Abstract
The high genetic diversity of porcine reproductive and respiratory syndrome virus (PRRSV) has been an obstacle to developing an effective vaccine for porcine reproductive and respiratory syndrome (PRRS). This study was performed to assess the degree of genetic diversity among PRRSVs from Korean pig farms where wasting and respiratory syndrome was observed from 2005 to 2009. Samples from 786 farms were tested for the presence of PRRSV using reverse transcription PCR protocol. A total of 117 farms were positive for type 1 PRRSV while 198 farms were positive for type 2. Nucleotide sequences encoding the open reading frame (ORF) 5 were analyzed and compared to those of various published PRRSV isolates obtained worldwide. Sequence identity of the ORF 5 in the isolates was 81.6~100% for type 1 viruses and 81.4~100% for type 2 viruses. Phylogenetic analysis of the ORF 5 sequences showed that types 1 and 2 PRRSVs from Korea were mainly classified into three and four clusters, respectively. The analyzed isolates were distributed throughout the clusters independent of the isolation year or geographical origin. In conclusion, our results indicated that the genetic diversity of PRRSVs from Korean pig farms is high and has been increasing over time.
Keywords: Korea, open reading frame 5, phylogenetic analysis, porcine reproductive and respiratory syndrome virus
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is an infectious disease that results in significant economic losses in the pig industry worldwide [9,21,30]. Since its first recognition during the late 1980s in the USA and Europe, PRRS has spread throughout the world [1,3,5]. The causative agent, the porcine reproductive and respiratory syndrome virus (PRRSV), is a member of the order Nidovirales, family Arteriviridae, and genus Arterivirus [6,26]. PRRSVs are categorized into two genotypic groups based on the prototypic European (type 1) and North American (type 2) strains also known as Lelystad virus (LV) and VR-2332, respectively. Both strains share an approximately 60% sequence identity at the nucleotide level [23].
The PRRSV genome is approximately 15 kb in length and consists of at least nine open reading frames (ORFs) including ORFs 1a and 1b, ORFs 2a and 2b, and ORFs 3-7 [2,11]. ORFs 1a and 1b encode the enzymes responsible for replication, ORFs 2a and 3-5 encode membrane-associated glycoproteins, ORFs 2b and 6 encode non-glycosylated membrane proteins, and ORF 7 encodes the N protein [29]. GP5, encoded by ORF 5, is a commonly recognized antigen in animals showing a protection against PRRSV and an excellent candidate protein for producing a recombinant vaccine [4,11-13,24,31]. This protein also exhibits the highest degree of diversity within the same genotypic groups [32].
Since the emergence of type 2 PRRSV in Korea during 1993, the virus has spread widely [7,10,16,17,20,30]. Genetic analyses of type 2 PRRSVs have been performed for the ORF 5 region of 27 viruses isolated from 2002 to 2003 [7]. Type 1 PRRSV was first detected in Korea in 2005 [17]. Several studies have recently reported that the genetic diversity of type 1 PRRSVs has increased in Korea [17,20]. So far, few genetic or phylogenetic analyses of type 1 and type 2 PRRSV in Korea have been performed. More representative samples and extensive sequence libraries are needed to better understand the genetic diversity of Korean PRRSVs. The current study was performed to examine the genetic diversity of the ORF 5 sequence in types 1 and 2 PRRSVs originated from pig farms in Korea where wasting and respiratory syndrome had been observed between 2005 and 2009.
Materials and Methods
Samples
Tissue samples (lung and lymph node) were collected from animals at a total of 786 pig farms nationwide where wasting and/or respiratory symptoms had been observed between 2005 and 2009. This included 201 pig farms in 2005, 93 farms in 2006, 96 farms in 2007, 250 farms in 2008, and 146 farms in 2009. Geographical distribution of the 786 farms was as follows: 194 farms in Gyeonggi, 29 in Gangwon, 29 in Chungbuk, 170 in Chungnam, 65 in Jeonbuk, 30 in Jeonnam, 132 in Gyeongbuk, 50 in Gyeongnam, and 87 in Jeju. One to three animals from each farm were tested. Several tissue samples were collected from a single animal. Samples from the same animal were pooled.
Reverse transcription (RT)-PCR for PRRSV detection
RNA was extracted from the tissue samples using an RNeasy mini kit (Qiagen, Germany) according to the manufacturer's protocol. To detect and differentiate types 1 and 2 PRRSVs, RT-PCR was performed using a OneStep RT-PCR kit (Qiagen, Germany) and primers based on sequences of ORF 7 and the 3´ non-coding region (NCR) of type 1 and type 2 PRRSVs (Table 1). The primer sets were designed to detect and differentiate types 1 and 2 PRRSVs using CLC Main Workbench 6.8.2 (CLC bio, Denmark). The reaction mixture contained 5 µL of 5× RT-PCR buffer (including 2.5 mM MgCl2), 0.4 mM dNTPs, 0.5 µM of each of the four primers (synthesized in Bioneer, Korea), 1 µL of the enzyme mix, and 5 µL of RNA in a final volume of 25 µL. The RT-PCR conditions were as follows: a reverse transcription step at 50℃ for 30 min, reverse transcriptase inactivation and initial PCR activation at 95℃ for 15 min; 35 cycles of denaturation at 94℃ for 20 seconds, annealing at 55℃ for 20 seconds, and extension at 72℃ for 30 seconds; and a final elongation step at 72℃ for 10 min. T3000 Thermocycler (Biometra, Germany) was used for the RT-PCR. The amplicons were separated using electrophoresis in 1.5% agarose gels and stained with ethidium bromide.
