Abstract
Background:
Glioma stem-like cell (GSC) properties are responsible for gliomagenesis and recurrence. GSCs are invasive but its mechanism remains to be elucidated. Here, we attempted to identify the molecules that promote invasion in GSCs.
Methods:
Neurospheres and CD133+ cells were collected from glioblastoma (GBM) specimens and glioma cell lines by sphere-formation method and magnetic affinity cell sorting, respectively. Differential expression of gene candidates, its role in invasion and its signaling pathway were evaluated in glioma cell lines.
Results:
Neurospheres from surgical specimens attached to fibronectin and laminin, the receptors of which belong to the integrin family. Integrin α3 was overexpressed in CD133+ cells compared with CD133− cells in all the glioma cell lines (4 out of 4). Immunohistochemistry demonstrated the localisation of integrin α3 in GBM cells, including invading cells, and in the tumour cells around the vessels, which is believed to be a stem cell niche. The expression of integrin α3 was correlated with migration and invasion. The invasion activity of glioma cells was linked to the phosphorylation of extracellular signal–regulated kinase (ERK) 1/2.
Conclusion:
Our results suggest that integrin α3 contributes to the invasive nature of GSCs via ERK1/2, which renders integrin α3 a prime candidate for anti-invasion therapy for GBM.
Keywords: glioma, stem-like cell, invasion, integrin
Gliomas are the most common primary tumours of the central nervous system, with glioblastomas (GBMs) being the most malignant entity. The poor prognosis of GBM patients is largely due to the highly invasive nature of these tumours. These invading cells are extremely resistant to radiation and chemotherapy, and currently, there are no anti-invasive therapies available (Nakada et al, 2007, 2013). A better understanding of the glioma invasion mechanism will help in developing therapeutic strategies to combat GBM.
An increasing body of evidence suggests that a subpopulation of tumour stem-like cell properties in glioma, called either glioma stem-like cells (GSCs) or glioma-initiating cells, is responsible for tumour formation, maintenance, and malignant progression (Singh et al, 2003; Tamase et al, 2009; Natsume et al, 2011). These rare tumour cells are characterised by their strong tumourigenic properties and self-renewal ability. It is critical to understand how the properties of GSCs make them particularly difficult to eradicate. From our data and data from other studies, it is clear that GSCs are primarily responsible for invasion (Liu et al, 2006; Beier et al, 2007; Tamase et al, 2009). However, the molecular features of GSCs that orchestrate the invasion process remain to be elucidated. If we can identify the responsible molecules that mediate GSC invasion, these molecules may represent promising targets for the development of novel anti-invasive therapies.
In GSCs, the expression of some neural stem markers such as nestin, SOX2, and Musashi-1 has been reported (Ignatova et al, 2002). In addition, CD133 has been evaluated as an enrichment marker for GSCs; however, several studies have demonstrated their limitations as specific markers (Beier et al, 2007; Wang et al, 2008). To date, there is no perfect GSC marker that can isolate these cells alone. GSCs tend to form neurospheres in the specific culture medium containing various kinds of growth factors, such as epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). The neurosphere assay is one of the reasonable methods for isolating GSCs. However, a previous report showed that the microenvironment in the neurosphere induces cell differentiation, suggesting that the neurosphere cannot maintain the stem cell properties on its own (Pollard et al, 2009). Therefore, it is difficult to obtain pure cell groups of GSC. However, it is reasonable to investigate the molecular characteristics of GSCs using cell groups that containing high numbers of GSCs. In this study, we used neurosphere methods and cell sorting with CD133 as a marker in order to investigate the biology of GSCs.
Integrins are cell surface migration-promoting receptor glycoproteins that mediate various intracellular signals through interaction with the extracellular matrix (ECM). Integrins also have a significant role in the attachment of cells to the ECM, through the formation of cell adhesion complexes, consisting of integrins and many cytoplasmic proteins. In particular, in GBMs, integrins participate in the regulation of complex processes, such as invasion, tumour growth, and angiogenesis by interacting with the ECM in the brain (Nakada et al, 2007). A phase II clinical trial demonstrated that combining the integrin inhibitor cilengitide with standard chemoradiation improved the survival of patients with newly diagnosed GBMs (Nabors et al, 2012). Currently, cilengitide is under phase III clinical trials for patients with GBM and the results will be reported soon (Kurozumi et al, 2012).
In this study, we report that GSCs attach to fibronectin and laminin and highly express integrin α3. The immunohistochemistry demonstrated that integrin α3 is localised in GBM cells, especially in invading cells and cells surrounding vessels in vivo. Additionally, overexpression of integrin α3 increases glioma migration and invasion, whereas downregulation of integrin α3 inhibits glioma invasion concomitant with a change in the phosphorylation level of the extracellular signal–regulated kinase (ERK) 1/2 pathway. These results suggest that integrin α3 have significant roles in the invasive behavior of GSCs through the activation of ERK1/2.
Materials and methods
Clinical samples and histology
Under an institutional review board-approved protocol and an informed consent from patients, fresh human GBM tissues were obtained from two patients who underwent therapeutic removal. Histological diagnosis was made by standard light-microscopic evaluation of the sections stained with hematoxylin and eosin. The classification of human brain tumours used in this study is based on the revised World Health Organisation criteria for tumours of the central nervous system (Louis et al, 2007).
Tumour neurospheres
For GBM neurosphere formation, cells were cultured in DMEM/F12-based serum-free growth medium containing insulin (25 μg ml−1), transferrin (100 μg ml−1), progesterone (20 nℳ), sodium selenate (30 nℳ), EGF (20 ng ml−1), and bFGF (20 ng ml−1) as described previously (Tamase et al, 2009; Muraguchi et al, 2011). All reagents were from Sigma-Aldrich (St Louis, MO, USA) except for EGF and bFGF, which were obtained from Invitrogen (Carlsbad, CA, USA).
Antibodies and reagents
Primary antibodies recognising the following proteins were used for immunostaining assays: CD133 (Miltenyi Biotec, Auburn, CA, USA), Nestin (Abcam, Cambridge, England), integrin α3 (monoclonal; Abcam), β-actin (Sigma-Aldrich), ERK1/2 (Cell Signaling Technology, Danvers, MA, USA), and phosphorylated ERK1/2 (Thr 202/Tyr 204; Cell Signaling Technology). PD98059, a mitogen-activated protein kinase (MAPK) kinase inhibitor, was purchased from Cell Signaling Technology.
