Abstract
Background:
Prostate cancer (PCa) is characterised by great heterogeneity of the disease progression rate. Tumours range from insignificant and not life threatening to high risk for relapse ones. Consequently, a large number of patients undergo unnecessary treatment. miR-145 is a well-documented tumour suppressor and its expression, which is regulated by the p53 pathway, has been found to be decreased in the majority of human malignancies. The aim of our study was to evaluate the clinical utility of miR-145 for the prognostication of PCa.
Methods:
Total RNA was isolated from 137 prostate tissue specimens obtained from 73 radical prostatectomy-treated PCa patients and 64 transurethral- or open prostatectomy-treated benign prostate hyperplasia (BPH) patients. Following polyadenylation and reverse transcription, miR-145 levels were determined by quantitative real-time PCR assay, using SNORD48 (RNU48) for normalisation purposes.
Results:
Downregulated miR-145 expression was found in PCa compared with BPH patients. The reduction of miR-145 expression in PCa was correlated with higher Gleason score, advanced clinical stage, larger tumour diameter and higher prostate-specific antigen (PSA) and follow-up PSA levels. In addition, higher risk for biochemical recurrence and significantly shorter disease-free survival (DFS) was found for the PCa patients expressing lower miR-145. Focusing on ‘low- and intermediate-recurrence risk' PCa patients, miR-145 loss was revealed to be a reliable predictor of biochemical relapse and poor DFS independent from Gleason score, clinical stage, PSA and patients' age.
Conclusion:
The loss of the tumour-suppressor miR-145 increases the risk for disease progression and predicts the poor survival of PCa patients.
Keywords: miR-145, molecular tumour markers, prostate cancer, disease-free survival, p53, risk stratification
Prostate cancer (PCa) prevails as the most common diagnosed malignancy in men and the third leading cause of cancer-related deaths in developed countries (Jemal et al, 2011). This is mainly attributed to the extension of the life expectancy and the widespread use of prostate-specific antigen (PSA) for population screening in the Western world.
One of the main features of PCa that physicians have to deal with is the heterogeneity of the disease, in terms of progression rate and whether it is life threatening. A large number of patients are suffering from clinically insignificant tumours, which would not have caused symptoms and death during the patient's life. This was further reinforced by the high rate of overdiagnosis and overtreatment introduced by the PSA screening of the male population (Andriole et al, 2009; Schroder et al, 2009; Diamandis, 2010). On the other hand, a significant portion of prostate tumours are rapidly progressive and responsible for the poor survival of the patients irrespective of active treatment (Trapasso et al, 1994; Zincke et al, 1994). As a consequence, the heterogeneity of prostate tumours is responsible for the diversity of outcomes of patients in the same risk group (Freedland, 2011) and the emerging need of novel biomarkers (Sardana et al, 2008).
MicroRNAs (miRNAs) are a rapidly growing family of small (∼22nt) endogenous non-coding RNA molecules able to regulate gene expression at the post-transcriptional level (Bartel, 2004). The mature miRNA binds usually the 3′-untranslated region (3′-UTR) of the mRNAs through complete or partially complementarity, resulting to the translational repression or/and the degradation of the target. Taking into account that miRNAs regulate gene expression, it becomes clear that the upstream regulation of their own levels is crucial for cellular homeostasis and behaviour. The deregulation of miRNA levels has been revealed as a hallmark of the majority of human malignancies (Calin and Croce, 2006). Consequently, the study of miRNAs is prominent in cancer-related research nowadays, aiming to clarify their role during tumourigenesis and to examine their clinical efficacy (Bartels and Tsongalis, 2009; Croce, 2009).
Among the large number of studied miRNAs, hsa-miR-145-5p (miR-145) has a well- characterised tumour-suppressor regulatory role in the cell microenvironment (Sachdeva and Mo, 2010b). Inhibition of cell growth, in terms of cell cycle arrest and apoptosis, is promoted by miR-145 through the silencing of MYC (c-Myc; Sachdeva et al, 2009), IRS-1 and -2 (Law et al, 2012), RTKN (Wang et al, 2009), EGFR and NUDT1 (Cho et al, 2011), TNSF10 (Zaman et al, 2010), SWAP70 (Chiyomaru et al, 2011), DFFA (DFF45; Zhang et al, 2010), CBFB, CLINT1 and PPP3CA (Ostenfeld et al, 2010). In addition, miR-145 has been found to protect from cancer cell invasion and metastasis throughout the repression of MUC1 (Sachdeva and Mo, 2010a), FSCN1 (Chiyomaru et al, 2010; Fuse et al, 2011), SERPINE1 (Villadsen et al, 2012) and RPS6KB1 (p70S6K1; Xu et al, 2012). Among the predicted targets of miR-145, SOX9 (Thomsen et al, 2010), IGF1R (Burfeind et al, 1996; Hellawell et al, 2002), ADAM17 (Karan et al, 2003) and DDC (Avgeris et al, 2008; Wafa et al, 2012) have already been found to have a crucial role during PCa establishment and progression, as well as to serve the clinical management of the patients.
MIR145 maps to the 5q32 chromosomal region close to MIR143, with which it is co-transcribed (Cordes et al, 2009). The expression of miR-145 is controlled by p53 (Sachdeva et al, 2009; Suzuki et al, 2009), FoxO (Gan et al, 2010), RREB1 (Kent et al, 2010) and C/EBP-β (Sachdeva et al, 2012) transcriptional factors, as well as by the methylation status of MIR145 promoter (Suh et al, 2011). Downregulated miR-145 levels has been documented in PCa (Porkka et al, 2007; Ozen et al, 2008; Schaefer et al, 2010a; Larne et al, 2012; Wach et al, 2012) and in the majority of human malignancies studied so far.
