Abstract
Monoclonal antibody (mAb, NVRQS-DON) against deoxynivalenol (DON) was prepared. DON-Ag coated enzyme linked immunosorbent assay (ELISA) and DON-Ab coated ELISA were prepared by coating the DON-BSA and DON mAb. Quantitative DON calculation ranged from 50 to 4,000 ng/mL for DON-Ab coated ELISA and from 25 to 500 ng/mL for DON-Ag coated ELISA. 50% of inhibitory concentration values of DON, HT-2, 15-acetyl-DON, and nivalenol were 23.44, 22,545, 5,518 and 5,976 ng/mL based on the DON-Ab coated ELISA. Cross-reactivity levels of the mAb to HT-2, 15-acetyl-DON, and nivalenol were 0.1, 0.42, and 0.40%. The intra- and interassay precision coefficient variation (CV) were both <10%. In the mAb-coated ELISA, mean DON recovery rates in animal feed (0 to 1,000 µg/kg) ranged from 68.34 to 95.49% (CV; 4.10 to 13.38%). DON in a buffer solution (250, 500 and 1,000 ng/mL) was isolated using 300 µg of NVRQS-DON and 3 mg of magnetic nanoparticles (MNPs). The mean recovery rates of DON using this mAb-MNP system were 75.2, 96.9, and 88.1% in a buffer solution spiked with DON (250, 500, and 1,000 ng/mL). Conclusively we developed competitive ELISAs for detecting DON in animal feed and created a new tool for DON extraction using mAb-coupled MNPs.
Keywords: deoxynivalenol, ELISA, magnetic nanoparticles, monoclonal antibody
Introduction
Deoxynivalenol (DON), a trichothecene mycotoxin, is readily produced by fungus of the Fusarium species in the field or during storage in the presence of low temperatures and high humidity. Sobrova et al. [23] previously reported that DON represents more than 90% of the total contaminants in animal feed and foodstuff samples that are analyzed. These findings suggest that DON can be a potential marker of contamination by other mycotoxins. In addition, DON can induce feed refusal, emesis, skin irritation, hemorrhage, and severe immunosuppression in animals [7]. The detection and surveillance of mycotoxins is therefore extremely important to prevent both animals and humans from consuming contaminated grain products.
DON can be quantitatively analyzed by high-performance liquid chromatography (HPLC)-tandem mass spectrometry or UV detection [12,18]. However, these methods require time-consuming extractions, sophisticated equipment, and skilled technicians, making them expensive and impractical for the routine screening of large numbers of samples in the field. The extraction efficacy of any method for mycotoxin testing is critical because it determines the accuracy and credibility of the measured mycotoxin contamination level. Immunochemical techniques such as immunochromatograpic assays and enzyme-linked immunosorbent assays (ELISAs) are simpler and less expensive methods that have been developed for DON quantitation. The usefulness of these immunoassays is dependent on the specificity or sensitivity of the antibody used. Immunoaffinity chromatography (IAC) combined with monoclonal antibodies (mAbs) is currently the most popular method for purifying mycotoxin contaminants from animal feed and foodstuffs [1]. Immunoaffinity columns use a solid phase matrix with a specific antibody. As a result, a large volume of solvent may be required for IAC. Additional disadvantages of this technique include a potential reduction in mycotoxin exposure to the antibody and the need for a relatively long washing time.
Many recent studies have reported on the application of nanoparticles in areas such as treatment for disease, drug delivery, and diagnostic techniques [5,14,19,20]. Unlike microbeads, nanoparticles can easily be dispersed in a liquid medium, thereby increasing the possibility of making contact with the nanoparticle. Recent studies have also pointed to the usefulness of nanoparticles for screening heavy metals in water [11] and isolating harmful microbes in livestock products [26,29]. Nanoparticles tagged with surface-enhanced Raman have also been used to detect human alpha-fetoprotein, a tumor marker, for diagnosing hepatocellular carcinoma [9]. Although previous studies have described the use of mAb-conjugated nanoparticles to detect mycotoxins [13,15,22], to our knowledge few investigations have been conducted on the extraction of DON in a liquid phase using antibodies and magnetic nanoparticles (MNPs).
In the present study, we developed a new anti-DON mAb using DON-1,1'-carbonyldiimidazole (CDI) conjugated to ovalubumin (OVA). This mAb was applied to an ELISA system to screen for DON in animal feed and foodstuffs. We also developed a technique for the rapid extraction of DON by utilizing the anti-DON mAb and MNPs to facilitate extraction by magnetism.
Materials and Methods
Chemicals and reagents
DON, 15-acetoxy-3α,4β-dihydroxy-8α-[3-methylbutyryloxy]-12,13-epoxytrichothec-9-ene(HT-2 toxin), 15-acetyl-deoxynivalenol(15-acetyl-DON), nivalenol, acetone, 1,1'-CDI, OVA, bovine serum albumin (BSA), DON-BSA, 25% glutaraldehyde, glycine, hypoxanthine-aminopterin-thymidine medium (HAT/HT), Tween 20, Carbonate-bicarbonate buffer, glutaraldehyde solution (Grade II, 25%), and tris (hydroxymethyl) amino-methane (A.C.S reagent), 8-azaguanine, PEG1500, Freud complete adjuvant/incomplete adjuvant were, and 3,3',5,5'-tetramethylbenzidine (TMB) solution were purchased from Sigma-Aldrich (USA). Skim milk (BD, USA), Tween 20 (Applichem, Germany) and pyridine (Wako, Japan) were purchased from each company. Micro BCA Protein Assay kit were purchased from Thermo scientific (USA). Goat anti-mouse IgG and 3,3',5,5'-TMB were purchased from KPL (USA). Amine-functionalized MNPs were obtained from Nanobric (Korea).