Table 1.
Sequences of primers used for the detection and differentiation of porcine reproductive and respiratory syndrome viruses (PRRSVs)
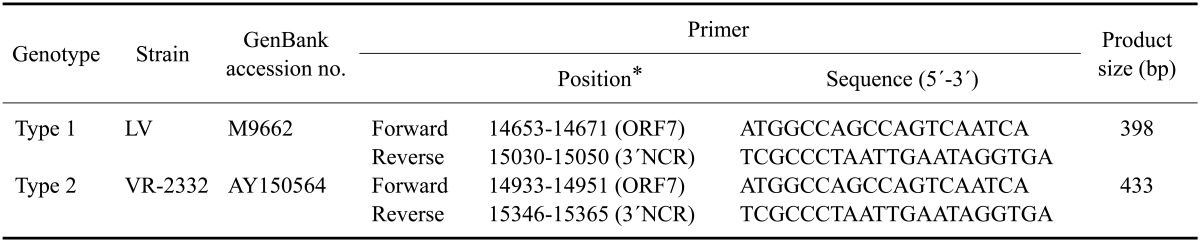
*Primer position: forward primers correspond to sequences in open reading frame (ORF) 7 and reverse primers correspond to the 3' non-coding region (NCR). LV: Lelystad virus.
Sequencing and phylogenetic analysis of the complete ORF 5 region
RT-PCR was performed on the samples positive for PRRSV type 1 or 2 using a SuperScript OneStep RT-PCR system with Platinum Taq (Invitrogen, USA) along with primer sets (Bioneer, Korea) specific for PRRSV ORF 4 and ORF 6 (Table 2). A separate primer set for each of the two PRRSV genotypes was designed (using CLC Main Workbench 6.8.2) to completely sequence ORF 5. The RT-PCR conditions were as follows: a reverse transcription step at 50℃ for 30 min, reverse transcriptase inactivation and initial PCR activation at 94℃ for 2 min; 35 cycles of denaturation at 94℃ for 15 seconds, annealing at 55℃ for 30 seconds, and extension at 72℃ for 30 seconds; and a final elongation step at 72℃ for 10 min. The amplified fragments were purified using a MinElute Gel extraction kit (Qiagen, Germany), and sequenced in both directions using a GenomeLabTMDTCS-Quick Start Kit (Beckman Coulter, USA) and CEQ8000 automated sequencer (Beckman Coulter, USA).
Table 2.
Sequences of primers used for amplification and sequencing of the complete ORF 5 of PRRSV

Individual sequences initially underwent multiple sequence alignment with CLUSTAL X ver. 1.81 [8], and the percent identity of the nucleotide sequences among the PRRSV isolates was calculated using Bioedit software (Ibis Biosciences, USA). Evolutionary history was inferred using the Neighbor-Joining method [25]. An optimal tree with the sum of branch length = 1.90543171 was created. The percentage of replicate trees in which the associated taxa clustered together in a bootstrap test (1,000 replicates) was also calculated. The tree was drawn to scale with branch lengths in the same units representing the evolutionary distances used to establish the phylogenetic tree. The evolutionary distances were computed using the Kimura 2-parameter method [18] and are shown as units of the number of base substitutions per site. The analysis involved 40 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 363 positions in the final dataset. Evolutionary analyses were conducted with MEGA5 [28].
Results
ORF 7 amplification and PRRSV genotyping
All samples obtained were first tested for the presence of PRRSV by amplifying a portion of ORF 7 using a primer pair specific for sequences in ORF 7 and the 3' NCR. Sizes of the amplicons from types 1 and 2 PRRSVs were 398 and 433 bp, respectively. PRRSV was detected in samples from 315 farms. Type 1 PRRSV was detected on 117 farms (eight in 2007, 55 in 2008, and 54 in 2009), and type 2 PRRSV was detected on 198 farms (51 in 2005, 54 in 2006, 20 in 2007, 13 in 2008, and 60 in 2009). Both types 1 and 2 PRRSVs were detected on 22 farms (two in 2008 and 20 in 2009). The following initials of each province from which the isolates originated were added at the end of the names of isolates: Gyeonggi (GG), Gangwon (GW), Chungbuk (CB), Chungnam (CN), Jeonbuk (JB), Jeonnam (JN), Gyeongbuk (GB), Gyeongnam (GN), and Jeju (JJ).
Genetic analysis of the PRRSV isolates
The PRRSV isolates were characterized by sequencing the complete ORF 5. Published ORF 5 sequences of PRRSVs isolated from 2004 to 2009 including those used as commercial vaccine strains were included in this analysis. Percent identity of the ORF 5 nucleotide sequence among the types 1 and 2 isolates ranged from 81.6 to 100.0% and from 81.4 to 100.0%, respectively (Table 3). The inferred amino acid sequences had an 81.0~100.0% identity for type 1 and 79.5~100.0% identity for type 2 (Table 3). The percent identity of the nucleotide/amino acid sequences between the type 1 isolates and a prototypic PRRSV LV strain or between type 2 isolates and a prototypic PRRSV VR-2332 strain was 85.6~90.8%/85.1~91.6% and 84.7~99.8%/83.1~99.5%, respectively (Table 4). Sequence comparison at the nucleotide level showed that the percent identity among type 1 isolates obtained in 2007 was 95.3~100.0%, and the percent identity among isolates recovered from 2008 and 2009 was 83.4~100.0% and 81.6~100.0%, respectively (Table 5). The type 2 isolates obtained in 2006, 2007, and 2008 showed 83.0~100.0%, 83.2~100.0%, and 83.5~100.0% identity with isolates collected during same year while isolates from 2005 and 2009 both showed 81.7~100.0% identity with isolates acquired during same year (Table 5). Among 12 groups of 25 type 1 PRRSV isolates with 100.0% identity in the ORF5 sequence, six groups of 12 isolates were from the same provinces and the remaining six groups of 13 isolates were from different provinces (Table 6). Two groups of four isolates were isolated during different years. For type 2 PRRSVs, 35 isolates from 15 groups showed 100.0% identity. Six groups with 16 isolates were from different provinces and nine groups with 19 isolates were from the same provinces. Four isolates from two groups were isolated during different years (Table 7).
