Abstract
In recent years, there have been unprecedented methodological advances in the dynamic imaging of brain activities. Electrophysiological, optical, and magnetic resonance methods now allow mapping of functional activation (or deactivation) by measurement of neuronal activity (e.g., membrane potential, ion flux, neurotransmitter flux), energy metabolism (e.g., glucose consumption, oxygen consumption, creatine kinase flux), and functional hyperemia (e.g., blood oxygenation, blood flow, blood volume). Properties of the glutamatergic synapse are used as a model to reveal activities at the nerve terminal and their associated changes in energy demand and blood flow. This approach reveals that each method measures different tissue- and/or cell-specific components with specified spatiotemporal resolution. While advantages and disadvantages of different methods are apparent and often used to supersede one another in terms of specificity and/or sensitivity, no particular technique is the optimal dynamic brain imaging method because each method is unique in some respect. Because the demand for energy substrates is a fundamental requirement for function, energy-based methods may allow quantitative dynamic imaging in vivo. However there are exclusive neurobiological insights gained by combining some of these different dynamic imaging techniques.
Keywords: fMRI, glia, GABA, glutamate, glutamine, lactate, multi-modal
1. Introduction
The brain is a highly complex organ, both anatomically [1] and physiologically [2], requiring an impressive arsenal of technological tools to study it. In recent years, neuroscientists and neurophysiologists have benefited from the emergence of several dynamic imaging techniques. Currently a variety of electrophysiological, optical, and magnetic resonance methods allow in vivo probing of brain activities in terms of transients in neuronal activity as well as their fundamentally associated energetic and hyperemic events [3]. Since these functional imaging methods either measure directly or exploit relationships between electrical, metabolic, and/or hemodynamic changes, it is important to classify the underlying basic processes with appropriate spatial and temporal scales.
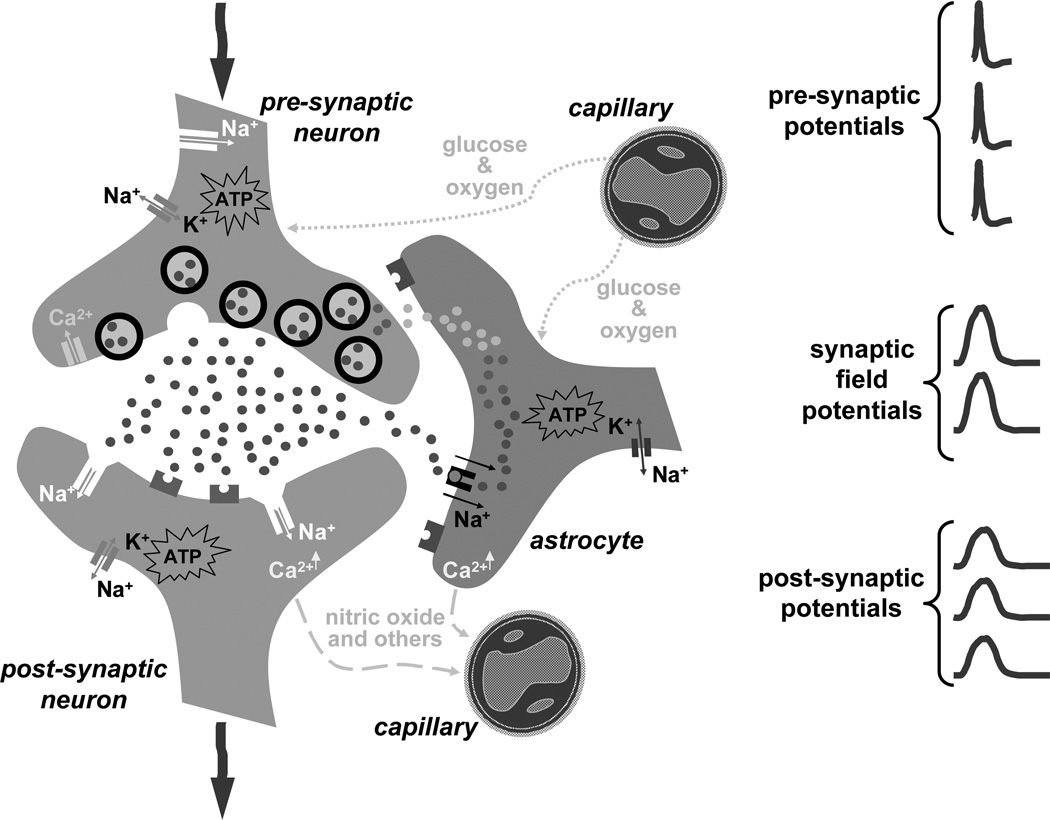
Functional integrity of the working brain is maintained by electrical communication amongst an enormous number of neurons with active partnership provided by astrocytes [4,5]. Cytological association of neurons and astrocytes with the microvasculature (Fig. 1) provides the framework that links activities at the nerve terminal to energy demand [6,7] and blood flow [8,9]. In the mammalian cerebral cortex, glutamate is the major excitatory neurotransmitter whereas γ-amino butyric acid (GABA) is its conjugate inhibitory partner. Together they constitute nearly 90% of cortical neurons [10]. Glutamate metabolism plays a central role in both glutamatergic and GABAergic synapses because glutamate is a precursor of GABA and it is a constituent of both neurons and astrocytes [11,12]. Thus featuring properties of the glutamatergic system seem to be an appropriate starting point for this discourse.
Figure 1.
Cytological association between microvasculature with neurons and astrocytes in the glutamatergic synapse.
2. Activities at the nerve terminal
Electrical communication between cells at the glutamatergic nerve terminal is characterized by 1–2 ms epochs of cellular discharge (or depolarization) which are followed by quiescent charging periods lasting a few ms [2]. Cellular excitability (or signaling) depends on ionic distribution across the cell membrane. The cell maintains a large difference in ionic distribution (~10:1) between the intra- and extracellular compartments: higher concentrations of sodium (Na+), calcium (Ca2+), and chloride (Cl−) ions outside and higher but unbalanced concentration of potassium (K+) ions inside. Since the resting cell membrane is only marginally leaky to ions, passage of ions between intra- and extracellular compartments is mediated predominantly by ion channels and pumps [13]. Both voltage- and ligand-gated ion channels allow ion movement down large chemical gradients, whereas ion pumps (e.g., Na+/ K+ ATPase) help restore the ionic distributions (back to that in the resting cell) by moving ions against large chemical gradients. Restoration of ion gradients (requiring energy input) is needed to keep neurons ready to discharge (or fire) whenever needed.