Immunocytostaining
Spheres were grown in precoated chamber slides and fixed with 4% paraformaldehyde for 30 min at room temperature, and permeabilised for 5 min in 0.1% TX-100 PBS buffer. After washing with PBS, cells were blocked with 2% BSA and 3% goat serum and incubated with following antibodies: anti-CD133/2 (1 : 10), anti-nestin (1 : 200), and integrin α3 (1 : 200) for 1 h at 25 °C. Controls were stained with a 1 : 50 dilution of preimmunisation goat sera. Primary antibodies were detected using Alexa Fluor 488-labeled goat anti-mouse IgG or Alexa Fluor 594-labeled rabbit anti-goat IgG (Molecular Probes, Eugene, OR, USA). Fluorescence was monitored by inverted confocal laser microscopy (LSM510 META SP; Carl Zeiss AG, Oberkochen, Germany).
Adhesion of tumour spheres
Tumour neurospheres were seeded onto a six-well plate coated by fibronectin (10 μg ml−1; Sigma-Aldrich) or laminin (10 μg ml−1; Sigma-Aldrich) and incubated in the sphere medium described above at 37 °C with 5% CO2. They were observed after 72 h.
Cell culture
Human astrocytoma cell lines U87, U251, T98G, and SNB19 (American Type Culture Collection, Manassas, VA, USA) were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) at 37 °C.
Isolation of CD133+ cells
The cells from surgical specimens and cell lines were suspended in labeling buffer (0.5% bovine serum albumin and 2 mℳ EDTA in PBS). The cells were incubated with indirect CD133 MicroBead Kit (Miltenyi Biotec) for 30 min at 4 °C. Cell sorting was performed by autoMACS (Miltenyi Biotec), and later, the CD133 positively labeled with the magnetic microbeads were separated to obtain the positive fraction as previously described (Ohtsuki et al, 2007).
Real-time quantitative reverse transcription (QRT)-PCR
QRT-PCR was carried out in a LightCycler (Roche Diagnostics, Indianapolis, IN, USA) as described previously (Nakada et al, 2006). PCR was performed with the following primers: CD133(NM_006017.1): sense, 5′-TTGTGGCAAATCACCAGGTA-3′ antisense, 5′-TCAGATCTGTGAACGCCTTG-3′ (amplicon size, 162 bp); integrin α2(NM_002203): sense, 5′-TTGACCTATCCACTGCCACA-3′ antisense, 5′-GTCAGAACACACACCCGTTG-3′ (amplicon size, 200 bp); integrin α3(NM_002204): sense, 5′-GGAAGGAACAAAGACAGGCAAAC-3′ antisense, 5′-GGTAGTGGTGAGTGAGAAGTGGC-3′ (amplicon size, 150 bp); integrin α5(NM_002205): sense, 5′-CTACAATGATGTGGCCATCG-3 -3′ antisense, 5′-GGATATCCATTGCCATCCAG-3′ (amplicon size, 198 bp); integrin αv(NM_002210): sense, 5′-AACTCAAGCAAAAGGGAGCA-3′ antisense, 5′-TGCAAGCCTGTTGTATCAGC-3′ (amplicon size, 223 bp); and β-actin(NM_001101): sense, 5′-CTACAATGAGCTGCGTGTGGC-3′ antisense, 5′-CAGGTCCAGACGCAGGATGGC-3′ (amplicon size, 271 bp). The LightCycler analysis software was used to analyse the PCR data, as described previously (Nakada et al, 2010).
Immunohistochemistry
Immunohistochemistry was performed using an avidin–biotin immunoperoxidase technique as previously described (Nakada et al, 2005). Anti- integrin α3 antisera or rabbit preimmune sera were used at a dilution of 1 : 200.
Immunoblot analysis
Western blot analysis was performed according to the standard procedure as described previously (Nakada et al, 2005). All antibodies were used at a dilution of 1 : 500.
Expression plasmid and cell transfection
An expression plasmid for human integrin α3 was designed as described previously (Mizuno et al, 2008). Transient transfection into U87, SNB19, and U251 cells was done using Effectene (Qiagen, Valencia, CA, USA). The overexpression by transient transfection using Effectene lasted for at least 96 h as described previously (Nakada et al, 2006). Cells transfected with empty plasmid vector were used as controls.
Silencing of endogenous integrin α3 with small interfering RNA (siRNA)
Purified, duplexed siRNAs for integrin α3 and control luciferase were purchased from Qiagen. The two target sequences of human integrin α3 (Genbank accession number NM_002204) were designed to be unique relative to the sequences of other integrin members; siTGA3-1 and siTGA3-2, catalogue number SI02628094 and SI02664095, respectively. Twenty nanomolar siRNA was transfected into cells cultured in 60-mm-diameter dishes using Lipofectamine 2000 (Invitrogen). Transfected cells were cultured for 48 h before use. The effects of siRNA lasted for at least 96 h as described previously (Nakada et al, 2006).
Cell migration and invasion assay
Chemotactic migration on fibronectin and laminin and invasion into Matrigel (Becton Dickinson, Franklin Lakes, NJ) of cells was measured in a modified Boyden chamber (Neuroprobe, Gaithersburg, MD, USA) as described previously (Yoshida et al, 2010).
Statistics
Statistical analyses were done using the unpaired Student's t-test for comparison between the two groups. Statistical significance among the three groups was assessed using Bonferroni Dunn test. P<0.05 was considered significant.