Experimental animals
Five female BALB/c mice (6 weeks old) were purchased from Orient Bio (Korea). The animals were given tap water and a commercial diet (Purina, USA) ad libitum. They were maintained at 24 ± 2℃ with 50 ± 20% relative humidity and a 12-h light/dark cycle. All animals were cared for according to the Code of Laboratory Animal Welfare and Ethics of the Animal and Plant Quarantine Agency (QIA), Korea. The experimental design was approved by the QIA Animal Welfare Committee.
Production of anti-DON mAbs
DON was converted into DON-CDI containing a reactive group for coupling to the carrier proteins (OVA or BSA) as previously described [17]. The DON-CDI conversion was confirmed by high-performance liquid chromatography (HPLC) with the following conditions: a reverse-phase C18 column (3.9 × 150 mm, 5.0 µm; Waters, USA), water-acetonitrile (90:10, v/v) at a flow rate of 1 mL/min, and a HPLC system (Waters 2695; Waters, USA) with a photodiode array (Waters 2296; Waters, USA) set at a wavelength of 220 nm. DON-OVA and DON-BSA conjugation prepared by previously described [17] were confirmed by a competitive ELISA. Following desalting with a PD-10 column (GE healthcare, UK) according to the manufacturer's instructions, the DON protein conjugates were lyophilized and stored at -20℃ prior to use. To determine the antibody specificity and competiveness of the prepared immunogens, 100 ng each of DON-BSA, DON-OVA, and commercial DON-BSA (Sigma-Aldrich, USA) were coated, and their capacities for binding to the polyclonal antibody purchased from Sigma-Adrich (USA) and inhibiting the binding by DON (1,000 ppb) were determined. The BALB/c mice were acclimated to a specific pathogen-free animal room for 1 week and then intraperitoneally injected with a 100 µg DON-OVA conjugate emulsified in an equal volume of Freund's complete adjuvant. A booster with Freund's incomplete adjuvant and 100 µg DON-OVA conjugate was injected intraperitoneally 6, 8, and 10 weeks later. Four days prior to cell fusion, a high-titer mouse that produced antibody with high affinity for DON was given an intraperitoneal injection of 100 µg DON-OVA conjugate without adjuvant. The ratio of spleen cells from the immunized mouse fused with SP2/0 myeloma cells in was about 5 : 1. Following HAT selection, supernatant from the hybridoma cells was analyzed by an indirect competitive ELISA. Isotype of the immunoglobulin secreted from the cloned cell was determined using a mouse mAb isotyping kit (Roche, Switzerland). The DON mAb was conjugated to horseradish peroxidase (HRP) using an HRP labeling kit (Roche, Switzerland).
An indirect competitive ELISA for screening the mouse sera and culture supernatants was used to identify the presence of DON antibodies. A total 100 µL of serum or culture supernatant were placed into the wells of 96 well microplate. One hundred µL of 5,000 ng/mL DON was then added and incubated at room temperature for 1 h. After washing three times, 100 µL of anti-mouse IgG-HRP conjugate (1 : 2,000) was added and incubated for 1 h. After washing, TMB substrate solution was added to each well. Following incubation for 15 min at room temperature, the reaction was stopped by adding 2N H2SO4 (100 µL per well). The absorbance was measured at 450 nm using FLEXstation3 (Molecular Devices, USA). To produce the ascites fluid, the cultured cells (2 × 106) in medium were intraperitoneally injected into mice that had been previously injected with 0.5 mL pristane. After 7 ~ 14 days, the ascites fluid was collected and purified with a HiTrap protein IgG column (GE Heathcare, UK) according to the manufacturer's instructions.
ELISA
The DON mAb (1.5 µg per well, 100 µL) for the DON-Ab coated ELISA or the DON-BSA (0.3 µg per well, 100 µL) for the DON-Ag coated ELISA in 0.05 M carbonate buffer was immobilized on the 96-well plate overnight at 4℃. After washing with phosphate buffered saline (PBS) containing Tween 20, standard DON (50 µL) or sample DON (50 µL) was diluted in distilled water (DW) and a 50 µL DON-HRP solution (1 : 200 in 10% skim milk) or in 50 µL of a DON antibody-HRP conjugate solution (1 : 100 in 10% skim milk). Following incubation for 5 min at room temperature, each well was washed with PBS and developed with 100 µL of the TMB solution for 5 min. To stop the color development, 100 µL of 2N H2SO4 was added and the absorbance was measured.
