Abstract
The renal glomeruli of 12 male Osborne-Mendel (OM) rats 3 to 24 weeks old were examined by electron microscopy. Effacement of podocyte foot processes (FPs) developed at 3 weeks of age and became progressively worse over time. Loss or dislocation of the slit membrane was also found. Vacuoles and osmiophilic lysosomes appeared in the podocytes starting at 6 weeks of age. Podocyte detachment from the glomerular basement membrane (GBM) was apparent at 18 weeks of age. Laminated GBM was occasionally observed in all animals. These features might lead to the development of spontaneous proteinuria and glomerulosclerosis in OM rats.
Keywords: glomerular basement membrane, kidney glomerulus, podocyte, proteinuria, rat
We recently described early-onset podocyte injury and glomerulosclerosis spontaneousely developed in Osborne-Mendel (OM) rats [12]. Characteristic light microscopic lesions in male OM rats with these conditions included glomerular hypertrophy, progressive podocytopathy, and a reduction in podocyte number and area. These findings suggest that podocyte injury is closely linked with the development of glomerulosclerosis. We also described ultrastructural abnormalities of the podocytes such as effacement of foot processes (FPs) and actin cytoskeleton rearrangement that were first observed at 5 weeks of age in the male OM rats. Glomerular injury was associated with selective proteinuria (albuminuria) starting at 5 weeks of age and non-selective proteinuria from 7 weeks of age.
Podocytes serve as the final barrier against urinary protein loss; therefore, injury of these structures results in proteinuria [3,10,11]. Detachment of podocytes from the glomerular basement membrane (GBM) leads to the development of segmental glomerular sclerosis [5,6]. FPs of the podocytes anchor the GBM that is composed of several extra-cellular matrices, primarily made of type IV collagen and laminin, and plays a role in selective glomerular filtration [3].
The present study was performed to see early ultrastructural abnormalities of the renal glomeruli that appeared prior to sclerotic changes, especially those in the GBM and podocytes, spontaneously developed in male OM rats. Twelve kidney samples (two samples per rat) from male OM rats aged 3, 6, 8, 9, 18, or 24 weeks originally from a commercial source (Japan SLC, Japan) were fixed in 10% neutral-buffered formalin and embedded in paraffin. Tissue sections (3-µm thick) were cut and stained with hematoxylin and eosin or periodic acid-Schiff (PAS) stain for light microscopy. For electron microscopy, renal cortical tissues were cut into 1 mm3 cubes, fixed in 2.5% glutaraldehyde, and post-fixed in 1% OsO4 (Nisshin EM, Japan). The fixed specimens were then dehydrated with graded ethanol and embedded in epoxy resin (Quetol-812 set; Nisshin EM, Japan). Semi-thin sections (1-µm thick) were cut, stained with 1% toluidine blue and non-sclerotic glomeruli were selected for examination by light microscopy. Ultra-thin sections (0.06 ~ 0.09-µm thick) were cut by an ultramicrotome (Leica EM UC7; Leica Microsystems, Germany), double-stained with uranyl acetate and lead citrate, and examined with a JEOL 1210 transmission electron microscope (JEOL, Japan) at 80 kV. All animal procedures in this study were performed in accordance with the guidelines for animal experiments approved by the Animal Research Committee of Azabu University.
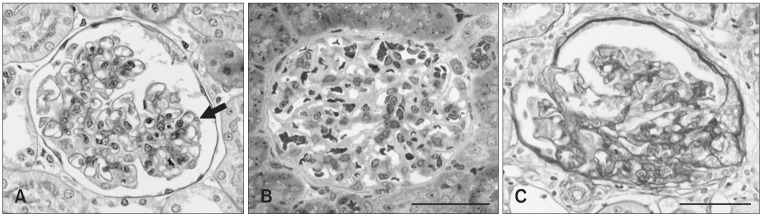
Characteristic glomerular lesions (Fig. 1) similar to ones previously reported [12] were observed in all rats examined with light microscopy as follows. No apparent glomerular abnormalities were detected at 3 weeks of age. Hyaline droplet formation and vacuolation appeared in the podocytes at 6 weeks of age (Figs. 1A and B). Adhesion of the glomerular tufts to Bowman's capsule along with focal and segmental sclerosis were observed in rats 18 and 24 weeks old (Fig. 1C).