Table 3.
Sequence identity among PRRSV isolates collected between 2005 and 2009
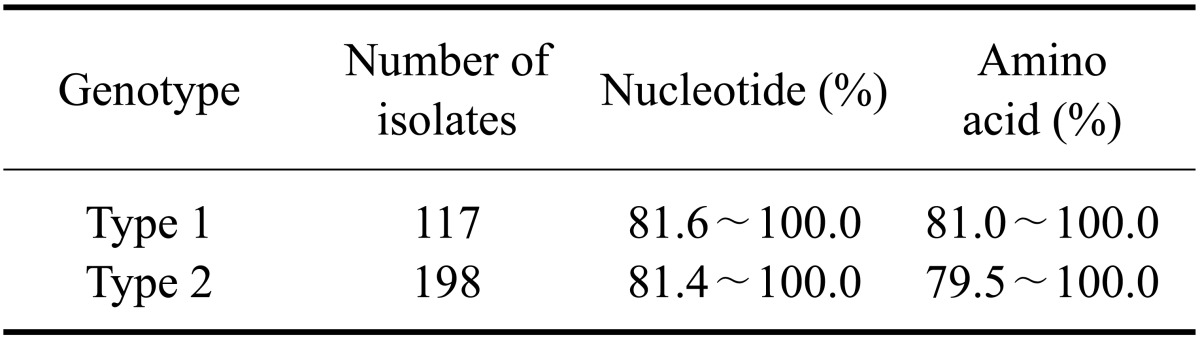
Table 4.
Sequence identity between each prototype virus and the respective PRRSV isolates
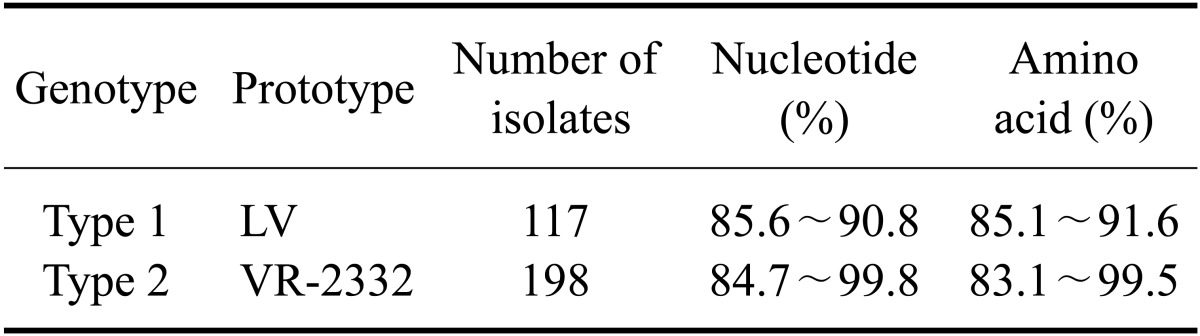
Table 5.
Nucleotide sequence identity of ORF 5 among the PRRSV isolates according to the year isolated
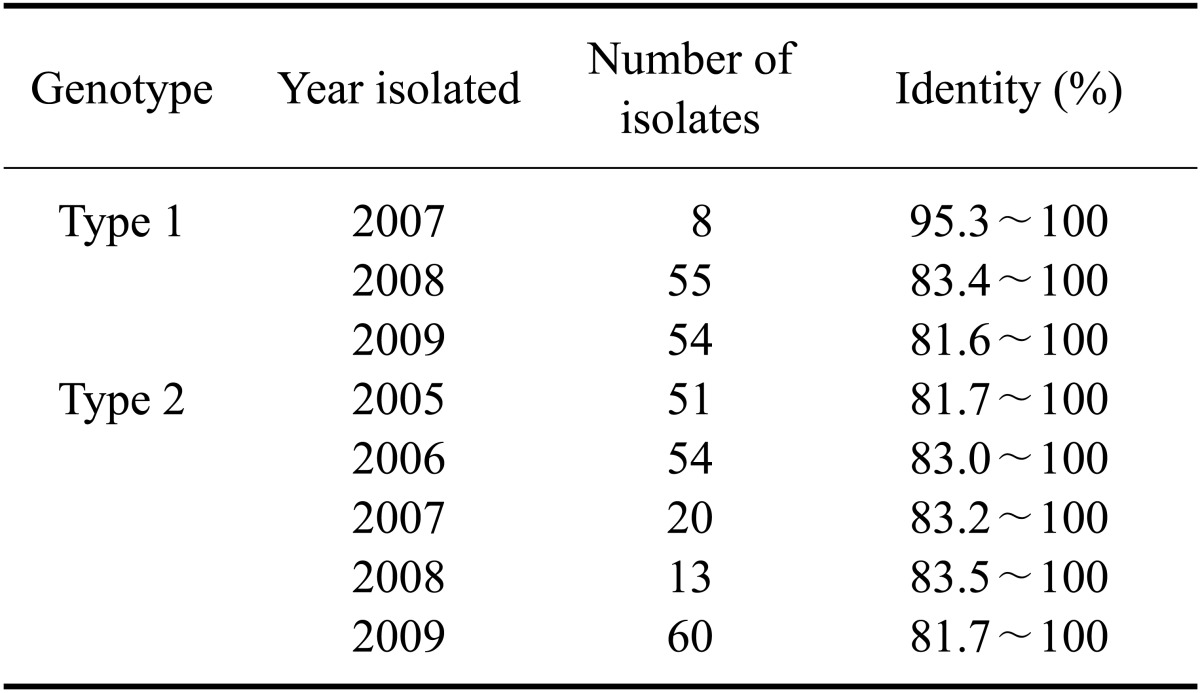
Table 6.
