Abstract
It has been demonstrated that dentin matrix protein 1 (DMP1) is an essential regulator in the formation of bone and tooth. In addition to the mineralized tissues, DMP1 is also expressed in the non-mineralized tissues such as kidney, brain and salivary glands. Some studies have shown that the expression of DMP1 is significantly elevated in cancerous glands, while details about the expression and localization patterns of DMP1 in these glandular tissues still remain largely unknown. In this study, with multiple approaches, we systematically analyzed the expression and localization of DMP1 in mouse sub-mandibular glands (SMGs). The results showed that although DMP1 was expressed in both female and male mouse SMGs, the mRNA levels of DMP1 in male mice were higher than those in female mice after the appearance of granular convoluted tubule (GCT). In mouse SMGs, DMP1 was primarily present as the 46 kDa C-terminal fragment and the 37 kDa N-terminal fragment. The C-terminal fragment was mainly localized in the nuclei of acinar and ductal cells, while the N-terminal fragment was restricted to the cytoplasm of ductal cells. This study showed the expression of DMP1 in the GCT of male mice, a novel finding different from the result of previous reports. Collectively, the differential localization patterns of DMP1 fragments indicate that different forms of DMP1 may play distinct roles in the SMGs.
Keywords: Dentin matrix protein 1, Submandibular gland, Granular convoluted tubule, Localization, Posttranslational modification
Introduction
Dentin matrix protein 1 (DMP1), an acidic phosphoprotein, belongs to the family of small integrin-binding ligand, N-linked glycoprotein (SIBLING) (Fisher et al. 2001). As a member of the family, DMP1 shares some common features with other SIBLING proteins, such as the same chromosomal localization of their genes, similar exon-intro structures, the same Arg–Gly–Asp (RGD) motif within the last one or two exons and similar posttranslational modifications (Fisher and Fedarko 2003). DMP1 was originally postulated as a dentin-specific protein (George et al. 1993) and then was later detected in the matrices of other mineralized tissue as well as in their forming cells, including osteocytes, osteoblasts, ameloblasts and cementoblasts (Hirst et al. 1997; Macdougall et al. 1998; Toyosawa et al. 2001). In dentin and bone, DMP1 seems to actively participate in regulating matrix mineralization. Both Dmp1-null mice and patients with inactivating mutations in DMP1 develop osteomalacia and dentin hypomineralization, indicating that DMP1 plays crucial roles in osteogenesis and odontogenesis (Ye et al. 2004; Feng et al. 2006).
In the extracellular matrix (ECM) of tooth and bone, DMP1 mainly exists as proteolytically processed fragments including the N-terminal fragment and the C-terminal fragment. Phosphate analysis demonstrated that the C-terminal fragment is more highly phosphorylated than the N-terminal fragment (Qin et al. 2003). Based on the evident differences in biochemical structures, it is tempting to postulate that the two DMP1 fragments may have different functions in biomineralization. It had been found that the C-terminal fragment of DMP1 promoted hydroxyapatite nucleation (Tartaix et al. 2004; He et al. 2005; Gajjeraman et al. 2007). In addition, the proteoglycan form of the N-terminal fragment (DMP1–PG) and the full-length form of DMP1 had also been detected in bone and dentin (Qin et al. 2006; Huang et al. 2008). In contrast to the 57 kDa C-terminal fragment and the 37 kDa N-terminal fragment of DMP1, the full-length form of DMP1 and DMP1–PG inhibit the nucleation of hydroxypatite crystals (Tartaix et al. 2004; Gericke et al. 2010).
In addition to the mineralized tissues, studies showed that DMP1 is also expressed in the non-mineralized tissues such as kidney, brain, pancreas and salivary glands (Ogbureke and Fisher 2004; Terasawa et al. 2004). As the largest pair of salivary glands in mice, submandibular gland (SMG) functions in lubrication, wound healing and immunization regulation by secreting saliva (Sabbadini and Berczi 1995). From an initial bud in embroynic stage, SMG gradually develops into a complicated secretory system after birth. In the SMG of mice, acini are connected to the oral cavity through a branching duct system consisting of intercalated ducts (ID), granular convoluted tubules (GCT), striated ducts (SD) and excretory ducts (ED). Previous studies showed that DMP1 was expressed in seromucous acini and ducts except for the GCT of male mice (Ogbureke and Fisher 2004). However, details about the expression patterns of DMP1 fragments in mouse SMG are lacking. On the other hand, several studies have shown that the expression of DMP1 was significantly elevated in some cancerous glands (Chaplet et al. 2003; Fisher et al. 2004). This indicates DMP1 may have other functions in the SMGs in addition to regulation of biomineralization in tooth and bone. The main objective of this investigation is to systematically analyze the expression and localization patterns of DMP1 fragments in mouse SMG, which may provide novel clues to explore the roles of DMP1 in mouse SMG.
