Abstract
Objective
We aimed to investigate the effects of silanation time on the shear bond strength (SBS) of metal brackets on gold alloy in a silicoating procedure and compare the SBS of metal brackets on gold alloy and enamel.
Methods
Type III gold alloy plates were sandblasted with 30-µm silicon dioxide. Excess particles were removed with gentle air after silica coating, and silane was applied. Maxillary central-incisor metal brackets were bonded to each conditioned alloy surface with a light curing resin adhesive for 1 s, 30 s, 60 s, or 120 s after applying silane. The brackets were also bonded to 36 upper central incisors with the same adhesive. All samples were cured for 40 s with a light emitting diode curing light. The SBS was tested after 1 h and after 24 h. The adhesive remnant index (ARI) of the samples was also compared.
Results
The 60-s and 120-s silanation time groups showed a higher SBS than the other groups (p < 0.05). Samples tested after 24 h showed a significantly higher SBS than did the samples tested after 1 h (p < 0.05). The 1-s group showed higher ARI scores. The one-way analysis of variance and Student-Newman-Keuls test showed that the SBS values of the 60-s and 120-s silanation time groups were not significantly different from the SBS values of enamel.
Conclusions
Adequate silanation time is required to produce sufficient bond strength during silicoating.
Keywords: Bonding, Biomaterial science
INTRODUCTION
Clinicians are often faced with the problem of bonding orthodontic brackets to teeth that already have undergone different types of restorations, especially in adult patients. The prevalence of malocclusion in adults is equal to or greater than the prevalence in children and adolescents;1 therefore, the percentage of adult orthodontic patients is high.2 Stress with a bonding procedure such as posterior tooth banding (which is more prone to gingival inflammation and attachment loss3) has become a matter of concern to orthodontists. Metallic surfaces can be encountered on the labial surfaces of the molars and premolars and the lingual surfaces of the anterior or posterior teeth.
Conventional acid etching is ineffective for preparing metal surfaces for mechanically retaining orthodontic attachments. Surface roughening before bracket bonding is therefore a prerequisite for sufficient bracket-to-alloy bonding.4,5 Sandblasting, the most common method for surface preparation, creates scratch-like irregularities that increase the surface area, thereby enhancing mechanical bond strength.6
A recently introduced silica coating method facilitates mechanical retention by sandblasting and chemicophysical bonding between a composite resin and an alloy surface with a silane.6 By using an intraoral sandblaster, alloy surfaces are treated with aluminum oxide particles that are modified with silicic acid. The particles form a reactive silica layer on the substrate. Silane must thereafter be applied to allow chemical bonding with a resin-based system such as composite adhesives.7
Silica coating has been used in many dental applications such as repairing intraoral fractured ceramic surfaces,8,9 treating ceramic bracket bases for rebonding, 10 and repairing resin-bonded prostheses.11 Several studies were recently published in regard to bracket bonding on metal surfaces.12-14
In silicoating procedures, the ideal timing of silane application before bracket bonding remains equivocal. Manufacturer recommendations for silanation are typically 5 min for extraoral use (3M ESPE Sil; 3M ESPE Dental Products. St. Paul, MN, USA).15 However, 5 min is excessively long in clinical situations. For that reason, manufacturers also recommend 30 s (3M ESPE Sil)15 or 1 min (Pore-Etch and porcelain conditioner; Reliance Orthodontic Products Inc., Itasca, IL, USA) in intraoral use.16 However, there has been no data concerning bond strength differences in regard to these time differences. The objectives of this in vitro study were to measure the shear bond strength (SBS) of metal brackets on gold alloys (treated by using a silica coating) at different time intervals after applying silane and compare these SBS values with the SBS values on an enamel surface.