Results
GSCs attach to fibronectin and laminin
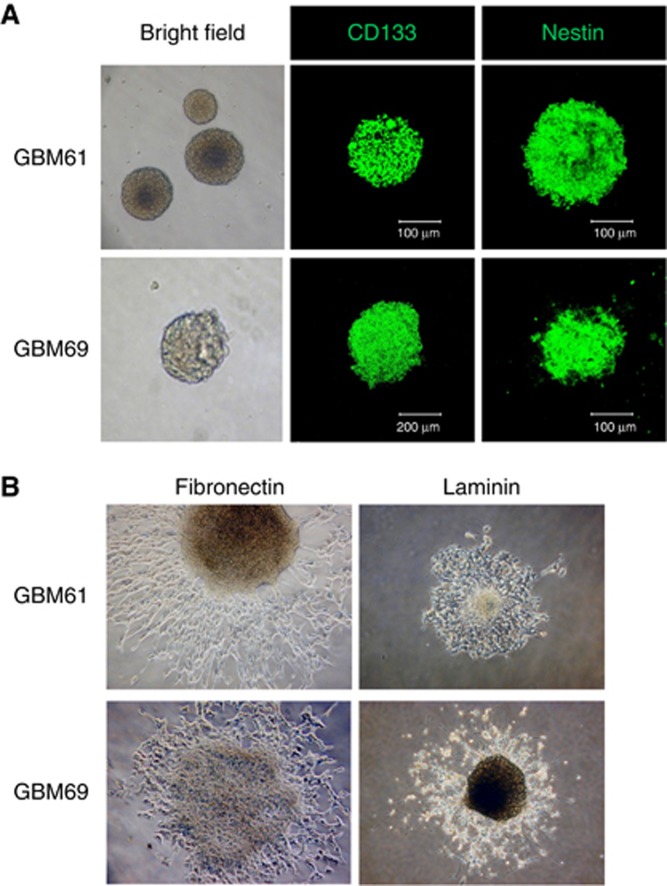
To confirm the expression of stem cell markers such as CD133 and nestin in GSCs extracted from GBM surgical specimens, immunocytochemistry was performed for the tumour neurospheres. CD133 and nestin were found to be expressed in the tumour neurospheres originating from two GBM specimens (Figure 1A). Additionally, differentiation of neurospheres was induced by adding 10% FBS. Immunocytochemical analysis with glial fibrillary acidic protein (GFAP), Olig2, and βIII-tubulin antibodies demonstrated positive staining, suggesting that the neurospheres obtained had multi-lineage potential, which is a characteristic of GSCs (Supplementary Data S1).
Figure 1.
Neurospheres formed by glioblastoma surgical specimens. (A) Neurospheres formed by glioblastoma surgical specimens (GBM61 and GBM69) in serum-free culture media containing 20 ng ml−1 of EGF and bFGF were immunostained with antibodies against glioma stem-like cell marker (CD133 and nestin). Note that the CD133 and nestin were immunostained in the cells. (B) Neurospheres from glioblastoma surgical specimens (GBM61 and GBM69) were cultured on plates coated with fibronectin (10 μg ml−1) or laminin (10 μg ml−1) in serum-free culture media containing 20 ng ml−1 of EGF and bFGF for 72 h. (original magnification × 100).
It has been reported that glioma-initiating cells are maintained in a tumour vascular microenvironment as the niche (Li and Neaves, 2006; Calabrese et al, 2007). As adhesion to the ECM is important for glioma-initiating cells to remain in the vascular niche, we investigated whether tumour spheres are capable of attaching to fibronectin and laminin, representative components of the ECM. As shown in Figure 1B, both tumour spheres attached to fibronectin and laminin. This suggests that glioma-initiating cells express the receptors for fibronectin and laminin.
Integrin α3 is overexpressed in CD133+ cells
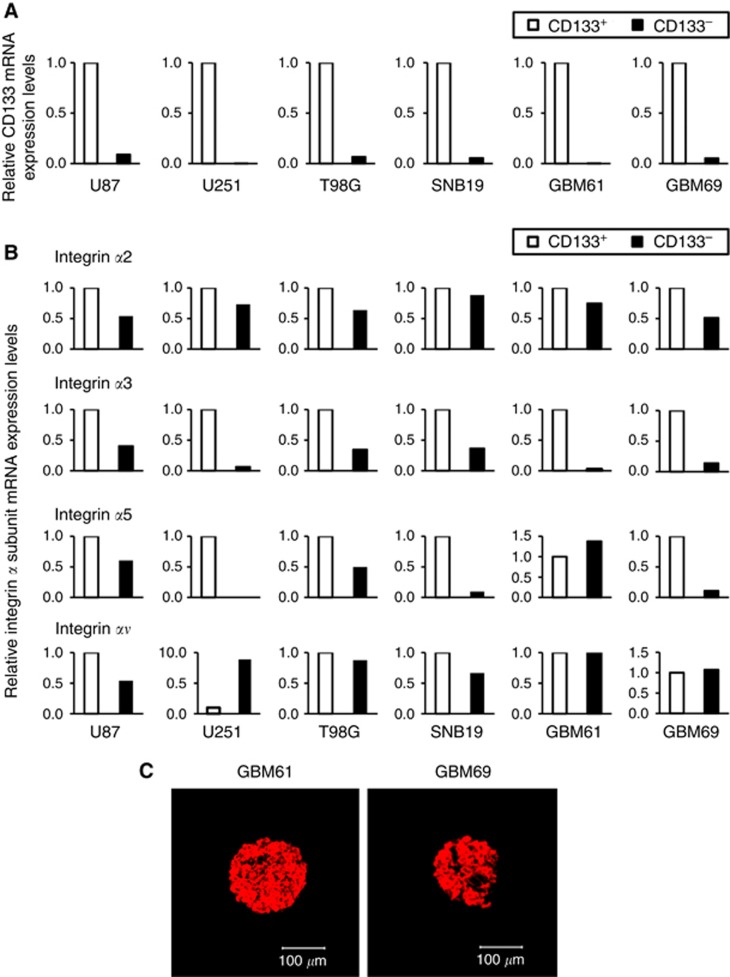
Because the integrin family has been described as having a role in cell adhesion, we evaluated the expression of the integrin α family members, especially α2, 3, 5, and v, which are known to be expressed in glioma (D'Abaco and Kaye, 2007), in CD133+ and CD133− cells isolated from four glioma cell lines and two GBM specimens. RNA was isolated from each cell population, and the expression levels of CD133 and integrin α family members were determined by QRT-PCR using β-actin mRNA as an internal quantitative reference. We confirmed that the levels of CD133 expression (CD133 mRNA/β-actin mRNA ratios) were significantly higher in the cells extracted as CD133+ cells by autoMACS than in CD133− cells in all the cell lines and surgical specimens (Figure 2A). Among the integrin α family members tested, only integrin α3 was overexpressed in CD133+ cells relative to CD133− cells (2.4–240-fold) in all the cell lines and surgical specimens (Figure 2B). Consistent with this observation, the expression of integrin α3 was confirmed in tumour neurospheres by immunocytochemistry (Figure 2C). Intriguingly, it has been previously reported that integrin α3 can be a receptor for both fibronectin and laminin (D'Abaco and Kaye, 2007). Accordingly, we focused on integrin α3 for further experiments.
