Abstract
Plants and herbivores have co-evolved in their natural habitats for about 350 million years, but since the domestication of crops, plant resistance against insects has taken a different turn. With the onset of monoculture-driven modern agriculture, selective pressure on insects to overcome resistances has dramatically increased. Therefore plant breeders have resorted to high-tech tools to continuously create new insect-resistant crops. Efforts in the past 30 years have resulted in elucidation of mechanisms of many effective plant defenses against insect herbivores. Here, we critically appraise these efforts and – with a focus on sap-sucking insects – discuss how these findings have contributed to herbivore-resistant crops. Moreover, in this review we try to assess where future challenges and opportunities lay ahead. Of particular importance will be a mandatory reduction in systemic pesticide usage and thus a greater reliance on alternative methods, such as improved plant genetics for plant resistance to insect herbivores.
Keywords: phloem-feeding insects, crop pests, breeding, genetically modified crops, natural insecticides
EVOLUTIONARY PERSPECTIVE OF PLANT–INSECT INTERACTIONS
Around 350 m years ago, the first insects evolved to feed on plant material (Labandeira, 2007), after which plants evolved mechanisms to deter herbivores. These mechanisms include antibiosis, compounds toxic to insects, antixenosis, the deterrence of insect, physiological defensive properties, such as thorns and trichomes and tolerance (Smith and Clement, 2012). Insects on their part have evolved to detoxify or efficiently sequester these toxic metabolites. As early as 1888, Ernst Stahl elegantly demonstrated that extractable plant-based chemicals are responsible for defining host-specificity in plant–herbivore interactions (Stahl, 1888). It took until 1964 before the role of secondary metabolites in plants were again associated with insect host suitability, with Ehrlich and Raven (1964) in their landmark “plants and butterflies” paper. Here, the theory of co-evolution between plants and their herbivorous pests was laid out, and their paper was an important basis for subsequent plant–insect research.
CROP DOMESTICATION AND MODERN AGRICULTURE
Over 4000 years, humans have been domesticating a large variety of crops; primarily selecting for “easy” traits, such as fruit size and yield. Evidently, in for example strawberries, the wild ancestors have much smaller berries and a completely different taste than the currently cultivated big, juicy, and often very sweet strawberry varieties (Aharoni et al., 2004). During this selection process and before global spread and subsequent outbreaks of pests and diseases, little or no attention was given to resistance beyond those required for locally occurring biotic and abiotic conditions. Therefore, many naturally occurring resistances have probably been lost (de-selected) during the cultivation of our current staple crops.
During the last century’s green revolution, crops were developed that are adapted to large-scale, high-input agriculture. This has driven an industrial-scale global agriculture and has, logically, resulted in industrial-sized seed production, for which a few suppliers in the EU and the USA provide seeds to a multitude of countries worldwide. The focus on high-input monocultures has advantages for industrial-sized agriculture, e.g., crops are easier to harvest, highly uniform, and produce predictably stable yields. However, such crop production also provides concerns and has drawbacks. Besides its high cost in energy input per unit arable land, one can also foresee that the use of these crop practices exert a tremendous selection pressure on pests and diseases, implying that resistances can easily be broken. In order to fight destructive herbivorous insects, humankind has heavily relied on the use of insecticides. However, in the last 15 years a large number of them, mostly systemic pesticides, have been banned because of their harmfulness toward consumers (e.g., parathion, dinitro-o-cresol), non-target organisms, or the environment [e.g., dichlorodiphenyltrichloroethane (DDT)]. More recently, neonicotinoids have come under fire because of harmful effects to non-target species such as bees and bumblebees (Henry et al., 2012; Whitehorn et al., 2012). Neonicotinoids are very effective pesticides as they are able to spread systemically throughout the plant, ensuring easy application and extending their usage in the formulations for seed coating. Overall, the EU and other countries worldwide have banned the use of many systemic pesticides1 because; (i) concerns about insecticide retention in food crops; (ii) effects on off-target organisms; (iii) broader negative impact on ecosystems, and (iv) higher risk of insecticide resistance in key insect pests.
INSECT RESISTANCE IN MODERN-DAY BREEDING
With the current reduction in the range of pesticides that are available to farmers, efforts to find alternative methodologies for insect resistance have been on the rise. As a result, breeding for insect-resistant crops has received increased attention and many seed companies advertise their insect-resistant varieties. These insect resistance traits have come from a variety of sources, including plants and micro-organisms. For instance, broad resistance to Lepidoptera and Coleoptera is attained by the use of genetically modified (GM) plants, expressing a “Cry” toxin from Bacillus thuringiensis (Vaeck et al., 1987) in a number of important row crops, including corn, soybean, and cotton (Bohorova et al., 1996; Lambert et al., 1996; Stewart et al., 1996). Different Cry variants have been used in crops that exhibit differing spectra of efficacy against various groups of herbivorous insects, and are used widely in agriculture throughout the USA and other parts of the world2.Resistance or insensitivity to Bt in target insects has been observed in the laboratory (Meihls et al., 2012; Zhang et al., 2012) and the field (Gassmann et al., 2011). However, issues of insect resistance to Bt will be at least partly overcome in the latest generation Bt-crops, in which several Cry toxins, that do not show cross-resistance, are stacked or combined with other methodologies such as RNA interference (RNAi; Bhatia et al., 2012; Kumar et al., 2012; Tang et al., 2012) or the production of secondary metabolites. Evidently, the usage of Bt-crops has re-shaped the need for insecticide use, but has also allowed other, previously less economically important, insect pests to flourish. In particular, Bt-insensitive insects, such as aphids, whiteflies, and scale insects populations might increase in abundance. Hence, if GM strategies are to be used, these insect pests require other GM resistance strategies. GM approaches using plant-derived lectins, agglutinin, and protease inhibitors have been shown to provide high levels of resistance to aphids and other phloem-feeding insect species (Fitches et al., 2008; Alvarez-Alfageme et al., 2011; Carrillo et al., 2011). In addition, in planta expression of RNAi-vectors that target physiologically important insect transcripts for degradation, have been shown to result in crop protection against a number of insect pests, including phloem-feeding aphids (Price and Gatehouse, 2008; Upadhyay et al., 2011; Zha et al., 2011). Although potentially effective, none of these GM methodologies have been commercially marketed. A variety of reasons can underlie their lack of success on the market, these include (i) high risk of limited durability, particularly if less than 99% mortality is achieved; (ii) potential negative effects on non-target insects, ecosystems, or consumers; (iii) narrow target-specificity, i.e., high cost of deregulation of a GM does not pay off compared to the reduction in yield loss resulting from an economically minor pest or a niche market crop.