At rest, the cell membrane can be considered to be nearly impermeable to Na+ ions with a membrane potential of approximately −70 mV (resting potential). Upon depolarization, however, the membrane becomes almost freely permeable to Na+ ions and the potential is nearly reversed in an action potential (i.e., when the neuron has fired). Therefore complete depolarization of the pre-synaptic axon (>1 mm long) can be approximated by increased permeability of Na+ ions across the cell membrane. However complimentary roles of K+ and Ca2+ voltage-gated ion channels are important. Delayed increase in K+ conductance (in conjunction with delayed decrease in Na+ conductance) causes the membrane potential of the pre-synaptic neuron to ultimately move back towards the resting value following depolarization [14]. Rapid pre-synaptic Ca2+ influx (~200 µM) within 1–2 ms after onset of depolarization triggers vesicles to release glutamate into the extracellular space [15].
Glutamate discharge into the synaptic cleft (10–100 nm wide) is a vital step in transmission of the pre-synaptic signal onto post-synaptic elements [2]. Depolarization of the post-synaptic neuron is initiated by raising extracellular glutamate concentration (from nM to µM). Glutamate activation, mediated by ligand-gated ion channels on post-synaptic elements, occurs within 10 ms [16]. During transient binding of glutamate with these ionotropic receptors, Na+ ions enter post-synaptic dendrites. The pyramidal dendritic branching patterns (spanning several mm) are thought to play a role in amplification of post-synaptic depolarization [1,2].
What is the fate of extracellular glutamate after pre-synaptic release? There is a large glutamate concentration gradient between pre-synaptic and extracellular compartments (mM vs. nM) [17]. Also there is a high density of glutamate receptors on astrocytic end feet [13]. Together these favor extracellular glutamate release followed by glial uptake of glutamate (which is co-transported with Na+ ions). Astrocytic glutamate uptake prevents excitotoxicity, and in doing so, helps maintain glutamate homeostasis [11,12]. Glutamate and glutamine are more concentrated respectively in neurons and astrocytes. However glutamate is used in astrocytes to synthesize glutamine which in turn is a precursor of glutamate synthesis in neurons. Reestablishment of glutamate reserves (requiring energy input) is essential for continuous signaling at the glutamatergic nerve terminal. The glutamate-glutamine cycle, therefore, is a necessary part of overall cellular excitability [17], but more importantly, links membrane depolarization to synaptic activity [11,12]. A prudent advisory, however, to the highlighted events at the nerve terminal is that synaptic activity is not exclusively mediated by digital (i.e., all or none) signaling alone [2]. Membrane potential changes can also be quite graded (and slow) to produce analog signals which may also influence activities at the nerve terminal [18].
3. Energy demand and blood flow
Signaling at the glutamatergic nerve terminal, spanning wide bandwidths [19], depends on synchronized electrical as well as chemical events where neurons and astrocytes play complimentary roles [6,7]. Removal of any one step from the rest compromises function of the entire system [11,12]. While most of the energy is expended for moving Na+ and K+ ions against large chemical gradients, a small but non-negligible fraction is needed for intracellular Ca2+ homeostasis and neurotransmitter recycling/synthesis/repackaging [6,7]. Because activities at the nerve terminal are in continuous need of energy, demand for it is a fundamental requirement [20].
In no other organ is the continuous energy supply more imperative than in the brain. In humans, the brain is merely 2% of the body’s weight but it consumes more than 20% of the oxidative fuels in the entire body [21] and receives nearly 15% of the cardiac output [22] to supply nutrients (i.e., glucose and oxygen). Furthermore endogenous energy reserves in the brain – glucose (1–3 mM [23]), oxygen (50–100 µM [24]), glycogen (2–4 mM [25]), and creatine (8–10 mM [26]) – are minimal [27]. Normal function needs, therefore, blood circulation to efficiently provide nutrients (and remove waste) throughout the brain [28]. Energy demand and blood flow are well correlated over different brain regions [29].
The energetic costs for brain work are mainly met by ATP derived exclusively from glucose oxidation [30]. Glucose metabolism is limited by phosphorylation, not transport [31]. Glucose is rapidly delivered [32] by a variety of transporters [33] located at the blood-brain barrier and plasma membranes and then broken down by non-oxidative (~5% ATP) and oxidative (~95% ATP) pathways to maintain ATP concentration (2–4 mM [27]). Some glucose is stored by glial cells as glycogen [34]. Because astrocytes lack the enzyme to generate glucose from glycogen, glucose-6-phosphate generated in glia may be transferred to neurons to serve as an additional energy buffer [35]. Total creatine, which represents the quantities of phosphocreatine (PCr) and creatine (Cr), can undergo phosphorylation-dephosphorylation reaction catalyzed by creatine kinase (i.e., ADP + PCr ↔ ATP + Cr). Therefore PCr may provide an additional energy reserve when oxidative phosphorylation cannot maintain constant ATP supply [36]. These alternate energy reserves together can provide energy support for a short time (a couple of minutes) under ischemic conditions [27]. It is possible, however, that these extra non-oxidative pathways may provide faster ATP (in ms range) than from mitochondrial respiration [35,36].
To date, in spite of several decades of research, it remains unclear by what mechanism(s) nutrient supply (of glucose and oxygen) adjusts to changing energy demanded by neurons and astrocytes [37]. Many vasoactive agents have been implicated in mechanism(s) leading to functional hyperemia. Given the extremely low and high oxygen contents, respectively, in brain and blood (µM vs. mM [24]) but at the same time its ubiquitous need for oxidation of glucose (and other carbohydrates), should blood flow be tightly coupled to oxidative energy use, and thereby, suggest potential mechanism(s) for functional hyperemia [38]? Although theoretical [39] and experimental [40] studies show that these parameters are indeed well coupled, oxygen is not a candidate for a vasoactive agent because its excess has no impact on the hemodynamic response (within 500 ms) during sensory stimulation [41]. In other words, the system’s use of energy substrates is only based on demand [20], not availability [42]. Since suggestions of other agents (e.g., H+, K+, Ca2+, adenosine) have lacked clear evidence of an impartial role with functional hyperemia [37], new proposals [43] have shifted some attention to astrocytes as a key participant, thereby revising the century-old neurovascular coupling idea [44] to include glia (i.e., “neurogliovascular” coupling).
Recent opinions [5,9,12] suggest that neuronal glutamate release not only induces metabolic responses in glial cells but it may also even trigger hemodynamic events through them. This seems plausible given that astrocytes are proximal to both cerebral microvasculature [4,5] as well as glutamatergic and GABAergic neurons [45,46]. Past experiments [47] suggested that astrocytes are activated by glutamate uptake (and increase glial glucose utilization as well as glial lactate production; see refs. 11 and 12 for details). Recent immunohistochemical and molecular studies [48–51] have identified receptors for a variety of neurotransmitters (including glutamate) on specific cellular components of the microvasculature. Furthermore small increases (in nM range) in cytosolic Ca2+, both in the post-synaptic neuron and astrocytes mediated respectively by activation of ionotropic [52,53] and metabotropic [54,55] glutamate receptors, may stimulate enzymes like nitric oxide synthase (and others) to generate strong vasoactive agents like nitric oxide (and others). Together these new findings shed some preliminary [56,57] but not always complete [58] understanding about complexities of “neurogliovascular” coupling.