Type 1 PRRSV isolates with 100.0% homology (12 groups of 25 isolates)
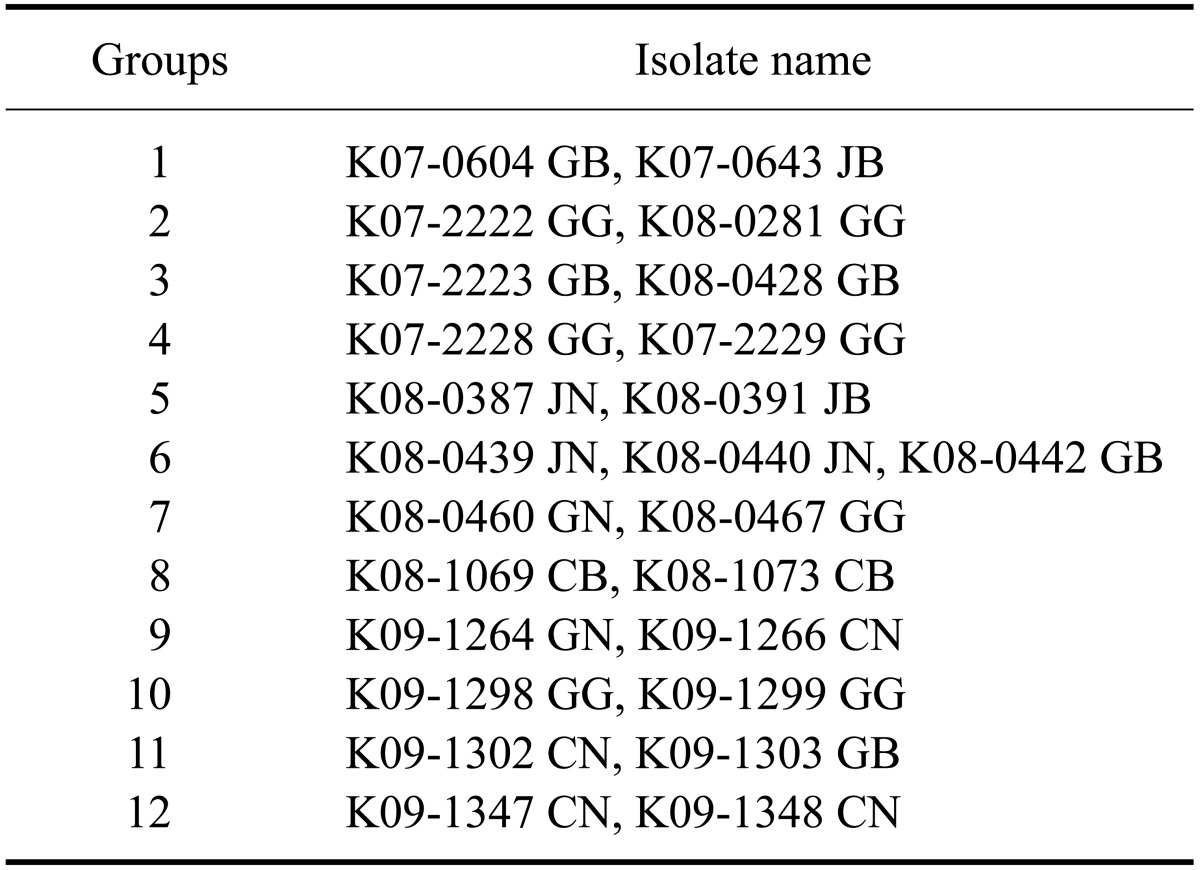
Table 7.