Although the tumour-suppressor role of miR-145 is well documented, there is no complete evaluation of its clinical utility for PCa patients. The aim of this study was the analysis of the miR-145 expression profile in prostate tumours, the correlation of its levels with the clinicopathological features of the disease and the evaluation of its prognostic significance for the patients.
Materials and methods
Study population
For the purpose of our study, 137 consecutive tissue samples were obtained from 73 patients diagnosed with PCa (Table 1) and 64 patients with benign prostate hyperplasia (BPH) at the ‘Laiko' General Hospital, Athens, Greece. Transurethral or open prostatectomy was performed in BPH patients, whereas PCa patients underwent radical retropubic prostatectomy. Following the removal of the prostate gland from the PCa patients, a tissue sample of approximately 200 mg was sectioned from the peripheral zone based on the preoperative features of the biopsy and on macroscopic findings. Thereafter, the tissue sample was divided into two mirror-image specimens, one of which was tested by a pathologist for the presence of malignancy, whereas the remaining one was immediately frozen in liquid nitrogen and stored at −80 °C until the analysis. Patients who had received hormonal therapy or radiotherapy before the surgery were not included in our study. Our study was approved by the ethics committee of ‘Laiko General Hospital' and performed with respect to the ethical standards of the Declaration of Helsinki, as revised in 2008.
Table 1. Correlation of miR-145 expression profile with PCa patients' Gleason score, Clinical stage, PSA, DRE and age.
| |
|
No. of patients (%) |
|
|
|---|---|---|---|---|
| Variable | No. of patients | miR-145-negativea | miR-145-positivea | P-value |
|
Gleason score | ||||
| 5 | 2 | 0 (0.0) | 2 (100.0) | 0.015b |
| 6 | 22 | 5 (22.7) | 17 (77.3) | |
| 7 (3+4) | 25 | 10 (40.0) | 15 (60.0) | |
| 7 (4+3) | 14 | 9 (64.3) | 5 (35.7) | |
| 8 | 7 | 5 (71.4) | 2 (28.6) | |
| 9 |
3 |
3 (100.0) |
0 (0.0) |
|
|
Gleason score | ||||
| ⩽6 | 24 | 5 (20.8) | 19 (79.2) | 0.004b |
| 7 | 39 | 19 (48.7) | 20 (51.3) | |
| ⩾8 |
10 |
8 (80.0) |
2 (20.0) |
|
|
Clinical stage | ||||
| pT2a | 17 | 4 (23.5) | 13 (76.5) | 0.047b |
| pT2b | 18 | 6 (33.3) | 12 (66.7) | |
| pT2c | 10 | 5 (50.0) | 5 (50.0) | |
| pT3a | 15 | 7 (46.7) | 8 (53.3) | |
| pT3b |
13 |
10 (76.9) |
3 (23.1) |
|
|
Clinical stage | ||||
| ⩽ pT2c | 45 | 15 (33.3) | 30 (66.7) | 0.030c |
| ⩾ pT3a |
28 |
17 (60.7) |
11 (39.3) |
|
|
PSA (ng ml–1) | ||||
| <4.0 | 7 | 0 (0.0) | 7 (100.0) | <0.001b |
| 4.0–10.0 | 43 | 14 (32.6) | 29 (67.4) | |
| ⩾ 10.0 | 22 | 18 (81.8) | 4 (18.2) | |
| Unknown |
1 |
|
|
|
|
DRE | ||||
| Negative | 27 | 9 (33.3) | 18 (66.7) | 0.145b |
| Positive | 44 | 23 (52.3) | 21 (47.7) | |
| Unknown |
2 |
|
|
|
|
Age (years) | ||||
| <65 | 36 | 15 (41.7) | 21 (58.3) | 0.887b |
| 65–74 | 32 | 15 (46.9) | 17 (53.1) | |
| ⩾75 | 4 | 2 (50.0) | 2 (50.0) | |
| Unknown | 1 | |||
Abbreviations: DRE=digital rectal examination; PCa=prostate cancer; RQ=relative quantification.
Cutoff =2.97 × 103 RQ units, equal to the 45th percentile of the PCa patients' cohort.
Calculated by χ2 test.
Calculated by Fisher's exact test.
For the evaluation of miR-145 prognostic significance, 62 PCa patients were successfully followed-up, whereas 11 patients were excluded from the monitoring plan either because of insufficient and unclear monitoring data or because they had received adjuvant therapy before any biochemical recurrence because of the fact that they were considered of high risk (e.g., positive surgical margins). National Comprehensive Cancer Network (NCCN) guidelines for PCa were used for the stratification of PCa patients according to their recurrence risk (Mohler et al, 2010). Biochemical relapse was defined by two consecutive measurements of serum PSA ⩾0.2 ng ml–1 (Boccon-Gibod et al, 2004). Disease-free survival (DFS) was defined as the interval between the radical prostatectomy and the time of biochemical relapse, or the time period between the surgery and the most recent measurement of serum PSA for the patients who did not present biochemical recurrence. As pre-treatment PSA was used as the last measurement prior the radical prostatectomy, whereas follow-up PSA was used as the most recent measurement of the non-relapsed patients or the PSA measurement at the time of biochemical recurrence of the relapsed ones.
Extraction and polyadenylation of total RNA
Following the pulverisation of 50–120 mg of prostate tissue, total RNA was isolated using TRI-Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA). Polyadenylation at the 3′-end of the RNAs was performed in a 15 μl reaction containing 1 μg of total RNA, 800 μℳ ATP and 1 U of E. coli Poly(A) Polymerase (New England Biolabs Inc., Ipswich, MA, USA), at 37 °C for 60 min (Shi and Chiang, 2005; Mavridis et al, 2013). Total RNA concentration and purity were determined spectrophotometrically at 260 and 280 nm, whereas RNA integrity was evaluated by agarose gel electrophoresis.