Hence, there is a strong incentive to develop alternative strategies against these pests. In that respect, combined approaches seem particularly attractive. For instance, the use of (non-GM) genetic crop resistance, combined with biological control using predatory insects or practical solutions that limit the build-up of high population densities of herbivorous pests will likely result in effective pest control.
BENEFITTING FROM NATURAL VARIATION
An alternative to transgenic approaches is the use of wild relatives of crop plants, searching for desirable traits and then crossing those into the elite cultivars. This traditional way of plant breeding has been made substantially easier with the availability of novel sequence-based molecular approaches. For instance, genome-wide coverage of single nucleotide polymorphisms (SNPs; or other molecular markers) between wild and cultivated species are easily obtained and make marker assisted selection or marker assisted breeding for traits of interest feasible in many crops. Moreover, genome-wide association studies to identify SNPs linked to traits of interest and the subsequent use of novel breeding schemes (breeding by design) will further revolutionize crop breeding for insect resistance. All these methodologies are advanced by whole genome sequencing of crop plants, e.g., maize, rice, wheat, but also vegetable crops such as tomato, lettuce, and cabbage (Goff et al., 2002; Schnable et al., 2009; Brenchley et al., 2012; Sato et al., 2012), and re-sequencing of wild germplasm. However, a challenge remains when traits are polygenic, and the individual components have subtle effect. Moreover, the genetic background of elite cultivars might interfere with traits from wild relatives. There is a clear need to bridge the current gap in the understanding of these technological advances between bio-informaticians, bio-statisticians, entomologists, plant pathologists and (pre-) breeders. It is often overlooked that only their collective efforts will ensure important breakthroughs in pest and disease resistance in crops.
R-GENE-MEDIATED RESISTANCE TO INSECT PESTS
Although some resistances are effective against a broad range of pest species, most are highly herbivore-specific reactions. Exploitation of natural resistances, often found in wild relatives that are interbreedable with our current crops, is well-suited to combat pest species that consume a specific plant organ or tissue (e.g., aphids, whiteflies, and other phloem-feeding insects).
R-gene-based resistance relies on a “gene-for-gene” interaction, where a compound secreted by the insect is specifically recognized by the plant, thus enabling the plant to initiate a defense response.
Whereas R-gene-mediated resistance has not been established for tissue chewing insects (i.e., Lepidoptera and Coleoptera), several examples of strong monogenic natural resistance to phloem-feeding pests have been reported in literature. Only a few of these dominant R-genes – that provide resistance against phloem-feeders – have been cloned (e.g., Mi-1.2, VAT, and BPH16) and many more are extensively used in agricultural settings through the use of marker assisted breeding (for a recent review, see Broekgaarden et al., 2011; Table 1).
Table 1.
Overview of R-genes mediating insect resistance (adapted from Broekgaarden et al., 2011, with permission).
| Plant species | Gene | Insect | Resistance broken | Reference |
|---|---|---|---|---|
| Triticum aestivum | H genes | Mayetiola destructor | yes | Wang et al.(2006);Yu et al. (2009), and Harris et al. (2012) |
| Dn genes | Diuraphis noxia | yes | Liu et al. (2005),Peng et al. (2009), and Tolmay et al. (2007) | |
| Oryza sativa | Bph genes | Nilaparvata lugens | yes | Du et al. (2009),Qiu et al. (2010) and Peñalver Cruz et al. (2011) |
| Gm genes | Gall midge | Himabindu et al. (2010), and Kumar et al. (2009) | ||
| Solanum lycopersicum | Mi-1.2 | Macrosiphum euphorbiae, Bemisia tabaci | yes | Rossi et al. (1998),Nombela et al. (2003),and Goggin et al. (2001) |
| Cucumis melo | Vat | Aphis gossypii | yes | Klingler et al. (2001) and Lombaert et al. (2009) |
| Medicago truncatula | AIN | Acyrthosiphon kondoi | yes | Klingler et al. (2009) and Humphries et al. (2010) |
| Glycine max | Rag genes | Aphis glycines | yes | Li et al. (2007); Zhang et al. (2009,2010) and Kim et al. (2008) |
Therefore, it is tempting to draw a general conclusion about R-gene-mediated insect resistances found in nature: only those pests, such as phloem-feeding insects, that require an intimate relationship with their host plant to successfully colonize are likely to be contained using R-gene-mediated defenses.
Interestingly, even in crops where the R-gene is cloned and characterized, the mode-of-action of these resistances is unclear. It should involve attacker recognition and down-stream signal transduction leading to an effective defense response that results in the inability of phloem-feeding insects to establish prolonged feeding.
Similarly to plant–pathogen interactions, the cloned insect resistance genes are members of the family of nucleotide-binding, leucine-rich repeat (NBS-LRR). Therefore, in analogy with pathogen recognition, it is expected that recognition of insect herbivores by NBS-LRR proteins takes place through direct or indirect binding of insect effector molecules (Dodds and Rathjen, 2010). Effector molecules of phloem-feeding insects are thought to be secreted into the host plant during probing (testing phase) or subsequent prolonged feeding (ingestion of phloem sap; Will et al., 2007; Moreno et al., 2011). Although several candidate effector molecules, e.g., secreted from the salivary glands of aphids, have been identified (Ramsey et al., 2007; Harmel et al., 2008; Carolan et al., 2009; Bos et al., 2010; Rodriguez and Bos, 2013), none of these have been associated with the binding by R-genes directly, or to known so-called virulence targets “guarded” by R-genes. It is expected that this field of research will take an enormous flight and shows a promise for plant breeding for insect-resistant crops.
