viallard J-F, Goulvestre C, Pellegrin J-L, Weil B, Moreau J-F, Batteux F, Blanco P. Platelet CD154 potentiates interferon-α secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3001001. 47ra63. [DOI] [PubMed] [Google Scholar]
- 6.Bergtold A, Gavhane A, D’Agati V, Madaio M, Clynes R. FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis. J. Immunol. 2006;177:7287–7295. doi: 10.4049/jimmunol.177.10.7287. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J. Immunol. 2003;171:489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 8.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 9.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 10.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N. Engl. J. Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 12.Preble OT, Black RJ, Friedman RM, Klippel JH, Vilcek J. Systemic lupus erythematosus: Presence in human serum of an unusual acid-labile leukocyte interferon. Science. 1982;216:429–431. doi: 10.1126/science.6176024. [DOI] [PubMed] [Google Scholar]
- 13.Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982;25:401–406. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- 14.Bengtsson AA, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, Rönnblom L. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 15.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 18.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 19.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 20.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 21.Bauer M, Redecke V, Ellwart JW, Scherer B, Kremer JP, Wagner H, Lipford GB. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 2001;166:5000–5007. doi: 10.4049/jimmunol.166.8.5000. [DOI] [PubMed] [Google Scholar]
- 22.Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur. J. Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Cederblad B, Blomberg S, Vallin H, Perers A, Alm GV, Rönnblom L. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-alpha-producing cells. J. Autoimmun. 1998;11:465–470. doi: 10.1006/jaut.1998.0215. [DOI] [PubMed] [Google Scholar]
- 24.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-α activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenberg RA, Craven SY, Warren RW, Cohen PL. Stochastic control of anti-Sm autoantibodies in MRL/Mp-lpr/lpr mice. J. Clin. Invest. 1987;80:691–697. doi: 10.1172/JCI113123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 28.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, Zal T, Mellman I, Schröder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 30.Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, Barrat FJ, Zal T, Gilliet M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol. Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 32.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, Soumelis V, Banchereau J, Coffman RL, Pascual V, Barrat FJ. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal S, Elliott JR, Manzi S. Atherosclerosis risk factors in systemic lupus erythematosus. Curr. Rheumatol. Rep. 2009;11:241–247. doi: 10.1007/s11926-009-0034-0. [DOI] [PubMed] [Google Scholar]
- 35.Joseph JE, Harrison P, Mackie IJ, Isenberg DA, Machin SJ. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br. J. Haematol. 2001;115:451–459. doi: 10.1046/j.1365-2141.2001.03101.x. [DOI] [PubMed] [Google Scholar]
- 36.Petri MA, Kiani AN, Post W, Christopher-Stine L, Magder LS. Lupus Atherosclerosis Prevention Study (LAPS), Lupus Atherosclerosis Prevention Study (LAPS) Ann. Rheum. Dis. 2010 doi: 10.1136/ard.2010.136762. [DOI] [PubMed] [Google Scholar]
- 37.Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grammer AC, Slota R, Fischer R, Gur H, Girschick H, Yarboro C, Illei GG, Lipsky PE. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J. Clin. Invest. 2003;112:1506–1520. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: The dark side of a great activator. Semin. Immunol. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gourley MF, Austin 3rd. HA, Scott D, Yarboro CH, Vaughan EM, Muir J, Boumpas DT, Klippel JH, Balow JE, Steinberg AD. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann. Intern. Med. 1996;125:549–557. doi: 10.7326/0003-4819-125-7-199610010-00003. [DOI] [PubMed] [Google Scholar]
- 41.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, Appel GB. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N. Engl. J. Med. 2005;353:2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]