4. Functional brain imaging
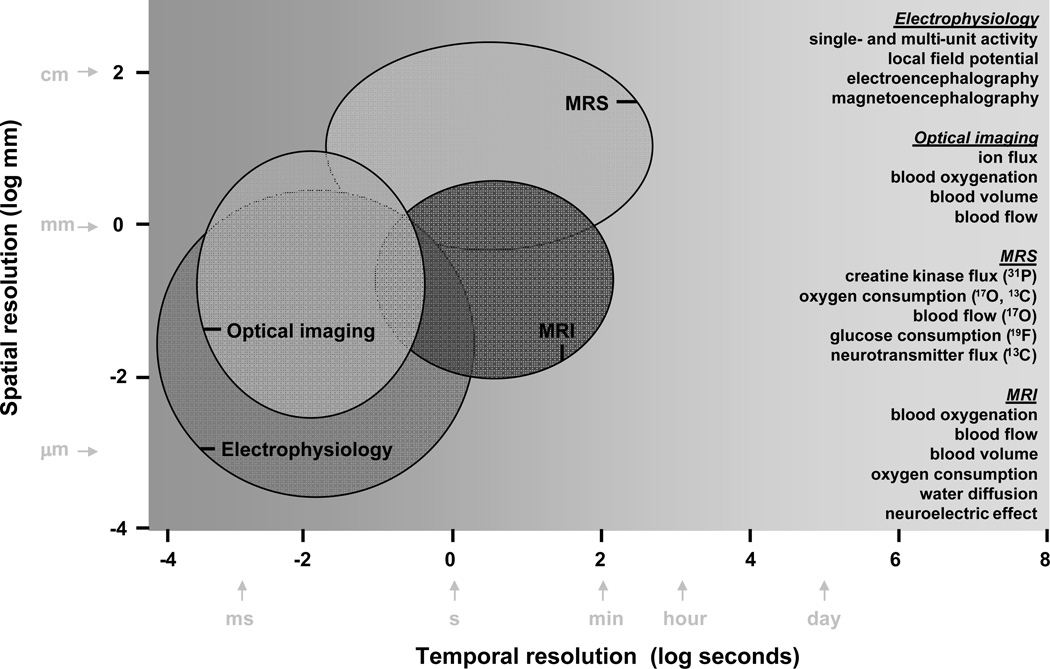
The goal of the ideal functional imaging technique is to map task-induced changes in activity in the brain in vivo. The task may be sensory or cognitive and the extent of the activated region may be localized (as generally observed with sensory paradigms [59]) or delocalized (as often found with cognitive paradigms [60]). To what extent do different methods allow dynamic identification of task activated brain region(s)? The answer depends on the spatiotemporal resolution of the method. There are at least three relevant factors. Spatial coverage of the brain by the method is a practical limitation. Specificity depends on the physiological basis of the measurement. Sensitivity if enhanced can improve either the spatial or temporal resolution, but rarely both. Together these factors describe the spatiotemporal resolution of the method (Fig. 2). Other (e.g., hardware) factors that affect spatiotemporal resolution of a given method are beyond the scope of discussion here.
Figure 2.
Comparison of spatiotemporal resolutions of current methods used in neuroscience.
Schematic illustration of spatial and temporal resolution ranges from a few experimental techniques is shown in Fig. 2. Since it is impractical to refer to the several dozen studies that were used to develop this figure, three reports [61–63] with similar comparisons are highlighted. But these may be questioned for several reasons. Techniques which did not reflect dynamic activity changes in their measurements (i.e., static or steady-state) were included with methods that were strictly designed for measuring transient signal changes. Methods specifically designed for use in humans were not separated from those applied in small animals. Similarly differences between in vivo and in vitro methods were not distinguished. Thus the apparent superiority of one method vs. another is debatable. Because there have also been technological advancements that influence this type of cross comparison across methods since then, up-to-date results were compiled from in vivo dynamic imaging studies of primarily small animals. Unfortunately these criteria excluded two dominant techniques in functional brain imaging: 14C-deoxy-glucose (14C-DG) autoradiography and positron emission tomography (PET).
14C-DG, a glucose analog, measures glucose consumption by the amount of metabolically trapped 14C-DG-6-phosphate throughout the brain [64]. However about an hour is required for radioactivity to build up in the tissue. 14C-DG autoradiography provides superior spatial resolution (50–100 µm) but it is a terminal experiment and therefore used only in animals.
PET, fashioned after the 14C-DG method but designed for application to humans [65], has several methods for functional imaging. Glucose consumption measurement is analogous to the 14C-DG method except that 18F-fluoro-deoxy-glucose (18F-FDG) is the glucose analog for PET. 15O-water is used to measure blood flow. This is done by injecting 15O-water into the blood stream and then detecting the rate of appearance of 15O-water in the brain tissue. Carbon monoxide with 11C or 15O, which mimics binding of oxygen to hemoglobin in red blood cells, is used to measure blood volume. The principle is that carbon monoxide is blood borne and therefore the detected radioactivity is only representative of the vascular compartment. The blood flow and blood volume methods in combination with inhaled 15O-oxygen can be used to measure oxygen consumption by detecting the rate of metabolized 15O-water in brain tissue. In all of these studies the radioactive labels are at trace levels (µM or less). Most PET methods use radioactive isotopes that are relatively short-lived (<30 min), but as in 14C-DG autoradiography, require long accumulation times and/or repeated scans (5–30 min) for radioactivity to build up. PET provides spatial resolution appropriate for human brain imaging (3–5 mm) and is well suited for clinical use. Recent advancements have improved PET resolution further (1–3 mm) to image brains of smaller animals [66].
The techniques shown in Fig. 2 include a variety of electrophysiological, optical, and magnetic resonance methods which are now widely used in many neuroscience laboratories, both in animals and in humans. These methods dynamically measure everything from changes in neuronal activity (e.g., membrane potential, ion flux, neurotransmitter flux) to the coupled alterations in energy metabolism (e.g., glucose consumption, oxygen consumption, creatine kinase flux) and hemodynamics (e.g., blood oxygenation, blood flow, blood volume).
4.1 Electrophysiology
Electrophysiology deals with the study of the electrical properties of cells and tissues [2]. Classical electrophysiological methods, using different types of microelectrodes, measure changes in voltage (or current). Microelectrodes are inherently designed to cover a few specific regions because it is impractical to perforate very large areas of the brain. Microelectrode arrays cover slightly larger (e.g., 4×4 mm2) superficial regions of the brain.