The Mi-1.2 gene in tomato, arguably most researched, is extensively used for control of root-knot nematodes [Meloidogyne species (Milligan et al., 1998; de Vos et al., 2008)], but also is effective against some clones of the tomato–potato aphid (Macrosiphum euphorbiae; Rossi et al., 1998), whiteflies (Bemisia tabaci; Nombela et al., 2003), and the potato psyllid (Casteel et al., 2006)]. This broad effectiveness of the Mi gene toward several tomato phloem-feeding pests is striking and suggests recognition of several species-specific effector molecules. As an alternative – and more likely – hypothesis one would expect these insect species use a similar gateway, guarded by Mi-1.2, to successfully colonize tomato. To date, no such effector from either of the insect, nor Meloidogyne species, has been identified that causes the hypersensitive response in Mi1.2 tomato plants. Mi-mediated resistance to root-knot nematodes in characterized by a local hypersensitive response that takes place within 24 h upon feeding by Meloidogyne species. The Mi-mediated response to aphids is clone-specific and requires common signaling components characterized for pathogen defenses (Bhattarai et al., 2007,2010; Atamian et al., 2012).
Over the past decades plant breeding companies have exploited natural variation for dominant monogenic insect resistance genes. The genes described above are extensively used in horticulture. Other dominant loci, such as those required for resistance against wheat against the Russian wheat aphid or Hessian flies have been extensively used in agricultural settings. The large-scale usage of these dominant loci has resulted in newly arisen insect populations (virulent biotypes). For example, aphid biotypes of Nasonovia ribisnigri have been identified in Europe that are able to feed from cultivated lettuce carrying a dominant monogenic resistance introgressed from Lactuca virosa (Thabuis et al., 2011). Other examples, include virulent biotypes of the Russian wheat aphid that break through Dn resistance in wheat (Haley et al., 2004).
Pyramiding of R-genes (similar to Bt-approaches), where more than one resistance trait is stacked, can possibly prevent, or at least delay, the formation of new insect biotypes that can evolve to feed on resistant crops and this strategy can contribute to increased durability of these resistances. This may be a more responsible use of the currently limited set of available resistance traits. Ultimately, the decision to pyramid resistance genes will depend on several, often economic, factors, including (i) the availability of natural germplasm; (ii) the current and future economic threat of a pest; (iii) the population characteristics of the pest and its ability to evolve counter measures that lead to insensitivity; (iv) the time-to-market for the crop at hand, and (v) the level of resistance in current (competitive) commercial varieties.
METABOLITE-MEDIATED RESISTANCE
As described above, R-gene-based defense can render strong species-specific resistance to a limited set of herbivores, but is certainly not effective against all herbivores. The constitutive or induced production of secondary metabolites can provide an alternative resistance strategy. These compounds, which may be specific for the plant genus or family, often accumulate in leaf tissue where they occur in specialized structures on the plant’s surface or are compartmentalized within the host cell.
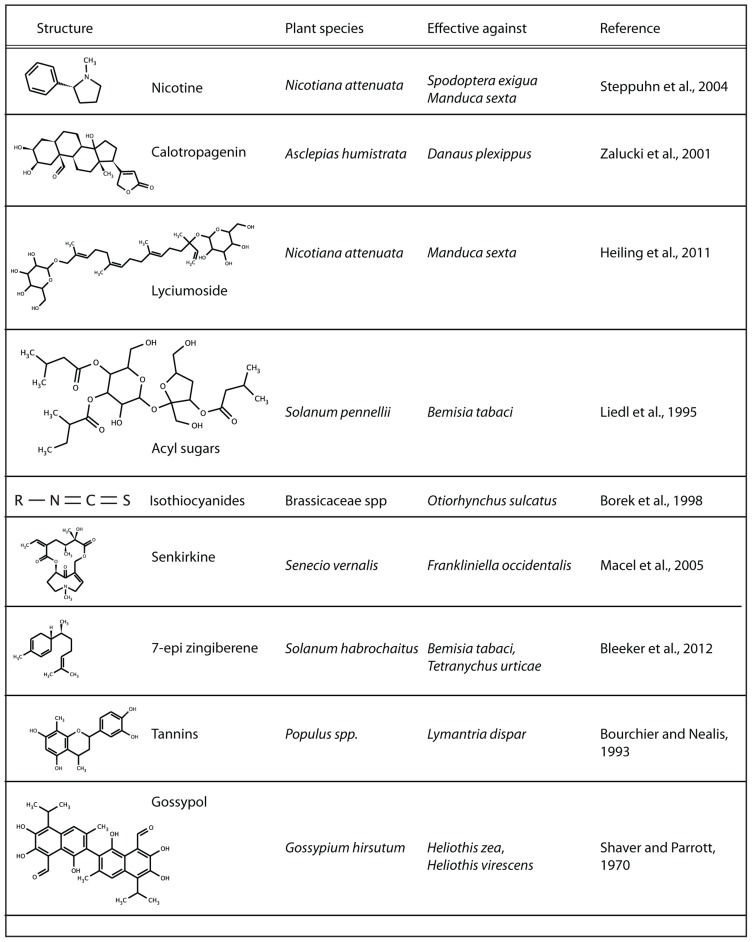
There is an incredible natural diversity of compounds present in plants (Figure 1). Whereas some of the biosynthetic pathways are restricted to a certain family, others are spread throughout the plant kingdom. Examples of specialized plant metabolites are glucosinolates in brassicaceae, from which toxic and anti-feedant compounds are enzymatically formed as soon as the cells are ruptured by herbivore feeding (Lüthy and Matile, 1984). Moreover, a wide variety of alkaloids have been identified, such as the neurotoxin nicotine in Nicotiana attenuata (Steppuhn et al., 2004), saponin glycoalkaloids in tomato (Chan and Tam, 1985) and pyrrolizidine alkaloids in chrysanthemum (Macel et al., 2005) that are related to resistance to generalist insect pests.