Materials and methods
Animals and tissue samples
C57BL/6J mice (The Jackson Laboratory; Bar Harbor, ME, USA) at the ages of 2, 4 or 12 weeks after birth were used in this study. SMGs from 4-week-old wild type male or female mice were used to extract total RNA for reverse transcription polymerase chain reaction (RT-PCR). SMGs from the 2-, 4- or 12-week-old male and female mice were used to perform quantitative real time reverse transcription polymerase chain reaction (qRT-PCR) and immunohistochemistry (IHC) analyses. SMGs from ten 12-week-old mice were used to extract proteins for sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and western immunoblotting analyses. To verify the expression of DMP1 in the GCT of male mouse SMG, 4-week-old Dmp1 knockout mice (The Feng Laboratory; Dallas, TX, USA) and 12-week-old C57BL/6J mice were used for β-galactosidase expression assays and in situ hybridization (ISH), respectively. The animal protocol and procedures used in this study were approved by the Animal Welfare Committee of Texas A&M Health Science Center Baylor College of Dentistry (Dallas, TX, USA).
Isolation of RNA, RT-PCR and qRT-PCR
For the total RNA extraction, the SMGs from 4-week-old male or female mice were dissected and frozen in liquid nitrogen. Total RNA was isolated from mouse SMG using RNeasy mini kit (Qiagen; Germantown, MD, USA) according to the manufacturer’s instructions. The total RNA extracted from mouse incisor with the same protocol was used as the positive control. The extracted RNA was reversely transcribed into cDNA using the QuantiTect Rev Transcription Kit (Qiagen; Germantown, MD, USA). The PCR amplification of the DMP1 cDNA was performed using a forward primer 5′-GACCACGAGCCCACGAG-CAC-3′ and a reverse primer 5′-TGGAGCCCCCAA-GACTCCGT-3′. The house-keeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used as the internal control. The PCR conditions were as follows: initial denaturation was at 95 °C for 10 min, followed by 35 cycles of 94 °C for 30 s, 60 °C for 45 s and 72 °C for 1 min, and then ended with the final extension for 10 min at 72 °C. After amplification, the PCR products were separated by electrophoresis in 1 % agarose gel with 1× TAE (Tris-acetate-EDTA) buffer, stained with ethidium bromide and visualized under ultraviolet light.
Real-time PCR was performed to assess the relative levels of DMP1 expressed in male and female mouse SMG at ages of 2, 4 or 12 weeks after birth. The reagents used for RNA extraction and reverse transcription were the same as for RT-PCR. For quantitative comparison, the same amount of cDNA (1 μg/μl) was used as the template for each sample. The qRT-PCR analyses were performed on a Bio-Rad CFX96 system (Bio-Rad; Hercules, CA, USA) using Brilliant SYBR Green Master Mix (Applied Biosystems; Foster City, CA, USA). A forward primer 5′-AGTGAGTCAT-CAGAAGAAAGTCAAGC-3′ and a reverse primer 5′-CTATACTGGCCTCTGTCGTAGCC-3′ were used for the real-time PCR. The PCR conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 45 cycles of 95 °C for 30 s, 60 °C for 1 min, and 72 °C for 30 s. All results were normalized to GAPDH that served as the reference gene. The PCR product accumulation was monitored by the increase in fluorescence intensity caused by the binding of SYBR Green to double-stranded DNA. The amplification cycle (Ct value) was determined on the fluorescence detected above the threshold.
SDS–PAGE and western immunoblotting
To extract DMP1 protein from mouse SMG, ten 12-week-old mice were used. The samples were ground carefully using mortar and pestle, and then placed into 4 M guanidium–HCl (Gdm–HCl) solution with pH 7.2 (Acros Organics; Fairlawn, NJ, USA) containing proteinase inhibitors for 48 h. The Gdm–HCl extracts were subjected to Q-Sepharose (Amersham Biosciences; Uppsala, Sweden) ion-exchange chromatography with a gradient ranging from 0.1 to 0.8 M NaCl in 6 M urea solution (pH 7.2) (Qin et al. 2001). Acidic proteins from this extraction were eluted into sequential fractions. The samples from chromatographic fractions were treated with 3 % β-mercaptoethanol (β-ME), and 60 μl of sample from each chromatographic fraction was separated by 10 % SDS–PAGE followed by western immunoblotting analyses to examine the presence of DMP1 proteins. For western immunoblotting, the polyclonal antibody anti-DMP1-C-857 and the monoclonal antibody anti-DMP1-N-9B6.3 (Table 1) were used for the detection of the C-terminal and the N-terminal fragment of DMP1, respectively (Huang et al. 2008; Qin et al. 2006). Western immunoblotting was performed as previously described (Qin et al. 2001).
Table 1.