MATERIALS AND METHODS
Thirty-two 1.5-mm thick and 10-mm long gold alloy square plates (type III gold consisting of 50% gold, 5% palladium, 32.5% silver, and 11.45% copper; Argen Co., San Diego, CA, USA) and 36 human central incisors, which were extracted for periodontal purposes and stored in thymol solution (0.1% wt/vol), were used in this study. The criterion for tooth selection was that a tooth had to be free of restoration, cracks, caries, attrition, or white spot lesion. The upper central incisors were randomly divided into 2 groups: 1 h group and 24 h group. Institutional Review Board of the Seoul National University Dental Hospital authorized the authors to proceed with this experimental study.
All plates and teeth were embedded in a cold-curing acrylic resin (Leocryl; Leone, Sesto Fiorentino, Italy) with acrylic rings (Taejin Acrylic, Seoul, Korea). Each plate was oriented so that its surface was parallel to the force applied during the shear bond tests.
The silica coating process was performed on all alloy plates after embedding. A sandblasting device (Air-Flow Handy II; EMS Corp., Dallas, TX, USA) was filled with 30-µm aluminum oxide grain that was modified with silicic acid (Cojet-Sand; 3M ESPE, Seefeld, Germany). The grain was used for surface roughening. In accordance with the manufacturer's instructions, the abrasive was applied perpendicularly to the metal surface at a distance of 10 mm under 2.5 bar pressure for 15 seconds. Excess particles were removed with gentle air after the silica coating procedure. A silane coupling agent (ESPE-Sil; 3M ESPE) was then applied to the plate surface and air-dried with oil-free air.
A power analysis (alpha level, p < 0.05; beta level, < 0.20; 2-tailed) was performed in this study by using the mean and standard deviation, on the basis of the results of a previously conducted pilot study. As a result of this analysis, 18 samples were used for each group.
Because 2 factors (i.e., silanation time and test timing) had to be evaluated, we assigned alloy plates to 8 groups. In the 1-s silanation time group, brackets were bonded immediately after the silane application. We tried to immediately bond the brackets, but bracket positioning and removal of excessive adhesive takes time; therefore, we named the group the "1-s silanation time group," for the sake of convenience. In the 30-s, 60-s, and 120-s silanation time groups, the specimens were air-dried for 30 s, 60 s, and 120 s, respectively, after the silane application to allow chemical adhesion between the silane and the silica-coated surface and to acquire a dry field before bracket bonding. To evaluate the effect of test timing, specimens were stored in water in a thermostatic chamber at 37℃ for 1 h or for 24 h before SBS testing.
Immediately after surface conditioning and air drying, maxillary central-incisor metal brackets (item number 017-875; 3M Unitek, Monrovia, CA, USA) were bonded to each conditioned alloy surface with a light-cured composite adhesive (Transbond XT; 3M Unitek). To maximize the bond strength, a thin uniform coat of adhesive primer was applied to the bracket base and was light cured for 10 s with light emitting diode (LED) curing lights (Ortholux LED; 3M Unitek), as demonstrated in previous research.12 Resin adhesives were then applied to the bracket bases. According to the manufacturer, the average surface area of each bracket base is 10.56 mm2.
Each bracket was positioned on the prepared alloy surface with sufficient pressure to expel excess adhesive, which was then carefully removed. Previous study suggests that 40 s of curing time is required to obtain proper bond strength of metal brackets on metal plates when using LED curing lights.14 Therefore, the brackets were light cured for 40 s. As the manufacturer recommends, the light source was held 1 - 2 mm above the bracket, and the mesial and distal edges were cured for an equal amount of time on each side. A minimum light intensity of at least 2,000 mW/cm2 was verified by using a handheld curing radiometer (Demetron 100; Demetron Research, Danbury, CT, USA).
After photopolymerization, the plates were stored in water in a thermostatic chamber at 37℃ for 1 h or for 24 h. A universal testing device was used to determine the bracket SBS (LF Plus; Ametek, Albany, NY, USA). For this test, acrylic rings were mounted in a jig with the brackets positioned vertically. Shear force at a cross-head speed of 1 mm/min was transmitted to the bracket by means of a square plate of the same size. The force required to shear the bracket was recorded and converted into units of stress (MPa), based on a known bracket area.