FIGURE 1.
Overview of insecticidal secondary metabolites and their plant origin.
On the contrary, compounds such as terpenes occur ubiquitously throughout the plant world, and are synthesized through common pathways present in most plants. However, there is also immense structural variation in these terpenes themselves, with an estimated 30,000 different structures occurring in plants (Connolly and Hill, 1991; Degenhardt et al., 2009b; Pichersky and Lewinsohn, 2011). Small changes in the final biosynthetic enzymes (terpene synthases), the availability of substrates and the biosynthetic conditions in the cells play a defining role in determining which terpenes are produced (Degenhardt et al., 2009a). This has provided plants with an enormous evolutionary flexibility to fine-tune the chemical responses to herbivory.
Because many terpenes are volatile, and many plants induce their production when attacked by herbivores, they provide an opportunity for predators to locate herbivore-infested plants, and serve a role as semiochemicals (information-conveying chemicals; Turlings et al., 1990; Kessler and Baldwin, 2001). A very diverse set of terpenoids has been suggested to play a role in indirect defense, such as bergamotene in wild tobacco, and a blend of mono- and sesquiterpens in tomato (Kessler and Baldwin, 2001; Kant et al., 2004).
Terpenes have also been shown to act as direct toxins to a suite of insects and pathogens [e.g., 7-epi-zingiberene against whiteflies (Bleeker et al., 2011), resins against bark beetles in confiners (Phillips and Croteau, 1999), and terpenoid lactones against Colorado potato beetles (Szczepanik et al., 2005)], but are at high concentrations also toxic to the plant itself (Aharoni et al., 2003). Therefore, plants sequester and compartmentalize terpenes, transport them to the leaf surface, or produce and store terpenoids in trichomes. The latter allows a terpene coating toward the outside environment of the plant without the need to adapt to high intercellular concentrations of these compounds.
A major pest in commercial tomato cultivation is the whitefly Bemisia tabaci, mainly because it is a vector for begomo viruses, causing substantial losses in commercial vegetable cultivation (Navas-Castillo et al. , 2011). Although some promising sources of resistance have been identified (Firdaus et al., 2013), to date no R-gene-based resistance has been identified for Bemisia tabaci, a highly polyphagous phloem-feeding insect with a host-range spanning over 100 plant species (Mound and Halsey, 1978). Although a whitefly population can quickly reach enormous numbers, their direct impact on crop yield is limited. In contrast, indirect damage from whitefly vectored viruses is a major threat to crop production. To prevent virus vectoring by sap-sucking pests one should ideally rely on a complete avoidance response of the insect toward the host plant. Volatile-mediated repellency of whiteflies might just provide such an opportunity in tomato, where tomato yellow leaf curl virus (TYLCV) is a major agricultural disease transmitted by Bemisia tabaci.
By screening a number of wild tomato plants for repellence against whiteflies, Bleeker et al. (2011) found that Solanum habrochaites showed strong repellency to whiteflies. Subsequently, the repellency was shown to be mediated by a sesquiterpene, namely 7-epi-zingiberene (Bleeker et al., 2011). 7-epi-zingiberene is exclusively produced in the glandular trichomes of S. habrochaites (Bleeker et al., 2012). In the offspring of interspecific crosses between S. habrochaites and cultivated tomato (S. lycoperiscum) were made, the F2 plants showed a strong correlation between 7-epi-zingiberene production and whitefly resistance. Surprisingly, this compound did not only confer resistance against whiteflies, but also against other herbivorous pests with entirely different modes of feeding, these include single-cell feeders (the spider mite T. urticae) and caterpillars (Manduca sexta; Bleeker et al., 2012). The above-described approach is very promising for multiple (vegetable) crop species. The repellent and toxic effects of such compounds produced at the plant–environment interface (e.g., in glandular trichomes) directly functions as an alarm bell that signals “inedible” to approaching herbivorous pests, but will be particularly important in fighting off virus vectoring insect species.
WHAT CHALLENGES ARE AHEAD
Preventing pre- and post-harvest damage caused by insects is a very challenging, but economically important, issue for plant breeders. Particularly, the proposed and partly implemented reductions in the use of systemic pesticides will further increase the need of genetic host resistance in the near future. GM approaches have been extremely successful in controlling some insect species, but their implementation, particularly in the EU, face heavy political opposition. Moreover, due to the de-regulatory process, GM introduction is expensive, thereby making it less feasible for the smaller vegetable crop markets, which are often locally tailored and also diversified to achieve specific consumer traits.
In order to have a chance against insect species that have multiple generations in a year, it is of crucial importance to widen our understanding of resistances in wild relatives of our current crops against insect herbivores. This will be an essential responsibility for plant pathologists, entomologists, breeders, and the entire research community. It has been estimated that for crops such as tomato, there is a multitude of gene-information “buried” in wild species that can be crossed with elite varieties. This genetic reservoir represents a largely untapped treasure for new or improved traits that could make our current crops significantly more productive. Because every day more genomic sequences are becoming available, this enables a quicker trait-to-gene path, thus providing a good academic opportunity to look beyond model plants and provide an insight in unique traits of wild species. Large efforts will need to be made to understand what genes are underlying the traits of future importance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the two reviewers for their valuable comments.