Type 2 PRRSV isolates with 100.0% homology (15 groups of 35 isolates)
Phylogenetic analysis of the ORF 5 gene in type 1 PRRSV isolates
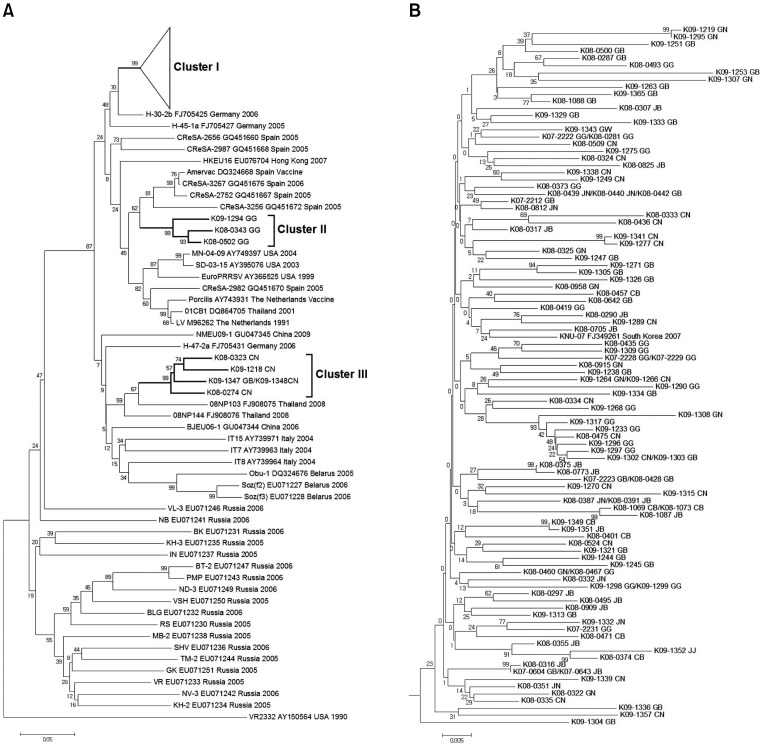
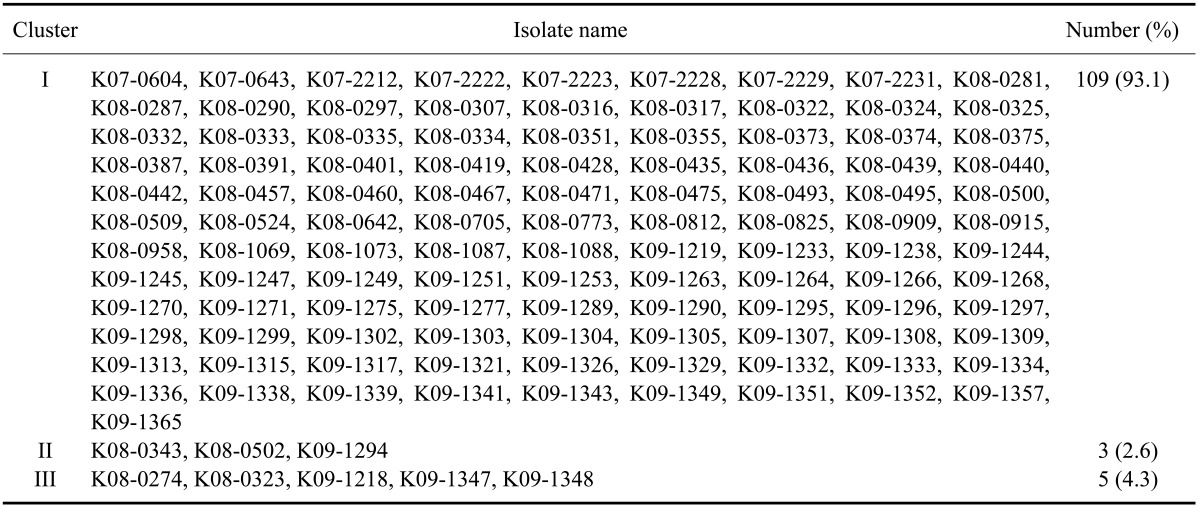
A phylogenetic analysis was conducted using sequences of the ORF 5 gene from reference PRRSVs deposited in GenBank (National Center for Biotechnology Information, USA). Korean type 1 PRRSV isolates were assembled into three genetic groups: cluster I, cluster II, and cluster III (Fig. 1A). Phylogenetic analysis of the ORF 5 gene of type 1 PRRSVs revealed that cluster I contained a diverse assemblage of viruses for which the ORF 5 nucleotide identity varied from 92.4 to 100.0% (Fig. 1B). Cluster II had a lower degree of genetic diversity (nucleotide identity of 93.8~96.8%) and contained several viruses that were closely related to vaccine strains (Amervac; HIPRA, USA or Porcilis; Merck Animal Health, the Netherlands) and type 1 strain prototypic LV (Fig. 1A and B). Cluster III had greater genetic diversity (nucleotide identity of 92.2~100.0%). Based on the 117 ORF 5 sequences from Korean type 1 PRRSVs, 109 isolates (93.1%) belonged to cluster I, three (2.6%) were assigned to cluster II, and five (4.3%) were grouped into cluster III (Table 8).
Fig. 1.
(A) Phylogenetic analysis of the open reading frame (ORF) 5 in Korean type 1 porcine reproductive and respiratory syndrome viruses (PRRSVs). The phylogenetic tree was constructed with 117 PRRSV isolates from Korea and 46 PRRSV strains isolated from around the world. The VR-2332 strain was used as the outgroup. Gray boxes and bundle lines indicate genetic clusters (I, II, and III) of Korean isolates. Bootstrap values greater than 500 of 1,000 replicates are indicated. PRRSV strains are denoted as follows: name of the PRRSV strain/GenBank accession no./country name/collection time, year published, or vaccine. Isolates are denoted by serial numbers (Table 6). (B) Korean type 1 PRRSV isolates collected from 2007 to 2009 belonging to cluster I.
Table 8.