Intracellular recordings involve direct access into the intracellular milieu so that membrane potential can be measured with microelectrodes. The obvious advantage with intracellular recording is the unrivaled information about the individual cell, but the disadvantage is the difficulty in sampling large number of neurons. Extracellular recordings are made with the microelectrode tip (1–3 µm) situated next to cell bodies so that the extracellular voltage can be measured. Depending on the impedance and location of the electrode tip, extracellular recordings can capture the activity from either a single neuron (i.e. single-unit activity (SUA) recording) or from several neurons (i.e., multi-unit activity (MUA) recording). The clear benefit of extracellular recording is that several individual neurons can be assayed simultaneously. However only the largest signals from neurons closest to the microelectrode tip can be reliably identified individually.
Since microelectrodes are designed to measure the high frequency action potentials (or spikes), the data are collected with high temporal resolution (< 100 µs). The high signal-to-noise ratio of action potentials can be sorted out from the lower amplitude signals (by spike sorting). The extracellular signal, however, is also susceptible to the slower waves representing the local field potential (LFP) which may arise from graded events at the nerve terminal as shown in Fig. 1. Thus appropriate filtering is applied to separate the MUA (102–104 Hz) from the LFP (<102 Hz) signals. SUA and MUA signals are typically representative of signals from neuron(s) in the microelectrode’s vicinity (spanning 10–100 µm). LFP signals can integrate potentials over much larger distances (µm to mm) to represent the aggregate cellular activity in that region.
Both electroencephalography (EEG; [67]) and magnetoencephalography (MEG; [68]) allow mapping with much better brain coverage because the measurement devices nearly surround the entire head. EEG measures summed activity of post-synaptic currents, whereas MEG measures tiny magnetic fields (in fT range) produced by synchronized ionic currents flowing in dendrites. Since EEG and MEG signals originate from slightly different cortical locations and are acquired with different temporal resolutions (ms vs. µs), the types of information obtained vary. Because both EEG and MEG suffer from the inverse problem (i.e., difficulty localizing origin of signal), the spatial resolution (in cm range) is compromised. EEG signals are susceptible to body movements and MEG signals from the brain sometimes compete with higher magnitude environmental noise. However non-invasive use of EEG and MEG in humans is invaluable for basic science and clinical research.
4.2 Optical imaging
Optical imaging techniques are based on the discovery [69] that optical properties of cells, and therefore tissue, change during activity. Sensitivity of all optical techniques is limited by the magnitude of the changed optical properties of the system. At present, four dynamic optical techniques dominate: optical intrinsic signal (OIS), voltage sensitive dye (VSD), near infra red spectroscopy (NIRS), and laser-Doppler flowmetry (LDF). These methods can be used to measure a variety of dynamic events in cells (e.g., ion flux) and vessels (e.g., blood oxygenation). OIS and VSD are primarily applied in animals because of the need for a window (e.g., 4×4 mm2) on the skull and typically have high spatial resolution (10–50 µm). The data are limited to superficial regions of the brain because of limited light penetration in tissue (mm range). NIRS is a non-invasive technique and suited for use in humans. The spatial resolution of NIRS and LDF is limited by the distance between the emitting and detecting probes. Most of these techniques allow two dimensional images of brain activity with relatively high temporal resolution (10–100 ms).
OIS is perhaps the most widely used optical technique in neuroscience [70], in part, because it does not require exogenous optical probes. However the origin of the task-dependent changes in the intrinsic signal is complex. Changes in blood oxygenation (increase or decrease in deoxyhemoglobin), blood volume (vessel dilation, capillary recruitment, etc.), and light-scattering that accompany cell swelling with activation, all can affect the intrinsic signal in different ways and to various extents. Because these optical properties of the tissue vary in time, multiple illumination wavelengths (e.g., 600–630 nm for oxymetry) are necessary to calculate the desired parameters. The intrinsic signals are based on small reflectance signal changes (0.1–1%) which peak shortly (2–3 sec) after stimulus onset.
VSD uses small exogenous probes (weight <500 Da; length < 200 nm) that are designed to preferentially bind to the periphery of the cell membrane, and therefore, act as molecular transducers [71]. Changes in the membrane potential or ion concentration can then be transformed into an optical signal. There are two key factors to a successful dye used in VSD imaging. The optical yield (or contrast-to-noise ratio) when the molecule binds to the membrane is obviously critical. Another factor, which partly limits its application more widely, is successful delivery of the dye to the site of interest. Since the blood-brain barrier limits most foreign molecules to enter the extracellular milieu, direct injection of the dye into the site of interest is preferred. Because different events at the nerve terminal are linked to intracellular Ca2+ ion, dyes sensitive to Ca2+ voltage-gated ion channels have wide use in imaging activities both neurons and astrocytes with good temporal resolution (<102 ms). The VSD signals changes can be quite large (2–4%) which peak quite rapidly (<1 sec) after stimulus onset.
NIRS is a spectroscopic method which utilizes the near infra-red region (650–950 nm) of the electromagnetic spectrum to sense changes in the hemoglobin oxygenation state [72]. Because NIRS is non-invasive and has better tissue penetration (cm instead of mm) than visible light, the method has found wide use in both clinical monitoring of tissue oxygenation and functional imaging in humans and small animals [73]. Since near-infrared absorption spectra of oxyhemoglobin and deoxyhemoglobin allow separation of these two species, NIRS measurements commonly utilize three separate wavelengths to measure these components for blood oxygenation, and later sum them to get blood volume. The spatial resolution (in mm range) is lower than other optical methods because of the diffuse nature of photon migration through tissue which can affect sensitivity and quantitation of oxyhemoglobin and deoxyhemoglobin [74]. The NIRS signals changes larger than intrinsic signal changes (>2%) and peak slowly (within 5 sec) after stimulus onset.
LDF is an optical technique for measuring movement of red blood cells in the microvascular bed [75]. The principle is based on the “Doppler” frequency shift that arises in light that is scattered by moving red blood cells. By illuminating the tissue with single-frequency (low power) laser light and detecting the frequency distribution of the back-scattered light, red blood cell velocity and/or flux can be estimated. Since the LDF device is usually standardized with suspensions (e.g., latex spheres undergoing Brownian motion), it is a relative method and cannot easily provide absolute units of blood flow. LDF is a widely used technique in laboratories because of its minimal invasiveness and relatively high sensitivity for stimulation-induced blood flow/volume changes [76]. LDF has found popular applications in the clinical environment (e.g., neonatal care [77]) because it is quite simple to use. The LDF signals changes can be quite large (10–100%) and peak faster than NIRS signals (within 4 sec) after stimulus onset.