Footnotes
EU directive 2009/128/EC for sustainable use of pesticides in plant protection. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0071:0086:en:PDF
ISAAA Brief 43-2011. Global Status of Commercialized Biotech/GM Crops in 2011. Or summary at http://www.isaaa.org/resources/publications/pocketk/16/default.asp
REFERENCES
- Aharoni A., Giri A. P., Deuerlein S., Griepink F., De Kogel W.-J., Verstappen F. W. A., et al. (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 15 2866–2884 10.1105/tpc.016253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A., Giri A. P., Verstappen F. W. A., Bertea C. M., Sevenier R., Sun Z. K., et al. (2004) Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16 3110–3131 10.1105/tpc.104.023895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Alfageme F., Maharramov J., Carrillo L., Vandenabeele S., Vercammen D., Van Breusegem F., et al. (2011) Potential use of a serpin from Arabidopsis for pest control. PLoS ONE 6: e20278 10.1371/journal.pone.0020278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamian H. S., Eulgem T., Kaloshian I. (2012) SlWRKY70 is required for Mi-1-mediated resistance to aphids and nematodes in tomato. Planta 235 299–309 10.1007/s00425-011-1509-6 [DOI] [PubMed] [Google Scholar]
- Bhatia V., Bhattacharya R., Uniyal P. L., Singh R., Niranjan R. S. (2012) Host generated siRNAs attenuate expression of serine protease gene in Myzus persicae. PLoS ONE 7: e46343 10.1371/journal.pone.0046343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai K. K., Atamian H. S., Kaloshian I., Eulgem T. (2010) WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J. 63 229–240 10.1111/j.1365-313X.2010.04232.x [DOI] [PubMed] [Google Scholar]
- Bhattarai K. K., Li Q., Liu Y., Dinesh-Kumar S. P., Kaloshian I. (2007) The Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 144 312–323 10.1104/pp.107.097246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker P. M., Diergaarde P. J., Ament K., Schütz S., Johne B., Dijkink J., et al. (2011) Tomato-produced 7-epizingiberene and R-curcumene act as repellents to whiteflies. Phytochemistry 72 68–73 10.1016/j.phytochem.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Bleeker P. M., Mirabella R., Diergaarde P. J., Vandoorn A., Tissier A., Kant M. R., et al. (2012) Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. U.S.A. 109 20124–20129 10.1073/pnas.1208756109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohorova N., Maciel A. M., Brito R. M., Aguilart L., Ibarra J. E., Hoisington D. (1996) Selection and characterization of Mexican strains of Bacillus thuringiensis active against four major lepidopteran maize pests. Entomophaga 41 153–165 10.1007/BF02764243 [DOI] [Google Scholar]
- Borek V., Elberson L. R., Mccaffrey J. P., Morra M. J. (1998) Toxicity of isothiocyanates produced by glucosinolates in Brassicaceae species to black vine weevil eggs. J. Agric. Food Chem. 46 5318–5323 10.1021/jf9805754 [DOI] [Google Scholar]
- Bos J. I., Prince D., Pitino M., Maffei M. E., Win J., Hogenhout S. A. (2010) A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 6: e1001216 10.1371/journal.pgen.1001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourchier R. S., Nealis V. G. (1993) Development and growth of early-and late-instar gypsy moth (Lepidoptera: Lymantriidae) feeding on tannin-supplemented diets. Environ. Entomol. 22 642–646 10.1007/BF00333222 [DOI] [Google Scholar]
- Brenchley R., Spannagl M., Pfeifer M., Barker G. L. A., D’Amore R., Allen A. M., et al. (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491 705–710 10.1038/nature11650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekgaarden C., Snoeren T. A., Dicke M., Vosman B. (2011) Exploiting natural variation to identify insect-resistance genes. Plant Biotechnol. J. 9 819–825 10.1111/j.1467-7652.2011.00635.x [DOI] [PubMed] [Google Scholar]
- Carolan J. C., Fitzroy C. I., Ashton P. D., Douglas A. E., Wilkinson T. L. (2009) The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics 9 2457–2467 10.1002/pmic.200800692 [DOI] [PubMed] [Google Scholar]
- Carrillo L., Martinez M., Alvarez-Alfageme F., Castanera P., Smagghe G., Diaz I., et al. (2011) A barley cysteine-proteinase inhibitor reduces the performance of two aphid species in artificial diets and transgenic Arabidopsis plants. Transgenic Res. 20 305–319 10.1007/s11248-010-9417-2 [DOI] [PubMed] [Google Scholar]
- Casteel C. L., Walling L. L., Paine T. D. (2006) Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2gene. Entomol. Exp. Appl. 121 67–72 10.1111/j.1570-8703.2006.00458.x [DOI] [Google Scholar]
- Chan H. T, Tam S. Y. T. (1985) Toxicity of -tomatine to larvae of the Mediterranean fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 78 305–307 10.1653/024.095.0110 [DOI] [Google Scholar]
- Connolly J. D., Hill R. A. (1991) Dictionary of Terpenoids. London: Chapman and Hall; 10.1002/ffj.2730070418 [DOI] [Google Scholar]
- de Vos M., Kriksunov K. L., Jander G. (2008) Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiol. 146 916–926 10.1104/pp.107.112185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt J., Köllner T. G., Gershenzon J. (2009a) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70 1621–1637 10.1016/j.phytochem.2009.07.030 [DOI] [PubMed] [Google Scholar]
- Degenhardt J., Köllner T. G., Gershenzon J. (2009b) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70 1621–1637 10.1016/j.phytochem.2009.07.030 [DOI] [PubMed] [Google Scholar]