Antibodies used in this study
| Antibody | Antibody type | Immunizing antigen | Immunoreactivity in western immunoblotting | Immunoreactivity in immunohistochemistry |
|---|---|---|---|---|
| Anti-DMP1-C-857a | Polyclonal | Oligopeptide (residues 471–485) | Yes | Yes |
| Anti-DMP1-N-9B6.3b | Monoclonal | 37 kDa (N-terminal) | Yes | Very weak |
| Anti-DMP1-N-859c | Polyclonal | Oligopeptide (residues 101–121) | Yes | Yes |
| Anti-DMP1-C-8G10.3d | Monoclonal | 57 kDa (C-terminal) | No | Yes |
This polyclonal antibody was used to detect the C-terminal fragment of DMP1 by western blotting analysis (Huang et al. 2008)
This monoclonal antibody was used to detect the N-terminal fragment of DMP1 by western blotting analysis (Qin et al. 2006)
This polyclonal antibody was used to detect the N-terminal fragment of DMP1 by immunohistochemistry analysis (Maciejewska et al. 2009)
This monoclonal antibody was used to detect the C-terminal fragment of DMP1 by immunohistochemistry analysis (Baba et al. 2004)
Immunohistochemistry (IHC)
IHC was carried out to analyze the expression and distribution of DMP1 fragments in the SMG of six male or female mice at postnatal 2, 4 or 12 weeks (n = 6 in each group). Under anesthesia, the mice at above ages were perfused from the ascending aorta with 4 % paraformaldehyde in 0.1 M phosphate buffer. To fix the SMGs, the samples were placed in the same fixative for 16 h. Tissues were processed for paraffin embedding, and serial 4 μm sections were prepared. For all paraffin sections, endogenous peroxidase activity was quenched with 3 % H2O2 for 10 min. Tissue sections were pretreated with hyaluronidase solution for 1 h at 37 °C, rinsed in PBS and incubated overnight with blocking solution. For the immunohistochemical detection of DMP1, the monoclonal antibody anti-DMP1-C-8G10.3 and the polyclonal antibody anti-DMP1-N-859 (Table 1), were used with the dilution of 1:200 for the detection of C- and N-terminal fragment of DMP1, respectively (Baba et al. 2004; Maciejewska et al. 2009). Mouse IgG at the same concentration was used to serve as the negative control. All the IHC experiments were performed using the MOM kit (Vector Laboratories; Burlingame, CA, USA). The immunoreactivity was visualized using ABC kit and DAB kit (Vector Laboratories; Burlingame, CA, USA) following the manufacturer’s instructions. Sections were counterstained with methyl green and viewed under light microscopy.
In situ hybridization (ISH)
To futher verify the expression of DMP1 in the GCT of male mice, six 12-week-old male mice were used for ISH staining following the protocol as previously described (Feng et al. 2002; Wang et al. 2010). Briefly, with DMP1 cDNA as the template, PCR amplification was performed to generate an 891-bp fragment using a forward primer 5′-GTCAAGCTAGCCCAGAGGGACA-3′ and a reverse primer 5′-TGGACTCGCTGGTCACCCCT-3′. The PCR product was subcloned into the PCR II-TOPO transcription vector (Invitrogen; Carlsbad, CA, USA) and then linearized to synthesize antisense and sense RNA probes using Sp6 and T7 RNA polymerase, respectively, according to the manufacturer’s instructions. The probes were labeled with digoxigenin (DIG) using a RNA labeling kit (Roche; Indianapolis, IN, USA). Serial 5 μm sections from SMG of 12-week-old male mice underwent fixation in 4 % cold fresh paraformadehyde, digestion with 20 μg/ml proteinase K and acetylation with 0.1 M triethanolamine and acetic anhydride. In a humidified chamber, the sections were hybridized overnight at 62 °C with DIG labeled RNA probes in hybridization buffer. After washing and blocking, DIG labeled RNA probes were localized by an alkaline-phosphate anti-DIG Fab fragment (Roche). Positive staining was then observed using a vector substrate red kit (Vector Laboratories) following the manufacturer’s instructions. Methyl green was used for counterstaining.
β-galactosidase (lacZ) expression assay
The β-galactosidase expression assay was carried out to analyze the expression of DMP1 in Dmp1 knockout mice. The Dmp1 knockout mice (The Feng laboratory; Dallas, TX, USA) were generated by using gene targeting to replace exon 6 of Dmp1 with a lacZ gene (Feng et al. 2003). For the β-galactosidase expression assay, six 4-week-old male Dmp1 knockout mice were used. The β-galactosidase activity was assessed in the SMGs of 4-week-old mice as previously described (Feng et al. 2002; Simmer et al. 2011). Briefly, mouse SMG was dissected, and fixed in 4 % paraformaldehyde for 3 h at 4 °C. After washing in 1× PBS 3 times at 4 °C, the SMGs were cryprotected by immersing in 15 % and then 30 % sucrose until the sample sank to the bottom of the bottle. The tissues were embedded in OCT medium and stored at −80 °C. The sample was cryosectioned at 8 μm at −20 °C on a Leica cryostat. The slides was stained with freshly made X-Gal solution (1 mg/ml) at 37 °C for 5 h and then washed in 1× PBS 3 times. Hematoxylin was used for counterstaining. SMGs from six wild-type C57BL/6J mice of the same age were used as the negative control.
Statistical analysis
All quantitative data were expressed as mean ± standard deviation (SD) of 6 individual determinations in this study, statistical difference was determined using the Student’s t test. Differences were considered significant if P < 0.05 (*) and highly significant if P < 0.01 (**).