4.3 Magnetic resonance
Magnetic resonance (MR) is based upon the quantum mechanical properties of an atom’s nucleus [78]. Nuclei that contain odd numbers of protons or neutrons have an intrinsic magnetic moment. Some MR visible and biologically relevant non-radioactive isotopes are 1H, 13C, 15N, 17O, 19F, 23Na, and 31P. Sensitivity of MR detection for a given isotope depends on the physical intrinsic property of the isotope (cannot be changed, easily) and the abundance of the isotope prescribed by nature (can be enhanced by enrichment). The most sensitive and weak isotopes that are commonly used for in vivo MR experiments are 1H and 13C, respectively. 1H has four times stronger intrinsic sensitivity than 13C. Also 1H is ~91% more naturally abundant than 13C. Together, this makes 1H orders of magnitude more sensitive than 13C.
MR imaging (MRI) is primarily used in diagnostic imaging to map structure, but lately also function. MRI is predominantly based on 1H signal from water. MRI data have good spatial resolution and the temporal resolution depends on the specific type of function being measured (see below). MR spectroscopy (MRS) is a powerful technique used to obtain rich chemical information about a wide range of biomolecules (other than water) based on their variable chemical shifts. MRS detection of an isotope depends on the experimental purpose (see below). MRS data can also be viewed in two or three dimensions but the images do not appear as crisp as MRI data because the MRS voxels are much larger. The lower spatial resolution of MRS (mm to cm) compared to MRI (µm to mm) is mainly because of the orders of magnitude lower concentration of biomolecules (mM range) compared to water (>10 M) being detected. Since a wide range of MR methods can be used for neurobiological studies [79], the few that meet the criteria of dynamic functional imaging in Fig. 2 is discussed.
4.3.1 MRS methods
In the past, 31P MRS had been mainly used to detect high energy phosphates (e.g., ATP, ADP) which lead to assessment of tissue pH [80]. Since these signals do not change significantly with modest functional challenges, 31P MRS found little use in functional brain imaging. Recently, due to better sensitivity at high magnetic fields, 31P MRS has been able to measure forward and backward fluxes of creatine kinase [81]. Although the temporal resolution of this approach is still low (seconds to min), there is great potential of its use because comparable techniques do not exist yet.
Similar to 31P MRS, 17O MRS was also demonstrated nearly two decades ago [82] and due to recent advancements, the method has been revitalized [83]. The 17O MRS method for oxygen consumption follows almost the same approach as 15O PET (i.e., inhale labeled-oxygen and detect labeled-water). However a key difference between the two methods is that 17O MRS can distinguish between labeled-water (very strong MRS signal) and labeled-oxygen (exceptionally weak MRS signal), whereas PET cannot. This allows for removal of a few assumptions for modeling oxygen consumption with 17O MRS. The 17O MRS method can also measure blood flow and follows the same principles as 15O PET (i.e., inject labeled-water in blood and detect appearance of labeled-water in brain).
Although 19F is intrinsically almost as sensitive as 1H and it is naturally 100% abundant [78], there are no biomolecules in vivo (of relevance) with fluorine. Therefore FDG, as in PET, has been applied with 19F MRS for glucose consumption measurement [84], but the MRS tracer is not radioactive. Furthermore 19F MRS can distinguish between FDG-6-phosphate created by metabolism of FDG from FDG itself, whereas 18F PET cannot. Dynamic separation between the amount of FDG-6-phosphate and FDG helps quantify the glucose consumption rate. For 19F MRS detection of these signals with modest signal-to-noise ratio, either very high FDG dose (>10 mM) have been used with acceptable acquisition times or very long acquisition times (minutes to hours) have been used with low FDG dose (<1 mM). More recently, due to superior sensitivity at high magnetic fields, 19F MRS with reasonable spatial (mm range) and temporal resolution (<10 min) has been demonstrated using a low FDG dose [85].
Glucose consumption measured by the FDG method (MRS or PET) has a non-oxidative and oxidative component, where the latter can be measured by 13C MRS. Although 13C detection sensitivity is quite low (see above), using 13C-labeled exogenous biomolecules provides a viable alternative because of compartment/substrate specific information. 13C MRS is a very powerful tool to study metabolism when it is used in combination with a 13C-labeled substrate, such as [1-13C]glucose [86]. Infusion of [1-13C]glucose (into the blood stream) results in 13C turnover into glucose, glutamate, glutamine, GABA, lactate, aspartate, etc, in brain tissue [25,42]. Since 13C turnover into these pools is time dependent, coupled differential equations can be used to estimate a range of fluxes (including neuronal/glial glucose oxidation, glutamate-GABA-glutamine cycling; see refs. 11, 17, and 79 for details). But this is a steady-state method where the measured turnover times are on the order of about half an hour (or slightly more). Recently, however, by dynamic nuclear polarization (DNP) the MR detection sensitivity can be artificially improved by orders of magnitude [87]. To date, this method (using injection of hyperpolarized 13C or 15N biomolecules) has primarily been used to demonstrate perfusion through organs with quite high temporal (within seconds) and spatial (below mm range) resolutions. Although technical challenges remain [88], study of metabolism with 13C and/or 19F MRS in conjunction with DNP is quite promising.
4.3.2 MRI methods
There are several hemodynamic-based functional MRI (fMRI) methods that are sensitive to functional hyperemic events. The blood oxygenation level dependent (BOLD) method [89] is the most popular, in part, due to its simplicity. Both BOLD and NIRS methods are sensitive to changing concentrations of oxyhemoglobin and deoxyhemoglobin (i.e., diamagnetic or red blood, and paramagnetic or black blood, respectively). With NIRS both moieties are detected (by different absorption bands), with BOLD the magnetic properties of blood changing with oxygenation influences the tissue water signal (i.e., less paramagnetic deoxyhemoglobin, higher MRI signal).
Just as hemoglobin is nature’s endogenous MRI contrast agent to measure blood oxygenation changes, blood borne exogenous MRI contrast agents are used to measure alterations in blood volume with almost the same assumptions (i.e., more paramagnetic agent, lower MRI signal) [90]. Principles of blood flow measured by MRI are similar to that in PET. Following magnetic labeling of arterial blood (water), subsequent dynamic MRI maps reflect the degree to which the label has decayed (by mixing with unlabeled water), thereby reflecting tissue perfusion [91].
Since its discovery, the biophysical understanding of BOLD has improved significantly. Better specificity of the BOLD effect at higher magnetic fields [92] allows changes in oxygen consumption to be titrated out by combining the contributing changes in blood flow and blood volume with the BOLD signal [93]. At steady-state, the oxygen consumption predicted by BOLD calibration has been shown to be in good agreement with oxygen consumption measured by 13C MRS [94] and which in turn concurs with ensemble neuronal activity measured by extracellular recordings [95]. Since these multi-modal MRI methods can be applied dynamically [96], there is potential for transient energetics with higher temporal resolution (200–500 ms) [97].