- Dodds P. N., Rathjen J. P. (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11 539–548 10.1038/nrg2812 [DOI] [PubMed] [Google Scholar]
- Du B., Zhang W. L., Liu B. F., Hu J., Wei Z., Shi Z. Y., et al. (2009) Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. U.S.A. 106 22163–22168 10.1073/pnas.0912139106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. R., Raven P. H. (1964) Butterflies and plants: a study in co-evolution. Evolution 18 586–608 10.2307/2406212 [DOI] [Google Scholar]
- Firdaus S., Heusden A., Hidayati N., Supena E., Mumm R., Vos R. H., et al. (2013) Identification and QTL mapping of whitefly resistance components in Solanum galapagense. Theor. Appl. Genet. 1–15 10.1007/s001220050803 [DOI] [PubMed] [Google Scholar]
- Fitches E., Wiles D., Douglas A. E., Hinchliffe G., Audsley N., Gatehouse J. A. (2008) The insecticidal activity of recombinant garlic lectins towards aphids. Insect Biochem. Mol. Biol. 38 905–915 10.1016/j.ibmb.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Gassmann A. J., Petzold-Maxwell J. L., Keweshan R. S., Dunbar M. W. (2011) Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE 6: e22629 10.1371/journal.pone.0022629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Ricke D., Lan T.-H., Presting G., Wang R., Dunn M., et al. (2002) A draft sequence of the rice genome (Oryza sativa L.ssp. japonica).Science 296 92–100 10.1126/science.1068275 [DOI] [PubMed] [Google Scholar]
- Goggin F. L., Williamson V. M., Ullman D. E. (2001) Variability in the response of Macrosiphum euphorbiae and Myzus persicae (Hemiptera: Aphididae) to the tomato resistance gene Mi. Environ. Entomol. 30 101–106 10.1603/0046-225X-30.1.101 [DOI] [Google Scholar]
- Haley S. D., Peairs F. B., Walker C. B., Rudolph J. B., Randolph T. L. (2004) Occurrence of a new Russian wheat aphid biotype in Colorado research supported through funding from Colorado Agric. Exp. Stn. Projects795 and 646 and the Colorado Wheat Administrative Committee. Crop Sci. 44 1589–1592 10.2135/cropsci2004.1589 [DOI] [Google Scholar]
- Harmel N., Létocart E., Cherqui A., Giordanengo P., Mazzucchelli G., Guillonneau F., et al. (2008) Identification of aphid salivary proteins: a proteomic investigation of Myzus persicae. Insect Mol. Biol. 17 165–174 10.1111/j.1365-2583.2008.00790.x [DOI] [PubMed] [Google Scholar]
- Harris M. O., Freeman T. P., Anderson K. M., Harmon J. P., Moore J. A., Payne S. A., et al. (2012) Hessian fly Avirulence gene loss-of-function defeats plant resistance without compromising the larva’s ability to induce a gall tissue. Entomol. Exp. Appl. 145 238–249 10.1111/eea.12010 [DOI] [Google Scholar]
- Heiling S., Schuman M. C., Schoettner M., Mukerjee P., Berger B., Schneider B., et al. (2010) Jasmonate and ppHsystemin regulate key malonylation steps in the biosynthesis of 17-hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell 22 273–292 10.1105/tpc.109.071449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M., Béguin M., Requier F., Rollin O., Odoux J.-F., Aupinel P., et al. (2012) A common pesticide decreases foraging success and survival in honey bees. Science 336 348–350 10.1126/science.1215039 [DOI] [PubMed] [Google Scholar]
- Himabindu K., Suneetha K., Sama V., Bentur J. S. (2010) A new rice gall midge resistance gene in the breeding line CR57-MR1523, mapping with flanking markers and development of NILs. Euphytica 174 179–187 10.1007/s10681-009-0106-2 [DOI] [Google Scholar]
- Humphries A., Peck D., Robinson S., Oldach K., Glatz R., Howie J. (2010) “A new highly virulent bluegreen aphid causes severe damage in previously tolerant pasture and grain legumes,” in Food Security from Sustainable Agriculture Proceedings of 15th Agronomy Conference 2010, 15-18 November 2010, Lincoln eds Dove H., Culvenor R. A. (Gosford NSW: The Regional Institute Ltd) [Google Scholar]
- Kant M. R., Ament K., Sabelis M. W., Haring M. A., Schuurink R. C. (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 135 483–495 10.1104/pp.103.038315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A., Baldwin I. T. (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291 2141–2144 10.1126/science.291.5511.2141 [DOI] [PubMed] [Google Scholar]
- Kim K.-S., Hill C. B., Hartman G. L., Mian M., Diers B. W. (2008) Discovery of soybean aphid biotypes. Crop Sci. 48 923–928 10.2135/cropsci2007.08.0447 [DOI] [Google Scholar]
- Klingler J., Kovalski I., Silberstein L., Thompson G. A., Perl-Treves R. (2001) Mapping of cotton-melon aphid resistance in melon. J. Am. Soc. Hortic. Sci. 126 56–63 10.1007/s11032-006-9039-9 [DOI] [Google Scholar]
- Klingler J. P., Nair R. M., Edwards O. R., Singh K. B. (2009) A single gene, AIN, in Medicago truncatula mediates a hypersensitive response to both bluegreen aphid and pea aphid, but confers resistance only to bluegreen aphid. J. Exp. Bot. 60 4115–4127 10.1093/jxb/erp244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Jain A., Sahu R. K., Shrivastava M. N., Nair S., Mohan M. (2005) Genetic analysis of resistance genes for the rice gall midge in two rice genotypes. Crop Sci. 45 1631–1635 10.2135/cropsci2004.0406 [DOI] [Google Scholar]
- Kumar L. V., Chakravarthy A., Patil S., Rajanna D. (2009) Changing scenario of Asian rice gall midge biotypes at Mangalore, coastal Karnataka and computation of their growth rates. J. Entomol. Res. 33 1–7 [Google Scholar]