Genetic cluster classification of Korean type 1 PRRSVs based on the phylogenetic analysis of ORF5
Phylogenetic analysis of the ORF 5 gene in type 2 PRRSV isolates
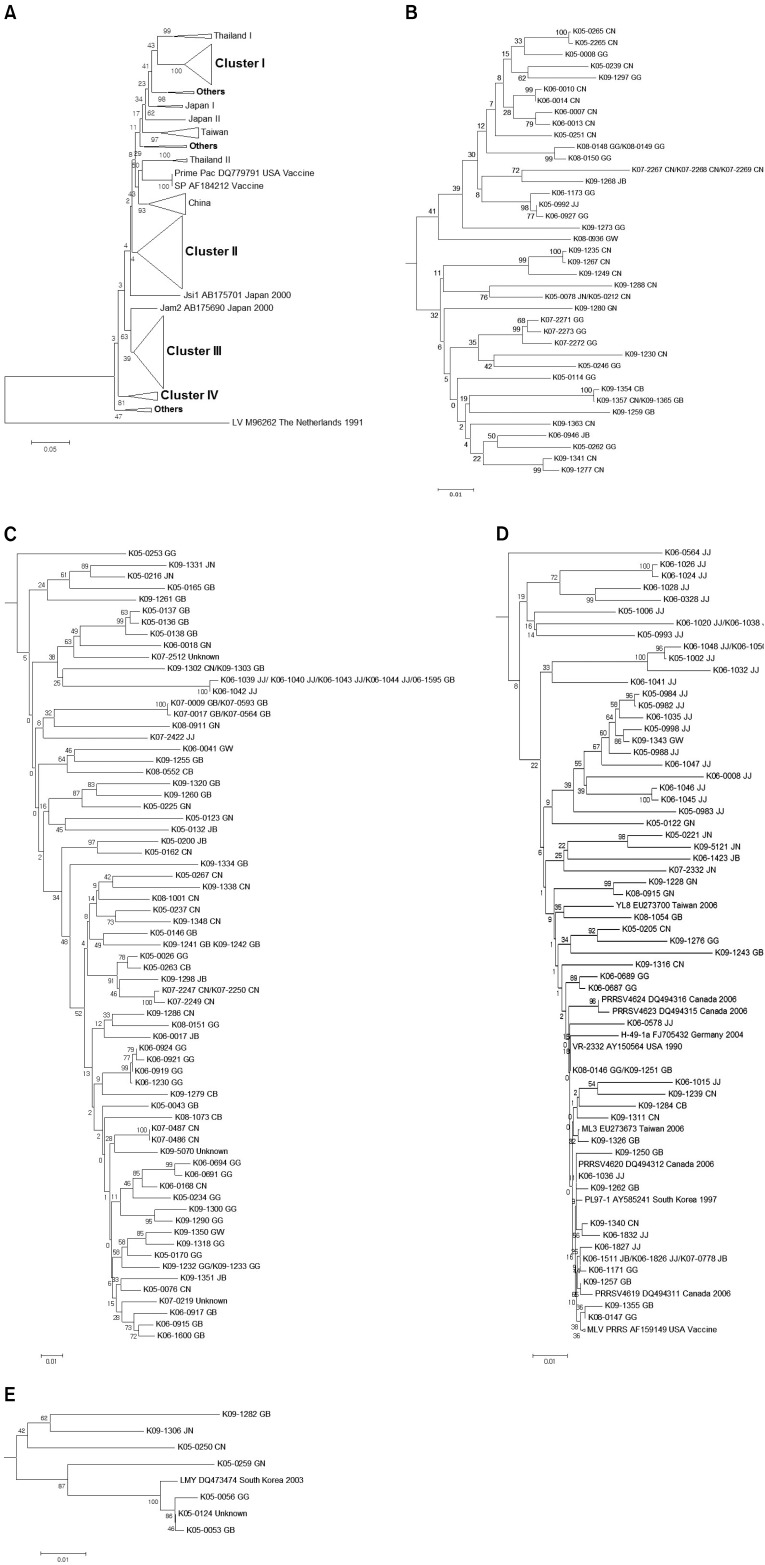
Based a phylogenetic analysis using sequences of the ORF 5 gene from reference PRRSVs deposited in GenBank (National Center for Biotechnology Information, USA), Korean type 2 PRRSV isolates were assembled into four genetic groups: cluster I, cluster II, cluster III, and cluster IV (Fig. 2A). Results of the phylogenetic analysis revealed that cluster I contained a diverse assemblage of viruses for which the ORF 5 nucleotide identities varied from 86.5 to 100.0% (Fig. 2B). The members of cluster II (Fig. 2C) had greater genetic diversity (nucleotide identity of 84.4~100.0%) and included several viruses closely related to vaccine strains (SP and Prime Pac). Cluster III PRRSVs (Fig. 2D) had the greatest level of genetic diversity (nucleotide identity of 83.2~100.0%). Finally, cluster IV (Fig. 2E) contained only isolates from Korea (nucleotide identity of 91.5~99.8%). Based on the 198 ORF 5 sequences from type 2 PRRSVs, 44 isolates (22.2%) belonged to cluster I, 79 (39.9%) were assigned to cluster II, 60 (30.3%) were grouped into cluster III, seven (3.5%) were placed in cluster IV, and eight (4.0%) to others (Table 9).
Fig. 2.
(A) Phylogenetic analysis of the ORF 5 in Korean type 2 PRRSVs. The phylogenetic tree was constructed with 198 Korean PRRSV isolates and 57 PRRSV strains isolated from around the world. The LV was used as the outgroup. Gray boxes and bundle lines indicate genetic clusters (I, II, III, and IV) of Korean isolates. Bootstrap values greater than 500 of 1,000 replicates are indicated PRRSV strains are denoted as follows: name of PRRSV strain/GenBank accession no./country name/collection time, year published, or vaccine. Korean isolates are denoted by serial numbers (Table 7). (B) Korean type 2 PRRSV isolates between 2005 and 2009 belonging to cluster I. (C) Korean type 2 PRRSV isolates collected from 2005 to 2009 belonging to cluster II. (D) Korean type 2 PRRSV isolates obtained from 2005 to 2009 belonging to cluster III. (E) Korean type 2 PRRSV isolates acquired from 2005 to 2009 belonging to cluster IV.
Table 9.