Results
Evaluation of DMP1 mRNA levels in male and female mouse SMG
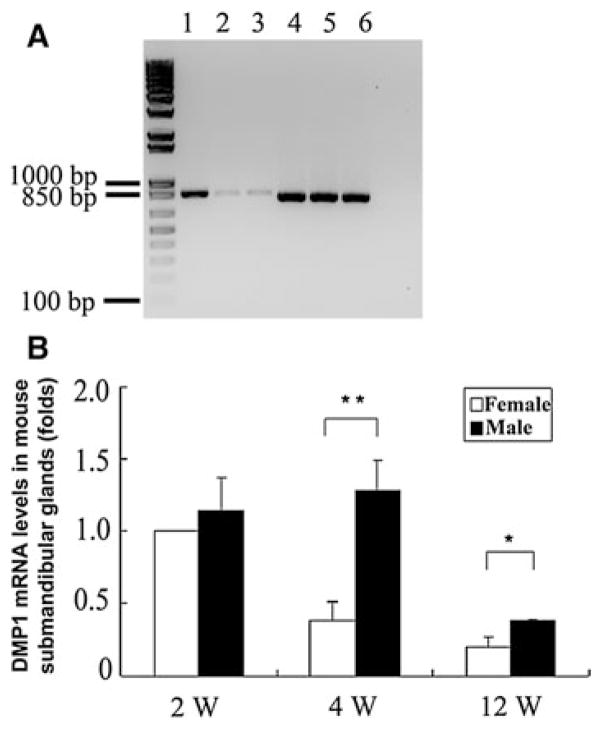
To examine the expression of DMP1 in mouse SMG, we initially performed RT-PCR to investigate the presence of DMP1 mRNAs in the SMG of both male and female mice. Gel electrophoresis of PCR products showed that specific 847 bp products were amplified in the SMG from both male and female mice (Fig. 1a, lanes 2, 3), whose size was identical to that when we used the RNA samples isolated from the mouse incisor as the template (Fig. 1a, lane 1). DNA sequencing confirmed that the PCR products in lanes 2 and 3 were from DMP1 mRNA. Using qRT-PCR, we confirmed the RT-PCR results and quantified the mRNA levels of DMP1 in SMG from male and female mice at different ages. For qRT-PCR analyses, the mRNA level in SMG from 2-week-old female mice was taken as the standard and its value was set at 1. All results were normalized with the loading control GAPDH. At postnatal 2 weeks, mRNA levels of DMP1 in female and male mouse SMG showed no significant difference (P > 0.05). However, the mRNA levels of DMP1 in male mouse SMG were significantly higher than those in female mouse SMG at ages of 4 and 12 weeks after birth (Fig. 1b).
Fig. 1.
Total DMP1 mRNA levels in male and female mouse SMG. a Agarose gel electrophoresis of reverse transcriptase-polymerase chain reaction (RT-PCR) products from the 4-week-old mouse submandibular glands. Lane 1 RNA from mouse incisor (positive control), lane 2 from submandibular glands of male mouse, lane 3 from submandibular glands of female mouse, lane 4 RT-PCR products for GAPDH with tooth RNA as the template, lane 5 RT-PCR products for GAPDH with male mouse submandibular gland RNA as the template, lane 6 RT-PCR products for GAPDH with female mouse submandibular gland RAN as the template. b Real-time quantitative PCR (qRT-PCR) using RNA samples extracted from 2-, 4-, or 12-week-old male and female mouse SMG. The mRNA level of DMP1 in 2-week-old female mouse SMG was set at 1. DMP1 mRNA levels in the SMG of male and female mice at different ages (4 or 12 weeks) were calculated relatively to that in the SMG of 2-week-old female mice. All the quantitative data were expressed as mean ± standard deviation (SD) of 6 individual determinations (n = 6), statistical difference was determined using the Student’s t test. *P < 0.05; **P < 0.01
The proteolytic fragments of DMP1 in mouse SMG
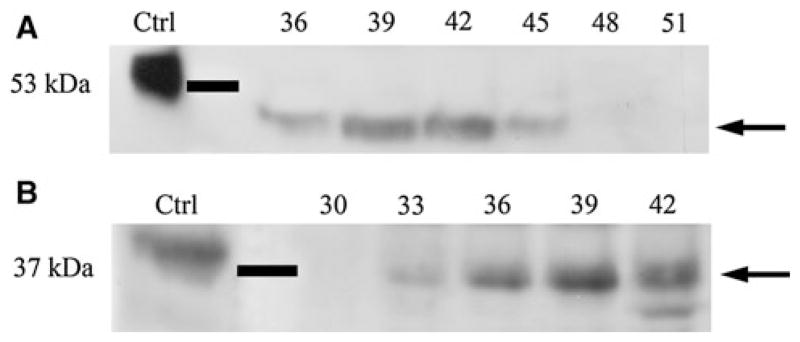
Previous studies showed that in the mineralized tissues, DMP1 mainly exists as its proteolytically processed fragments including 57 kDa C-terminal fragment, 37 kDa N-terminal fragment and the proteoglycan form of DMP1 referred to as DMP1-PG. In this study, Q-Sepharose ion-exchange chromatography separated Gdm–HCl extracts of the SMGs into 120 fractions. Proteins separated on SDS–PAGE were assayed by western immunoblotting. As shown in Fig. 2a, different from the 57 kDa C-terminal fragment in bone and dentin, the migration rate of C-terminal fragment in mouse SMG was about 46 kDa (Fig. 2a, arrow). The migration rate (~37 kDa) for the N-terminal fragment of DMP1 in the SMG was same as that in the mineralized tissues (Fig. 2b, arrow).
Fig. 2.