Bond strength testing was performed on 4 alloy plates in each group. The tested alloy surfaces were polished with a Shofu Gold Polishing Kit (HP 0303; Shofu Inc., Tokyo, Japan) and were cleaned for 10 min in an ultrasonic bath (Bransonic; Ultrasonic Cleaner, Shelton, CT, USA) containing ethylacetate. The plates were then air dried with oil-free air before reusing them for the next experiment. These procedures followed the methods used in previous studies.13,14 The polished plates were randomly reassigned to a group for the next experiment. This procedure was repeated for the alloy plates until the whole test was completed.
To compare the SBS of the brackets on a gold surface and on an enamel surface, the brackets were bonded to the tooth surface of each group by using the same adhesive after 30 s of etching with 37% phosphoric acid; the brackets were light cured for 40 s. After 1-h and 24-h storage in a thermostatic chamber, SBS testing was performed.
After bond strength testing, the gold alloy plates were examined under 10× magnification to detect adhesive remnants on the alloy surfaces, on the basis of ARI system.17 The ARI scale ranges from 1 to 5 to define the sites of bond failure. The ARI assessment was performed with a Zeiss OPMI 111 microscope (Mednet Locator Inc., Memphis, TN, USA).
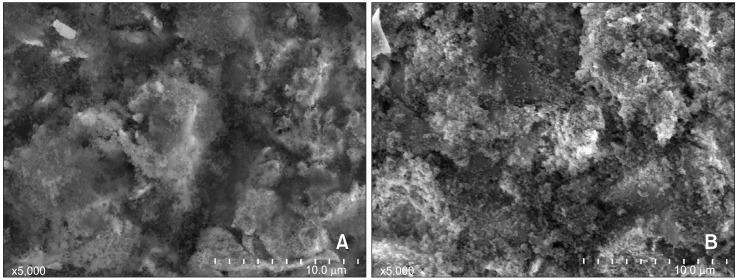
After applying a CoJet silica coating (3M ESPE) and silane, the specimens were sputter-coated with carbon evaporation (SCD-005; Leica Microsystems, Wetzlar, Germany) and examined at 5,000× magnification with a scanning electron microscope (SEM) (JSM-6380; JEOL, Akishima, Japan).
Statistical analysis
The programming language R was used for all statistical analyses. Descriptive statistics (i.e., mean, standard deviation) and inferential statistical analyses were performed.
After checking the normality assumption and the equality of variance, two-way analysis of variance (ANOVA) was implemented to deduce the significant influential factors on the SBS of the alloy plates with regard to two variables: silanation time and testing timing. Differences between the groups were assessed by using the Student-Newman-Keuls (S-N-K) multiple comparisons test with a level of significance at p less than 0.05. One-way ANOVA and S-N-K test were used to compare the SBS of enamel and gold surfaces after 24 h.
The Kruskal-Wallis test for the silanation time and the Wilcoxon rank sum test for the test time were used to determine whether differences in ARIs existed between the groups. For the silanation time, a pairwise comparison test using the Wilcoxon rank sum test with Bonferoni correction was performed to determine whether there were differences between 1 s and 30 s, 30 s and 60 s, and 60 s and 120 s.