Recently there has been a surge in fMRI that are based on non-hemodynamic events. Two prominent directions are effects of electrical currents directly or indirectly on the MRI signal [98] and task-related structural changes which may lead to alteration of diffusion of water molecules [99]. Both of these emerging fMRI approaches have potential to improve temporal resolution because the signals are not delayed by hemodynamics. However the greater challenge is to enhance the magnitude of the non-hemodynamic effects (<1%) which are much smaller than hemodynamic-based fMRI (>3%).
5. Future
The brain is mapped by several dynamic imaging methods sensitive to a variety of events at the glutamatergic nerve terminal (Fig. 1). These methods span wide ranges of spatial and temporal resolutions (Fig. 2) where each technique has obvious limitations, but also definite advantages. No one method can cover the several orders of magnitude in temporal and spatial resolutions and at the same time capture the many cellular and vascular events. Any such promise [100] discounts potential reachable insights, even at present, gained by combining different techniques which compliment each other [101]. The recent trend to combine fMRI [102–105] or optical imaging [106–109] with electrophysiology is in accord with this suggestion. Since energy is the currency of trade between cellular events and the substrate that those activities demand, dynamic functional imaging with energetics may be a crucial direction to pursue in the future [79]. In functional imaging, the qualitative way by which the baseline signal is conveniently differenced away to reveal stimulation-induced focal area(s) of interest ignored the high energy utilization in the resting brain [6] for a long time. Recent awareness [6,110] of the high baseline activity, which slowly varies in time, has been investigated by multi-modal methods like fMRI [111], optical imaging [112], and electrophysiology [113]. The anatomical [1] and physiological [2] complexities of the brain, both at rest and during activation, reward an extraordinary set of dynamic imaging [2,3,79] and analytical [114,115] tools.
Acknowledgements
This work was supported, in part, by NIH grants from NIMH (R01 MH-067528), NIDCD (R01 DC-003710), and NINDS (P30 NS-52519).
References
- 1.Shepherd GM. The Synaptic Organization of the Brain. New York, NY, USA: Oxford University; 2004. [Google Scholar]
- 2.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York, NY, USA: McGraw-Hill; 2000. [Google Scholar]
- 3.Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sunderland, MA, USA: Sinauer; 2004. [Google Scholar]
- 4.Jakovcevic D, Harder DR. Role of astrocytes in matching blood flow to neuronal activity. Curr Top Dev Biol. 2007;79:75–97. doi: 10.1016/S0070-2153(06)79004-4. [DOI] [PubMed] [Google Scholar]
- 5.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 6.Shulman RG, Rothman DL. Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc Natl Acad Sci USA. 1998;95:11993–11998. doi: 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 9.Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Nicholls DG. The glutamatergic nerve terminal. Eur J Biochem. 1993;212:613–631. doi: 10.1111/j.1432-1033.1993.tb17700.x. [DOI] [PubMed] [Google Scholar]
- 11.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 12.Bonvento G, Sibson N, Pellerin L. Does glutamate image your thoughts? Trends Neurosci. 2002;25:359–364. doi: 10.1016/s0166-2236(02)02168-9. [DOI] [PubMed] [Google Scholar]
- 13.Meldrum BS. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J Nutr. 2000;130(4S Suppl):1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 14.Hodgkin AL, Huxley AF. Propagation of electrical sickals along giant nerve fibres. Proc R Soc Lond B Biol Sci. 1952;140:177–183. doi: 10.1098/rspb.1952.0054. [DOI] [PubMed] [Google Scholar]
- 15.Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 16.Dzubay JA, Jahr CE. Kinetics of NMDA channel opening. J Neurosci. 1996;16:4129–4134. doi: 10.1523/JNEUROSCI.16-13-04129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman DL, Sibson NR, Hyder F, Shen J, Behar KL, Shulman RG. In vivo nuclear magnetic resonance spectroscopy studies of the relationship between the glutamate-glutamine neurotransmitter cycle and functional neuroenergetics. Philos Trans R Soc Lond B Biol Sci. 1999;354:1165–1177. doi: 10.1098/rstb.1999.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marder E. Neurobiology: Extending influence. Nature. 2006;441:702–703. doi: 10.1038/441702a. [DOI] [PubMed] [Google Scholar]
- 19.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 20.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 21.Sokoloff L. Relationship between functional activity and energy metabolism in the nervous system: Whether, where and why? In: Lassen NA, Ingvar DH, Raichle ME, Friberg L, editors. Brain Work and Mental Activity. Copenhagen, Denmark: Munksgaard; 1991. pp. 52–64. [Google Scholar]
- 22.Williams LR, Leggett RW. Reference values for resting blood flow to organs of man. Clin Phys Physiol Meas. 1989;10:187–217. doi: 10.1088/0143-0815/10/3/001. [DOI] [PubMed] [Google Scholar]
- 23.Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: Continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman P, Trubel HK, Hyder F. A multiparametric assessment of oxygen efflux from the brain. J Cereb Blood Flow Metab. 2006;26:79–91. doi: 10.1038/sj.jcbfm.9600165. [DOI] [PubMed] [Google Scholar]
- 25.Choi IY, Gruetter R. In vivo 13C NMR assessment of brain glycogen concentration and turnover in the awake rat. Neurochem Int. 2003;43:317–322. doi: 10.1016/s0197-0186(03)00018-4. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Zhang YP, Xiao ZW, Li H, Shen ZW, Chen XK, Huang K, Wu RH. Quantification of brain creatine concentration using PRESS sequence and LCModel: Comparison with HPLC method. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1928–1931. doi: 10.1109/IEMBS.2006.260498. [DOI] [PubMed] [Google Scholar]
- 27.Siesjo BK. Brain Energy Metabolism. New York, NY, USA: Wiley; 1978. [Google Scholar]
- 28.Trubel HK, Sacolick LI, Hyder F. Regional temperature changes in the brain during somatosensory stimulation. J Cereb Blood Flow Metab. 2006;26:68–78. doi: 10.1038/sj.jcbfm.9600164. [DOI] [PubMed] [Google Scholar]
- 29.Roland PE, Eriksson L, Stone-Elander S, Widen L. Does mental activity change the oxidative metabolism of the brain? J Neurosci. 1987;7:2373–2389. [PMC free article] [PubMed] [Google Scholar]