First-strand cDNA synthesis
The polyadenylated total RNA was thereafter used as template for the first-strand cDNA synthesis. Reverse transcription was carried out in a 20 μl reaction using 50 U MMLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA), 40 U recombinant ribonuclease inhibitor (Invitrogen) and 0.25 μℳ poly(T) adapter 5′-GCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTTTTTTTVN-3′ (V=G, A, C and N=G, A, T, C) as reaction primer, at 37 °C for 60 min (Shi and Chiang, 2005; Mavridis et al, 2013).
Quantitative real-time PCR (qPCR)
A SYBR-Green fluorescent-based qPCR assay was applied for the quantification of miR-145 levels (Shi and Chiang, 2005; Mavridis et al, 2013). Specific forward primers for the miR-145 and the small nucleolar RNA, C/D box 48 (SNORD48), also known as RNU48, were designed according to their published sequences (NCBI Reference Sequence: NR_029686.1 and NR_002745.1, respectively) and in silico specificity analysis. Specifically, the combination of the 5′-CCAGTTTTCCCAGGAATCCCTAA-3′ forward primer for miR-145 or the 5′-TGATGATGACCCCAGGTAACTCT-3′ forward primer for SNORD48 with the universal reverse primer 5′-GCGAGCACAGAATTAATACGAC-3′, which hybridises the above mentioned poly(T) adapter, amplifies a 65 bp miR-145- or a 105 bp SNORD48-specific amplicon, respectively (Supplementary Figure 1).
The qPCR was performed in the 7500 Real-Time PCR System using the sequence detection software (Applied Biosystems, Carlsbad, CA, USA). The 10 μl reaction mixture consists of Kapa SYBR Fast Universal 2X qPCR Master Mix (Kapa Biosystems Inc., Woburn, MA, USA), 200 nℳ of each PCR primer and 0.2 ng of cDNA. The thermal protocol consists of a 3-min polymerase activation step at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s and the primer annealing and extension step at 60 °C for 1 min.
Melting curve analysis and agarose gel electrophoresis were performed following the amplification in order to distinguish the accumulation of the specific reaction products from nonspecific ones or primer-dimers. Calibration curves for miR-145 and SNORD48 amplification were generated by a validation experiment using as template serial dilutions of a control cDNA covering eight orders of magnitude (10–10−6 ng cDNA) (Supplementary Figure 1). The linear increases of miR-145 (y= −3.57x+13.93; r2=0.999) and SNORD48 (y= −3.53x+18.88; r2=0.996) amplification indicate the 90.5% and 92.1% reaction efficiencies, respectively, as well as the absence of PCR inhibition by the template.
The expression analysis of miR-145 was carried out using the 2−ΔΔCT relative quantification (RQ) method (Livak and Schmittgen, 2001). Duplicate reactions were performed for each tested sample and the average CT (Avg. CT) was calculated for the quantification analysis. SNORD48 was used as an endogenous reference control and the LNCaP PCa cell line as our assay calibrator. More precisely, using the formula ΔCT=Avg. CTmiR-145–Avg. CTSNORD48, the miR-145 expression of each tested sample was normalised to the SNORD48 endogenous reference control of the sample. Thereafter, the normalised miR-145 expression levels of the tested samples were quantified relative to the expression of the calibrator (RQ unitscalibrator=1), using the formula RQ unitssample=2−ΔΔCT, where ΔΔCT= ΔCTsample–ΔCTcalibrator. No template controls, reverse transcriptase-negative controls, Poly(A) polymerase-negative controls and DNA template controls generated undetectable CT. The intra-assay %CV was calculated to be 3.7%.
Statistical analysis
The analysis of the differences in miR-145 levels between PCa and BPH patients was performed by the non-parametric Mann–Whitney U-test. To evaluate the ability of miR-145 to discriminate PCa patients from BPH ones, logistic regression and ROC analysis were used. Univariate and multivariate logistic regression models were used for the calculation of the odds ratio (OR) of log10miR-145, serum PSA levels, patient's age and digital rectal examination (DRE). The ROC curve was developed by plotting %sensitivity versus (100%-specificity) and the area under curve (AUC) was calculated by the Hanley and McNeil method.
To evaluate the miR-145 expression differences between the different Gleason score or clinical stage groups of patients, the non-parametric Mann–Whitney U and Kruskal–Wallis tests were used appropriately. Using the X-tile algorithm, the 2.97 × 103 RQ units of miR-145 (equal to the 45th percentile of the PCa patients' cohort) was adopted as an optimal cutoff value to classify PCa patients into miR-145 (+) and miR-145 (−) cohorts, expressing higher and lower miR-145 levels, respectively. The distribution of miR-145 (+) and miR-145 (−) patients to Gleason score, clinical stage, serum PSA levels, DRE and patients' age was analysed using the χ2 and Fisher's exact tests. The correlation of miR-145 levels with tumour diameter, pre-treatment PSA and follow-up PSA levels of PCa patients was determined by the Spearman's correlation coefficient (rs).
The significance of miR-145 expression for the DFS of PCa patients was assessed with Cox proportional hazards regression analysis as well as Kaplan–Meier survival analysis using the log-rank test. Hazard ratio (HR) was calculated for miR-145, Gleason score, clinical stage, serum PSA levels, patient's age and DRE, using both univariate and multivariate regression models. Kaplan–Meier survival curves were generated by plotting the %DFS probability versus the time period following the radical prostatectomy.
Results
miR-145 expression is downregulated in PCa patients
Statistically significant (P=0.037) underexpression of miR-145 was detected in the PCa tissues (range: 38.36–1.67 × 105 RQ units; median: 3.80 × 103 RQ units) compared with the BPH ones (range: 898.57–3.37 × 105 RQ units; median: 6.07 × 103 RQ units).