RESULTS
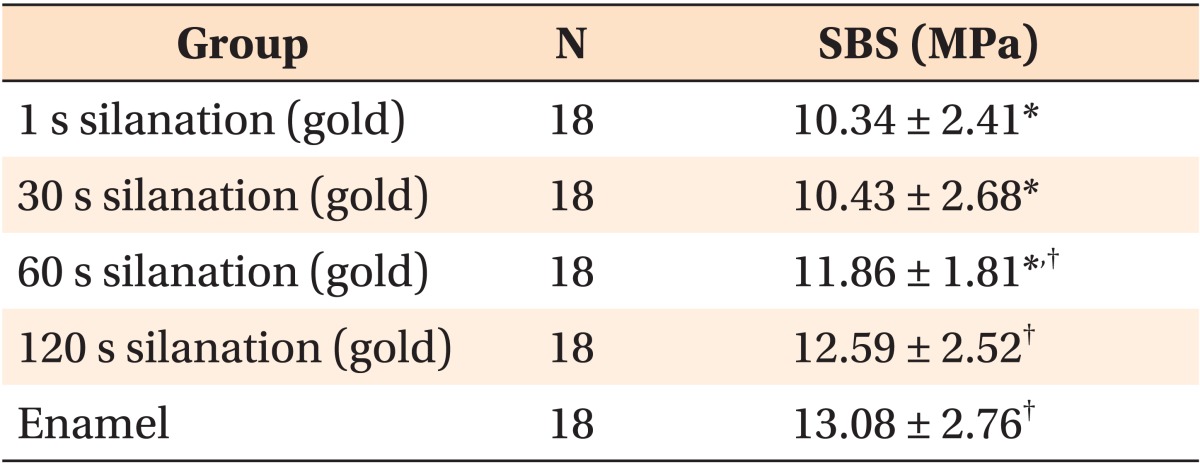
Table 1 shows the means, standard deviations, and range of SBS values, based on silanation time and test timing. The results of the two-way ANOVA test revealed significant differences between the groups (p < 0.05). A significantly greater SBS was observed in samples subjected to 60 s and 120 s silanation time that were examined after 24 h, compared to the SBS of the other groups (p < 0.05). Figure 1 shows the distribution of the SBS values in relation to silanation time and test timing factor.
Table 1.
Shear bond strength of the specimens after 1 hour and after 24 hours
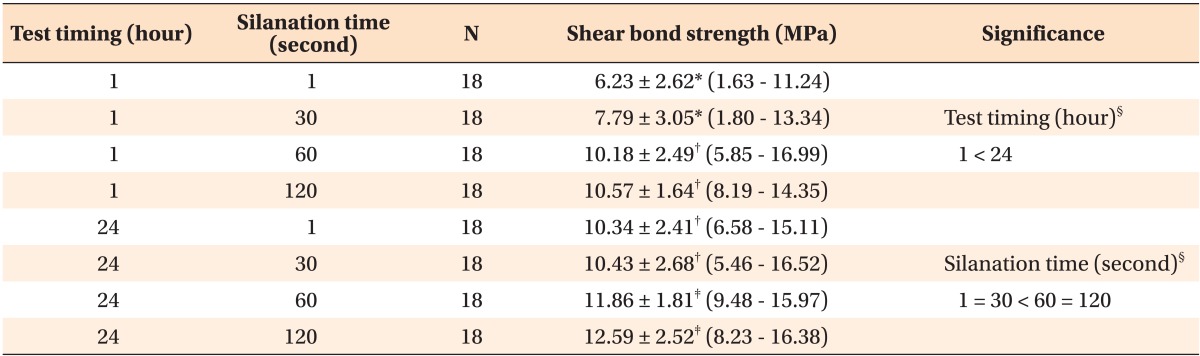
Values are presented as number only or mean ± standard deviation (range).
*-‡Items with the same superscripts indicate a homogenous subset, after performing the Student-Newman-Keuls multiple comparisons test (p < 0.05).
§p < 0.05 indicates a significant result after two-way analysis of variance.
Figure 1.
A, Scanning electron micrographs of a specimen after sandblasting with silica (5,000× magnification) and B, after silane application.
The results of the Kruskal-Wallis test revealed significant differences in the ARI scores between the different silanation time groups (χ2 = 44.0465, degree of freedom = 3, p < 0.001). The Wilcoxon rank sum test showed no significant difference between the 1-h and the 24-h silanation time groups (Table 2). The pairwise comparison test revealed a difference in the ARI score between 1 s and 30 s, but there was no significant difference between 30 s and 60 s or between 60 s and 120 s.