- 30.Ames A., 3rd CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 31.Pardridge WM. Brain metabolism: A perspective from the blood-brain barrier. Physiol Rev. 1983;63:1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- 32.Lund-Andersen H. Transport of glucose from blood to brain. Physiol Rev. 1979;59:305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- 33.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: The role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magistretti PJ, Allaman I. Glycogen: A Trojan horse for neurons. Nat Neurosci. 2007;10:1341–1342. doi: 10.1038/nn1107-1341. [DOI] [PubMed] [Google Scholar]
- 35.Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: Neurochemical basis of functional imaging. Proc Natl Acad Sci USA. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- 37.Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: Basis of functional neuroimaging. Cerebrovasc. Brain Metab Rev. 1995;7:240–276. [PubMed] [Google Scholar]
- 38.Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Hyder F, Shulman RG, Rothman DL. A model for the regulation of cerebral oxygen delivery. J Appl Physiol. 1998;85:554–564. doi: 10.1152/jappl.1998.85.2.554. [DOI] [PubMed] [Google Scholar]
- 40.Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman DL. Dependence of oxygen delivery on blood flow in rat brain: A 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–498. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Wolf T, Lindauer U, Villringer A, Dirnagl U. Excessive oxygen or glucose supply does not alter the blood flow response to somatosensory stimulation or spreading depression in rats. Brain Res. 1997;761:290–299. doi: 10.1016/s0006-8993(97)00354-5. [DOI] [PubMed] [Google Scholar]
- 42.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: Implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Lou HC, Edvinsson L, MacKenzie ET. The concept of coupling blood flow to brain function: Revision required? Ann Neurol. 1987;22:289–297. doi: 10.1002/ana.410220302. [DOI] [PubMed] [Google Scholar]
- 44.Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol. 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rancillac A, Rossier J, Guille M, Tong XK, Geoffroy H, Amatore C, Arbault S, Hamel E, Cauli B. Glutamatergic control of microvascular tone by distinct GABA neurons in the cerebellum. J Neurosci. 2006;26:6997–7006. doi: 10.1523/JNEUROSCI.5515-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen Z, Bouchelet I, Olivier A, Villemure JG, Ball R, Stanimirovic DB, Hamel E. Multiple microvascular and astroglial 5-hydroxytryptamine receptor subtypes in human brain: molecular and pharmacologic characterization. J Cereb Blood Flow Metab. 1999;19:908–917. doi: 10.1097/00004647-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Elhusseiny A, Cohen Z, Olivier A, Stanimirovic DB, Hamel E. Functional acetylcholine muscarinic receptor subtypes in human brain microcirculation: Identification and cellular localization. J Cereb Blood Flow Metab. 1999;19:794–802. doi: 10.1097/00004647-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Gillard SE, Tzaferis J, Tsui HC, Kingston AE. Expression of metabotropic glutamate receptors in rat meningeal and brain microvasculature and choroid plexus. J Comp Neurol. 2003;461:317–332. doi: 10.1002/cne.10671. [DOI] [PubMed] [Google Scholar]
- 51.Parfenova H, Fedinec A, Leffler CW. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J Cereb Blood Flow Metab. 2003;23:190–197. doi: 10.1097/01.WCB.000004823561824.C4. [DOI] [PubMed] [Google Scholar]
- 52.Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 53.Ledo A, Barbosa RM, Gerhardt GA, Cadenas E, Laranjinha J. Concentration dynamics of nitric oxide in rat hippocampal subregions evoked by stimulation of the NMDA glutamate receptor. Proc Natl Acad Sci USA. 2005;102:17483–17488. doi: 10.1073/pnas.0503624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 55.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 56.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 57.Peppiatt C, Attwell D. Neurobiology: Feeding the brain. Nature. 2004;431:137–138. doi: 10.1038/431137a. [DOI] [PubMed] [Google Scholar]
- 58.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 59.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raichle ME. Functional brain imaging and human brain function. J Neurosci. 2003;23:3959–3962. doi: 10.1523/JNEUROSCI.23-10-03959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Churchland PS, Sejnowski TJ. Perspectives on cognitive neuroscience. Science. 1988;242:741–745. doi: 10.1126/science.3055294. [DOI] [PubMed] [Google Scholar]
- 62.Cohen MS, Bookheimer SY. Localization of brain function using magnetic resonance imaging. Trends Neurosci. 1994;17:268–277. doi: 10.1016/0166-2236(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 63.Grinvald A, Hildesheim R. VSDI: A new era in functional imaging of cortical dynamics. Nat Rev Neurosci. 2004;5:874–885. doi: 10.1038/nrn1536. [DOI] [PubMed] [Google Scholar]
- 64.Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 65.Raichle ME. Handbook of Physiology - The Nervous System V. New York, NY, USA: Springer-Verlag; 1988. Circulatory and metabolic correlates of brain function in normal humans; pp. 633–674. [Google Scholar]
- 66.Chatziioannou AF. Molecular imaging of small animals with dedicated PET tomographs. Eur J Nucl Med Mol Imaging. 2002;29:98–114. doi: 10.1007/s00259-001-0683-3. [DOI] [PubMed] [Google Scholar]
- 67.Freeman WJ. Origin, structure, and role of background EEG activity. Part 1. Analytic amplitude. Clin Neurophysiol. 2004;115:2077–2088. doi: 10.1016/j.clinph.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 68.Ioannides AA. Magnetoencephalography as a research tool in neuroscience: State of the art. Neuroscientist. 2006;12:524–544. doi: 10.1177/1073858406293696. [DOI] [PubMed] [Google Scholar]
- 69.Cohen LB. Changes in neuron structure during action potential propagation and synaptic transmission. Physiol Rev. 1973;53:373–418. doi: 10.1152/physrev.1973.53.2.373. [DOI] [PubMed] [Google Scholar]
- 70.Roe AW. Long-term optical imaging of intrinsic signals in anesthetized and awake monkeys. Appl Opt. 2007;46:1872–1880. doi: 10.1364/ao.46.001872. [DOI] [PubMed] [Google Scholar]
- 71.Baker BJ, Kosmidis EK, Vucinic D, Falk CX, Cohen LB, Djurisic M, Zecevic D. Imaging brain activity with voltage- and calcium-sensitive dyes. Cell Mol Neurobiol. 2005;25:245–282. doi: 10.1007/s10571-005-3059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jobsis FF. Noninvasive infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 73.Hoshi Y. Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology. 2003;40:511–520. doi: 10.1111/1469-8986.00053. [DOI] [PubMed] [Google Scholar]
- 74.Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: Approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage. 2004;23(Suppl 1):S275–S288. doi: 10.1016/j.neuroimage.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 75.Stern MD. In vivo evaluation of microcirculation by coherent light scattering. Nature. 1975;254:56–58. doi: 10.1038/254056a0. [DOI] [PubMed] [Google Scholar]