Figure 2.
Expression of integrin in glioma cells. The relative mRNA expression levels of (A) CD133 and (B) integrin family members were calculated by QRT-PCR. Each mRNA level is expressed as a proportion of the CD133+ glioma cell mRNA level, which was given a value of 1. The QRT-PCR procedure was performed at least two times for each sample and the difference between obtained values was <3%. Results shown are typical of at least two replicate experiments. (C) Neurospheres derived from glioblastoma surgical specimens (GBM61 and GBM69) were immunostained for integrin α3. The spheres positive for integrin α3 were stained in red. (original magnification × 100).
Integrin α3 is expressed in invading glioma cells and in glioma cells surrounding vessels
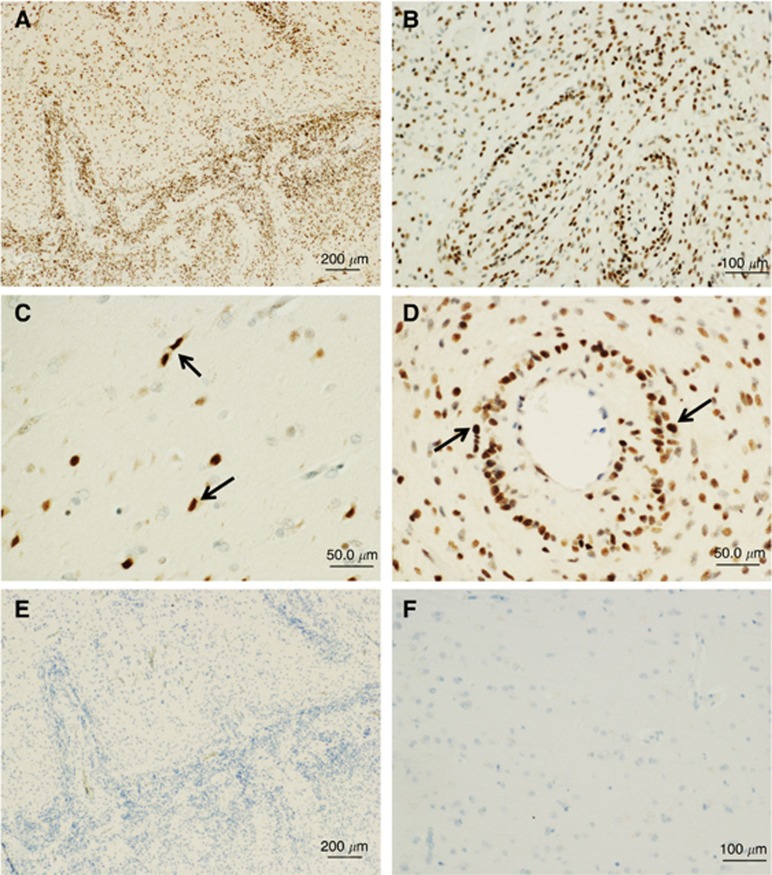
Cells expressing integrin α3 in the normal brain and GBM specimens were identified using immunohistochemical analysis. Integrin α3 was localised predominantly to neoplastic astrocytes in GBM specimens (21 out of 25 cases; Figures 3A and B). Invading neoplastic cells as well as glioma cells surrounding vessels also showed significant staining for integrin α3 (Figures 3C and D). Neoplastic astrocytes were identified by nuclear atypia in H&E-stained sections and were confirmed by immunopositivity for GFAP staining (data not shown). No staining was observed when the primary antibody was substituted with normal serum (Figure 3E). Normal cells showed no immunopositivity (Figure 3F). According to the well-known hypothesis that GSCs are highly invasive and are localised surrounding vessels, the so-called tumour niche (Liu et al, 2006; Calabrese et al, 2007), these data suggest that GSCs express integrin α3 in vivo.
Figure 3.
Immunolocalisation of integrin α3 in glioblastoma tissues and normal brain tissues. Paraffin sections were immunostained with (A–D, F) monoclonal antibodies against integrin α3 or (E) non-immune rabbit IgG, as described in the Materials and Methods section. (A) Invading front in low magnification (upper: invading area, lower: tumour core), (B) tumour core, (C) invading area in high magnification, (D) tumour vessels in high magnification, (E) tumour border, same portion as in A, and (F) normal brain. Note that integrin α3 is immunostained in the invading glioblastoma cells (C, arrows) and in the tumour cells surrounding vessels (D, arrows), while no staining is observed with non-immune IgG. Hematoxylin counterstaining was used. Bars=200 μm (A and E); 100 μm (B and F); 50 μm (C and D).
Depletion of integrin α3 decreases the migration of glioma cells
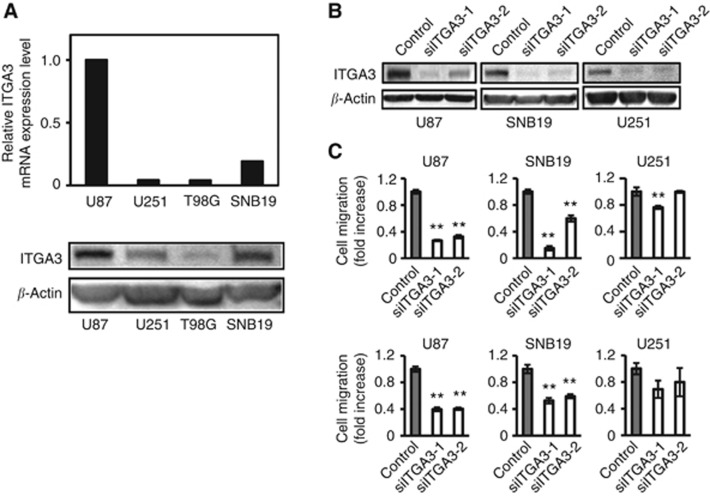
To determine the function of integrin α3 in common glioma cell lines, the endogenous level of integrin α3 in four glioma cell lines was assessed by QRT-PCR and western blotting. As shown in Figure 4A, integrin α3 expression is highest in U87, which has previously been shown to be the most rapidly migrating cell line among the four glioma cell lines tested (Nakada et al, 2004, 2006).
Figure 4.