Fig. 1.
Representative features of renal glomeruli in male Osborne-Mendel (OM) rats observed with light microscopy. (A) Non-sclerotic glomerulus of a 6-week old male OM rat showing a few hyaline droplets (arrow) in the podocyte. Periodic acid-Schiff (PAS) staining. (B) Non-sclerotic glomerulus of a 6-week old male OM rat with no significant changes. Semi-thin section stained with toluidine blue. (C) Adhesion of the glomerular tufts to the Bowman's capsule with segmental sclerosis in the kidney of a 24-week old rat. PAS staining. Scale bars = 50 µm.
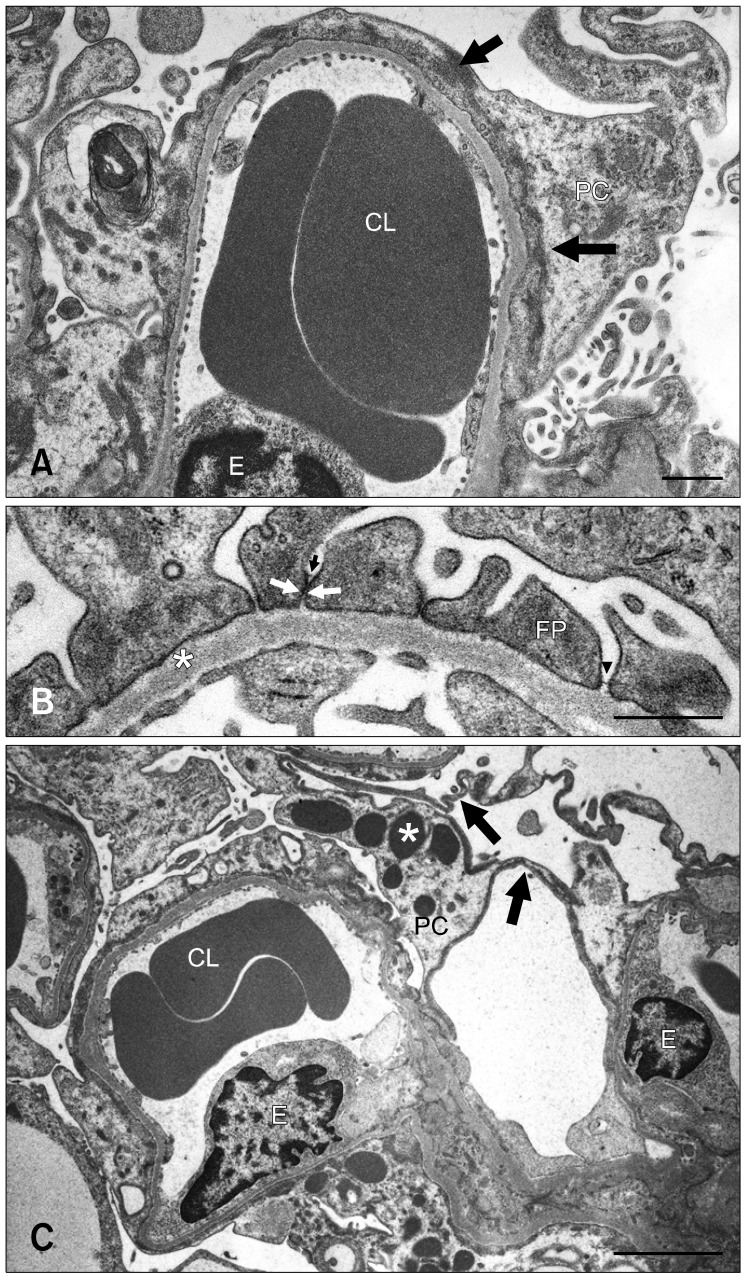
Effacement of podocyte FPs and rearrangement of the actin filaments were observed as early as 3 weeks of age. These conditions worsened and became more widespread from 8 or 9 weeks of age (Fig. 2A). In addition, FPs frequently came into close contact with one another as a result of the loss or dislocation of the slit membrane (Fig. 2B). Cytoplasmic vacuoles or pockets, attenuation of the cytoplasm, and osmiophilic lysosomes were found in the podocytes starting at 6 weeks of age; these features became to be more prominent over time (Fig. 2C). FP detachment or cell bodies of effaced podocytes from the GBM appeared in rats 18 and 24 weeks old (Fig. 3A). An electron-dense matrix formed a bridge between parietal epithelial cells and the denuded GBM (Fig. 3B).