Determination of cross-reactivity of mAb with DON analogues
The cross-reactivity in the ELISA methods between the mAb and DON, HT-2 toxin, 15-acetyl-DON, and nivalenol was determined by dividing the 50% inhibitory concentration (IC50) of DON by the IC50 values of each analogue.
Feed sample preparation and DON recovery determination
A total of 10 g of pig grower feed (Livestock cooperative, Korea) was spiked with different concentrations of DON (0, 250, 500 and 1,000 ng/g) and extracted with 100 mL water for 3 min by vigorous agitation. The extracts were filtered through Whatman No. 1 filter paper (GE Heathcare, UK). The concentration of DON in the extracted samples was measured using both ELISA systems developed in the current study.
Conjugation of mAbs and MNPs
A total 3 mg of an MNP suspension was washed three times with coupling buffer (0.01 M pyridine, pH 6.0) using a magnet. A 5% aqueous glutaraldehyde solution (1 mL) was then added and incubated with the MNPs at room temperature for 30 min. The particles were again washed with coupling buffer and recovered by magnetic separation. mAb coupling with the MNPs was achieved by dissolving 300 µg of anti-DON mAb (NVRQS-DON) in 500 µL of the coupling buffer. The solution was mixed with the activated magnetic particles for 16 to 24 h at room temperature. MNPs coupled to NVRQS-DON were washed with coupling buffer and stored in the buffer (0.01M Tris, 0.15M NaCl, 0.1% BSA, pH 7.4) at 4℃ before use.
Separation of mycotoxins using mAb-coupled MNPs (mAb-MNPs)
mAb-MNPs were mixed with 500 µL of buffer solution (16% MeOH in PBS) containing 250, 500 and 1,000 ng/mL DON for 5 min at room temperature. Upon completion of the reaction, mAb-MNPs bound to DON were magnetically separated from the supernatant using a magnet, and the supernatant was then carefully discarded. DON was dissociated from the mAb-MNPs by adding 500 µL of 100% methanol with gentle shaking. After dissociation, the mAb-MNPs were magnetically removed perpendicular to gravity, and DON concentration in the supernatant was measured using the ELISA developed in this study.
Data analysis
The recovery rate of spiked DON was calculated with the formula: "recovery rate (%) = (amount of purified mycotoxin/amount of spiked mycotoxin) × 100". Graphs were prepared using SigmaPlot (ver. 8.0; Systat Software, USA). The intraplate and interwell assay variations for both ELISAs were compared by determining the coefficient of variation (CV).
Results
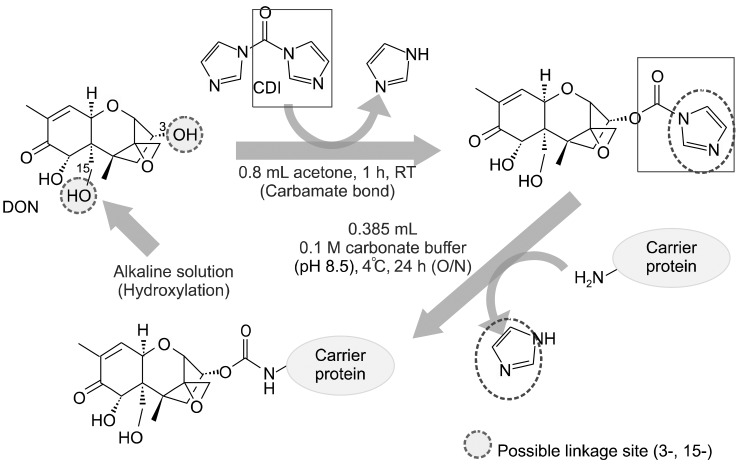
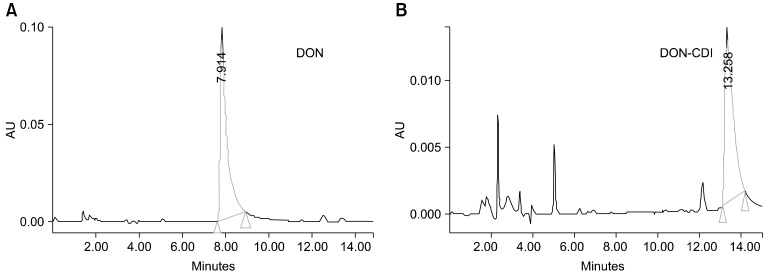
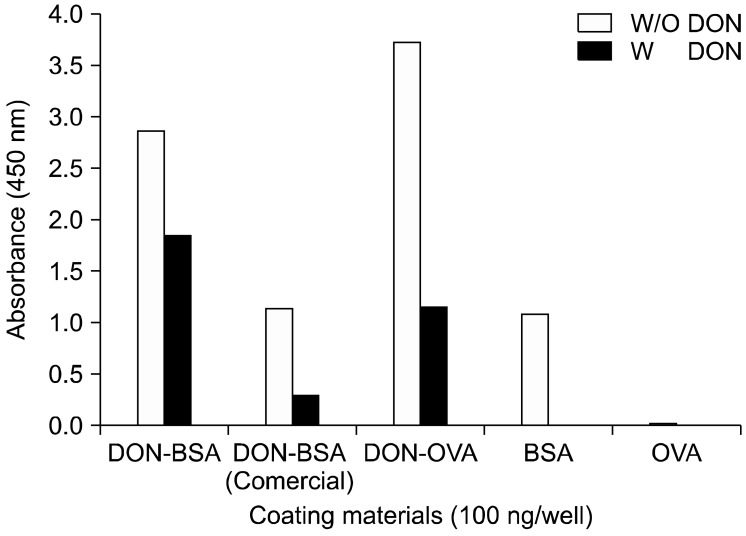
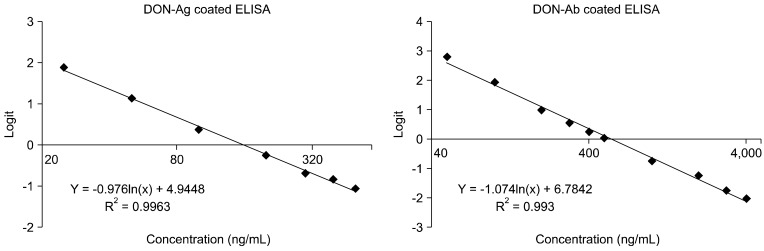
