Abstract
To evaluate radiosensitivity and the effects of radiation on the expression of vascular endothelial growth factor (VEGF) and VEGF receptors in the canine oral melanoma cell line, TLM 1, cells were irradiated with doses of 0, 2, 4, 6, 8 and 10 Gray (Gy). Survival rates were then determined by a MTT assay, while vascular endothelial growth factor receptor (VEGFR)-1 and -2 expression was measured by flow cytometry and apoptotic cell death rates were investigated using an Annexin assay. Additionally, a commercially available canine VEGF ELISA kit was used to measure VEGF. Radiosensitivity was detected in TLM 1 cells, and mitotic and apoptotic cell death was found to occur in a radiation dose dependent manner. VEGF was secreted constitutively and significant up-regulation was observed in the 8 and 10 Gy irradiated cells. In addition, a minor portion of TLM 1 cells expressed vascular endothelial growth factor receptor (VEGFR)-1 intracellularly. VEGFR-2 was detected in the cytoplasm and was down-regulated following radiation with increasing dosages. In TLM 1 cells, apoptosis plays an important role in radiation induced cell death. It has also been suggested that the significantly higher VEGF production in the 8 and 10 Gy group could lead to tumour resistance.
Keywords: cell line, radiation, TLM 1, vascular endothelial growth factor
Introduction
Melanoma is a common neoplastic disorder in dogs with variable presentation and biological behaviour. Melanomas account for 9~20% of all skin tumours [21] and are the most common tumour of the oral cavity [13] and on the digits [17]. Unlike cutaneous melanomas, which are often benign, melanomas within the oral cavity are highly malignant. They can be characterised by local infiltration and early metastasis to regional lymph nodes, lungs and other organs [27]. Although the pathogenesis is not completely understood, loss of functional tumour suppressor proteins such as p53, retinoblastoma susceptibility gene 1 (Rb) and phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is common and may contribute to their aggressive nature [20]. Despite local control with wide surgical excisions and radiation therapy, the median survival time is only 8 months, with greater than 80% of dogs developing distant metastasis [30].
Vaccines that use immune stimulation to target melanoma cells have recently become available and have shown promising results [2]. However, other treatment options are required to further improve the long term prognosis for this common canine neoplasm. Angiogenesis represents a fundamental step in the malignant growth of tumours and metastasis. Many pro-and anti-angiogenic factors influence the formation of new blood vessels, but the central growth factor in this process is vascular endothelial growth factor (VEGF) [11].
VEGF is a heparin-binding glycoprotein with five reported isoforms in mammals ranging from 126 to 206 amino acids [16]. VEGF binds two related receptor tyrosine kinases, vascular endothelial growth factor receptor (VEGFR)-1 and VEGFR-2, which are normally found on endothelial cells [9]. In many cases, neoplastic cells produce substantial amounts of VEGF and express VEGF receptors, thus utilizing VEGF as an autocrine growth stimulant [7]. Additionally, some tumour cells have been shown to protect themselves against therapeutic interventions such as radiotherapy by the release of VEGF [22]. Direct up-regulation of VEGF and its receptors after radiation in several tumour cell lines has been reported [14]. Furthermore, VEGF expression appears to be related to tumour grade and prognosis in some malignancies [6,23]. Over-expression of VEGF in human melanoma results in a phenotype that has increased malignant potential when compared to melanomas with low VEGF expression. Serum concentrations of VEGF in human patients with malignant melanomas are significantly elevated when compared to controls and have been shown to be correlated with poor outcome [28].
This study was conducted to evaluate the effects of radiation on the expression of VEGF and VEGF receptors in the canine melanoma cell line, TLM 1. Moreover, attempts to determine the radio-sensitivity of the TLM 1 cells and estimate the role of radiation induced apoptosis were made. The results should provide basic information to further exploit investigations of VEGF and VEGFR inhibitors in combination with radiotherapy.
Materials and Methods
The TLM 1 cell line was kindly provided by Dr. J. F. Modiano, who established the cell line in 1996 from a canine patient with oral malignant melanoma.
Iscove's Modified Dulbecco's medium (Gibco, Invitrogen, USA) supplemented with 10% foetal calf serum (FCS) (PAA laboratories, Austria) and antibiotics (penicillin/streptomycin 10 mg/mL and gentamycin 10 mg/mg) (PAA laboratories, Austria) was used to culture the cells under 5% CO2 at 37℃. Cells were passaged every 3~4 days and re-thawed from an original stock every 4 weeks. Cells from the 3rd to 6th passage were used for all experiments. Cell counts were obtained using a haemocytometer (Neubauer-Improved; LO-Laboroptik, Germany) and cell viability was confirmed by the Trypan blue exclusion method (Trypan blue 0.4%; Sigma-Aldrich, USA).