The proteolytically processed fragments of DMP1 in mouse SMG. Ten 12-week-old mice were used to extract proteins and then perform western immunoblotting. a Western immunoblotting for the C-terminal fragment of DMP1 in mouse SMG using the polyclonal antibody anti-DMP1-C-857. Positive control (Ctrl) was 1 μg of C-terminal fragment of DMP1 isolated from the rat incisors, which migrated at 57 kDa. Approximately 46 kDa protein band representing the C-terminal fragment of DMP1 (arrow) was observed in fractions 36–45 of the Q-Sepharose chromatography. b Western immunoblotting for the N-terminal fragment in mouse SMG using the monoclonal antibody anti-DMP1-N-9B6.3. Positive control (Ctrl) was 1 μg of DMP1 isolated from rat incisors. The 37 kDa N-terminal fragment of DMP1 (arrow) was detected in fractions 33–42
Different localization of DMP1 fragments in mouse SMG
Mouse SMG was primarily composed of seromucous acini and ductal system. The ductal system consists of ID, SD, GCT and ED. A variety of collagenous and non-collagenous proteins were synthesized, secreted and transported through above units within SMG. DMP1, an acidic non-collagenous protein, was mainly present in the mineralized tissues as proteolytically cleaved fragments. For IHC analyses, we used two different types of antibodies, anti-DMP1-C-8G10.3 and anti-DMP1-N-859, to detect the C-terminal fragment and the N-terminal fragment of DMP1, respectively. Based on the evident sexual dimorphism in the salivary glands of mice, the SMG from male and female mice at different developmental stages were analyzed by IHC.
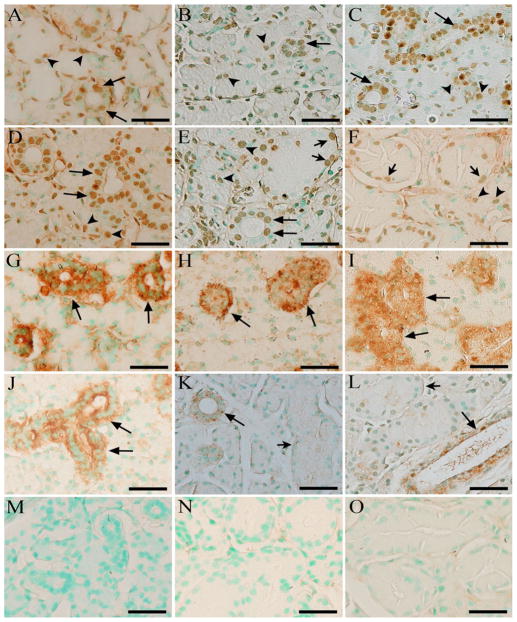
At 2 weeks after birth (Fig. 3a, d, g, j, m), GCT did not appear in the SMG and the SMG from male and female mice exhibited similar histological structures. The signals for the C-terminal fragment of DMP1 were observed in the nuclei of ID and SD cells, as well as in the seromucous acini cells (Fig. 3a, d), while the signals for the N-terminal fragment of DMP1 were limited to the cytoplasm of ductal cells (Fig. 3g, j). At postnatal 4 weeks (Fig. 3b, e, h, k, n), GCT could be evidently recognized in the female and male mouse SMG. Like those in 2-week-old mice, strong IHC reactions to the C-terminal fragment were also found in the nuclei of acinar and ductal cells (Fig. 3b, e). However, the signals for the N-terminal fragment were only observed in the ductal cells, except for the GCT in male mice (Fig. 3h, k). At 12 weeks old (Fig. 3c, f, i, l, o), the histological differences between male and female mouse SMG were more remarkable than those at the age of 4 weeks. The GCT in 12-week-old male mouse SMG was larger and more abundant than the GCT in female mice of the same age. Despite the marked changes in male mice, the positive immunostaining signals against the C-terminal fragment of DMP1 were still observed in the nuclei of acinar cells and ductal cells (Fig. 3c, f). On the contrary, the expression of the N-terminal fragment of DMP1 was not found in the GCT of male mice and acini (Fig. 3i, l).
Fig. 3.
IHC revealed different localization patterns for the C- and N-terminal fragments of DMP1 in mouse SMG. Column 1 2-week-old mouse SMG (a, d, g, j, m), column 2 4-week-old mouse SMG (b, e, h, k, n), column 3 12-week-old mouse SMG (c, f, i, l, o). Female (a–c, g–i) and male (d–f, j–o) mice were used for the analyses of DMP1 expression in different structures of the SMGs. Six male or female mice at postnatal 2, 4 or 12 weeks were used to for IHC assays (n = 6 in each group). The monoclonal antibody anti-DMP1-C-8G10.3 and the polyclonal antibody anti-DMP1-N-859 were used to recognize the C-terminal fragment (a–f) and N-terminal fragment (g–l) of DMP1, respectively, while mouse IgG at the same concentration was used as the negative control (m–o). Nuclei were stained by methyl green dye. At postnatal 2 weeks, GCTs were not observed in female (a, g) and male (d, j, m) mouse SMG. At 4 and 12 weeks after birth, GCT appeared in female and male mouse SMG, but more evidently identified in male mice (e, f, k, l, n, o). Immunohistochemistry reaction to the C-terminal fragment of DMP1 was detected in the nuclei of acinar cells (arrowheads) and ductal cells (arrows). Note that the localization of the C-terminal fragment of DMP1 was evidently observed in the basal region of the nuclei in GCT cells (e, f short arrows), central region of nuclei in SD or ID cells (a–e long arrows) and nuclei of acinar cells (a–f arrowheads) while the signals against the N-terminal fragment of DMP1 was restricted to ductal cells (g–l long arrows) except for the GCT cells of male mice (k, l short arrows). Scale bars 50 μm
Expression of DMP1 in the GCT of male mice
In previous studies, SIBLING proteins including DMP1 have been thought not to be expressed in the GCT cells of male mouse SMG. However, we revealed that DMP1 was expressed in the GCT cells of SMG using IHC staining (Fig. 3a–f) and we further confirmed the observation by ISH (Fig. 4) and β-galactosidase expression assay (Fig. 5). In IHC analysis, the signals for the C-terminal fragment of DMP1 were observed in the nuclei of GCT cells in the SMG of 4- and 12-week-old male mice. Strong immunohistochemical reactivity against the C-terminal fragment of DMP1 was observed within the basal nuclei of GCT cells, central nuclei of SD cells and nuclei of acinar cells (Fig. 3b, c, e, f). On the contrary, the signals for the N-terminal fragment of DMP1 were not detected in the GCT of 4- and 12-week-old male mouse SMG, but were observed in other ductal cells (Fig. 3k, l). On the other hand, positive signals of DMP1 mRNA were detectable in the GCT and other ductal cells, as well as the acinar cells of 12-week-old male mouse SMG with ISH staining (Fig. 4). In β-galactosidase expression assay, positive staining was detected in the GCT of 4-week-old male Dmp1 knockout mice (Fig. 5).