In the present study, we prepared the DON-CDI conjugate by linking CDI to the C3 or C15 carbon site of DON. DON was further conjugated with OVA or BSA by carbodimide condensation (Fig. 1). Following haptenization of DON with CDI, chromatographic mobility of the reaction product (DON-CDI) appeared faster than that of the parent compound. The DON peak appeared at 7.9 min while the DON-CDI peak appeared around 13.2 min (Fig. 2). The commercial polycolonal antibody did not bind to OVA as excepted. Binding of polyclonal antibody to DON-OVA conjugate was inhibited more markedly compared to that of the DON-BSA conjugate (Fig. 3). Since fused cells can produce many different types of antibodies not only against DON but also against OVA and CDI, screening the DON binding capacity of antibodies produced by these fused cells was performed by an indirect competitive ELISA using DON-BSA as the coating antigen. The cloned cells produced an IgG1 subclass with λ-type light chains. We selected the clone that produced antibodies with the highest specificity for DON and used it to produce mAbs from the mice. IC50 values of the mAb for DON, HT-2, 15-acetyl-DON, and nivalenol were 23.44, 22,545, 5,518 and 5,976 ng/mL, respectively. Based on the DON-mAb coated ELISA system, cross-reactivity levels of the mAb to HT-2, 15-acetyl-DON, and nivalenol were 0.1, 0.42, and 0.40%, respectively (Table 1). To obtain linear standard curves, a logistic curve expressing the y-axis was generated by calculating the binding percentage of the blank sample (B/B0). DON concentrations calculated from the DON-Ab coated ELISA and the DON-Ag coated ELISA data ranged from 50 to 4,000 ng/mL and 25 to 500 ng/mL (r2 > 0.99), respectively (Fig. 4). Two DON standard concentrations (500 and 1,000 ng/mL for the DON-Ab coated ELISA and 300 and 400 ng/mL for the DON-Ag coated ELISA) were analyzed. The intra- and interassay precision CVs were both < 10%, indicating a high degree of reproducibility (Table 2).
Fig. 1.
Strategy for preparing the deoxynivalenol-1,1'-carbonyldiimidazole (DON-CDI) and further conjugation with the carrier proteins.
Fig. 2.
High-performance liquid chromatography (HPLC) chromatogram of DON (A) and DON-CDI (B).
Fig. 3.
Binding specificity of the conjugated antigens to DON antibody and competiveness for DON binding. A polyclonal antibody specific for DON-bovine serum albumin (BSA) diluted 1:1,000 was used to evaluate the specificity for DON-ovalubumin (OVA) and DON-BSA. This specificity was compared to that for commercial DON-BSA. A total 100 ng each of DON-BSA, DON-OVA, and commercial DON-BSA was coated, and their capacity for binding to the polyclonal antibody and binding inhibition by DON (1,000 ng/mL) were determined.
Table 1.
Cross-reactivity (CR) and 50% inhibitory concentration (IC50) values of the mAb for DON, HT-2 toxin, 15-acetyl-DON, and nivalenol
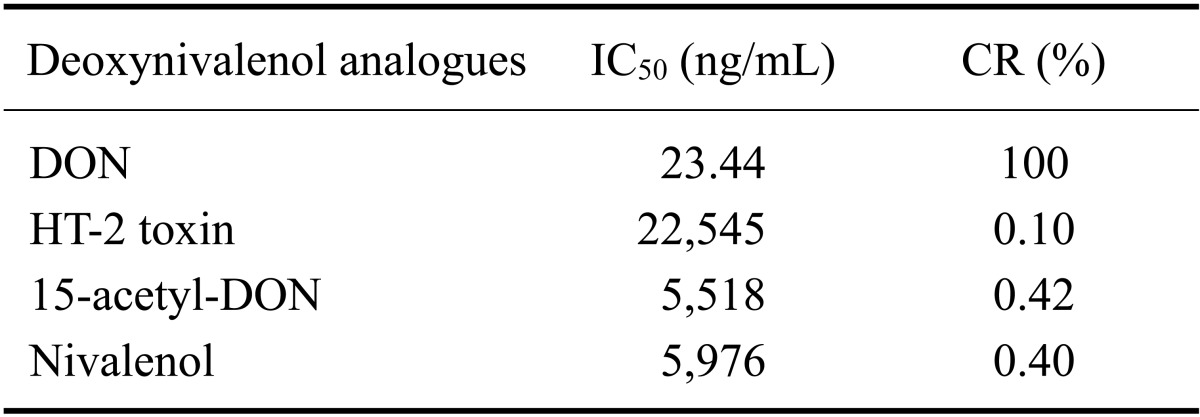
Fig. 4.