ROC analysis (AUC: 0.604; 95% CI: 0.509–0.698; P=0.037) illustrated that miR-145 loss in PCa can discriminate the malignant from the benign specimens. Moreover, the univariate logistic regression highlighted a statistically significant (P=0.020) lower risk of suffering from PCa for the patients with high miR-145 levels (OR: 0.484; 95% CI: 0.263–0.892). Multivariate logistic regression models showed that the abovementioned discriminatory value of miR-145 (OR: 0.917; 95% CI: 0.427–1.968; P=0.824) is not independent from the PSA serum concentration, patients' age and DRE.
The decreased miR-145 expression correlates with higher Gleason score, late-stage tumours, larger tumour diameter and higher PSA and follow-up PSA serum levels
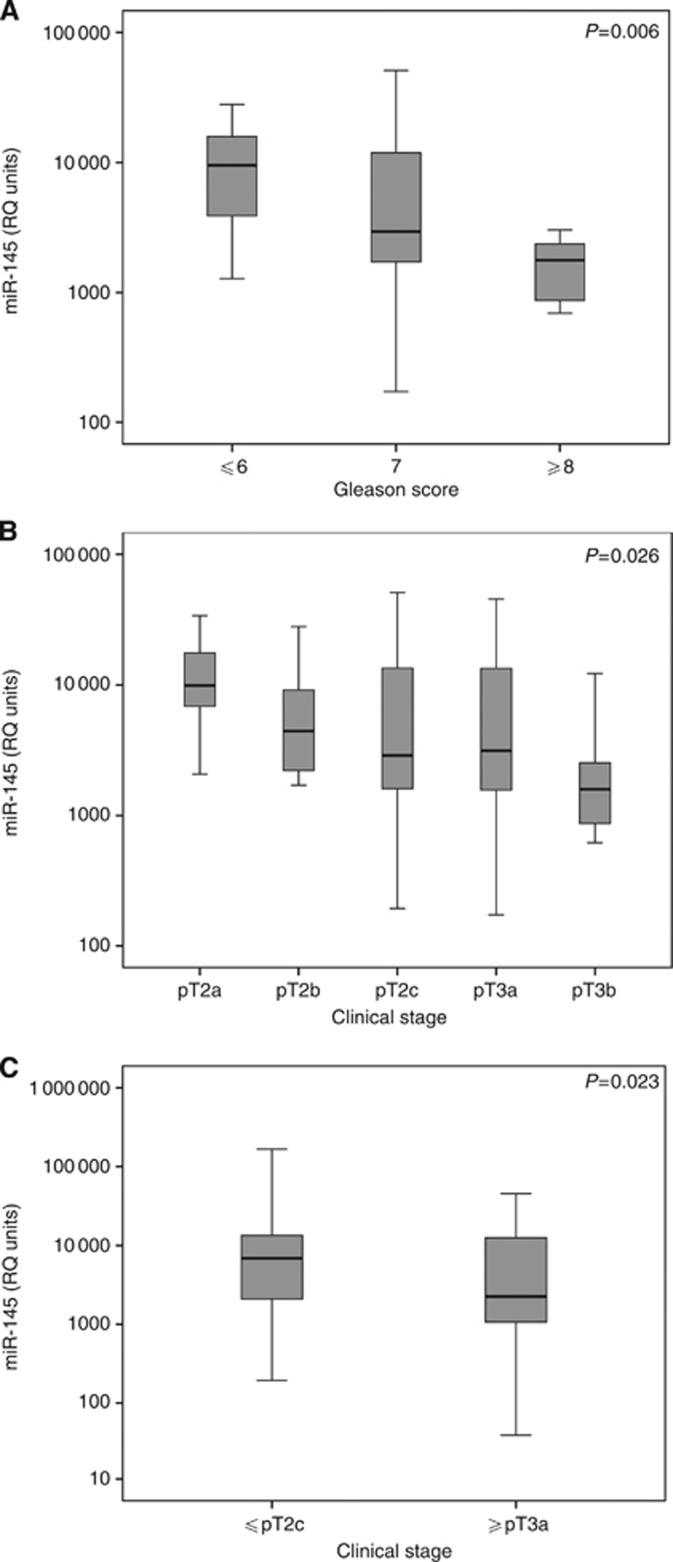
The downregulation of miR-145 levels in PCa patients was further associated with higher Gleason score tumours (Figure 1A). miR-145 expression was significantly (P=0.006) reduced in the groups with Gleason score ⩾8 (median: 1.78 × 103 RQ units) and Gleason score 7 (median: 2.95 × 103 RQ units) compared with the group with Gleason score ⩽6 (median: 9.52 × 103 RQ units). Analysing miR-145 as bivariate (Table 1), 80.0% of the patients with Gleason score ⩾8 were characterised as miR-145 (−) compared with the significantly lower 48.7% and 20.8% of the patients groups with Gleason score 7 and Gleason score ⩽6, respectively (P=0.004).
Figure 1.
Expression analysis of miR-145 related to PCa patients' Gleason score (A) and clinical stage (B and C). The bold lanes represent the median value (50th percentile) for each patient cohort. P-value was calculated by ‘Kruskal–Wallis test' (A and B) and by ‘Mann–Whitney U-test' (C).
Analysis of the miR-145 levels in relation to the clinical stage of the patients demonstrated the downregulation of miR-145 expression (P=0.026) as the disease progresses to late-stage tumours (Figure 1B). Classifying patients into ⩽pT2c and ⩾pT3a cohorts, significantly lower miR-145 expression (P=0.023) was detected in the ⩾pT3a (median: 2.26 × 103 RQ units) compared with the ⩽pT2c group (median: 6.88 × 103 RQ units; Figure 1C), resulting to the characterisation as miR-145 (−) of the 66.7% of ⩾pT3a patients compared with the significant lower percentage of 33.3% of the ⩽pT2c cohort (P=0.030; Table 1).