Fig. 2.
(A) Severe podocyte foot process (FP) effacement with rearrangement of the actin cytoskeleton (arrows) over broad areas of the glomerular basement membrane (GBM) in a 9-week old male OM rat. (B) FPs anchoring the GBM (*) in close contact with one another or adhered (arrows) with dislocation of the slit membrane in a 6-week old OM rat. Arrowhead indicates the slit membrane. (C) Osmiophilic lysosomes (*) and flattened podocytes (arrows) in a 24-week old male OM rat. CL: capillary lumen, E: endothelial cell, FP: foot process, PC: podocyte. Scale bars = 1 µm (A and B) and 3 µm (C).
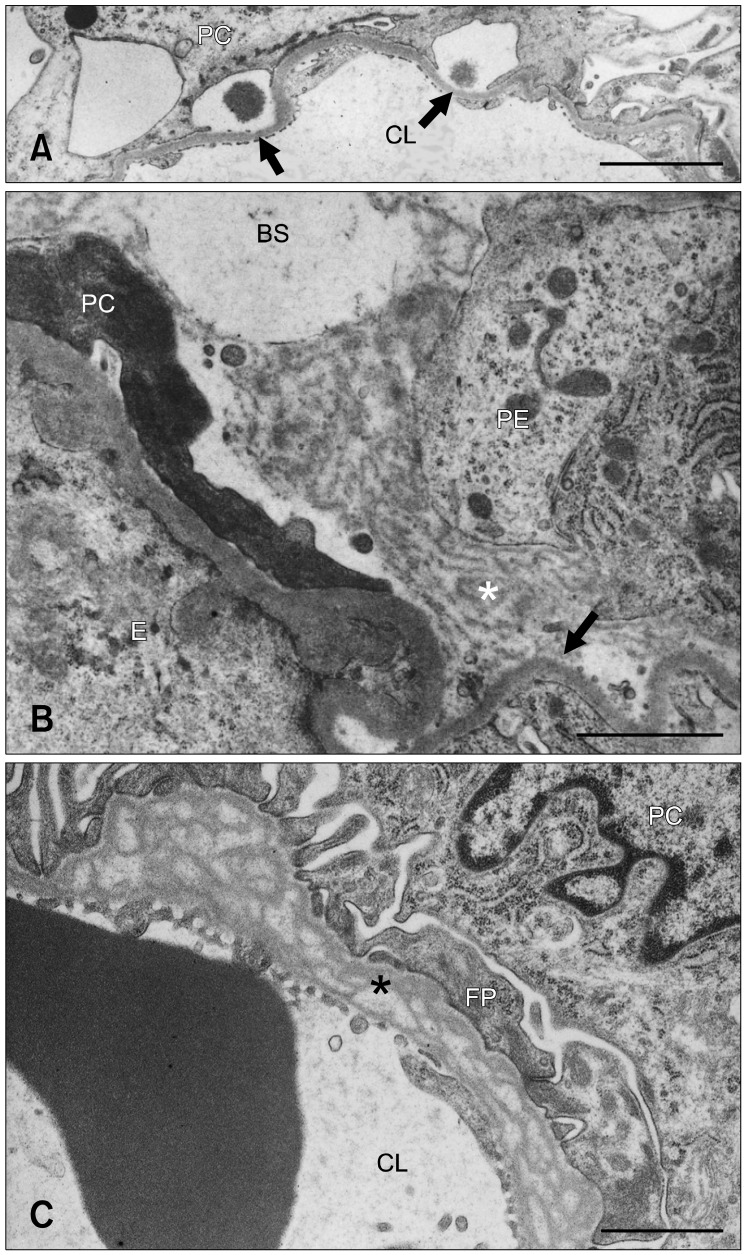
Fig. 3.