Standard curves for DON for the DON-Ag coated ELISA (left) and DON-Ab coated ELISA (right). Microplates were coated with 0.3 ug DON-BSA/well and 1.5 µg monoclonal antibody/well for each assay. Each point represents the mean of three duplicate experiments.
Table 2.
Recovery rates and coefficient variations of the ELISA assays coated with DON-BSA or the monoclonal antibody
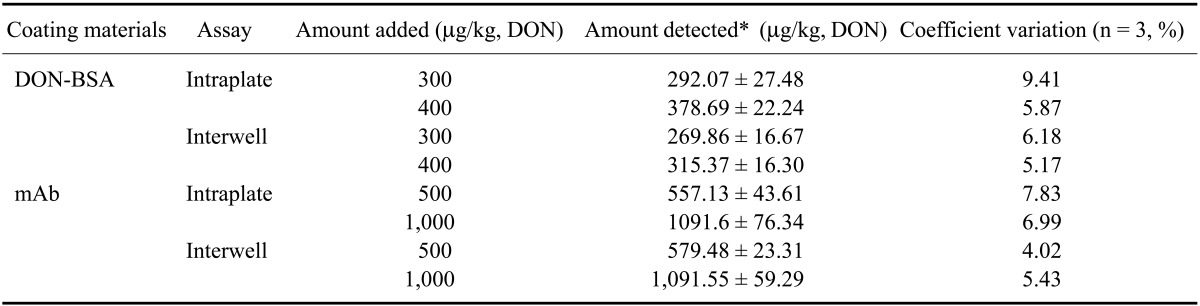
*Data are presented as the mean ± SD.
The DON toxin extracted with DW because DON can be extracted more easily from the solid matrices of animal feed with water than organic solvents like methanol. The Ab-coated ELISA was validated by spiking feed with DON at concentrations ranging from 0 to 1,000 µg/kg followed by DON extraction with DW. Recoveries rates ranged from 68.34 to 95.49% while CVs ranged from 4.10 to 13.38% (Table 3).
Table 3.
Recovery of DON from spiked feed samples with the DON-mAb coated ELISA
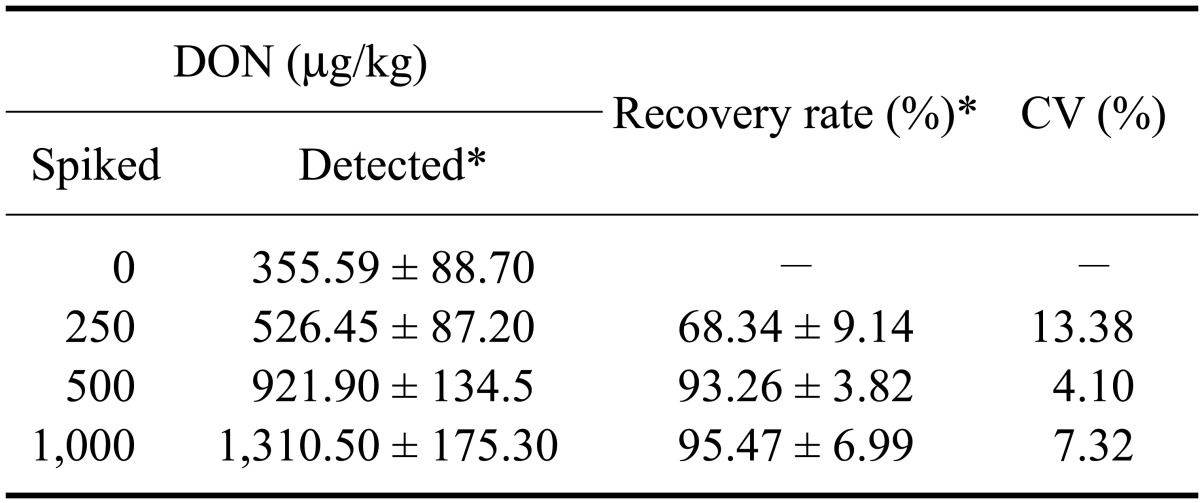
*Data are presented as the mean ± SD. To measure recovery rates, the amount of DON in the blank feed samples was deducted from that in each of the spiked samples (n = 3). CV: coefficient variation.
The mAb specific for DON was coupled to MNPs in an antibody concentration-dependent manner. The binding capacity was 95.44 ± 0.51% when 300 µg of the mAb was bound to 3 mg of the MNPs (data not shown). DON recovery rates using this novel mAb-MNP system were 75.2, 96.9, and 88.1% in a buffer solution spiked with DON at a concentration of 250, 500 or 1,000 ng/mL, respectively (Table 4).