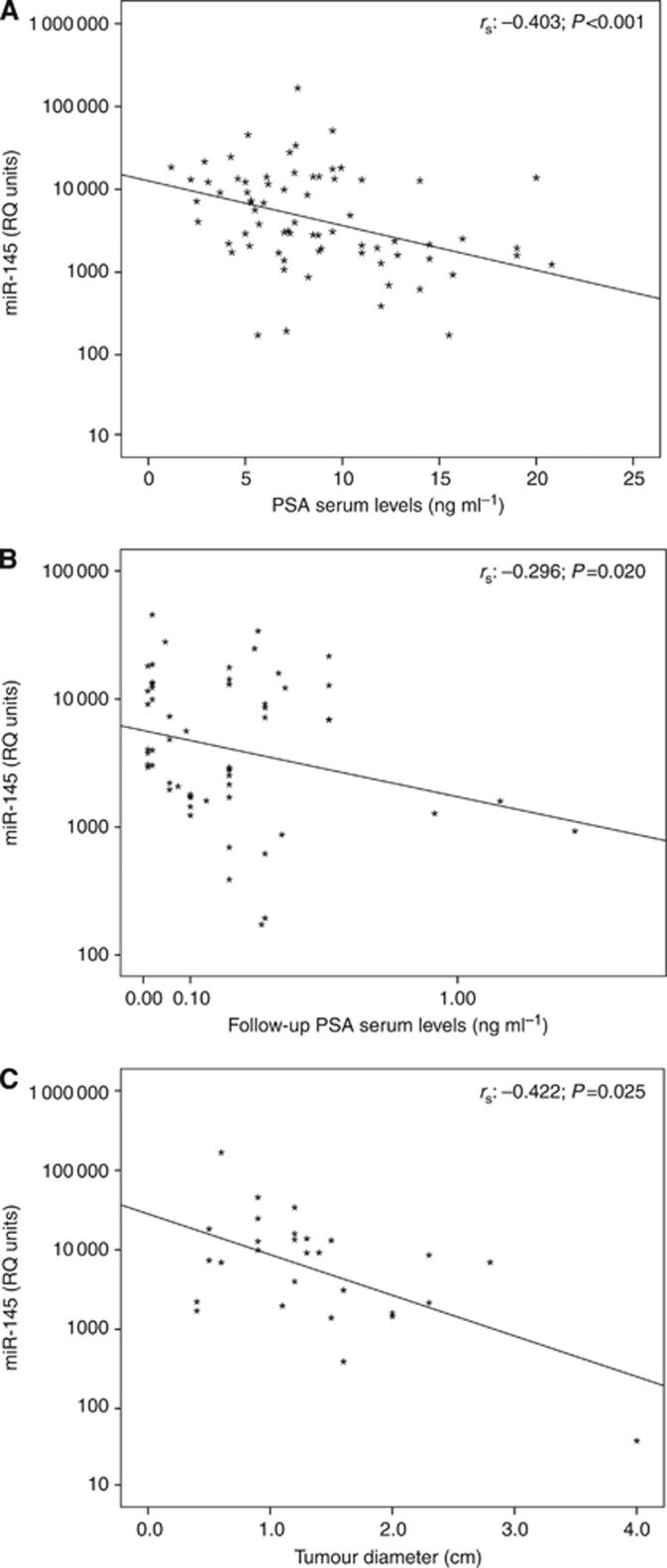
Apart from the most significant prognostic factors of Gleason score and clinical stage, the loss of miR-145 was found to correlate with higher pre-treatment PSA and follow-up PSA serum levels as well as with tumours of larger diameter (Figure 2). As pre-treatment PSA was used as the last measurement prior the radical prostatectomy, whereas follow-up PSA was used as the most recent measurement of the non-relapsed patients or the PSA measurement at the time of biochemical recurrence of the relapsed ones. A statistically significant negative correlation was highlighted between the miR-145 levels and the pre-treatment PSA of PCa patients using Spearman's correlation coefficient (rs: −0.403, P<0.001; Figure 2A) or χ2 test (P<0.001; Table 1). Moreover, the reduction of miR-145 expression levels were also associated with higher follow-up PSA (rs: −0.296, P=0.020; Figure 2B) as well as with larger tumour diameter (rs: −0.422, P=0.025; Figure 2C). The analysis of miR-145 levels with patients' DRE and age did not highlight any statistically significant correlation (Table 1).
Figure 2.
Correlation of miR-145 levels with PCa patients' pre-treatment PSA serum levels (A), follow-up PSA serum levels (B) and tumour diameter (C). rs: Spearman correlation coefficient. P-value was calculated by ‘Spearman correlation'.
The downregulation of miR-145 expression correlates with the shorter DFS of PCa patients
Elevated risk for biochemical recurrence was designated for the miR-145 (−) PCa patients by the univariate Cox proportional regression analysis (HR: 2.533; 95% CI: 1.249–5.137; P=0.010), highlighting their shorter DFS period (Table 2). Along with miR-145, the univariate analysis indicated the unfavourable clinical value of Gleason score, clinical stage and PSA serum levels for the outcome of PCa patients.
Table 2. Cox proportional regression analysis for the prediction of the PCa patients' DFS.
| |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Covariant | HR | 95% CIa | P-valueb | HR | 95% CIa | P-valueb |
|
miR-145 | ||||||
| Positive | 1.00 | 1.00 | ||||
| Negative |
2.533 |
1.249–5.137 |
0.010 |
1.264 |
0.489–3.273 |
0.629 |
| Gleason score |
2.244 |
1.407–3.578 |
0.001 |
1.660 |
0.944–2.921 |
0.078 |
| Clinical stage |
1.504 |
1.178–1.922 |
0.001 |
1.151 |
0.818–1.621 |
0.419 |
| PSA |
1.092 |
1.035–1.151 |
0.001 |
1.034 |
0.957–1.117 |
0.397 |
| Age |
0.996 |
0.944–1.051 |
0.877 |
0.983 |
0.930–1.039 |
0.548 |
| DRE | 2.285 | 1.104–4.727 | 0.026 | 1.535 | 0.679–3.469 | 0.303 |
Abbreviations: CI=confidence interval; DFS=disease-free survival; DRE=digital rectal examination; HR=hazard ratio; PCa=prostate cancer.