(A) Denuded GBM (arrows) resulting from FP detachment in a 24-week old male OM rat. (B) An electron-dense matrix (*) formed a bridge between the denuded GBM (arrow) and parietal epithelial cells rich in polysomes and rough endoplasmic reticula in a 24-week old male OM rat. A degenerated podocyte is shown on the left side. (C) Prominent splitting or lamination of the GBM (*) in a 6-week old male OM rat. BS: Bowman's space, CL: capillary lumen, E: endothelial cell, FP: foot process, PC: podocyte, PE: parietal epithelial cell. Scale bars = 3 µm (A) and 1 µm (B and C).
Distinct lamination or splitting of the GBM was occasionally observed in the glomeruli in any age of rats examined; however, these lesions did not worsen with age (Fig. 3C). Mild thickening of the GBM was also detected in all rats 18 and 24 weeks old as previously reported [12].
In the present study, we identified early ultrastructural lesions in the podocytes and GBM of non-sclerotic glomeruli in OM rats. Aside from FP effacement that is a common feature of proteinuric nephropathies [3,10,11], we observed close contact between neighboring FPs of podocytes occasionally associated with loss and dislocation of the slit membranes. These changes in the filtration slits have been found in cases of puromycin aminonucleoside nephrosis as well as protamine sulfate-treated rats, and are linked with newly formed occluding-type intercellular junctions [7]. The tight junction protein zonula occudens-1 (ZO-1), a major slit membrane protein, is expressed in dislocated slit membranes and along newly formed intercellular junction of podocytes in proteinuric animals [7]. We did not examine the localization of ZO-1 in the present study. However, tight junction-like structures might have developed at sites between FPs in close contact with one another. Although the pathologic significance of close contact between neighboring FPs of podocytes observed in this study was unclear.
The appearance of osmiophilic lysosomes in the podocytes corresponding to hyaline droplet degeneration might represent endocytosis of proteins (such as albumin) leaked from capillary walls. It has been demonstrated that protein overload injures podocytes in vitro and in vivo, mainly via the transforming growth factor-β1/p38 mitogen-activated protein kinase pathway [13]. Morigi et al. [8] also determined that the actin cytoskeleton is reorganized in podocytes in response to protein load and can modulate endothelin-1 gene expression, resulting in the alteration of glomerular permselectivity. In our investigation, effaced FPs and attenuated cytoplasm of the podocytes were found in conjunction with dense lysosome formation along with dense actin cytoskeleton aggregations. Proteins leaked from capillary walls might contribute to the formation of further functional podocyte abnormalities in OM rats. Podocyte vacuolization was frequently found in these animals, which is considered a typical podocyte lesion regardless of the cause of injury [11] and associated with the development glomerulosclerosis [14].