Fig. 4.
ISH for DMP1 mRNA in 12-week-old male mouse SMG. a Negative control using sense RNA probe of DMP1 mRNA. Sample size: 6. Scale bar 50 μm. ISH staining (red) for 12-week-old male mouse SMG using antisense RNA probe of DMP1 indicated DMP1 mRNA in acinar cells (arrowheads) and GCT cells (arrows). Nuclei were stained by methyl green dye. Sample size: 6. Scale bar 50 μm
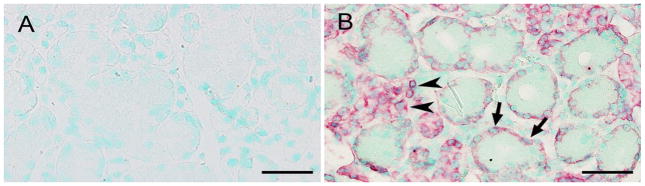
Fig. 5.
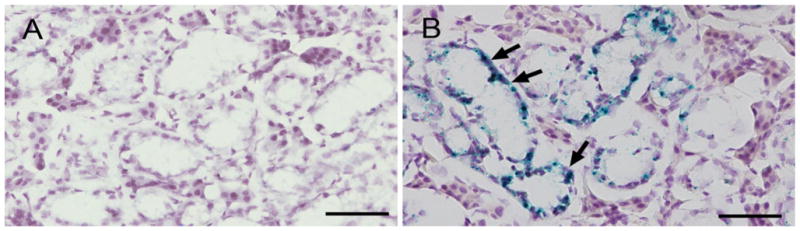
DMP1–lacZ expression pattern in 4-week-old male homozygous Dmp1 knockout mouse SMG. a Negative control of SMG from 4-week-old wild type C57BL/6J male mice. Sample size: 6. Scale bar 50 μm. b Positive signals were observed in the GCT of 4-week-old male Dmp1 knockout mouse SMG (arrows). Hematoxylin was used for counterstaining. Sample size: 6. Scale bar 50 μm
Discussion
GCT has been proven to be a specialized segment of striated duct in the ductal system of rodent SMGs (Gresik 1980). The most striking feature of GCT distinct from other salivary gland ducts is that GCT cells synthesize and secrete a large number of biologically active polypeptides such as epidermal growth factor (EGF), nerve growth factor (NGF), renin and members of the kallikrein gene family (Gresik 1980). In male mice, GCT contains pyramidal cells with secretion granules in the upper half of cells, basal nuclei and lightly basophilic cytoplasm, while the GCT in female mice have smaller and fewer secretion granules, and central nuclei. The GCT in male mice is larger and more abundant than those in female mice, which characterizes a marked sexual dimorphism (Gresik 1994; Jayasinghe et al. 1990).
Previous studies demonstrated that DMP1 was expressed in the acinar cells and ductal cells, but not in the GCT of male mice (Ogbureke and Fisher 2004). However, in the present study, we observed the evident expression of DMP1 C-terminal fragment in the GCT cells of male mice at postnatal 4 and 12 weeks. Like in other ductal cells, the signals for the C-terminal fragment of DMP1 were also found in the nuclei of GCT cells. Moreover, both ISH and β-galactosidase expression assay verified the expression of DMP1 in the GCT of male mouse SMG. Generally, GCT is situated between ID and SD but more close to SD from its origin. In the SMG of rodent animals, GCT are absent in the ductal system at birth untill SD cells are transformed into GCT cells at postnatal 25th day (Gresik 1980). SD cells are often columnar with a centrally placed nucleus and acidophilic cytoplasm. It has been postulated that major histological and microenviromental changes take place in the cytoplasm of SD when the transformation proceeds, whereas their nuclei are rarely affected. Thus it is possible that the signals for the C-terminal fragment of DMP1 were still observed in the nuclei of ductal cells even after SD cells have converted into GCT cells, while the signals for the N-terminal fragment observed in the cytoplasm of SD were not detected in the GCT cells. On the other hand, the mRNA levels of DMP1 in 2-week-old male and female mice did not show significant differences, while the mRNA levels of DMP1 in 4- and 12-week-old male mouse SMG were obviously higher than those in the female mouse SMG of the same age (Fig. 1b). The observation that the male mouse SMG had larger amounts of GCT than the female mouse SMG but did not show a lower level of DMP1 mRNA, further strengthens our conclusion that DMP1 must be expressed in the GCT of male mice.