Table 2.
Frequency distribution of the ARI scores
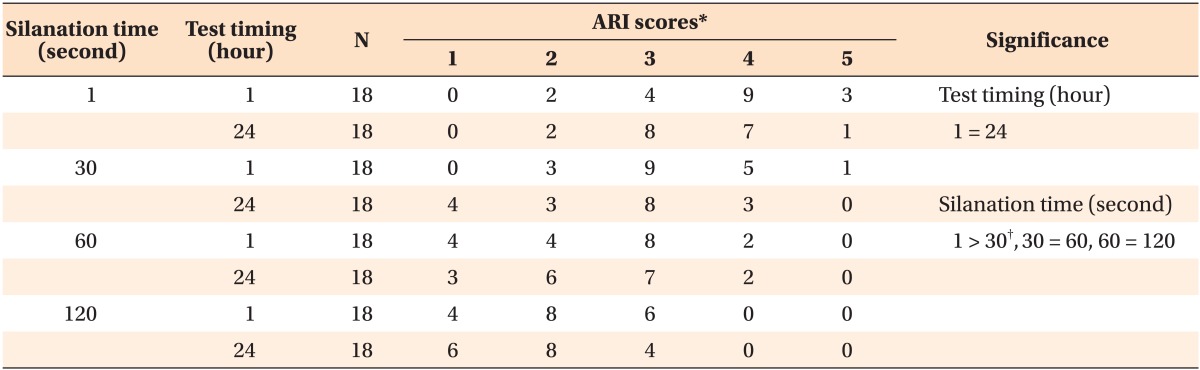
*Adhesive remnant index (ARI) scores: 5, no composite remains on the specimen; 4, less than 10% of the composite remains on the specimen surface; 3, more than 10% but less than 90% of the composite remains on the specimen; 2, more than 90% of the composite remains on the specimen; 1, all of the composite, with an impression of the bracket base, remains on the specimen.
†Indicates a significant result, after performing the pairwise comparison test with Bonferoni correction (p < 0.0167).
The one-way ANOVA and the S-N-K test showed that the SBS values of the 60 s and 120 s silanation time groups were not significantly different from SBS values of enamel after 1 h or 24 h (Tables 3 and 4, respectively).
Table 3.
Comparison of SBS of the specimens after 1 hour by using one-way ANOVA
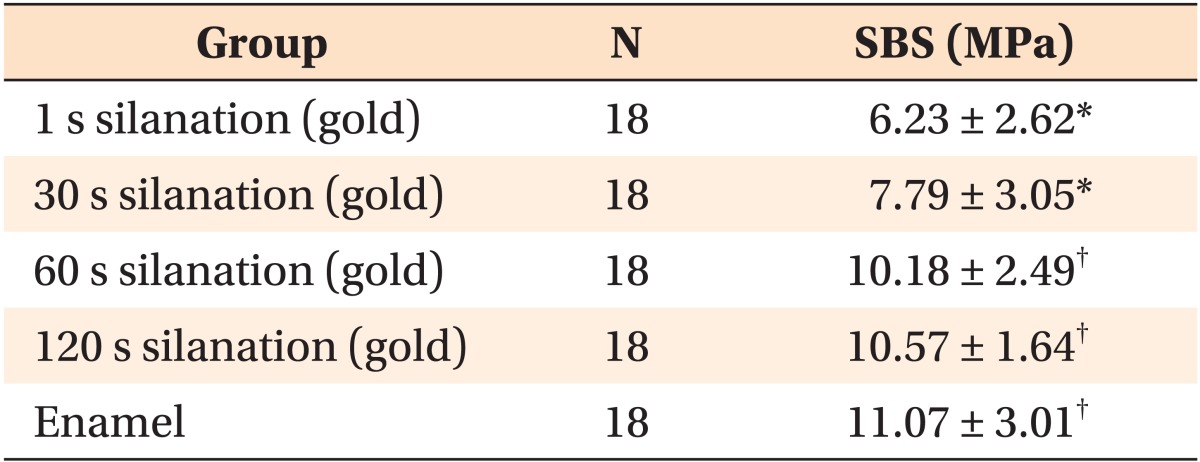
Values are presented as number only or mean ± standard deviation.