Analysis of cell migration of glioma cell lines with knock down of integrin α3. (A) The relative mRNA expression levels of integrin α3 (ITGA3) were calculated by quantitative RT-PCR. The value in U87 cells was taken as 1. Extracts of U87, U251, T98G, and SNB19 cells were immunoblotted with antibodies against integrin α3 (ITGA3) or β-actin. (B) Extracts of U87, SNB19, or U251 cells treated by siRNA for integrin α3 (siITGA3-1 and siITGA-2) or control luciferase (control) were immunoblotted with antibodies against integrin α3 (ITGA3) or β-actin. (C) Cells treated by siRNA were plated on membranes coated with fibronectin (upper panel) or laminin (lower panel) in a chemotaxis chamber. Cells migrated to the underside of membrane for 6–8 h, and the migrated cells were stained with Diff-Quick and the absorbance measured at 490 nm as described in the Materials and Methods section. The mean absorbance (490 nm) value from mock cells in each cell line is shown as 1. Bar: mean±s.e. (n=6). Double asterisks indicate a significant difference from the control at a P-value of <0.01.
To evaluate the potential role of integrin α3 in the invasive behavior of human gliomas and to verify that integrin α3 could provide a potential therapeutic target, the migration assay was performed using an siRNA technique. We used two different siRNA sequences (siITGA3-1 and siITGA-2) specifically to silence endogenous integrin α3 expression in U87, U251, and SNB19 cells, which exhibit evident integrin α3 protein expression (Figure 4A). No obvious phenotypic changes were observed upon knock down of the integrin α3 gene in glioma cell lines (data not shown). The inhibition of integrin α3 mRNA by both siITGA3-1 and siITGA-2 was approximately 90% this did not affect the expression levels of other integrin family members (data not shown). Figure 4B illustrates the reduction of integrin α3 protein expression in integrin α3 siRNA-transfected U87, SNB19, and U251 cells as compared with its expression in luciferase siRNA-transfected controls. U87 and SNB19 cells transfected with integrin α3 siRNA showed significantly lesser cell migration on fibronectin (40%–82%) and laminin (40%–63%) than cells transfected with control siRNA. By contrast, U251 cells, which express lower levels of endogenous integrin α3 than seen in U87 and SNB19 cells, showed a smaller effect on the reduction of migration by siRNA on fibronectin (0%–23%) and laminin (20%–27%) relative to control cells (Figure 4C). These data confirm the role of integrin α3 in the promotion of migration in glioma cells.
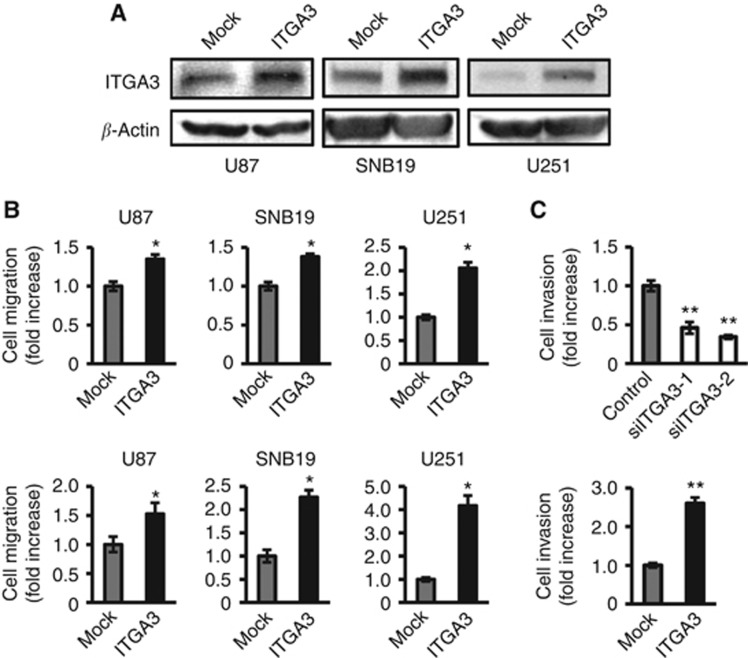
Integrin α3 promotes migration of glioma cells
To further examine the functional effects of integrin α3, the expression vector of integrin α3 was transiently transfected into the glioma cell lines utilised for siRNA experiments. As shown in Figure 5A, integrin α3 was successfully transfected into U87, SNB19, and U251 cells. No obvious phenotypic change was observed upon overexpression of the integrin α3 gene in glioma cell lines (data not shown). As expected, integrin α3 overexpression in all the three cell lines resulted in increased cell migration on both fibronectin and laminin, relative to that for mock-transfected cells (Figure 5B). U251, which has the lowest expression of integrin α3, showed the highest effect on invasion promotion on fibronectin and laminin (2.1-fold and 4.2-fold, respectively) upon the forced expression of integrin α3, whereas U87, which has the highest expression of integrin α3, showed the lowest effect of invasion promotion by integrin α3 overexpression (1.3-fold and 1.5-fold, respectively). These data support the specific role of integrin α3 signaling in the promotion of cell migration.
Figure 5.
Analysis of cell migration and invasion of glioma cell lines upon manipulating the gene expression of integrin α3. (A) Extracts of U87, SNB19, or U251 cells transiently transfected with integrin α3 (ITGA3) or empty plasmid vector (Mock) were immunoblotted with antibodies against ITGA3 or β-actin. (B) Transfected cells were plated on membranes coated with fibronectin (upper panel) or laminin (lower panel) in a chemotaxis chamber. Bar: mean±s.e. (n=6). Asterisk indicates a significant difference from the control at a P-value of <0.05. (C) U87 cells transfected with integrin α3 (ITGA3) or empty plasmid vector (Mock) (upper panel) and treated by siRNA for integrin α3 (siITGA3-1 and siITGA3-2) or luciferase (lower panel) were plated on membranes coated with Matrigel in a chemotaxis chamber. Cells were allowed to migrate to the underside surface of membranes for 24 h; invading cells were stained with Diff-Quick and the absorbance was measured at 490 nm, as described in Materials and Methods. The mean absorbance (490 nm) value from mock or control cells in each figure is shown as 1. Bar: mean±s.e. (n=6). Single and double asterisks indicate significant differences between mock and control at P-values of <0.05 and <0.01, respectively.