- Kumar P., Pandit S. S., Baldwin I. T. (2012) Tobacco rattle virus vector: a rapid and transient means of silencing Manduca sexta genes by plant mediated RNA interference. PLoS ONE 7: e31347 10.1371/journal.pone.0031347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira C. (2007) The origin of herbivory on land: initial patterns of plant tissue consumption by arthropods. Insect Sci. 14 259–275 10.1111/j.1744-7917.2007.00141.x-i1 [DOI] [Google Scholar]
- Lambert B., Buysse L., Decock C., Jansens S., Piens C., Saey B., et al. (1996) A Bacillus thuringiensis insecticidal crystal protein with a high activity against members of the family noctuidae. Appl. Environ. Microbiol. 62 80–86 10.1046/j.1365-2672.2002.01545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Hill C. B., Carlson S. R., Diers B. W., Hartman G. L. (2007) Soybean aphid resistance genes in the soybean cultivars Dowling and Jackson map to linkage group M. Mol. Breed. 19 25–34 10.1007/s11032-006-9039-9 [DOI] [Google Scholar]
- Liedl B. E., Lawson D. M., White K. K., Siiapiho J. A., Cohen D. E., Carson W. G., et al. (1995) Acylsugars of wild tomato Lycopersicon pennellii alters settling and reduces oviposition of Bemisia argentifolii (Homoptera: Aleyrodidae). J. Econ. Entomol. 88 742–748 10.1371/journal.pone.0033064 [DOI] [Google Scholar]
- Liu X. M., Smith C. M., Friebe B. R., Gill B. S. (2005) Molecular mapping and allelic relationships of Russian wheat aphid-resistance genes. Crop Sci. 45 2273–2280 10.2135/cropsci2004.0704 [DOI] [Google Scholar]
- Lombaert E., Carletto J. R. M., Piotte C., Fauvergue X., Lecoq H., Vanlerberghe-Masutti F., et al. (2009) Response of the melon aphid, Aphis gossypii, to host-plant resistance: evidence for high adaptive potential despite low genetic variability. Entomol. Exp. Appl. 133 46–56 10.1111/j.1570-7458.2009.00904.x [DOI] [Google Scholar]
- Lüthy B., Matile P. (1984) The mustard oil bomb: rectified analysis of the subcellular organisation of the myrosinase system. Biochem. Physiol. Pflanz. 179 5–12 10.1016/S0015-3796(84)80059-1 [DOI] [Google Scholar]
- Macel M., Bruinsma M., Dijkstra S., Ooijendijk T., Niemeyer H., Klinkhamer P. L. (2005) Differences in effects of pyrrolizidine alkaloids on five generalist insect herbivore species. J. Chem. Ecol. 31 1493–1508 10.1007/s10886-005-5793-0 [DOI] [PubMed] [Google Scholar]
- Meihls L. N., Higdon M. L., Ellersieck M. R., Tabashnik B. E., Hibbard B. E. (2012) Greenhouse-selected resistance to Cry3Bb1-producing corn in three western corn rootworm populations. PLoS ONE 7: e51055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan S. B., Bodeau J., Yaghoobi J., Kaloshian I., Zabel P., Williamson V. M. (1998) The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10 1307–1319 10.1105/tpc.10.8.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A., Garzo E., Fernandez-Mata G., Kassem M., Aranda M. A., Fereres A. (2011) Aphids secrete watery saliva into plant tissues from the onset of stylet penetration. Entomol. Exp. Appl. 139 145–153 10.1111/j.1570-7458.2011.01117.x [DOI] [Google Scholar]
- Mound L. A., Halsey S. H. (1978) Whitefly of the World, A Systematic Catalog of the Aleyrodidae (Homoptera) with Host Plant and Natural Enemy Data. New York: John Wiley &Sons [Google Scholar]
- Navas-Castillo J., Fiallo-Olivé E., Sánchez-Campos S. (2011) Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 49 219–248 10.1146/annurev-phyto-072910-095235 [DOI] [PubMed] [Google Scholar]
- Nombela G., Williamson V. M., Muñiz M.(2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol. Plant Microbe Interact. 16 645–649 10.1094/MPMI.2003.16.7.645 [DOI] [PubMed] [Google Scholar]
- Peñalver Cruz A., Arida A., Heong K. L., Horgan F. G. (2011) Aspects of brown planthopper adaptation to resistant rice varieties with the Bph3 gene. Entomol. Exp. Appl. 141 245–257 10.1111/j.1570-7458.2011.01193.x [DOI] [Google Scholar]
- Peng J., Bai Y., Haley S., Lapitan N. (2009) Microsatellite-based molecular diversity of bread wheat germplasm and association mapping of wheat resistance to the Russian wheat aphid. Genetica 135 95–122 10.1007/s10709-008-9262-x [DOI] [PubMed] [Google Scholar]
- Peng J., Wang H., Haley S. D., Peairs F. B, Lapitan N. L. V. (2007) Molecular mapping of the Russian wheat aphid resistance gene Dn2414 in wheat. Crop Sci. 47 2418–2429 10.2135/cropsci2007.03.0137 [DOI] [Google Scholar]
- Phillips M. A., Croteau R. B. (1999) Resin-based defenses in conifers. Trends Plant Sci. 4 184–190 10.1016/S1360-1385(99)01401-6 [DOI] [PubMed] [Google Scholar]
- Pichersky E., Lewinsohn E. (2011) Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 62 549–566 10.1146/annurev-arplant-042110-103814 [DOI] [PubMed] [Google Scholar]
- Price D. R., Gatehouse J. A. (2008) RNAi-mediated crop protection against insects. Trends Biotechnol. 26 393–400 10.1016/j.tibtech.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Qiu Y. F., Guo J. P., Jing S. L., Zhu L. L., He G. C. (2010) High-resolution mapping of the brown planthopper resistance gene Bph6 in rice and characterizing its resistance in the 9311 and Nipponbare near isogenic backgrounds. Theor. Appl. Genet. 121 1601–1611 10.1007/s00122-010-1413-7 [DOI] [PubMed] [Google Scholar]
- Ramsey J., Wilson A., De Vos M., Sun Q., Tamborindeguy C., Winfield A., et al. (2007) Genomic resources for Myzus persicae: EST sequencing, SNP identification, and microarray design. BMC Genomics 8: 423 10.1186/1471-2164-8-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P. A., Bos J. I. (2013) Toward understanding the role of aphid effectors in plant infestation. Mol. Plant Microbe Interact. 26 25–30 10.1094/MPMI-05-12-0119-FI [DOI] [PubMed] [Google Scholar]