Radiation experiments
For all radiation experiments, the required number of cells was suspended in IMDM medium and then transferred into clear 12 mL tubes. To achieve a homogenous radiation dose, attention was paid to ensure that the tubes were filled completely. Cells were then irradiated with 2, 4, 6, 8 and 10 Gray (Gy) with a dose rate of 200 monitor units per min from a linear accelerator source (Primus; Siemens, Germany). Post radiation, cells were centrifuged, re-suspended, and plated onto different plates depending on the experiment. The negative control, hereafter referred to as the 0 Gy group, was treated identically to irradiated cells.
Growth curves
To evaluate growth curves and the population doubling time of TLM 1 cells, 1 × 105 cells were cultured in six well plates and counted on day 1, 2, 3, 4, 6, 8 and 10. The same steps were performed with cells following radiation. All samples were obtained in duplicate and all experiments were conducted in triplicate.
MTT assay
An MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Sigma-Aldrich, USA), which measures changes in the function of mitochondrial dehydrogenases in viable cells [19], was used to determine metabolic cell activity after radiation. After radiation, 100 µL of cell suspension containing 1 × 104 cells were inoculated into 96 well round bottom microtiter plates and incubated for 5 days under 5% CO2 at 37℃. The number of cultured cells was adjusted to a density that allowed the negative control group to grow exponentially in the microtiter plates. The optimal concentration of MTT, incubation time and cell number were established in pilot studies. On day 5, cell counts were performed and 100 µL of MTT solution was added to each well. Cells were then re-suspended and incubated for 1 h at 37℃. The MTT solution was then discarded and cells were solubilised in 100 µL of dimethyl sulfoxide (Merck, Germany). Finally, plates were placed in a photo-resistant foil and incubated at room temperature for at least 1.5 h, but not longer than 24 h. After ensuring the precipitate had dissolved, the plates were analysed with an enzyme linked immunosorbent assay (ELISA) plate reader (GENios, Switzerland) at a wavelength of 570 nm and a reference wavelength of 690 nm.
VEGF ELISA
VEGF protein was measured in cell free supernatant and in cell lysate using canine VEGF development kits (R&D Systems, UK) according to manufacturer's guidelines. Briefly, after radiation, 5 × 104 cells per well were plated into 24-wells plates and incubated at 37℃. At 2, 24, 48, and 72 h after radiation, cells were harvested and cell numbers were recorded. Samples of each group were then centrifuged at 2,000 × g force for 8 min, after which supernatants were collected. The cell pellets were then re-suspended in distilled water, a protease inhibitor mix diluted 1 : 100 was added and samples were stored at -80℃ until assayed. The optical density of each well was then determined using a microplate reader and the respective software (Sunrise, Switzerland) at a set wavelength of 450 nm. Measured VEGF concentrations were corrected for cell number in each group. Concentrations of VEGF are expressed as pg/mL/105 cells.
Evaluation of apoptosis
Flow cytometry studies were conducted using the Annexin-V FLUOS staining kit (Roche, Germany) to detect radiation induced apoptosis. Following radiation, cells were plated in 6 well plates and assayed at different time points (6, 24, 48, 72 and 96 h). Annexin staining was then carried out according to the manufacturer's guidelines. Cells stained by annexin alone were analyzed first to determine the spectral overlap of annexin stained cells with the emission region of propidium iodide staining. Propidium iodide was then added to the sample and annexin and propidium iodide staining were analyzed in combination. A total of 30,000 cells was analyzed for each sample and all experiments were performed in triplicate. For all flow cytometry (FCM) experiments, data were analyzed with a FACSAria (BD Biosciences, USA) equipped with the FACSDiva Software, Version 6.1.3. (BD Bioscience, USA).
VEGFR-1 and VEGFR-2 expression
FCM analysis was used to evaluate the effects of radiation on VEGFR-1 and VEGFR-2 expression. Cross-reactive polyclonal rabbit anti-human VEGFR-1 antibodies (Santa Cruz Biotechnology, USA) were used for detection of VEGFR-1 expression. The monoclonal antibody A-3 (Santa Cruz Biotechnology, USA) was used to identify VEGFR-2 expression. Binding of primary antibodies was visualized by fluorochrome-labelled secondary antibodies. For VEGFR-1 expression analysis, this was achieved using goat anti-canine IgG-R-Phycoerythrin (Southern Biotechnology, USA). For VEGFR-2 expression analysis, a goat anti-mouse IgG1-R-Phycoerythrin (Southern Biotechnology, USA) was used. Isotype control samples were incubated with polyclonal rabbit IgG and monoclonal mouse IgG1 antibodies (Dianova, Germany) for VEGFR-1 and VEGFR-2, respectively. Irradiated cells were plated in 6 well plates, incubated and harvested 2, 24, 48, 72 and 144 h after radiation. The expression of VEGFR-1 and VEGFR-2 of non-irradiated cells served as a baseline. To discriminate viable cells with flow cytometry, a Live/Dead Fixable NearIR Stain Kit (Invitrogen, USA) was used according to the manufacturer's instructions. Briefly, cells were incubated with primary antibodies against VEGFR-1 and -2 or respective isotype control antibodies on ice for 20 min. After washing with a wash solution (phosphate buffered saline), cells were incubated for another 20 min with fluorochrome-labelled secondary antibodies and Live/Dead stain, then subjected to two final washing steps.