Expression of integrin α3 correlates with invasion activity of U87 cells
Next, an invasion assay was performed in U87 cells. The data showed similar outcomes to those seen in the migration assay. Two independent integrin α3 siRNAs inhibited cell invasion through membranes coated with Matrigel (50% less than that of control). By contrast, invasion was greater in cells overexpressing integrin α3 in U87 cells than in mock-transfected cells (2.6-fold) (Figure 5C). Taken together, these data indicate that interference with or forced expression of integrin α3 retards or accelerates glioma cell migration and invasion, respectively, suggesting a role of integrin α3 as an invasion promoter.
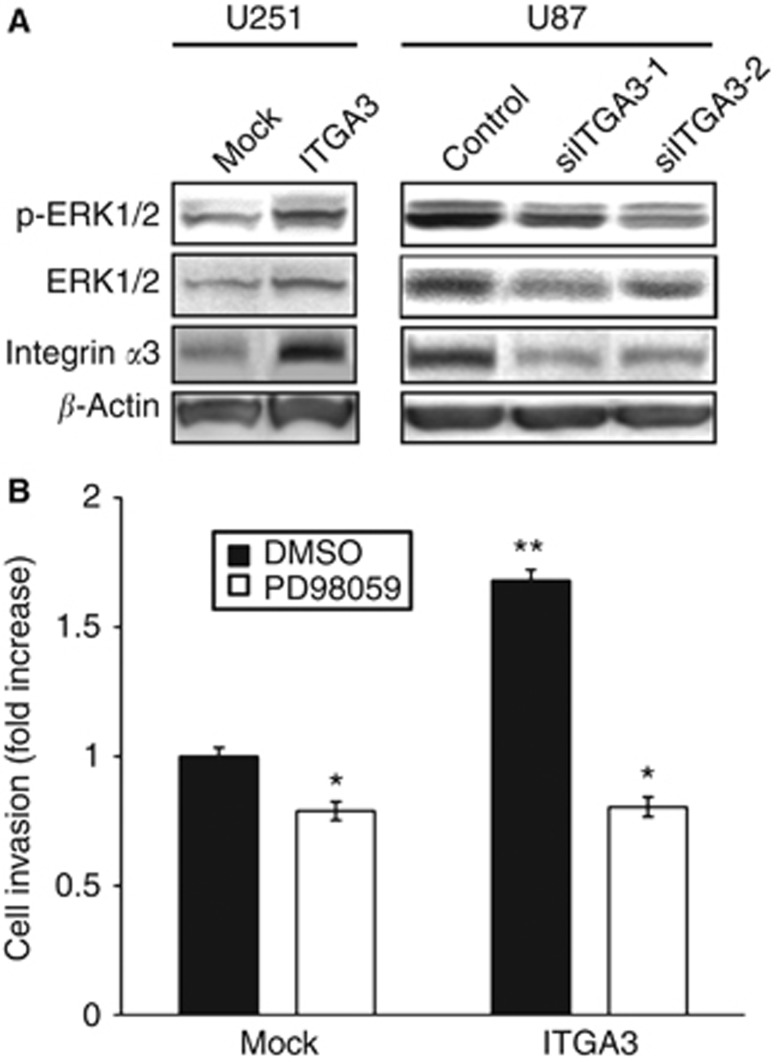
Integrin α3 promotes invasion via ERK1/2
As a possible mechanism by which integrin α3 regulates glioma cell invasion, we investigated the correlation between the expression of integrin α3 and ERK1/2, whose signaling was tightly linked to glioma invasion (Nakada et al, 2013). The total and phosphorylated protein levels of ERK1/2 in glioma cell lines manipulating integrin α3 expression were examined by western blotting, and the association of ERK1/2 signaling with integrin α3 levels was analysed. To observe the maximum change in signaling, we used U251 cells, which express low levels of integrin α3, for overexpression, and U87 cells, which express high integrin α3 levels, for siRNA treatment. In integrin α3-overexpressing U251 cells, phosphorylated ERK1/2 levels were higher along with increased total protein level, as compared with those in the control vector-transfected cells (Figure 6A). Thus, the production and activation of ERK1/2 were induced by integrin α3 signaling. Consistent with these data, the protein expression and phosphorylation levels of ERK1/2, along with inhibition of integrin α3 expression in U87 cells transfected with siRNA against integrin α3, were clearly lower than those for control siRNA treatment (Figure 6A). The expression and activation of other molecules that are involved in glioma invasion, such as Akt and matrix metalloproteinases, did not change upon manipulating the expression of the integrin α3 gene in glioma cells (Supplementary Data S2).
Figure 6.
Effect of integrin α3 on ERK1/2 and Rac activation. (A) U251 cells transiently transfected with integrin α3 (ITGA3) or empty plasmid vector (Mock) and U87 cells treated with siRNA against integrin α3 (siITGA3-1, siITGA3-2) or control luciferase (Control) were grown for 24 or 48 h. After starvation for 12 h, the cells were seeded on plates coated with fibronectin (10 μg ml−1) and were incubated for 30 min. Extracts of the cells were immunoblotted with antibodies against phosphorylated ERK1/2 (p-ERK1/2), total ERK1/2 (ERK1/2), integrin α3 (ITGA3), or β-actin. (B) U251 cells transiently transfected with integrin α3 (ITGA3) or empty plasmid vector (Mock) were plated on membranes coated with Matrigel in a chemotaxis chamber. Cells were allowed to migrate for 24 h in the absence or presence of 20 μℳ PD98059 to the underside of the membranes, and the invading cells were measured. Mean cell counts from at least six fields in each of the four experiments are shown. Bars represent s.e. *P<0.05; **P<0.01 vs Mock without PD98059.
We hypothesised that ERK1/2 is a major signaling molecule linked to integrin α3 in glioma invasion. To confirm this, we examined the role of ERK1/2 downstream of integrin α3 in glioma cells. An invasion assay was performed using U251 cells transfected with empty vector or integrin α3 with PD98059, which is a potent and selective inhibitor of MAPK kinase (MEK), the kinase immediately upstream of ERK1/2. Inhibition of MEK/ERK signaling suppressed the migration of mock-transfected U251 cells by 0.8-fold. Moreover, PD98059 treatment completely abrogated the integrin α3-induced invasion of U251 cells (Figure 6B). Taken together, these data indicate that integrin α3 is strongly associated with ERK1/2 production and activation and that this interaction is critical to the glioma cell invasion induced by integrin α3.