CI of the estimated HR.
Test for trend.
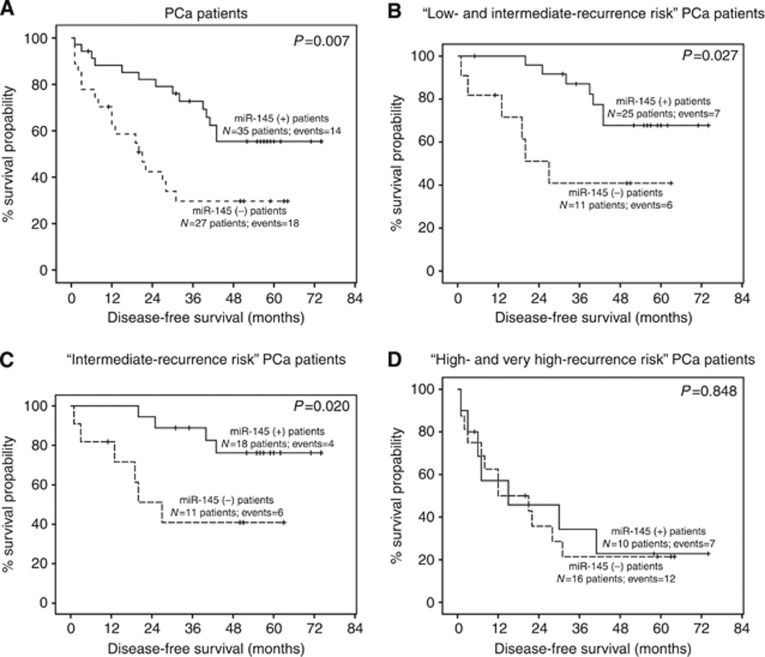
The prognostic significance of miR-145 for radical prostatectomy PCa patients was further assessed with Kaplan–Meier survival analysis (Figure 3). Shorter DFS period was demonstrated for miR-145 (−) patients (average DFS: 24.52 months) compared with miR-145 (+) (average DFS: 40.69 months) group (P=0.007; Figure 3A). Overall, these findings strengthen the case for an unfavourable outcome, in terms of shorter DFS, of PCa patients with downregulated miR-145 expression.
Figure 3.
Kaplan–Meier survival curves for the DFS of PCa patients (A), low- and intermediate-recurrence risk PCa patients (B), intermediate-recurrence risk PCa patients (C) and high- and very high-recurrence risk PCa patients (D) comparing with miR-145 expression levels. P-value was calculated by ‘log-rank test'.
miR-145 loss represents an independent unfavourable prognostic marker for the DFS of low- and intermediate-risk PCa patients treated with radical prostatectomy
Considering the need for more accurate prediction of the low- and intermediate-recurrence risk patients' outcome, PCa patients were further grouped according to their recurrence risk using NCCN guidelines for PCa (Mohler et al, 2010).
A significantly higher risk for biochemical relapse was indicated by the univariate Cox proportional analysis (Table 3) for the miR-145 (−) patients of the ‘low- and intermediate-recurrence risk' cohort (HR: 3.211; 95% CI: 1.071–9.622; P=0.037). For the same patients' group, Gleason score, clinical stage, PSA serum levels and DRE failed to have a statistically significant prognostic value for the biochemical recurrence of the patients. This unfavourable outcome of the miR-145 (−) patients was revealed to be independent from patients' Gleason score, clinical stage, PSA levels, age and DRE, as indicated by the multivariate models (HR: 4.467; 95% CI: 1.268–15.736; P=0.020). The abovementioned independent predictive significance of miR-145 for the ‘low- and intermediate-recurrence risk' patients' outcome was further verified and confirmed by multivariate Cox regression analysis (Table 3) for the ‘intermediate-recurrence risk' cohort (HR: 4.425; 95% CI: 1.112–17.613; P=0.035).
Table 3. Cox proportional regression analysis for the prediction of the ‘low- and intermediate-recurrence risk' and the ‘intermediate-recurrence risk' PCa patients' DFS.
| |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Covariant | HR | 95% CIa | P-valueb | HR | 95% CIa | P-valueb |
|
Low- and intermediate-recurrence risk PCa patients | ||||||
|
miR-145 | ||||||
| Positive | 1.00 | 1.00 | ||||
| Negative |
3.211 |
1.071–9.622 |
0.037 |
4.467 |
1.268–15.736 |
0.020 |
| Gleason score |
1.067 |
0.423–2.691 |
0.890 |
0.926 |
0.306–2.801 |
0.891 |
| Clinical stage |
0.711 |
0.309–1.634 |
0.422 |
0.435 |
0.133–1.419 |
0.167 |
| PSA |
1.101 |
0.885–1.370 |
0.386 |
1.110 |
0.847–1.428 |
0.475 |
| Age |
1.024 |
0.944–1.110 |
0.569 |
1.021 |
0.929–1.123 |
0.666 |
| DRE |
1.166 |
0.404–3.364 |
0.777 |
2.820 |
0.643–12.368 |
0.169 |
|
Intermediate-recurrence risk PCa patients | ||||||
|
miR-145 | ||||||
| Positive | 1.00 | 1.00 | ||||
| Negative |
4.048 |
1.131–14.479 |
0.032 |
4.425 |
1.112–17.613 |
0.035 |
| Gleason score |
1.235 |
0.411–3.706 |
0.707 |
1.225 |
0.323–4.646 |
0.766 |
| Clinical stage |
0.707 |
0.265–1.884 |
0.488 |
0.392 |
0.081–1.900 |
0.245 |
| PSA |
1.143 |
0.898–1.454 |
0.278 |
1.131 |
0.861–1.485 |
0.376 |
| Age |
1.002 |
0.916–1.097 |
0.957 |
0.986 |
0.879–1.106 |
0.812 |
| DRE | 1.249 | 0.380–4.099 | 0.714 | 3.205 | 0.576–17.848 | 0.169 |
Abbreviations: CI=confidence interval; DFS=disease-free survival; DRE=digital rectal examination; HR=hazard ratio; PCa=prostate cancer.