To evaluate intracellular receptor expression, TLM 1 cells were first incubated with Live/Dead stain for 20 min on ice, after which they were fixed and permeabilized with buffer containing 3% formaldehyde and 0.1% saponin for another 20min on ice. After two washing steps with phosphate buffered saline (PBS) containing 2% FCS, 0.1% saponin labelling with primary and secondary antibody was performed as described above. Samples were again washed with PBS containing 2% FCS and 0.1% saponin and a total of 20,000 cells per sample was analyzed by flow cytometry.
Data analysis
Data were analyzed using SPSS version 17 (SPSS, IL). All means were calculated from at least three experiments. Error bars represent the standard error (SE) of the mean. Statistical significance was determined using one way ANOVA, as appropriate. A p < 0.05 was considered to be significant.
Results
Growth curves
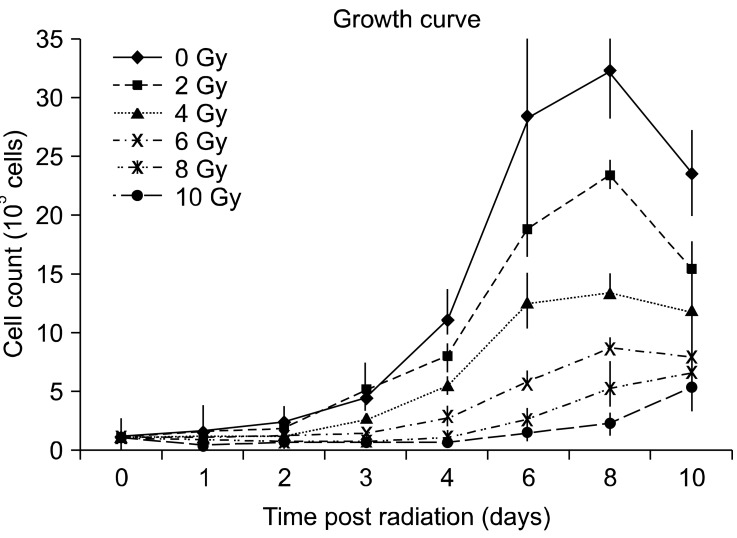
Growth curves for the different treatment groups (0, 2, 4, 6, 8 and 10 Gy) of radiated TLM 1 cells are shown in Fig. 1. Absolute cell numbers decreased in a radiation dose dependent manner.
Fig. 1.
Growth curves of irradiated TLM 1 cells. Dose dependent growth inhibition was observed after single doses of 0, 2, 4, 6, 8 and 10 Gy. Each data point represents the mean ± standard deviation. Gy: Gray.
MTT assay
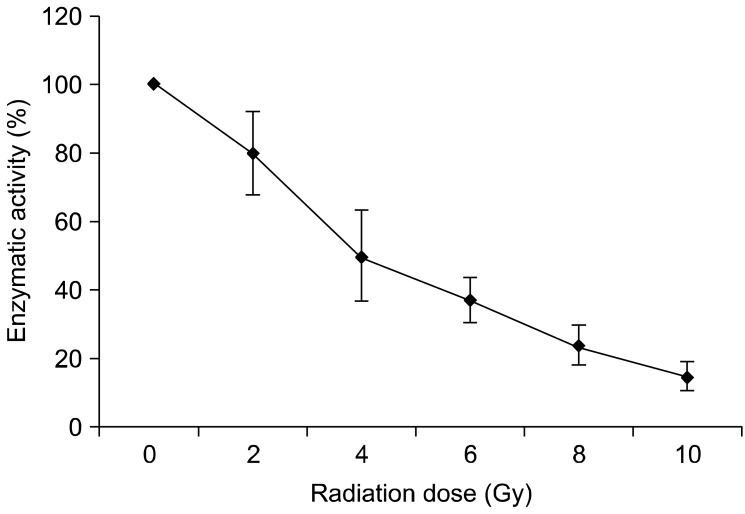
Cell survival curves were determined by MTT assay on day 5 after radiation. In all treatment groups, significantly reduced survival rates were measured when compared with non-radiated controls. The dose dependent survival curve is shown in Fig. 2.
Fig. 2.