Discussion
Previous studies have shown that GSCs have high invasive potential (Liu et al, 2006; Beier et al, 2007). We aimed to reveal invasion-promoting genes in GSCs. Based on our data that GSCs attached to the ECM components, the expression of integrin family members was screened. Our data demonstrate that integrin α3 is specifically overexpressed in GSCs obtained from glioma cell lines and GBM surgical specimens by two different methods. Consistent with this observation, the immunohistochemical results indicated that integrin α3 is localised in invading glioma cells and glioma cells surrounding vessels, where the stem cell niche is believed to be located in vivo. Additionally, integrin α3 promotes the migration and invasion of glioma cells via the ERK1/2 pathway in vitro. Taken together, these data suggest that integrin α3 has a crucial role in GSC invasion.
Accumulated evidence indicated that integrins are expressed in stem cells and promote cell migration via ECM stimulation and the subsequent induction of migration signaling (Hynes, 2002; Desgrosellier and Cheresh, 2010; Ellis and Tanentzapf, 2010). Similarly, neurospheres originated from neuronal stem cells attach to the ECM through integrin, and its signaling contributes to neuronal migration and differentiation (Kearns et al, 2003; Leone et al, 2005; Flanagan et al, 2006). Our study revealed that GSCs also attach to the ECM. The specific pattern of integrin expression suggests that GSCs, similar to neuronal stem cells, are dependent on integrin for attachment to the ECM. Among the integrin family members, we focused on integrin α3 because it was overexpressed in CD133+ cells compared with CD133− cells in all the cell lines and GBM neurospheres tested, and spheres of GSCs attached to fibronectin and laminin, for which integrin α3 could be a receptor (D'Abaco and Kaye, 2007). Immunocytochemical analysis confirmed the expression of integrin α3 in spheres of GSCs. Coincidentally, recent work has identified integrin α6, which was not included in our investigation, as a glioma stem-cell marker and invasion promoter (Lathia et al, 2010). Taking these findings into consideration, we propose that integrin has a crucial role in the stem cell properties of glioma cells, such as their high invasiveness potential. Additional studies will be required to dissect the role of integrin α3 and α6 in glioma stem cell biology.
The data in the present study using siRNA and expression vector in vitro have demonstrated that integrin α3 is an invasion-promoting molecule in glioma cells. Our results are consistent with previous work using a blocking antibody against integrin α3, which indicated that integrin α3 is involved in the invasive character of glioma (Fukushima et al, 1998; Fujiwara et al, 2001). Moreover, integrin α3-specific expression in invading GBM cells shown by immunohistochemical analysis agrees with the previous finding of positive staining for integrin α3 in invading glioma cells in a mouse glioma model (Mahesparan et al, 2003). The expression level of integrin α3 in glioma cell lines is positively correlated with its invasive capacity as shown by our previous reports (Nakada et al, 2006, 2010). Taken together, these observations indicate that integrin α3 has major role in glioma invasion.
As integrins are obligate heterodimers containing two distinct chains, called the α and β subunits, we should consider not only the α but also the β subunits. Among the β subunit members, the β1 subunit has an important role in glioma biology, and its expression has been correlated with the invasive behavior of gliomas (Paulus et al, 1996). Previous data showed that integrin β1 controls proliferation, differentiation, and migration in neural stem cells (Campos et al, 2004; Tate et al, 2004). Integrin α3 can form a specific heterodimer with the β1 subunit (Fukushima et al, 1998). This may support the importance of integrin α3 and β1 subunit heterodimers in GSCs. Analysis of the expression of integrin β subunits in GSCs is ongoing in our laboratory.
Previous studies have reported that integrin αvβ3 cooperates with platelet-derived growth factor receptor and activates the Ras-ERK/MAPK signaling pathway, inducing glioma cell proliferation (Guo and Giancotti, 2004). Integrin α3β1 has been shown to activate ERK in some epithelial cells (Gonzales et al, 1999). However, the signal transduction of integrin α3 in the context of glioma migration and invasion remains unclear. This study is the first to demonstrate that integrin α3 promotes glioma invasion via ERK1/2. Similarly, it has been reported that ERK1/2 regulates glioma stem cell invasion through the co-localisation of N-cadherin and integrin α6 (Velpula et al, 2012). In our study, the change in ERK1/2 phosphorylation seems to be attributed to the downregulation of ERK1/2 expression, although many reports have suggested that integrin can alter ERK1/2 phosphorylation levels without any change in ERK expression. Phosphorylated ERK can dimerise and translocate into the nucleus, where it phosphorylates many substrates, including transcription factors (Ramos, 2008), suggesting that ERK might possibly regulate its own expression. Further analysis will be necessary to investigate the molecular mechanism of ERK regulation by integrin.
In summary, we have shown that GSCs have elevated levels of integrin α3, which is localised in invading cells and at the stem cell niche and can promote glioma cell invasion via ERK1/2. Our discovery of the involvement of integrin α3 in glioma stem cell invasion provides the insight that targeting integrin α3 has profound consequences on the glioma stem cell phenotype and may thus provide a therapeutic target for GBMs.