Items with the same superscript indicate a homogenous subset, after performing the Student-Newman-Keuls multiple comparisons test (p < 0.05).
SBS, Shear bond strength; ANOVA, analysis of variance.
Table 4.
Comparison of SBS of the specimens after 24 hours by using one-way ANOVA
Values are presented as number only or mean ± standard deviation.
Items with the same superscript indicate a homogenous subset, after performing the Student-Newman-Keuls multiple comparisons test (p < 0.05).
SBS, Shear bond strength; ANOVA, analysis of variance.
SEM images were used to examine the characteristics of the gold alloy surfaces, after undergoing silicoating procedures. After silica coating, bead-like structures were present on the irregular gold surfaces (Figure 1A). After applying silane, the bead-like structures apparently became more complex (Figure 1B).
DISCUSSION
Sandblasting can create surface irregularities that increase surface area, thereby enhancing mechanical bond strength. Previous studies have shown that a clinically acceptable bond strength can be achieved in bracket bonding procedures when sandblasting is combined with chemical bonding agents such as 4-META resins or primers.5,18
However, many clinicians still use bands in gold crowns to safeguard against bond failure. Usually gold surfaces are located in the posterior teeth, which receive a great amount of masticatory force, which can range above 30 kg.19 Unlike enamel surfaces, strong bonds on alloy surfaces do not cause fractures or cracks on the surface of the substrate during debonding. Therefore, 6 - 8 MPa is usually the recommended minimum bond strength,20 although higher bond strengths are better for bracket bonding on alloy surfaces. A previous study has shown that silica coating can produce a very high bond strength (19 MPa) on a metal surface.13 Other researchers have observed a higher bond strength in silicoated samples, compared to sandblasted and 4-META-primed samples in bracket bonding on a gold alloy surface.14 The silica coating procedure may reduce the importance of the alloy composition and oxide formation on the bonding mechanism.9
In silicoating procedures, chemicophysical adhesion occurs between the resin monomer, silane, and silica particles. The silane agent is a bifunctional molecule - one end is connected to the hydroxyl groups in the silica molecule and the other end creates double bonds with the monomer in the adhesive. As for many other chemical reactions, this adhesion reaction requires time. Time for evaporation is also required for better bond since most of the silane uses alcohol as a solvent (Reliance porcelain conditioner, 3M ESPE Sil).15,16 The silanation reaction also produces alcohol as a by-product. Therefore, an appropriate time interval and air drying would be helpful in maximizing the success of the silicoating procedures.
Manufacturer recommendations encourage 5 min of silanation before applying the adhesive extraorally; therefore, most in vitro studies have incorporated silanation times of 5 min.11,13 However, 5 min of silanation is excessively long for clinical settings; therefore, manufacturer recommendations for intraoral use is 30 s (3M ESPE Sil; 3M ESPE Dental Products) or 1 min (Porcelain conditioner; Reliance Orthodontic Products Inc.). It is difficult to compare directly the bond strength values of different studies. However, a previous study21 assessed the bond strength of porcelain surfaces exposed to 1 min of silanation. It demonstrated lower bond strength than the bond strength observed in another study22 that used 2 min of silanation. On metal surfaces, a previous study that used 5 min of silanation time13 demonstrated higher bond strengths than studies that used 1 min12 or 2 min14 silanation time.
We tried to explore the most effective and efficient methods for bracket bonding on a gold alloy surface by using silicoating. Our results indicate that a silanation time of longer than 60 s is recommended, even though the manufacturer's recommendation is 30 s. The samples subjected to short silanation times showed increased bond strength after 24 h, but the initial bond strength was significantly lower than the bond strength of samples subjected to a longer silanation time.