Survival curve of irradiated TLM 1 cells. Following irradiation with 0, 2, 4, 6, 8 and 10 Gy, cells were incubated for 5 days and then harvested to measure cell viability with the MTT assay. Each data point represents the mean of three experiments (samples of each in duplicate) ± standard deviation.
VEGF ELISA
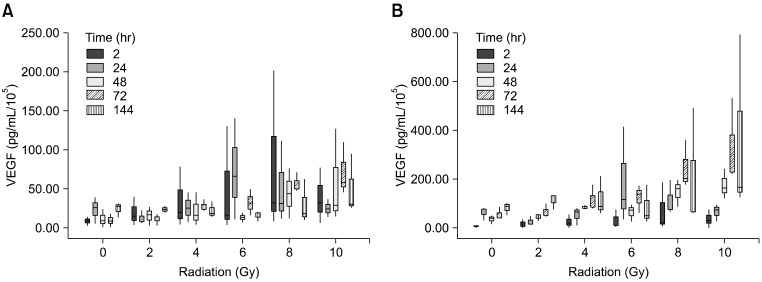
Canine VEGF ELISA kits were used to determine if radiation induces up-regulation of VEGF expression in TLM 1 cells. Cells in all treatment groups and non-irradiated cells produced VEGF constitutively. The main portion of VEGF was detected in the cell free supernatant (up to 2,800 pg/mL), whereas only low concentrations of VEGF were measured in the cell lysate (up to 980 pg/mL). VEGF concentrations were re-calculated to adjust them to the number of cells (pg/mL/105) present in the respective microcultures, as higher doses of radiation resulted in decreased numbers of viable secreting cells (Fig. 3A and B). Radiation dose had no effect on absolute VEGF concentrations in the cell lysate, but a significant increase over time was noted. Additionally, an interaction between time and dose was observed. The effect of radiation on VEGF production became lower as the radiation dose increased.
Fig. 3.
(A) Concentration of VEGF normalized to the number of viable cells (pg/mL/105) in the cell lysate. (B) Cell free supernatant. Means ± standard deviations of three independent experiments analysed in duplicate are shown. VEGF: vascular endothelial growth factor.
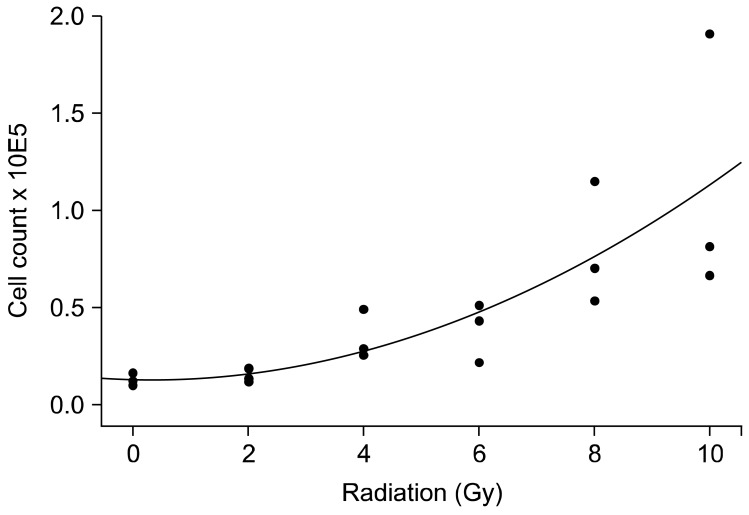
Absolute VEGF values corrected to the cell number (per 105) show that radiation has a significant effect on the amount of VEGF in the cell lysate. Cells in the 8 and 10 Gy group produced significantly more VEGF than untreated cells and cells treated with 2 Gy alone (Fig. 4). The same effect was seen in the cell free supernatant, with even greater differences being observed between the 0, 2 to 8 and 10 Gy groups. Absolute VEGF levels in the cell free supernatant increased significantly over time and with radiation dose (Fig. 3B).
Fig. 4.
VEGF expression in relation to radiation dose in cell free supernatant 72 hours after radiation. Mean ± standard deviation of three independent experiments analysed in duplicate are shown.