Another interesting finding in this study was that the N-terminal fragment and the C- terminal fragment of DMP1 had different localization patterns in mouse SMG. The localization of the N-terminal fragment was primarily observed in the cytoplasm of ductal system cells, while the localization of the C-terminal fragment was found in the nuclei of acinar and ductal cells. This observation was consistent with previous findings that DMP1 fragments have different distribution and localization in the mineralized tissues (Huang et al. 2008; Maciejewska et al. 2009). A more recent study provided additional solid evidence that DMP1 enters the nucleus and suggested that apart from its role as a constituent of dentin and bone matrix, DMP1 might play a regulatory role in a variety of cells and tissues (Siyam et al. 2012). The localization of the C-terminal fragment in the cell nuclei of mouse SMG indicates that DMP1 may play important regulatory roles in the development of salivary glands. DMP1 localized in the nucleus of osteoblasts was thought to be the unphosphorylated form, while DMP1 transported into the ECM was in the phosphorylated form (Narayanan et al. 2003). In the present study, the C-terminal fragment of DMP1 was observed in the nuclei of acinar and ductal cells in SMG; it is likely that the C-terminal fragment of DMP1 in the nuclei of acinar and ductal cells of mouse SMG was not phosphorylated, thus giving rise to a molecular weight of 46 kDa, lower than the C-terminal fragment of DMP1 isolated from dentin or bone matrix, which migrates at 57 kDa (Qin et al. 2003). The localization pattern of DMP1 N-terminal fragment in mouse SMG was in agreement with the distribution of matrix metalloproteinase 9 (MMP-9) previously reported (Nagel et al. 2004). MMP-9 is known as a partner of DMP1 and DMP1 may actively bind to pMMP-9 at Ser74 (Quarles 2008), resulting in co-localization of DMP1 and MMP-9 in many tissues. Based on the fact that DMP1 is primarily present as its proteolytic fragments and Ser74 is located in the N-terminal fragment of this protein (Qin et al. 2003), it is tempting to believe that DMP1 co-localized with MMP-9 in the ductal system of mouse SMG is actually the N-terminal fragment of this protein.
In summary, the expression of DMP1 mRNA and protein in the GCT ducts of male mice was confirmed with multiple approaches. DMP1 was not only present in mouse SMG but also showed some distinct expression patterns from other tissues. Although the main forms of DMP1 in SMG were similar to those in the mineralized tissues, different migration rate of the C-terminal fragment was observed in mouse SMG. The localization pattern of DMP1 in SMG was closely relevant to mouse age and gender. The N-terminal fragment of DMP1 was primarily limited to ductal system except for the GCT of male mice, while the C-terminal fragment of DMP1 was localized in the nuclei of acinar and ductal cells. Further studies need to be performed to explore the roles of DMP1 fragments in the SMGs.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant DE005092 and National Natural Science Foundation of China (NSFC) grant (No. 81171744). We thank Dr. Fei Liu of Texas A&M Health Science Center College of Medicine for his valuable suggestions in this study.
Contributor Information
Changcheng Li, Longjiang Scholar Laboratory, The First Affiliated Hospital of Harbin Medical University, 23 Youzheng Street, Harbin 150001, Heilongjiang, China. Department of Biomedical Sciences, Baylor College of Dentistry, Texas A&M University System Health Science Center, 3302 Gaston Ave. Room 400, Dallas, TX 75246, USA.
Xiaohua Xie, Longjiang Scholar Laboratory, The First Affiliated Hospital of Harbin Medical University, 23 Youzheng Street, Harbin 150001, Heilongjiang, China.
Xiaofang Wang, Department of Biomedical Sciences, Baylor College of Dentistry, Texas A&M University System Health Science Center, 3302 Gaston Ave. Room 400, Dallas, TX 75246, USA.
Yao Sun, Department of Biomedical Sciences, Baylor College of Dentistry, Texas A&M University System Health Science Center, 3302 Gaston Ave. Room 400, Dallas, TX 75246, USA.
Peihong Liu, Longjiang Scholar Laboratory, The First Affiliated Hospital of Harbin Medical University, 23 Youzheng Street, Harbin 150001, Heilongjiang, China.
Li Chen, Email: chenlihrbmu@163.com, Longjiang Scholar Laboratory, The First Affiliated Hospital of Harbin Medical University, 23 Youzheng Street, Harbin 150001, Heilongjiang, China.
Chunlin Qin, Email: cqin@bcd.tamhsc.edu, Department of Biomedical Sciences, Baylor College of Dentistry, Texas A&M University System Health Science Center, 3302 Gaston Ave. Room 400, Dallas, TX 75246, USA.