Lamination or splitting of the GBM is a characteristic ultrastructural feature of some hereditary glomerulopathies such as Alport's syndrome [2], Samoyed hereditary glomerulopathy [4], and progressive nephropathy in ICGN mice [9]. In the former two diseases, genetic abnormalities of type IV collagen, a major component of the GBM [3], have been identified as an important pathogenic factor [2,4]. Similar GBM lesions were frequently observed in the OM rats, but were limited to some segments of the capillary tufts and did not worsen with age. In developing glomeruli, the GBM is produced by fusion of endothelial and epithelial (podocyte) basement membranes, and its components are generated by podocytes [1,11]. Localized GBM abnormalities seen in the present study might be due to defective fusion of the two basement membrane layers during glomeruli development or abnormal production of GBM components by the podocytes. These GBM abnormalities could affect local permselectivity of the glomeruli. We also found that naked GBM was connected to parietal epithelial cells by an electron-dense matrix. This might represent early adhesion of the capillary tufts and Bowman's capsule that leads to focal segmental sclerosis [6].
In a previous study, we showed that spontaneous glomerulnephropathy in OM rats is associated with early-onset proteinuria and characterized by podocytopenia prior to the development of glomerulosclerosis [12]. We conclude that ultrastructural abnormalities of the podocytes and GBM observed in the present study closely relate to the development of proteinuria and podocytopenia followed by glomerulosclerosis in OM rats.
Acknowledgments
This research was supported by the Promotion and Mutual Aid Corporation for Private Schools of Japan, Grant-in-Aid for Matching Fund Subsidy for Private Universities. We wish to thank Japan SLC (Japan) for providing the OM rats.
References
- 1.Abrahamson DR. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol. 1985;100:1988–2000. doi: 10.1083/jcb.100.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler MC, Heidet L, Antignac C. Alport's syndrome, thin basement membrane nephropathy, nail-pattela syndrome and type III collagen glomerulopathy. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Haptinstall's Pathology of the Kidney. 6th ed. New York: Lippincott Williams & Wilkins; 2007. pp. 487–502. [Google Scholar]
- 3.Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 4.Jansen B, Thorner P, Baumal R, Valli V, Maxie MG, Singh A. Samoyed hereditary glomerulopathy (SHG). Evolution of splitting of glomerular capillary basement membranes. Am J Pathol. 1986;125:536–545. [PMC free article] [PubMed] [Google Scholar]
- 5.Kriz W. Progressive renal failure-inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant. 1996;11:1738–1742. [PubMed] [Google Scholar]
- 6.Kriz W. The pathogenesis of 'classic' focal segmental glomerulosclerosis-lessons from rat models. Nephrol Dial Transplant. 2003;18(Suppl 6):vi39–vi44. doi: 10.1093/ndt/gfg1064. [DOI] [PubMed] [Google Scholar]
- 7.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol. 1992;141:805–816. [PMC free article] [PubMed] [Google Scholar]
- 8.Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G. Benigni A. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogura A, Fujimura H, Asano T, Koura M, Naito I, Kobayashi Y. Early ultrastructural glomerular alterations in neonatal nephrotic mice (ICGN strain) Vet Pathol. 1995;32:321–323. doi: 10.1177/030098589503200317. [DOI] [PubMed] [Google Scholar]
- 10.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 11.Shankland SJ. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 12.Yasuno K, Ishihara S, Saito R, Ishikawa M, Kato T, Kobayashi R, Baba T, Kawano K, Ogihara K, Kamiie J, Shirota K. Early-onset podocyte injury and glomerular sclerosis in Osborne-Mendel rats. J Vet Med Sci. 2010;72:1319–1327. doi: 10.1292/jvms.10-0094. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida S, Nagase M, Shibata S, Fujita T. Podocyte injury induced by albumin overload in vivo and in vitro: involvement of TGF-beta and p38 MAPK. Nephron Exp Nephrol. 2008;108:e57–e68. doi: 10.1159/000124236. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa N, Ito H, Akamatsu R, Hazikano H, Okada S, Matsuo T. Glomerular podocytes vacuolation in focal segmental glomerulosclerosis. Arch Pathol Lab Med. 1986;110:394–398. [PubMed] [Google Scholar]