Radiation induced apoptosis in TLM 1 cells
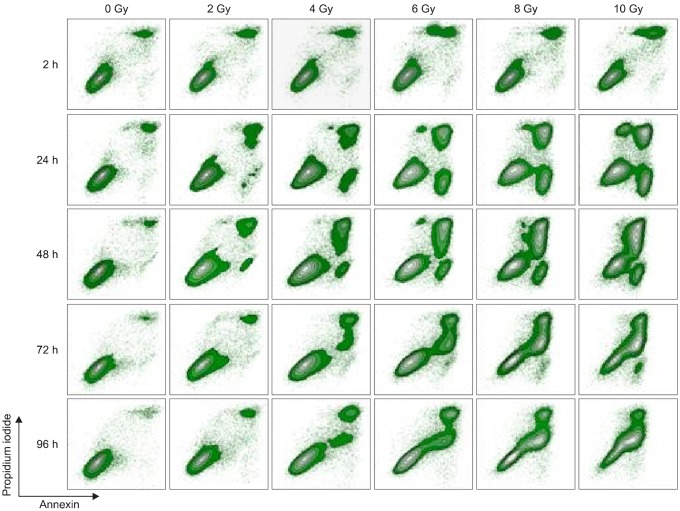
To examine the role of radiation induced apoptosis in TLM 1 cells, annexin expression in combination with propidium iodide staining was performed. Radiation induced apoptotic cell death is shown in one representative example (Fig. 5). Two hours after treatment, a slight increase in the frequency of apoptotic cells was induced by increasing doses of radiation. Over time (2 h~72 h), non treated cells showed a slight increase in the percentage of apoptotic cells (1.3~3.4%). Apoptosis in non treated cells did not change significantly throughout the experiment. The strongest increase in apoptotic cells was detected on day 1 after radiation (range 2.4~17.3%). On day 4 (96 h), apoptotic cell fractions approached percentages close to baseline. The same pattern was observed in two other representative experiments.
Fig. 5.
Radiation induced apoptosis of TLM 1 cells analyzed by FCM (annexin/PI iodide staining). Cells were gated according to FSC/SSC light scattering properties (not depicted). Results are displayed in contour plots. One representative sample of three is shown. FCM: flow cytometry, PI: propidium iodide, FSC/SSC: forward scatter/side scatter.
Expression of VEGFR-1 post radiation
FCM was used to evaluate whether TLM 1 cells express VEGFR-1 on their cell surface or in their cytoplasm before and after radiation. VEGFR-1 was not expressed on the surface of TLM 1 cells, nor did radiation have an effect on expression, regardless of time or radiation dose (data not shown). After permeabilisation, very few TLM 1 cells expressed VEGFR-1 intracellulary. A tendency for decreased expression was observed with increasing radiation dose. The percentage of VEGFR-1 positive cells was 8.7%, 6.8%, 6.8%, 3.5%, 6.9% and 2.2% for the 0, 2, 4, 6, 8 and 10 Gy groups, respectively (data not shown).
Expression of VEGFR-2 post radiation
No expression of VEGFR-2 was detected on the cell surface (data not shown). After fixation and permeabilisation of TLM 1 cells, VEGFR-2 was expressed in 87%, 88%, 80.6%, 54.3%, 26.3% and 10.4% of the 0, 2, 4, 6, 8 and 10 Gy irradiated cells, respectively, indicating down-regulation of VEGFR-2 expression with increasing radiation dose. Different time points did not have an effect on receptor expression. One representative example of day 4 after treatment is shown (Fig. 6). Mean fluorescence intensity was used to demonstrate the increase in expression of the marker compared to the isotype control. Sample and isotype controls were run under the same conditions and instrument settings. Down-regulation of the receptor was again observed in response to increasing radiation dose.
Fig. 6.
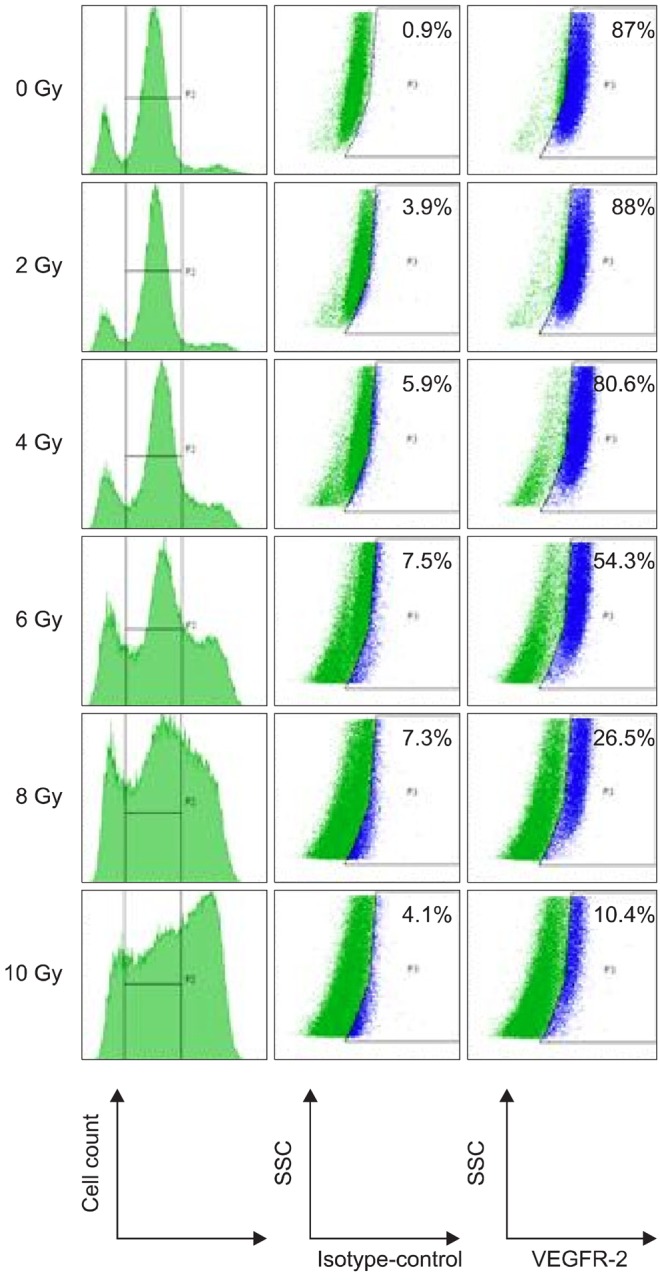
FCM analysis of VEGFR-2 in cytoplasm of TLM 1 cells four days post radiation (0, 2, 4, 6, 8 and 10 Gy). Cells were stained with live/dead stain, after which they were fixed, permeabilzed and stained with antibodies against VEGFR-2 or irrelevant isotype control antibodies. Only live cells were gated and analyzed for VEGFR-2 expression. VEGFR-2 positive cells are highlighted in blue. The percentage of VEGFR-2 positive cells is shown in the upper right corner. VEGFR: vascular endothelial growth factor receptor.
Discussion
Tumour incidence in small animal medicine is increasing. According to Brodey, 50% of dogs older than 10 years will develop a malignancy at some point in their life [5]. Despite early detection and aggressive treatments, often consisting of a multi-modality approach with surgery, radiation therapy and chemotherapy, control times and survival times are not satisfactory in many malignancies. In veterinary therapy, current therapies for oral malignant melanoma rarely achieve long term control of the disease [1,4,5].