Table 4.
Separation of DON from spiked buffer with the magnetic nanoparticles conjugated to the mAb
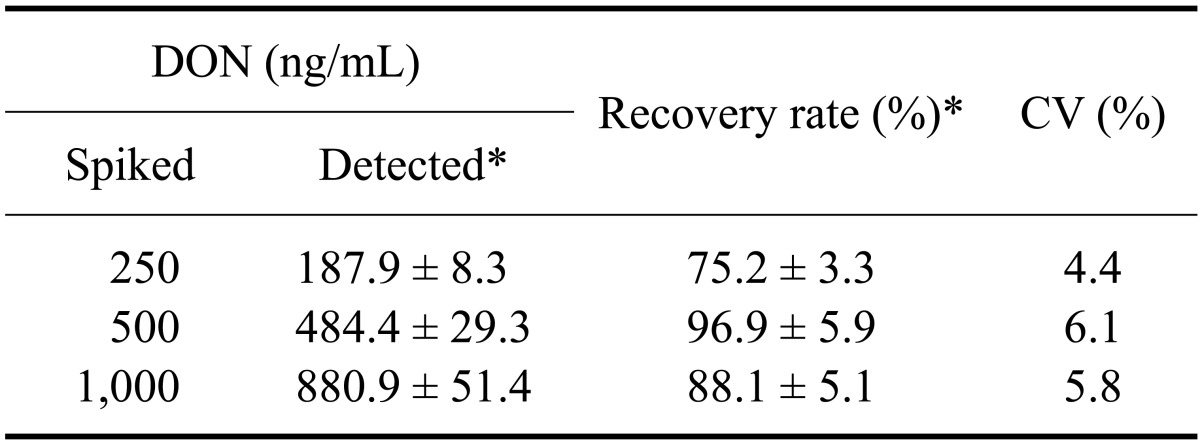
*Data are presented as the mean ± SD.
Discussion
Small molecules such as DON cannot on their own induce the production of antibodies. Therefore, DON must be conjugated to a carrier protein for the production of desirable antibodies. We prepared a DON-CDI conjugate by linking CDI to the C3 or C15 carbon site of DON. The DON peak appeared at 7.9 min and the DON-CDI peak appeared at around 13.2 min. This finding was expected due to the decreased polarity caused by the addition of a reactive group [3].
DON-BSA and DON-OVA prepared in this experiment were bound to a commercial polyclonal antibody and their bindings to antibody were inhibited by DON. Binding of polyclonal antibody to the DON-OVA conjugate to polyclonal antibody was inhibited more markedly compared to that of the DON-BSA conjugate. Therefore, DON-OVA was selected as the immunogen to produce mAbs.
IC50 values of the mAb developed for DON, HT-2, 15-acetyl-DON, and nivalenol were 23.44, 22545, 5518, and 5976 ng/mL. Based on the DON-mAb coated ELISA system, cross-reactivity values of the mAb for HT-2 toxin, 15-acetyl-DON, and nivalenol were 0.1, 0.42, and 0.40%, respectively. Our findings indicate that many clones which produce mAbs can be prepared using the present procedure we developed for generating DON-specific mAbs. The mAb produced in our study was an IgG1 λ-type and showed a very specific binding affinity for DON. Many studies have reported the production of mAbs specific for DON and the application of these antibodies in immunoassays for detecting DON in samples [4,6,10,16,28]. The IC50 for DON of mAb prepared in the present study was 23.4 ng/mL. Other previously reported IC50 values for other mAbs against DON are 25 ng/mL [10] and 15.8 ng/mL [16]. These data and our findings showed that the mAb developed in the present study has a similar binding capacity for DON compared to those reported in other studies. It has been found that IC50 values of the DON-specific mAb prepared by other to 3-Ac-DON, DON, nivalenol, 15-acetyl-DON, and fusarenon-X are 1.7, 15.8, 27.5, 68.9 and 1740 ng/mL, respectively [16]. However, IC50 values of the mAb in our study were 23.44, 22,545, 5,518 and 5,976 ng/mL for DON, HT-2, 15-acetyl-DON, and nivalenol, respectively. Based on the highly selective affinity of our mAb for DON, it is likely that the mAb can recognize DON-specific sites even though we did not include 3-acetyldeoxynivalenol as a DON analogue.
In the present study, DON concentrations detected by the DON-Ab coated ELISA and DON-Ag coated ELISA ranged from 50 to 4,000 ng/mL and 25 to 500 ng/mL, respectfully. The intra- and interassay precision CVs were both <10%. The DON-Ab coated ELISA was validated by spiking feed with DON at concentrations ranging from 0 to 1,000 µg/kg. Recovery rates ranged from 68.34 to 95.49% and the CVs ranged from 4.10 to 13.38%. Based on Codex Alimentarius guidelines (CAC/GL 71-2009; Food and Agriculture Organization and World Health Organization, Italy) for quantitative analytical methods, acceptable CVs for intra-laboratory testing are <15% while acceptable recovery rates are 70~110% for samples containing 100 to 1,000 ng/mL of the analyte. Our data suggest that the ELISA techniques developed in the current study satisfy these requirements. The new assays seem to be suitable for monitoring DON contamination in grain samples without the need to perform complicated extraction procedures.