- Rossi M., Goggin F. L., Milligan S. B., Kaloshian I., Ullman D. E., Williamson V. M. (1998) The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. U.S.A. 95 9750–9754 10.1073/pnas.95.17.9750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Tabata S., Hirakawa H., Asamizu E., Shirasawa K., Isobe S., et al. (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485 635–641 10.1038/nature11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnable P. S., Ware D., Fulton R. S., Stein J. C., Wei F., Pasternak S., et al. (2009) The B73 Maize genome: complexity, diversity, and dynamics. Science 326 1112–1115 10.1126/science.1178534 [DOI] [PubMed] [Google Scholar]
- Shaver T. N., Parrott W. L. (1970) Relationship of larval age to toxicity of gossypol to bollworms, tobacco budworms, and pink bollworms. J. Econ. Entomol. 63 1802–1804 [Google Scholar]
- Smith C. M., Clement S. L. (2012) Molecular bases of plant resistance to arthropods. Annu. Rev. Entomol. 57 309–328 10.1146/annurev-ento-120710-100642 [DOI] [PubMed] [Google Scholar]
- Stahl E. (1888) Pflanzen und Schnecken.Eine biologische Studie über die Schutzmittel der Pflanzen gegen Schneckenfra β Jena: G. Fischer. [Google Scholar]
- Steppuhn A., Gase K., Krock B., Halitschke R., Baldwin I. T. (2004) Nicotine’s defensive function in nature. PLoS Biol. 2: e217 10.1371/journal.pbio.0020217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. N., Adang M. J., All J. N., Raymer P. L., Ramachandran S., Parrott W. A. (1996) Insect control and dosage effects in transgenic canola containing a synthetic Bacillus thuringiensis crylAc gene. Plant Physiol. 112 115–120 10.1104/pp.112.1.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanik M., Dams I., Wawrzenczyk C. (2005) Feeding deterrent activity of terpenoid lactones with the p-menthane system against the Colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 34 1433–1440 10.1603/0046-225X-34.6.1433 [DOI] [Google Scholar]
- Tang T., Zhao C. Q., Feng X. Y., Liu X. Y., Qiu L. H. (2012) Knockdown of several components of cytochrome P450 enzyme systems by RNA interference enhances the susceptibility of Helicoverpa armigera to fenvalerate. Pest Manag. Sci. 68 1501–1511 10.1002/ps.3336 [DOI] [PubMed] [Google Scholar]
- Thabuis A. P. P., Teekens K. C., Van Herwijnen Z. O. (2011) Lettuce That is Resistant to the Lettuce Aphid Nasonovia ribisnigri Biotype1. WO Patent WO/2011/058 192 [Google Scholar]
- Tolmay V., Lindeque R., Prinsloo G. (2007) Preliminary evidence of a resistance-breaking biotype of the Russian wheat aphid, Diuraphis noxia (Kurdjumov)(Homoptera: Aphididae), in South Africa. Afr. Entomol. 15 228 10.4001/1021-3589-15.1.228 [DOI] [Google Scholar]
- Turlings T. C. J., Tumlinson J. H., Lewis W. J. (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250 1251–1253 10.1126/science.250.4985.1251 [DOI] [PubMed] [Google Scholar]
- Upadhyay S. K., Chandrashekar K., Thakur N., Verma P. C., Borgio J. F., Singh P. K., et al. (2011) RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. J. Biosci. 36 153–161 10.1007/s12038-011-9009-1 [DOI] [PubMed] [Google Scholar]
- Vaeck M., Reynaerts A., Hofte H., Jansens S., Debeuckeleer M., Dean C., et al. (1987) Trangenic plants protected from insect attack. Nature 328 33–37 10.1038/328033a0 [DOI] [Google Scholar]
- Wang T., Xu S. S., Harris M. O., Hu J. G., Liu L. W., Cai X. W. (2006) Genetic characterization and molecular mapping of Hessian fly resistance genes derived from Aegilops tauschii in synthetic wheat. Theor. Appl. Genet. 113 611–618 10.1007/s00122-006-0325-z [DOI] [PubMed] [Google Scholar]
- Whitehorn P. R., O’Connor S., Wackers F. L., Goulson D. (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336 351–352 10.1126/science.1215025 [DOI] [PubMed] [Google Scholar]
- Will T., Tjallingii W. F., Thönnessen A, Van Bel A. J. (2007) Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. U.S.A. 104 10536–10541 10.1371/journal.pone.0046903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. T., Cai X. W., Harris M. O., Gu Y. Q., Luo M. C., Xu S. S. (2009) Saturation and comparative mapping of the genomic region harboring Hessian fly resistance gene H26 in wheat. Theor. Appl. Genet. 118 1589–1599 10.1007/s00122-009-1006-5 [DOI] [PubMed] [Google Scholar]
- Zalucki M. P., Brower L. P., Alonso-M A. (2001) Detrimental effects of latex and cardiac glycosides on survival and growth of first-instar monarch butterfly larvae Danaus plexippus feeding on the sandhill milkweed Asclepias humistrata. Ecol. Entomol. 26 212–224 10.1046/j.1365-2311.2001.00313.x [DOI] [Google Scholar]
- Zha W., Peng X., Chen R., Du B., Zhu L., He G. (2011) Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect Nilaparvata lugens. PLoS ONE 6: e20504 10.1371/journal.pone.0020504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. R., Gu C. H., Wang D. C. (2009) Molecular mapping of soybean aphid resistance genes in PI 567541B. Theor. Appl. Genet. 118 473–482 10.1007/s00122-008-0914-0 [DOI] [PubMed] [Google Scholar]
- Zhang G. R., Gu C. H., Wang D. C. (2010) A novel locus for soybean aphid resistance. Theor. Appl. Genet. 120 1183–1191 10.1007/s00122-009-1245-5 [DOI] [PubMed] [Google Scholar]
- Zhang X., Tiewsiri K., Kain W., Huang L. H., Wang P. (2012) Resistance of Trichoplusia ni to Bacillus thuringiensis toxin Cry1Ac is independent of alteration of the Cadherin-like receptor for cry toxins. PLoS ONE 7: e35991 10.1371/journal.pone.0035991 [DOI] [PMC free article] [PubMed] [Google Scholar]