The present study attempted to describe radiation sensitivity and the effect of radiation on the expression of VEGF and VEGF receptor in the canine melanoma cell line TLM 1. Improved understanding of the interplay between radiation and angiogenic factors should help to identify appropriate targets for therapeutic interventions and aid in the development of new treatment regimens for oral malignant melanomas.
A constant constitutive secretion of VEGF was observed in non-irradiated and irradiated cells. The main portion of VEGF was detected in cell free supernatant, whereas only approximately half the amount of VEGF was measured in the cell lysate. This finding could be explained by the fact that VEGF is a secreted protein and seems to be exported out of the cell immediately. These results are supported by those of Sekis et al. [25], who found that VEGF was mainly in the cell free supernatant. The amount of intracellular VEGF increased significantly over time, but radiation dose had no impact. However, the effect of time decreased significantly with increasing radiation dose. Radiation induced significantly higher VEGF production in the 8 and 10 Gy group, which may have contributed to tumour resistance as the enhanced secretion of VEGF increases survival by decreasing apoptosis, stimulating proliferation and increasing angiogenic potential [14]. Overexpression of VEGF in human melanoma results in an angiogenic phenotype that has increased malignant potential compared with melanomas with little VEGF expression [28].
In the lower radiation dose groups, constitutive production of VEGF was observed and VEGF accumulated in the supernatant over time.
The same commercially available VEGF ELISA used for our measurements was employed in several clinical studies of VEGF levels in plasma, urine and body effusions of dogs with naturally occurring neoplasia. In a recent study by Wergin et al. [29], concentrations of VEGF were found to be highest with malignant melanoma, and high concentrations were thought to be associated with aggressiveness of the tumour. In another study conducted in 2007, VEGF levels were found to be higher in the plasma and serum of dogs with malignant melanomas than in a control population [26]. These findings support the evaluation of anti-angiogenic therapies in clinical trials. VEGF binding sites have been described on the cell surface and in the cell cytoplasm, and VEGFR-1 and VEGFR-2 are the most important receptors for canines. VEGFR-2 plays an important role in both physiological and pathological angiogenesis, the exact functions of VEGFR-1 are still unclear. Although it binds VEGF with higher affinity, VEGFR-1 is believed to only modulate the availability of VEGF to VEGFR-2, the principal receptor for VEGF [10]. VEGFR-1 has been found on the surface of many tumor cells [15]. In TLM 1 cells, no expression of VEGFR-1 was found extracellularly, and only a minor percentage expressed VEGFR-1 in the cell cytoplasm. VEGFR-2 was expressed in the cytoplasm of TLM-1 cells, but the receptor was down-regulated with increasing radiation dose. Although mechanisms regulating the expression of VEGF receptors are poorly understood, there is some evidence that stimuli that induce expression of VEGF may have the same effect on VEGF receptors or that VEGF itself may stimulate the expression of its own receptors in a paracrine way [3].