- 76.Kida I, Maciejewski PK, Hyder F. Dynamic imaging of perfusion and oxygenation by functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2004;24:1369–1381. doi: 10.1097/01.WCB.0000141501.12558.9B. [DOI] [PubMed] [Google Scholar]
- 77.Sudikoff S, Banasiak K. Techniques for measuring cerebral blood flow in children. Curr Opin Pediatr. 1998;10:291–298. doi: 10.1097/00008480-199806000-00012. [DOI] [PubMed] [Google Scholar]
- 78.Abragam A. Principles of Nuclear Magnetism. Oxford, UK: Oxford University; 1961. [Google Scholar]
- 79.Shulman RG, Rothman DL. Brain Energetics & Neuronal Activity: Applications to fMRI and Medicine. New York, NY, USA: Wiley; 2004. [Google Scholar]
- 80.Ackerman JJ, Grove TH, Wong GG, Gadian DG, Radda GK. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980;283:167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- 81.Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57:103–114. doi: 10.1002/mrm.21107. [DOI] [PubMed] [Google Scholar]
- 82.Fiat D, Dolinsek J, Hankiewicz J, Dujovny M, Ausman J. Determination of regional cerebral oxygen consumption in the human: 17O natural abundance cerebral magnetic resonance imaging and spectroscopy in a whole body system. Neurol Res. 1993;15:237–248. doi: 10.1080/01616412.1993.11740143. [DOI] [PubMed] [Google Scholar]
- 83.Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo 17O NMR approaches for brain study at high field. NMR Biomed. 2005;18:83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- 84.Nakada T, Kwee IL, Card PJ, Matwiyoff NA, Griffey BV, Griffey RH. Fluorine-19 NMR imaging of glucose metabolism. Magn Reson Med. 1988;6:307–313. doi: 10.1002/mrm.1910060309. [DOI] [PubMed] [Google Scholar]
- 85.Coman D, Sanganahalli BG, Cheng DW, McCarthy T, Rothman DL, Hyder F. In vivo 19F CSI of 2-fluoro-2-deoxy-D-glucose and 2-fluoro-2-deoxy-D-glucose-6-phosphate in rat brain. Proc Inter Soc Magn Reson Med. 2007;1:577. [Google Scholar]
- 86.Morris P, Bachelard H. Reflections on the application of 13C-MRS to research on brain metabolism. NMR Biomed. 2003;16:303–312. doi: 10.1002/nbm.844. [DOI] [PubMed] [Google Scholar]
- 87.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Molecular imaging with endogenous substances. Proc Natl Acad Sci USA. 2003;100:10435–10439. doi: 10.1073/pnas.1733836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klomp DW, Renema WK, van der Graaf M, de Galan BE, Kentgens AP, Heerschap A. Sensitivity-enhanced 13C MR spectroscopy of the human brain at 3 Tesla. Magn Reson Med. 2006;55:271–278. doi: 10.1002/mrm.20745. [DOI] [PubMed] [Google Scholar]
- 89.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kennan RP, Scanley BE, Innis RB, Gore JC. Physiological basis for BOLD MR signal changes due to neuronal stimulation: separation of blood volume and magnetic susceptibility effects. Magn Reson Med. 1998;40:840–846. doi: 10.1002/mrm.1910400609. [DOI] [PubMed] [Google Scholar]
- 91.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ugurbil K, Adriany G, Andersen P, Chen W, Garwood M, Gruetter R, Henry PG, Kim SG, Lieu H, Tkac I, Vaughan T, Van De Moortele PF, Yacoub E, Zhu XH. Ultrahigh field magnetic resonance imaging and spectroscopy. Magn Reson Imaging. 2003;21:1263–1281. doi: 10.1016/j.mri.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 93.Hoge RD, Pike GB. Oxidative metabolism and the detection of neuronal activation via imaging. J Chem Neuroanat. 2001;22:43–52. doi: 10.1016/s0891-0618(01)00114-4. [DOI] [PubMed] [Google Scholar]
- 94.Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14:413–431. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- 95.Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kida I, Rothman DL, Hyder F. Dynamics of changes in blood flow, volume, and oxygenation: Implications for dynamic functional magnetic resonance imaging calibration. J Cereb Blood Flow Metab. 2007;27:690–696. doi: 10.1038/sj.jcbfm.9600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanganahalli BG, Herman P, Hyder F. Transient energetics from fMRI: Single event to block design paradigms. J Cereb Blood Flow Metab. 2007 BP47-6W. [Google Scholar]
- 98.Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Appl Magn Reson. 2005;29:65–88. [Google Scholar]
- 99.Le Bihan D. The 'wet mind': Water and functional neuroimaging. Phys Med Biol. 2007;52:R57–R90. doi: 10.1088/0031-9155/52/7/R02. [DOI] [PubMed] [Google Scholar]
- 100.Jasanoff A. Bloodless FMRI. Trends Neurosci. 2007;30:603–610. doi: 10.1016/j.tins.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 101.Hyder F. Neuroimaging with calibrated fMRI. Stroke. 2004;35(11 Suppl 1):2635–2641. doi: 10.1161/01.STR.0000143324.31408.db. [DOI] [PubMed] [Google Scholar]
- 102.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2005;9:569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 103.Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, Shulman RG, Hyder F. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci USA. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharon D, Hamalainen MS, Tootell RB, Halgren E, Belliveau JW. The advantage of combining MEG and EEG: Comparison to fMRI in focally stimulated visual cortex. Neuroimage. 2007;36:1225–1235. doi: 10.1016/j.neuroimage.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stefanovic B, Schwindt W, Hoehn M, Silva AC. Functional uncoupling of hemodynamic from neuronal response by inhibition of neuronal nitric oxide synthase. J Cereb Blood Flow Metab. 2007;27:741–754. doi: 10.1038/sj.jcbfm.9600377. [DOI] [PubMed] [Google Scholar]
- 106.Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron. 2003;39:353–359. doi: 10.1016/s0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- 107.Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- 108.Jones M, Hewson-Stoate N, Martindale J, Redgrave P, Mayhew J. Nonlinear coupling of neural activity and CBF in rodent barrel cortex. Neuroimage. 2004;22:956–965. doi: 10.1016/j.neuroimage.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 109.Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: implications for functional imaging. J Neurosci. 2007;27:4572–4586. doi: 10.1523/JNEUROSCI.0326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 111.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 112.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 113.Leopold DA, Murayama Y, Logothetis NK. Very slow activity fluctuations in monkey visual cortex: Implications for functional brain imaging. Cereb Cortex. 2003;13:422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- 114.Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eke A, Herman P, Kocsis L, Kozak LR. Fractal characterization of complexity in temporal physiological signals. Physiol Meas. 2002;23:R1–R38. doi: 10.1088/0967-3334/23/1/201. [DOI] [PubMed] [Google Scholar]