Restorations usually remain in the mouth after the brackets are debonded. Damage to the alloy resulting from extreme roughening of the surfaces during the pretreatment should be avoided. Silicoating with 30-µm grains produces smoother alloy surfaces than does sandblasting with large grain aluminum oxide or high speed diamond bur roughening. Therefore, it will be easier to polish the debonded surface.
The ARI scores of samples subjected to a silanation time of longer than 30 s were lower than samples subjected to a silanation time of 1 s. The lower ARI score means that the adhesive resin had a stronger adhesion to the alloy surface than to the bracket base. Such ARI scores are more favorable since the weakest bond between the bracket base and alloy surface is usually at the adhesive-alloy interface and there is no risk of cracking or fracture during debonding procedures.
A direct transfer of in vitro values to clinical situations is not usually accepted since differences would exist between the in vitro and the in vivo bond strengths. The bond strength can be influenced by temperature, stress, humidity, and acidity and plaque - factors that cannot be reproduced in the laboratory.5 However, in vitro studies are required for testing recently developed materials or methods to provide reference data and guidelines.
Despite the many limitations caused by being unable to make direct comparisons with other studies, silicoating with more than 60 s of silanation time showed similar SBS of a bracket to an enamel surface in this study. This was a very encouraging result.
Bond strength is influenced by the type of resin composite used. Light curing and a highly filled adhesive, which are commonly used, were chosen for this investigation. However, further investigations should be performed for other types of adhesive agents.
Thermocycling is a widely accepted method for bond strength testing, although there is no international standard of thermocycling for 1 h testing. Thermocycling was not applied in this experiment because we were trying to determine time-related differences in SBS.
The results of this study indicated that bond strength is greater at 24 h after bonding than it is at 1 h after bonding. Such differences can easily be explained by the higher conversion rate of the monomer to a polymer over time, and are in accordance with the results of previous reports.17,22
CONCLUSION
The silanation procedure requires time for the chemical reaction and for solvent evaporation to occur. Therefore, more than 60 s of silanation time are required to initially achieve a sufficient bond strength when bonding brackets to gold alloy surfaces by using silicoating. The SBS of a bracket to a gold alloy when using more than 60 s of silanation time was not significantly different from the shear bond strength of conventional enamel bonding.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.McLain JB, Proffitt WR. Oral health status in the United States: prevalence of malocclusion. J Dent Educ. 1985;49:386–397. [PubMed] [Google Scholar]
- 2.Jung MH. Age, extraction rate and jaw surgery rate in Korean orthodontic clinics and small dental hospitals. Korean J Orthod. 2012;42:80–86. doi: 10.4041/kjod.2012.42.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd RL, Baumrind S. Periodontal considerations in the use of bonds or bands on molars in adolescents and adults. Angle Orthod. 1992;62:117–126. doi: 10.1043/0003-3219(1992)062<0117:PCITUO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Jost-Brinkmann PG, Böhme A. Shear bond strengths attained in vitro with light-cured glass ionomers vs composite adhesives in bonding ceramic brackets to metal or porcelain. J Adhes Dent. 1999;1:243–253. [PubMed] [Google Scholar]