We postulate that TLM-1 cells use VEGF in a paracrine and not an autocrine way. VEGF does not stimulate the producing cell itself, but is secreted into the immediate surrounding of the cell to act locally without entering the blood stream.
Gampel et al. [12] first showed the cellular distribution of endogenous VEGFR-2 in endothelial cells. Although the majority of receptors of other receptor tyrosine kinases, such as EGFR and PDGFR, are present on the cell surface, those of VEGFR-2 are kept in an endosomal storage pool. Two independent studies of the canine mastocytoma cell line, C2, also showed VEGFR-2 in the cell cytoplasma [24,25], which is in agreement with the results of the present study.
The fact that VEGFR-2 was detected in the TLM-1 cell line makes the use of a tyrosine kinase inhibitor interesting. It may be beneficial to treat canine patients with tumours that overexpress VEGF with specific inhibitors in conjunction with radiation. However, to the best of our knowledge, no studies testing the efficacy and tolerability of this combination treatment for canine oral malignant melanoma have been conducted to date.
This study also showed that radiation induces apoptosis in TLM 1 cells. Specifically, after a single dose of 2, 4, 6, 8, and 10 Gy, reduced numbers of TLM 1 cells were observed relative to a negative control group. The extent of apoptosis was correlated with radiation dose and first became detectable at 24 h after treatment. The first increase in apoptotic cell fraction was followed by a slow decrease on day 2. On day 4, the apoptotic cell fraction reached its baseline. Endlich et al. [8] also showed that, even for cells exhibiting late apoptosis, most cells had completed their apoptotic process at 72 h after treatment. This finding is in agreement with the results of the present study. The reduction in cell number is the result of very complex courses of action in the cell caused by irradiation. However, the major pathway of apoptosis cannot be determined from the results of the present study.
To the best of the author's knowledge, no previous data are available describing the radio-sensitivity of TLM 1 cells. However, a number of clinical reports on the response of oral malignant melanomas to radiation therapy suggest that melanoma cells are radiosensitive, which is in agreement with the results of the present study. In a clinical setting, response rates are better using higher single fractions of radiation [18]. This supports the importance of our finding that significantly more VEGF is produced in high dose (8 and 10 Gy) irradiated cells. Lower doses of radiation also had an effect on cell survival and cell activity, but not on VEGF expression in vitro.
In summary, this study shows that TLM 1 cells are radio-sensitive and that apoptosis is induced in a radiation dose dependent manner. VEGF was produced constitutively with significant up-regulation being observed in 8 and 10 Gy treated cells. A minor portion of TLM 1 cells expressed VEGFR-1 intracellularly, whereas no receptor was found extracellularly. VEGFR-2 was detected in the cytoplasm and down-regulated following radiation with increasing dosages. Given its central role in promoting many types of cancers, VEGF is a very attractive target for therapeutic interventions. Improved understanding of the effects of radiation on the expression of VEGF and VEGF receptor should facilitate the design of new treatment regimens.
References
- 1.Bateman KE, Catton PA, Penncock PW, Kruth SA. 0-7-21 radiation therapy for treatment of canine oral melanoma. J Vet Intern Med. 1994;8:267–272. doi: 10.1111/j.1939-1676.1994.tb03231.x. [DOI] [PubMed] [Google Scholar]
- 2.Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, Wulderk M, Jeffers Y, Sadelain M, Hohenhaus AE, Segal N, Gregor P, Engelhorn M, Riviere I, Houghton AN, Wolchok JD. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9:1284–1290. [PubMed] [Google Scholar]
- 3.Berkman RA, Merrill MJ, Reinhold WC, Monacci WT, Saxena A, Clark WC, Robertson JT, Ali IU, Oldfield EH. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. 1993;91:153–159. doi: 10.1172/JCI116165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwood L, Dobson JM. Radiotherapy of oral malignant melanomas in dogs. J Am Vet Med Assoc. 1996;209:98–102. [PubMed] [Google Scholar]
- 5.Brodey RS. Canine and feline neoplasia. Adv Vet Sci Comp Med. 1970;14:309–354. [PubMed] [Google Scholar]