CI of the estimated HR.
Test for trend.
The higher risk for biochemical recurrence of the miR-145 (−) ‘low- and intermediate-recurrence risk' patients was clearly illustrated by the Kaplan–Meier survival plots. Significantly shorter DFS expectancy (P=0.027) is attributed to the miR-145 (−) patients (average DFS: 28.00 months) in relation to the miR-145 (+) ones (average DFS: 47.36 months; Figure 3B). In addition, poor DFS of the patients expressing lower miR-145 levels was indicated by Kaplan–Meier DFS analysis (P=0.020) for the intermediate-recurrence risk patients group (Figure 3C). Evaluating the prognostic efficacy of miR-145 for the ‘high- and very high-recurrence risk' PCa patients, Kaplan–Meier survival analysis pointed out similar DFS intervals for the miR-145 (−) and miR-145 (+) patients (Figure 3D). Summarising, miR-145 proved to be an independent unfavourable marker for the disease progression and the DFS expectancy of the ‘low-and intermediate-recurrence risk' PCa patients.
Discussion
PCa represents a malignancy with good response to treatment, and therefore, guidelines and clinical practice support the active treatment of PCa in the local or regional disease stages (Heidenreich et al, 2011; Siegel et al, 2012). However, a significant portion of the patients undergo treatment without any benefits. The results from population-based PCa screening trials revealed that the reduction of PCa-specific mortality from PSA screening suffers from high risk of overdiagnosis, leading to treatment of clinically insignificant prostate tumours (Andriole et al, 2009; Schroder et al, 2009). Moreover, because of the heterogeneity of the disease, a big number of patients undergo useless invasive treatment because of the rapid progression and the immediate relapse of the disease (Trapasso et al, 1994; Zincke et al, 1994). The evolving role of miRNAs during tumourigenesis and cancer progression offers a big pool of candidates for the improvement of PCa patients' prognosis, risk assessment and treatment decisions (Schaefer et al, 2010b; Fendler et al, 2011).
Using the BPH patients' cohort as control group, decreased miR-145 expression was detected in the tissue specimens from PCa patients. ROC analysis highlighted the ability of miR-145 expression levels to discriminate the malignant from the benign specimens of the gland, whereas logistic regression analysis pointed out a greater risk of suffering from PCa for patients with reduced miR-145 levels. The loss of miR-145 expression in PCa appeared to follow the progression of the disease to late-stage and poorly differentiated tumours. Underexpressed miR-145 levels were detected in high Gleason score tumours compared with well-differentiated ones. Moreover, the patients in advanced stages were found to express lower miR-145 levels in relation to those suffering from early-stage tumours. Overall, these data support the tumour-suppressor function of miR-145 for PCa cell growth and invasion and point out the relatively higher risk of suffering from late-stage and poorly differentiate tumours for the patients expressing lower miR-145 levels.
Downregulated miR-145 levels have been documented in the majority of the studied malignancies so far. miR-145 has been proposed to be a crucial modulator of Akt and KRas pathways' tumourigenic role in cell microenvironment. The p53 (Sachdeva et al, 2009; Suzuki et al, 2009) and FoxO (Gan et al, 2010) transcription factors have been found to induce miR-145 expression. Thereafter, miR-145 silences MYC (c-Myc) leading to cell cycle arrest and apoptosis. In PCa, mutations of p53 and hypermethylation of the MIR145 promoter region, preventing p53 binding, were found to be responsible for the reduced miR-145 expression (Suh et al, 2011). In addition, miR-145 was found to further enhance activation of the p53 pathway and the expression of p53 transcriptional targets BBC3 (PUMA) and CDKN1A (P21), highlighting the existence of a tumour-suppressor loop between p53 and miR-145 (Spizzo et al, 2010). Focusing on KRas pathway, the repression of miR-145, through RREB1 transcription factor, revealed to be necessary for the KRas-mediated oncogenic cell transformation (Kent et al, 2010).
The tumour-suppressor role of miR-145 for PCa and the correlation of miR-145 loss with high Gleason score and late-stage tumours, prompted us to evaluate its significance for the prognostication of the disease. The loss of miR-145 was indeed further associated with higher PSA serum levels prior the surgery and during the follow-up period, as well as with larger tumour diameter. The Cox proportional regression analysis was further confirmed the elevated risk for biochemical recurrence for the radical prostatectomy-treated patients with lower miR-145. Moreover, Kaplan–Meier survival plot strongly illustrated the significantly shorter DFS of the miR-145 (−) patients compared with miR-145 (+) ones. Overall these findings support the unfavourable prognostic value of miR-145 loss for PCa, which was found to correlated with higher Gleason score, advance clinical stage, higher risk for biochemical recurrence and shorter DFS.