- 5.Zachrisson BU. Orthodontic bonding to artificial tooth surfaces: clinical versus laboratory findings. Am J Orthod Dentofacial Orthop. 2000;117:592–594. doi: 10.1016/s0889-5406(00)70211-3. [DOI] [PubMed] [Google Scholar]
- 6.Peutzfeldt A, Asmussen E. Silicoating: evaluation of a new method of bonding composite resin to metal. Scand J Dent Res. 1988;96:171–176. doi: 10.1111/j.1600-0722.1988.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 7.Jung MH. Direct bonding with composite resin; review. J Korean Found Gnatho-Orthod Res. 2005;7:61–117. [Google Scholar]
- 8.Ozcan M, Niedermeier W. Clinical study on the reasons for and location of failures of metal-ceramic restorations and survival of repairs. Int J Prosthodont. 2002;15:299–302. [PubMed] [Google Scholar]
- 9.Schneider W, Powers JM, Pierpont HP. Bond strength of composites to etched and silica-coated porcelain fusing alloys. Dent Mater. 1992;8:211–215. doi: 10.1016/0109-5641(92)90086-r. [DOI] [PubMed] [Google Scholar]
- 10.Toroglu MS, Yaylali S. Effects of sandblasting and silica coating on the bond strength of rebonded mechanically retentive ceramic brackets. Am J Orthod Dentofacial Orthop. 2008;134:181e1–181e7. doi: 10.1016/j.ajodo.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe I, Kurtz KS, Kabcenell JL, Okabe T. Effect of sandblasting and silicoating on bond strength of polymer-glass composite to cast titanium. J Prosthet Dent. 1999;82:462–467. doi: 10.1016/s0022-3913(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 12.Shon WJ, Kim TW, Chung SH, Jung MH. The effects of primer precuring on the shear bond strength between gold alloy surfaces and metal brackets. Eur J Orthod. 2012;34:72–76. doi: 10.1093/ejo/cjq163. [DOI] [PubMed] [Google Scholar]
- 13.Nergiz I, Schmage P, Herrmann W, Ozcan M. Effect of alloy type and surface conditioning on roughness and bond strength of metal brackets. Am J Orthod Dentofacial Orthop. 2004;125:42–50. doi: 10.1016/s0889-5406(03)00507-9. [DOI] [PubMed] [Google Scholar]
- 14.Jung MH, Chung SH, Shon WJ. Shear bond strength between gold alloy and orthodontic metal bracket using light emitting diode curing light. Korean J Orthod. 2010;40:27–33. [Google Scholar]
- 15.Instructions for use [Internet] St. Paul, MN: 3M ESPE Dental Products; [October 22, 2012]. Available from: http://multimedia.3m.com/mws/mediawebserver?mwsId=66666UF6EVsSyXTt4Xf6Mxf2EVtQEVs6EVs6EVs6E666666--&fn=espe_sil_ifu_we_R1.pdf. [Google Scholar]
- 16.Instructions for use [Internet] Itasca, IL: Reliance Orthodontic Products Inc; [October 22, 2012]. Available from: http://www.relianceorthodontics.com/store/files/instructions/PorcEtch_PorcelainConditioner_032609.pdf. [Google Scholar]
- 17.Bishara SE, VonWald L, Olsen ME, Laffoon JF. Effect of time on the shear bond strength of glass ionomer and composite orthodontic adhesives. Am J Orthod Dentofacial Orthop. 1999;116:616–620. doi: 10.1016/s0889-5406(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 18.Büyükyilmaz T, Zachrisson YO, Zachrisson BU. Improving orthodontic bonding to gold alloy. Am J Orthod Dentofacial Orthop. 1995;108:510–518. doi: 10.1016/s0889-5406(95)70051-x. [DOI] [PubMed] [Google Scholar]
- 19.Proffit WR, Fields HW, Nixon WL. Occlusal forces in normal- and long-face adults. J Dent Res. 1983;62:566–570. doi: 10.1177/00220345830620051201. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds IR. A review of direct orthodontic bonding. British J Orthod. 1975;2:171–178. [Google Scholar]
- 21.Trakyali G, Malkondu O, Kazazoğlu E, Arun T. Effects of different silanes and acid concentrations on bond strength of brackets to porcelain surfaces. Eur J Orthod. 2009;31:402–406. doi: 10.1093/ejo/cjn118. [DOI] [PubMed] [Google Scholar]
- 22.Nebbe B, Stein E. Orthodontic brackets bonded to glazed and deglazed porcelain surfaces. Am J Orthod Dentofacial Orthop. 1996;109:431–436. doi: 10.1016/s0889-5406(96)70125-7. [DOI] [PubMed] [Google Scholar]