Acknowledgments
We thank Dr. Yoshio Endo for providing primers for integrin; and Akiko Imamura for technical assistance. This work was supported by Grants-in-aid for young scientists research from the Japanese Ministry of Education, Science, Sports, Technology and Culture (A-21689038 to MN), Grant-in-Aid for Scientific Research (C-23592117 to MN), Japan Society for the Promotion of Science (JSPS), The Yasuda Medical Foundation (to MN), Foundation for Promotion of Cancer Research (to MN), Osaka Cancer Research Foundation (to MN), and Extramural Collaborative Research Grant of Cancer Research Institute, Kanazawa University (to HS and MN).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–3444. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- D'Abaco GM, Kaye AH. Integrins: molecular determinants of glioma invasion. J Clin Neurosci. 2007;14:1041–1048. doi: 10.1016/j.jocn.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SJ, Tanentzapf G. Integrin-mediated adhesion and stem-cell-niche interactions. Cell Tissue Res. 2010;339:121–130. doi: 10.1007/s00441-009-0828-4. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res. 2006;83:845–856. doi: 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem. 2001;276:17550–17558. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- Fukushima Y, Ohnishi T, Arita N, Hayakawa T, Sekiguchi K. Integrin alpha3beta1-mediated interaction with laminin-5 stimulates adhesion, migration and invasion of malignant glioma cells. Int J Cancer. 1998;76:63–72. doi: 10.1002/(sici)1097-0215(19980330)76:1<63::aid-ijc11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Gonzales M, Haan K, Baker SE, Fitchmun M, Todorov I, Weitzman S, Jones JC. A cell signal pathway involving laminin-5, alpha3beta1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol Biol Cell. 1999;10:259–270. doi: 10.1091/mbc.10.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- Kearns SM, Laywell ED, Kukekov VK, Steindler DA. Extracellular matrix effects on neurosphere cell motility. Exp Neurol. 2003;182:240–244. doi: 10.1016/s0014-4886(03)00124-9. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Ichikawa T, Onishi M, Fujii K, Date I. Cilengitide treatment for malignant glioma: current status and future direction. Neurol Med Chir (Tokyo) 2012;52:539–547. doi: 10.2176/nmc.52.539. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Relvas JB, Campos LS, Hemmi S, Brakebusch C, Fassler R, Ffrench-Constant C, Suter U. Regulation of neural progenitor proliferation and survival by beta1 integrins. J Cell Sci. 2005;118:2589–2599. doi: 10.1242/jcs.02396. [DOI] [PubMed] [Google Scholar]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahesparan R, Read TA, Lund-Johansen M, Skaftnesmo KO, Bjerkvig R, Engebraaten O. Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol. 2003;105:49–57. doi: 10.1007/s00401-002-0610-0. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Ogura M, Saito Y, Sekine W, Sano R, Gotou T, Oku T, Itoh S, Katabami K, Tsuji T. Changes in adhesive and migratory characteristics of hepatocellular carcinoma (HCC) cells induced by expression of alpha3beta1 integrin. Biochim Biophys Acta. 2008;1780:564–570. doi: 10.1016/j.bbagen.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Muraguchi T, Tanaka S, Yamada D, Tamase A, Nakada M, Nakamura H, Hoshii T, Ooshio T, Tadokoro Y, Naka K, Ino Y, Todo T, Kuratsu J, Saya H, Hamada J, Hirao A. NKX2.2 suppresses self-renewal of glioma-initiating cells. Cancer Res. 2011;71:1135–1145. doi: 10.1158/0008-5472.CAN-10-2304. [DOI] [PubMed] [Google Scholar]
- Nabors LB, Mikkelsen T, Hegi ME, Ye X, Batchelor T, Lesser G, Peereboom D, Rosenfeld MR, Olsen J, Brem S, Fisher JD, Grossman SA. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306) Cancer. 2012;118:5601–5607. doi: 10.1002/cncr.27585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada M, Anderson EM, Demuth T, Nakada S, Reavie LB, Drake KL, Hoelzinger DB, Berens ME. The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int J Cancer. 2010;126:1155–1165. doi: 10.1002/ijc.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada M, Drake KL, Nakada S, Niska JA, Berens ME. Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res. 2006;66:8492–8500. doi: 10.1158/0008-5472.CAN-05-4211. [DOI] [PubMed] [Google Scholar]
- Nakada M, Kita D, Teng L, Pyko IV, Watanabe T, Hayashi Y, Hamada J. Receptor tyrosine kinases: principles and functions in glioma invasion. Adv Exp Med Biol. 2013;986:143–170. doi: 10.1007/978-94-007-4719-7_8. [DOI] [PubMed] [Google Scholar]
- Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007;64:458–478. doi: 10.1007/s00018-007-6342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada M, Niska JA, Miyamori H, McDonough WS, Wu J, Sato H, Berens ME. The phosphorylation of EphB2 receptor regulates migration and invasion of human glioma cells. Cancer Res. 2004;64:3179–3185. doi: 10.1158/0008-5472.can-03-3667. [DOI] [PubMed] [Google Scholar]
- Nakada M, Niska JA, Tran NL, McDonough WS, Berens ME. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 2005;167:565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume A, Kinjo S, Yuki K, Kato T, Ohno M, Motomura K, Iwami K, Wakabayashi T. Glioma-initiating cells and molecular pathology: implications for therapy. Brain Tumor Pathol. 2011;28:1–12. doi: 10.1007/s10014-010-0011-3. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Yamaguchi H, Asashima T, Terasaki T. Establishing a method to isolate rat brain capillary endothelial cells by magnetic cell sorting and dominant mRNA expression of multidrug resistance-associated protein 1 and 4 in highly purified rat brain capillary endothelial cells. Pharm Res. 2007;24:688–694. doi: 10.1007/s11095-006-9188-x. [DOI] [PubMed] [Google Scholar]
- Paulus W, Baur I, Beutler AS, Reeves SA. Diffuse brain invasion of glioma cells requires beta 1 integrins. Lab Invest. 1996;75:819–826. [PubMed] [Google Scholar]
- Pollard SM, Yoshikawa K, Clarke ID, Danovi D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, Squire JA, Smith A, Dirks P. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Tamase A, Muraguchi T, Naka K, Tanaka S, Kinoshita M, Hoshii T, Ohmura M, Shugo H, Ooshio T, Nakada M, Sawamoto K, Onodera M, Matsumoto K, Oshima M, Asano M, Saya H, Okano H, Suda T, Hamada J, Hirao A. Identification of tumor-initiating cells in a highly aggressive brain tumor using promoter activity of nucleostemin. Proc Natl Acad Sci USA. 2009;106:17163–17168. doi: 10.1073/pnas.0905016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate MC, Garcia AJ, Keselowsky BG, Schumm MA, Archer DR, LaPlaca MC. Specific beta1 integrins mediate adhesion, migration, and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci. 2004;27:22–31. doi: 10.1016/j.mcn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Velpula KK, Rehman AA, Chelluboina B, Dasari VR, Gondi CS, Rao JS, Veeravalli KK. Glioma stem cell invasion through regulation of the interconnected ERK, integrin alpha6 and N-cadherin signaling pathway. Cell Signal. 2012;24:2076–2084. doi: 10.1016/j.cellsig.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Nakada M, Sugimoto N, Harada T, Hayashi Y, Kita D, Uchiyama N, Yachie A, Takuwa Y, Hamada J. Sphingosine-1-phosphate receptor type 1 regulates glioma cell proliferation and correlates with patient survival. Int J Cancer. 2010;126:2341–2352. doi: 10.1002/ijc.24933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.