In the present study, anti-DON mAbs were coupled with MNPs in an antibody concentration-dependent manner. The binding capacity was 95.44 ± 0.51% and the DON recovery rates for spiked buffer solutions using this novel mAb-MNP system were 75.2, 96.9, and 88.1%. Micronized beads have been widely used as immunosensors for separating glycoproteins, several kinds of cells, bacteria, and DNA from specific matrixes [2,8,21,24,25,27]. However, micro-sized beads have a low dispersion capacity in solution, can make the separation procedure laborious, and produce unreliable data. To separate chemicals from a biological sample using nanoparticles, a stable colloidal nanoparticle suspension should be prepared. Nanoparticles tend to agglomerate in liquid solutions. Despite this, MNPs (100~120 nm) using in the present study showed good dispersion. Our new method uses MNP-antibody conjugates to separate toxins from unbound materials by magnetism. The washing process is very fast and simple, and uses only a small amount of buffer. Furthermore, our novel technique requires only approximately 5 min to complete (from antibody binding to elution).
In conclusion, we prepared an anti-DON mAb with a very high affinity for DON compared to other analogues. This mAb could be used for the development of competitive ELISAs to detect DON in animal feed and foodstuffs. We also developed a new system for extracting DON from feed using mAb-coupled MNPs. Our extraction system could be a useful tool for detecting mycotoxin contamination in animal feed and food.
Acknowledgments
This study was conducted as an international collaborative research endeavor between the National Veterinary Research and Quarantine Service (NVRQS) in Korea and the Department of Population Medicine and Diagnostic Sciences of Cornell University (USA), and was funded by the NVRQS (I-AD-2008-11-01). Part of this work was supported by a Korea Research Foundation grant (2012M3A9C4048819) from the Ministry of Education, Science and Technology (MEST). M. H. Cho was also partially supported by the Research Institute for Veterinary Science, Seoul National University, Korea.
References
- 1.Böhm C, Cichna-Markl M, Brenn-Struckhofova Z, Razzazi-Fazeli E. Development of a selective sample clean-up method based on immuno-ultrafiltration for the determination of deoxynivalenol in maize. J Chromatogr A. 2008;1202:111–117. doi: 10.1016/j.chroma.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Bruno JG. In vitro selection of DNA to chloroaromatics using magnetic microbead-based affinity separation and fluorescence detection. Biochem Biophys Res Commun. 1997;234:117–120. doi: 10.1006/bbrc.1997.6517. [DOI] [PubMed] [Google Scholar]
- 3.Burkin AA, Kononenko GP, Soboleva NA. Group-specific antibodies against zearalenone and its metabolites and synthetic analogs. Prikl Biokhim Mikrobiol. 2002;38:194–202. [PubMed] [Google Scholar]
- 4.Choi GH, Lee DH, Min WK, Cho YJ, Kweon DH, Son DH, Park K, Seo JH. Cloning, expression, and characterization of single-chain variable fragment antibody against mycotoxin deoxynivalenol in recombinant Escherichia coli. Protein Expr Purif. 2004;35:84–92. doi: 10.1016/j.pep.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Debotton N, Zer H, Parnes M, Harush-Frenkel O, Kadouche J, Benita S. A quantitative evaluation of the molecular binding affinity between a monoclonal antibody conjugated to a nanoparticle and an antigen by surface Plasmon resonance. Eur J Pharm Biopharm. 2010;74:148–156. doi: 10.1016/j.ejpb.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Doyle PJ, Arbabi-Ghahroudi M, Gaudette N, Furzer G, Savard ME, Gleddie S, McLean MD, Mackenzie CR, Hall JC. Cloning, expression, and characterization of a single-domain antibody fragment with affinity for 15-acetyl-deoxynivalenol. Mol Immunol. 2008;45:3703–3713. doi: 10.1016/j.molimm.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Forsell JH, Witt MF, Tai JH, Jensen R, Pestka JJ. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem Toxicol. 1986;24:213–219. doi: 10.1016/0278-6915(86)90231-0. [DOI] [PubMed] [Google Scholar]
- 8.Gao P, Yao S, Zhang B, Li E, Chang J. On-chip magnetic separation of microcantilever immunosensor based on the CdSe QDs-tagged magnetic microbead. Sheng Wu Gong Cheng Xue Bao. 2008;24:315–322. [PubMed] [Google Scholar]
- 9.Gong JL, Liang Y, Huang Y, Chen JW, Jiang JH, Shen GL, Yu RQ. Ag/SiO2 core-shell nanoparticle-based surface-enhanced Raman probes for immunoassay of cancer marker using silica-coated magnetic nanoparticles as separation tools. Biosens Bioelectron. 2007;22:1501–1507. doi: 10.1016/j.bios.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Ji F, Chen Z, Xu J, Lu Q, Shi J. Development of the monoclonal antibody to deoxynivalenol. Wei Sheng Wu Xue Bao. 2008;48:929–934. [PubMed] [Google Scholar]