Genetic cluster classification of Korean type 2 PRRSVs based on the phylogenetic analysis of ORF5

Discussion
PRRS, porcine respiratory disease complex, and porcine multi-systemic wasting syndrome induced by PRRSV and other pathogens are currently recognized as major problems in the Korean swine industry [14,15]. Characterization of the genetic diversity of PRRSV may increase our understanding of the virus origin and epidemiology. This will in turn aid the development of new vaccines and improved diagnostic techniques [22].
We did not detect type 1 PRRSV from samples collected before 2007. It is presumed that type 1 PRRSV was present on a limited basis in Korean swine herds from 2005 to 2006 since this was around the time that the PRRSV was introduced to Korea. The sequence identity of ORF 5 among type 1 isolates from 2007 was 95.5~100.0% while the percent identity was lower among isolates collected in 2008 and 2009 (83.4~100.0% and 81.6~100.0%, respectively). This result may be due to the further introduction of different type 1 viruses or the genetic mutation of isolates introduced at an earlier time.
A previous comparison of ORF 5 sequences among type 2 Korean PRRSV isolates revealed a degree of genetic diversity between 1 and 15% [7]. The degree of ORF 5 genetic diversity among type 2 isolates obtained in the present study (0~18.6%) was greater than that previously observed [7]. These results indicate that the genetic variation of sequences among Korean type 2 isolates may be increasing. Antigenic variations among PRRSVs due to genetic variability and their adverse impact on vaccination efficacy are well documented [19,22]. Therefore, increasing heterogeneity among Korean PRRSVs raises a concern about the efficacy of the MLV vaccine that is widely used to control PRRS in Korea.
Sequence comparison showed that nucleotide identity among Korean type 1 isolates was 81.6~100.0% and 85.6~90.8% between these type 1 isolates and the type 1 prototypic LV strain. Genetic diversity of the Korean type 1 PRRSV ORF 5 sequence among the isolates obtained in this study was greater than that previously observed. Earlier investigations reported 82.5~100.0% [17] and 94.3~99.1% [20] identity according to nucleotide sequence comparisons.
Phylogenetic analysis indicated that there were three distinct clusters of ORF 5 nucleotide sequences among Korean type 1 PRRSVs. One hundred and nine type 1 isolates (93%) belonged to cluster I. Three isolates (2.5%) belonged to cluster II and five (2.1%) were assigned to cluster III. The ORF 5-based phylogenetic analysis demonstrated that type 1 isolates (except for ones belong to cluster III) appeared to be pan-European subtype 1 [20,27]. Further evaluation of the genetic characteristics of additional cluster III isolates may be required because we did not collect enough of these isolates for our study. Phylogenetic analysis of ORF 5 sequences also indicated that there were three major clusters of type 2 PRRSVs isolates [cluster I: 44 isolates (22.2%), cluster II: 79 (39.9%), and cluster III: 60 (30.3%)] and one minor cluster [cluster IV: seven isolates (3.5%)].
So far, vaccines for PRRSV have been developed using strains from cluster III including the MLV PRRS vaccine. It will be important to determine whether viruses from clusters I, II, and IV can be effectively controlled by this or any other PRRSV vaccine. Genetic analysis showed that there was no correlation between geographic proximity and genetic relatedness among PRRSVs in Korea, and that viruses isolated from different years were present in the same genetic clusters. These findings could be explained by geographical conditions. Korea is a country with a relatively small territory and dense animal populations. Furthermore, the movement of animals among farms or among provinces has not been a rare phenomenon. A large number of pigs are relocated for fattening or breeding. These two factors could be why a correlation between geographical proximity and genetic relatedness among PRRSVs was not observed in our study. It would be difficult for PRRSVs to develop unique regional characteristics within the Korean pig production system. Some isolates were identical despite being obtained in different locations. The introduction of PRRSV-infected animals into herds caused PRRSV to be spread throughout the country, and this might explain why identical viruses were found in different provinces. Nevertheless, adherence to a stringent biosecurity system would not completely hinder PRRSV transmission.
In conclusion, our results indicated that PRRSVs from pig farms in Korea have a high degree of genetic diversity. This diversity has been increasing over time. It is therefore important to detect new isolates from pig farms on a regular basis and analyze viral genetic diversity in order to effectively control the disease.