- 6.Chaudhry IH, O'Donovan DG, Brenchley PEC, Reid H, Roberts ISD. Vascular endothelial growth factor expression correlates with tumor grade and vascularity in gliomas. Histopathology. 2001;39:409–415. doi: 10.1046/j.1365-2559.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 7.Clifford CA, Hughes D, Beal MW, Mackin AJ, Henry CJ, Shofer FS, Sorenmo KU. Plasma vascular endothelial growth factor concentrations in healthy dogs and dogs with hemangiosarcoma. J Vet Intern Med. 2001;15:131–135. doi: 10.1892/0891-6640(2001)015<0131:pvegfc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Endlich B, Radford IR, Forrester HB, Dewey WC. Computerized video time-lapse microscopy studies of ionizing radiation-induced-rapid-interphase and mitosis-related apoptosis in lymphoid cells. Radiat Res. 2000;153:36–48. doi: 10.1667/0033-7587(2000)153[0036:cvtlms]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Gerber HP, Le Couter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Vis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 12.Gampel A, Moss L, Jones MC, Brunton V, Norman JC, Mellor H. VEGF regulates the mobilization of VEGFR2/KDR from an intracellular endothelial storage compartement. Blood. 2006;108:2624–2631. doi: 10.1182/blood-2005-12-007484. [DOI] [PubMed] [Google Scholar]
- 13.Goldschmidt MH. Benign and malignant melanocytic neoplasms of domestic animals. Am J Dermatopathol. 1985;7:203–212. doi: 10.1097/00000372-198501001-00039. [DOI] [PubMed] [Google Scholar]
- 14.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockade of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 15.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 16.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 17.Marino DJ, Matthiesen DT, Stefanacci JD, Mororff SD. Evaluation of dogs with digit masses: 117 cases (1981-1991) J Am Vet Med Assoc. 1995;207:726–728. [PubMed] [Google Scholar]
- 18.McEntee MC. Summary of Results of Cancer Treatment with radiation therapy. In: Morrison WB, editor. Cancer in Dogs and Cats: Medical and Surgical Management. 2nd ed. Jackson: Teton NewMedia; 2002. pp. 389–424. [Google Scholar]
- 19.Modiano JF, Ritt MG, Wojcieszyn J. The molecular basis of canine melanoma: pathogenesis and trends in diagnosis and therapy. J Vet Intern Med. 1999;13:163–174. doi: 10.1892/0891-6640(1999)013<0163:tmbocm>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Moultan JE. Tumors in Domestic Animals. 3rd ed. Berkeley: University of California Press; 1990. pp. 75–87. [Google Scholar]
- 22.Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, McKinstry R, Yacoub A, Duigou GJ, Young CSH, Grant S, Hagan MP, Ellis E, Fisher PB, Dent P. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene. 2001;20:3266–3280. doi: 10.1038/sj.onc.1204258. [DOI] [PubMed] [Google Scholar]
- 23.Platt SR, Scase TJ, Adams V, Wieczorek L, Miller J, Adamo F, Long S. Vascular endothelial growth factor expression in canine intracranial meningiomas and association with patient survival. J Vet Intern Med. 2006;20:663–668. doi: 10.1892/0891-6640(2006)20[663:vegfei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Rebuzzi L, Willmann M, Sonneck K, Gleixner KV, Florian S, Kondo R, Mayerhofer M, Vales A, Gruze A, Pickl WF, Thalhammer JG, Valent P. Detection of vascular endothelial growth factor (VEGF) and VEGF receptors Flt-1 and KDR in canine mastocytoma cells. Vet Immunol Immunopathol. 2007;115:320–333. doi: 10.1016/j.vetimm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Sekis I, Gerner W, Willmann M, Rebuzzi L, Tichy A, Patzl M, Thalhammer JG, Saalmüller A, Kleiter MM. Effect of radiation on vascular endothelial growth factor expression in the C2 canine mastocytoma cell line. Am J Vet Res. 2009;70:1141–1150. doi: 10.2460/ajvr.70.9.1141. [DOI] [PubMed] [Google Scholar]
- 26.Taylor KH, Smith AN, Higginbotham M, Schwartz DD, Carpenter DM, Whitley EM. Expression of vascular endothelial growth factor in canine oral malignant melanoma. Vet Comp Oncol. 2007;5:208–218. doi: 10.1111/j.1476-5829.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- 27.Todoroff RJ, Brodey RS. Oral and pharyngeal neoplasia in the dog: a retrospective survey of 361 cases. J Am Vet Med Assoc. 1979;175:567–571. [PubMed] [Google Scholar]
- 28.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 29.Wergin MC, Ballmer-Hofer K, Roos M, Achermann RE, Inteeworn N, Akens MK, Blattmann H, Kaser-Hotz B. Preliminary study of plasma vascular endothelial growth factor (VEGF) during low- and high-dose radiation therapy of dogs with spontaneous tumors. Vet Radiol Ultrasound. 2004;45:247–254. doi: 10.1111/j.1740-8261.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- 30.Withrow SJ, MacEwen EG. Withrow's and MacEwen's Small Animal Clinical Oncology. 3rd ed. St. Louis: W. B. Saunders; 2001. pp. 305–317. [Google Scholar]