- 11.Khaydarov RA, Khaydarov RR, Gapurova O. Water purification from metal ions using carbon nanoparticle-conjugated polymer nanocomposites. Water Res. 2010;44:1927–1933. doi: 10.1016/j.watres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Klinglmayr C, Nöbauer K, Razzazi-Fazeli E, Cichna-Markl M. Determination of deoxynivalenol in organic and conventional food and feed by sol-gel immunoaffinity chromatography and HPLC-UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:187–193. doi: 10.1016/j.jchromb.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Kolosova AY, Sibanda L, Dumoulin F, Lewis J, Duveiller E, Van Peteghem C, De Saeger S. Lateral-flow colloidal gold-based immunoassay for the rapid detection of deoxynivalenol with two indicator ranges. Anal Chim Acta. 2008;616:235–244. doi: 10.1016/j.aca.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Lin JJ, Chen JS, Huang SJ, Ko JH, Wang YM, Chen TL, Wang LF. Folic acid-pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials. 2009;30:5114–5124. doi: 10.1016/j.biomaterials.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Liu BH, Tsao ZJ, Wang JJ, Yu FY. Development of a monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Anal Chem. 2008;80:7029–7035. doi: 10.1021/ac800951p. [DOI] [PubMed] [Google Scholar]
- 16.Maragos C, Busman M, Sugita-Konishi Y. Production and characterization of a monoclonal antibody that cross-reacts with the mycotoxins nivalenol and 4-deoxynivalenol. Food Addit Contam. 2006;23:816–825. doi: 10.1080/02652030600699072. [DOI] [PubMed] [Google Scholar]
- 17.Maragos CM, MaCormick SP. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric Immunol. 2000;12:181–192. [Google Scholar]
- 18.Plattner RD. HPLC/MS analysis of Fusarium mycotoxins, fumonisins and deoxynivalenol. Nat Toxins. 1999;7:365–370. [PubMed] [Google Scholar]
- 19.Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, Oram DH, Jacobs IJ, Shepherd JH, Reznek RH. Diagnostic performance of nanoparticle-enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol. 2005;23:2813–2821. doi: 10.1200/JCO.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Hernández E, Baeza A, Vallet-Regí M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano. 2011;5:1259–1266. doi: 10.1021/nn1029229. [DOI] [PubMed] [Google Scholar]
- 21.Shim WB, Choi JG, Kim JY, Yang ZY, Lee KH, Kim MG, Ha SD, Kim KS, Kim KY, Kim CH, Eremin SA, Chung DH. Enhanced rapidity for qualitative detection of Listeria monocytogenes using an enzyme-linked immunosorbent assay and immunochromatography strip test combined with immunomagnetic bead separation. J Food Prot. 2008;71:781–789. doi: 10.4315/0362-028x-71.4.781. [DOI] [PubMed] [Google Scholar]
- 22.Shiu CM, Wang JJ, Yu FY. Sensitive enzyme-linked immunosorbent assay and rapid one-step immunochromatographic strip for fumonisin B1 in grain-based food and feed samples. J Sci Food Agric. 2010;90:1020–1026. doi: 10.1002/jsfa.3911. [DOI] [PubMed] [Google Scholar]
- 23.Sobrova P, Adam V, Vasatkova A, Beklova M, Zeman L, Kizek R. Deoxynivalenol and its toxicity. Interdiscip Toxicol. 2010;3:94–99. doi: 10.2478/v10102-010-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparbier K, Asperger A, Resemann A, Kessler I, Koch S, Wenzel T, Stein G, Vorwerg L, Suckau D, Kostrzewa M. Analysis of glycoproteins in human serum by means of glycospecific magnetic bead separation and LC-MALDI-TOF/TOF analysis with automated glycopeptide detection. J Biomol Tech. 2007;18:252–258. [PMC free article] [PubMed] [Google Scholar]
- 25.Tse LC, Dumaswala UJ, Greenwalt TJ. Separation of mixed red cell populations by using microbead columns. J Lab Clin Med. 1996;127:489–493. doi: 10.1016/s0022-2143(96)90066-9. [DOI] [PubMed] [Google Scholar]
- 26.Varshney M, Yang L, Su XL, Li Y. Magnetic nanoparticle-antibody conjugates for the separation of Escherichia coli O157 : H7 in ground beef. J Food Prot. 2005;68:1804–1811. doi: 10.4315/0362-028x-68.9.1804. [DOI] [PubMed] [Google Scholar]
- 27.Wynick D, Bloom SR. Magnetic bead separation of anterior pituitary cells. Neuroendocrinology. 1990;52:560–565. doi: 10.1159/000125644. [DOI] [PubMed] [Google Scholar]
- 28.Yuan Q, Pestka JJ, Hespenheide BM, Kuhn LA, Linz JE, Hart LP. Identification of mimotope peptides which bind to the mycotoxin deoxynivalenol-specific monoclonal antibody. Appl Environ Microbiol. 1999;65:3279–3286. doi: 10.1128/aem.65.8.3279-3286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao W, Wang L, Tan W. Fluorescent nanoparticle for bacteria and DNA detection. Adv Exp Med Biol. 2007;620:129–135. doi: 10.1007/978-0-387-76713-0_10. [DOI] [PubMed] [Google Scholar]