References
- 1.Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997;55:309–316. doi: 10.1016/s0378-1135(96)01322-3. [DOI] [PubMed] [Google Scholar]
- 2.Allende R, Lewis TL, Lu Z, Rock DL, Kutish GF, Ali A, Doster AR, Osorio FA. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J Gen Virol. 1999;80:307–315. doi: 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- 3.An TQ, Zhou YJ, Qiu HJ, Tong GZ, Wang YF, Liu JX, Yang JY. Identification of a novel B cell epitope on the nucleocapsid protein of porcine reproductive and respiratory syndrome virus by phage display. Virus Genes. 2005;31:81–87. doi: 10.1007/s11262-005-2203-1. [DOI] [PubMed] [Google Scholar]
- 4.Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. 2006;80:3994–4004. doi: 10.1128/JVI.80.8.3994-4004.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer J, Fichtner D, Schirrmeier H, Polster U, Weiland E, Wege H. Porcine reproductive and respiratory syndrome virus (PRRSV): kinetics of infection in lymphatic organs and lung. J Vet Med B Infect Dis Vet Public Health. 2000;47:9–25. doi: 10.1046/j.1439-0450.2000.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- 7.Cha SH, Choi EJ, Park JH, Yoon SR, Song JY, Kwon JH, Song HJ, Yoon KJ. Molecular characterization of recent Korean porcine reproductive and respiratory syndrome (PRRS) viruses and comparison to other Asian PRRS viruses. Vet Microbiol. 2006;117:248–257. doi: 10.1016/j.vetmic.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho JG, Dee SA. Porcine reproductive and respiratory syndrome virus. Theriogenology. 2006;66:655–662. doi: 10.1016/j.theriogenology.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Choi EJ, Lee CH, Song JY, Song HJ. Survey on porcine reproductive and respiratory disease virus infection in pig farms associated with wasting and respiratory syndrome during 2005~2009 in Korea. Korean J Vet Public Health. 2011;35:29–33. [Google Scholar]
- 11.Dea S, Gagnon CA, Mardassi H, Pirzadeh B, Rogan D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: comparison of the North American and European isolates. Arch Virol. 2000;145:659–688. doi: 10.1007/s007050050662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faaberg KS, Hocker JD, Erdman MM, Harris DLH, Nelson EA, Torremorell M, Plagemann PGW. Neutralizing antibody responses of pigs infected with natural GP5 N-glycan mutants of porcine reproductive and respiratory syndrome virus. Viral Immunol. 2006;19:294–304. doi: 10.1089/vim.2006.19.294. [DOI] [PubMed] [Google Scholar]
- 13.Fang L, Jiang Y, Xiao S, Niu C, Zhang H, Chen H. Enhanced immunogenicity of the modified GP5 of porcine reproductive and respiratory syndrome virus. Virus Genes. 2006;32:5–11. doi: 10.1007/s11262-005-5839-y. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Chung HK, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. 2003;166:251–256. doi: 10.1016/s1090-0233(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Chung HK, Jung T, Cho WS, Choi C, Chae C. Postweaning multisystemic wasting syndrome of pigs in Korea: prevalence, microscopic lesions and coexisting microorganisms. J Vet Med Sci. 2002;64:57–62. doi: 10.1292/jvms.64.57. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Lee SY, Sur JH, Lyoo YS. Serological and genetic characterization of the European strain of the porcine reproductive and respiratory syndrome virus isolated in Korea. Korean J Vet Res. 2006;46:363–370. [Google Scholar]
- 17.Kim SH, Roh IS, Choi EJ, Lee C, Lee CH, Lee KH, Lee KK, Song YK, Lee OS, Park CK. A molecular analysis of European porcine reproductive and respiratory syndrome virus isolated in South Korea. Vet Microbiol. 2010;143:394–400. doi: 10.1016/j.vetmic.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 18.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 19.Labarque G, Van Reeth K, Nauwynck H, Drexler C, Van Gucht S, Pensaert M. Impact of genetic diversity of European-type porcine reproductive and respiratory syndrome virus strains on vaccine efficacy. Vaccine. 2004;22:4183–4190. doi: 10.1016/j.vaccine.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Lee C, Kim H, Kang B, Yeom M, Han S, Moon H, Park S, Kim H, Song D, Park B. Prevalence and phylogenetic analysis of the isolated type I porcine reproductive and respiratory syndrome virus from 2007 to 2008 in Korea. Virus Genes. 2010;40:225–230. doi: 10.1007/s11262-009-0433-3. [DOI] [PubMed] [Google Scholar]
- 21.Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008;177:345–351. doi: 10.1016/j.tvjl.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng XJ. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet Microbiol. 2000;74:309–329. doi: 10.1016/S0378-1135(00)00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol. 1999;73:270–280. doi: 10.1128/jvi.73.1.270-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plagemann PGW. The primary GP5 neutralization epitope of North American isolates of porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2004;102:263–275. doi: 10.1016/j.vetimm.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 26.Snijder EJ, Meulenberg JJM. The molecular biology of arteriviruses. J Gen Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- 27.Stadejek T, Oleksiewicz MB, Scherbakov AV, Timina AM, Krabbe JS, Chabros K, Potapchuk D. Definition of subtypes in the European genotype of porcine reproductive and respiratory syndrome virus: nucleocapsid characteristics and geographical distribution in Europe. Arch Virol. 2008;153:1479–1488. doi: 10.1007/s00705-008-0146-2. [DOI] [PubMed] [Google Scholar]
- 28.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu WH, Fang Y, Farwell R, Steffen-Bien M, Rowland RRR, Christopher-Hennings J, Nelson EA. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology. 2001;287:183–191. doi: 10.1006/viro.2001.1034. [DOI] [PubMed] [Google Scholar]
- 30.Yoon SH, Song JY, Lee CH, Choi EJ, Cho IS, Kim B. Genetic characterization of the Korean porcine reproductive and respiratory syndrome viruses based on the nucleocapsid protein gene (ORF7) sequences. Arch Virol. 2008;153:627–635. doi: 10.1007/s00705-007-0027-0. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Q, Chen D, Li P, Bi Z, Cao R, Zhou B, Chen P. Co-expressing GP5 and M proteins under different promoters in recombinant modified vaccinia virus ankara (rMVA)-based vaccine vector enhanced the humoral and cellular immune responses of porcine reproductive and respiratory syndrome virus (PRRSV) Virus Genes. 2007;35:585–595. doi: 10.1007/s11262-007-0161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou YJ, Yu H, Tian ZJ, Li GX, Hao XF, Yan LP, Peng JM, An TQ, Xu AT, Wang YX, Wei TC, Zhang SR, Cai XH, Feng L, Li X, Zhang GH, Zhou LJ, Tong GZ. Genetic diversity of the ORF5 gene of porcine reproductive and respiratory syndrome virus isolates in China from 2006 to 2008. Virus Res. 2009;144:136–144. doi: 10.1016/j.virusres.2009.04.013. [DOI] [PubMed] [Google Scholar]