References
- Baba O, Qin C, Brunn JC, Wygant JN, McIntyre BW, Butler WT. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004;23:371–379. doi: 10.1016/j.matbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Chaplet M, De Leval L, Waltregny D, Detry C, Fornaciari G, Bevilacqua G, Fisher LW, Castronovo V, Bellahcene A. Dentin matrix protein 1 is expressed in human lung cancer. J Bone Miner Res. 2003;18:1506–1512. doi: 10.1359/jbmr.2003.18.8.1506. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, Owen M, Harris SE, MacDouquall M. Dentinmatrix protein 1, a target-molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 2002;17:1822–1831. doi: 10.1359/jbmr.2002.17.10.1822. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, Castranio T, Scott G, Bonewald LB, Mishina Y. The dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res. 2003;82:776–780. doi: 10.1177/154405910308201003. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tiss Res. 2003;44:33–40. [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Jain A, Tayback M, Fedarko NS. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501–8511. doi: 10.1158/1078-0432.CCR-04-1072. [DOI] [PubMed] [Google Scholar]
- Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–1204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- George A, Sabsay B, Simonian PA, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein: implications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, Butler WT, Boskey AL. Different forms of DMP1 play distinct roles in mineralization. J Dent Res. 2010;89:355–359. doi: 10.1177/0022034510363250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresik EW. Postnatal developmental changes in submandibular glands of rats and mice. J Histochem Cytochem. 1980;28:860–870. doi: 10.1177/28.8.6160181. [DOI] [PubMed] [Google Scholar]
- Gresik EW. The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microsc Res Tech. 1994;27:1–24. doi: 10.1002/jemt.1070270102. [DOI] [PubMed] [Google Scholar]
- He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, Hao J, George A. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst KL, Simmons D, Feng J, Aplin H, Dixon MJ, MacDougall M. Elucidation of the sequence and the genomic organization of the human dentin matrix acidic phosphoprotein 1 (DMP1) gene: exclusion of the locus from a causative role in the pathogenesis of dentinogenesis imperfecta type II. Genomics. 1997;42:38–45. doi: 10.1006/geno.1997.4700. [DOI] [PubMed] [Google Scholar]
- Huang B, Maciejewska I, Sun Y, Peng T, Qin DS, Lu Y, Bonewald L, Butler WT, Feng JQ, Qin C. Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tiss Int. 2008;82:401–410. doi: 10.1007/s00223-008-9140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasinghe NR, Cope GH, Jacob S. Morphometric studies on the development and sexual dimorphism of the submandibular gland of the mouse. J Anat. 1990;172:115–127. [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Gu TT, Luan X, Simmons D, Chen J. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 1998;13:422–431. doi: 10.1359/jbmr.1998.13.3.422. [DOI] [PubMed] [Google Scholar]
- Maciejewska I, Qin D, Huang B, Sun Y, Mues G, Svoboda K, Bonewald L, Butler WT, Feng JQ, Qin C. Distinct compartmentalization of dentin matrix protein 1 fragments in mineralized tissues and cells. Cells Tiss Organs. 2009;189:186–191. doi: 10.1159/000151372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel H, Laskawi R, Wahlers A, Hemmerlein B. Expression of matrix metalloproteinases MMP-2, MMP-9 and their tissue inhibitors TIMP-1, -2, and -3 in benign and malignant tumours of the salivary gland. Histopathology. 2004;44:222–231. doi: 10.1111/j.0309-0167.2004.01814.x. [DOI] [PubMed] [Google Scholar]
- Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho W, George A. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 2004;83:664–670. doi: 10.1177/154405910408300902. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. A comparative study of sialic acid-rich proteins in rat bone and dentin. Oral Sci. 2001;109:133–141. doi: 10.1034/j.1600-0722.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT. Evidence for the proteolytic processing of dentin matrix protein 1: identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- Qin C, Huang B, Wygant JN, Mcintyre BW, McDonald CH, Cook RG, Butler WT. A chondroitin sulfate chain attached to the bone dentin matrix protein 1 NH2-terminal fragment. J Biol Chem. 2006;281:8034–8040. doi: 10.1074/jbc.M512964200. [DOI] [PubMed] [Google Scholar]
- Quarles L. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbadini E, Berczi I. The submandibular gland: a key organ in the neuro-immunoregulatory network? Neuroimmunomodulation. 1995;2:184–202. doi: 10.1159/000097197. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Hu Y, Richardson AS, Bartlett JD, Hu JC. Why does enamel in Klk4-null mice break above the dentino–enamel junction? Cells Tiss Organs. 2011;194:211–215. doi: 10.1159/000324260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siyam A, Wang S, Qin C, Mues G, Stevens R, D’Souza RN, Lu Y. Nuclear localization of DMP1 proteins suggests a role in intracellular signaling. Biochem Biophys Res Commun. 2012;424:641–646. doi: 10.1016/j.bbrc.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab. 2004;22:430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- Toyosawa S, Shintani S, Fujiwara T, Ooshima T, Sato A, Ijuhin N, Komori T. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16:2017–2026. doi: 10.1359/jbmr.2001.16.11.2017. [DOI] [PubMed] [Google Scholar]
- Wang X, Hao J, Xie Y, Sun Y, Hernandez B, Yamoah AK, Prasad M, Zhu Q, Feng JQ, Qin C. Expression of FAM20C in the osteogenesis and odontogenesis of mouse. J Histochem Cytochem. 2010;58:957–967. doi: 10.1369/jhc.2010.956565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]