We thereafter hypothesised that miR-145 expression may also serve the prognostication of the low- and intermediate-risk group of patients. The poor outcome of the miR-145 (−) patients was confirmed for the ‘low- and intermediate-recurrence risk' cohort as well as for the ‘intermediate-recurrence risk' group alone. The Cox proportional regression analysis highlighted the higher risk for biochemical relapse of the miR-145 (−) patients. Moreover, this unfavourable prognostic significance was revealed to be independent from the patients' Gleason score, clinical stage, PSA levels and age. In addition, significantly shorter DFS was indicated for the miR-145 (−) patients, compared with miR-145 (+) patients of the same cohorts. Focusing on ‘high- and very high-risk' PCa patients group, similar DFS expectancy was indicated for the patients independently from the loss of miR-145, supporting that the accumulation of deregulated molecular pathways in late-stage and dedifferentiated tumours overcomes the tumour-suppressor role of miR-145; taking this into consideration, it would be intriguing to design a comprehensive large-scale analysis of high- and very high-risk PCa patients. These data clearly denote the independent and unfavourable nature of the miR-145 loss for the outcome of the intermediate-risk of radical prostatectomy-treated patients and support the use of miR-145 expression for the stratification of these patients' cohort according to their DFS expectancy. Future studies, focusing on low-recurrence risk PCa will further reinforce the prognostic significance of miR-145 for the outcome prediction of low-risk group of patients and their proper management.
Our data clearly support the tumour-suppressor role of miR-145 and highlight its clinical utility for PCa patients. The downregulation of miR-145 expression in PCa was further correlated with higher Gleason score, late-stage and larger diameter tumours, as well as higher pre-treatment and follow-up PSA serum levels. Moreover, PCa patients expressing lower miR-145 amounts are of significantly higher risk for disease recurrence and thus worse survival. Finally, we have analysed the miR-145 prognostic significance for ‘low- and intermediate-recurrence risk' patients, where miR-145 loss was revealed to be able to designate the PCa patients with high risk of relapse and poor survival expectancy independently from Gleason score, clinical stage, PSA levels and patient's age. Overall, these findings underline the central role of miR-145 loss for the progression of the disease and support the potential use of miR-145 for the improvement of patients' prognostication, risk assessment and treatment management.
Acknowledgments
This research has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program ‘Education and Lifelong Learning' of the National Strategic Reference Framework (NSRF) – Research Funding Program: THALES. Investing in knowledge society through the European Social Fund (THALES – UoA – BIOPROMO, MIS 377046).
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360 (13:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgeris M, Koutalellis G, Fragoulis EG, Scorilas A. Expression analysis and clinical utility of L-dopa decarboxylase (DDC) in prostate cancer. Clin Biochem. 2008;41 (14-15:1140–1149. doi: 10.1016/j.clinbiochem.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116 (2:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin Chem. 2009;55 (4:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- Boccon-Gibod L, Djavan WB, Hammerer P, Hoeltl W, Kattan MW, Prayer-Galetti T, Teillac P, Tunn UW. Management of prostate-specific antigen relapse in prostate cancer: a European Consensus. Int J Clin Pract. 2004;58 (4:382–390. doi: 10.1111/j.1368-5031.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- Burfeind P, Chernicky CL, Rininsland F, Ilan J. Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci USA. 1996;93 (14:7263–7268. doi: 10.1073/pnas.93.14.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6 (11:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Chiyomaru T, Enokida H, Tatarano S, Kawahara K, Uchida Y, Nishiyama K, Fujimura L, Kikkawa N, Seki N, Nakagawa M. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102 (5:883–891. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyomaru T, Tatarano S, Kawakami K, Enokida H, Yoshino H, Nohata N, Fuse M, Seki N, Nakagawa M. SWAP70, actin-binding protein, function as an oncogene targeting tumor-suppressive miR-145 in prostate cancer. Prostate. 2011;71 (14:1559–1567. doi: 10.1002/pros.21372. [DOI] [PubMed] [Google Scholar]
- Cho WC, Chow AS, Au JS. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol. 2011;8 (1:125–131. doi: 10.4161/rna.8.1.14259. [DOI] [PubMed] [Google Scholar]
- Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460 (7256:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10 (10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamandis EP. Prostate cancer screening with prostate-specific antigen testing: more answers or more confusion. Clin Chem. 2010;56 (3:345–351. doi: 10.1373/clinchem.2009.140046. [DOI] [PubMed] [Google Scholar]
- Fendler A, Stephan C, Yousef GM, Jung K. MicroRNAs as regulators of signal transduction in urological tumors. Clin Chem. 2011;57 (7:954–968. doi: 10.1373/clinchem.2010.157727. [DOI] [PubMed] [Google Scholar]
- Freedland SJ. Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 2011;117 (6:1123–1135. doi: 10.1002/cncr.25477. [DOI] [PubMed] [Google Scholar]
- Fuse M, Nohata N, Kojima S, Sakamoto S, Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, Naya Y, Ichikawa T, Seki N. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int J Oncol. 2011;38 (4:1093–1101. doi: 10.3892/ijo.2011.919. [DOI] [PubMed] [Google Scholar]
- Gan B, Lim C, Chu G, Hua S, Ding Z, Collins M, Hu J, Jiang S, Fletcher-Sananikone E, Zhuang L, Chang M, Zheng H, Wang YA, Kwiatkowski DJ, Kaelin WG, Jr., Signoretti S, DePinho RA. FoxOs enforce a progression checkpoint to constrain mTORC1-activated renal tumorigenesis. Cancer Cell. 2010;18 (5:472–484. doi: 10.1016/j.ccr.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni F. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59 (1:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62 (10:2942–2950. [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61 (2:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Karan D, Lin FC, Bryan M, Ringel J, Moniaux N, Lin MF, Batra SK. Expression of ADAMs (a disintegrin and metalloproteases) and TIMP-3 (tissue inhibitor of metalloproteinase-3) in human prostatic adenocarcinomas. Int J Oncol. 2003;23 (5:1365–1371. [PubMed] [Google Scholar]
- Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, Liu S, Leach SD, Maitra A, Mendell JT. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010;24 (24:2754–2759. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larne O, Martens-Uzunova E, Hagman Z, Edsjo A, Lippolis G, den Berg MS, Bjartell A, Jenster G, Ceder Y. miQ-A novel microRNA based diagnostic and prognostic tool for prostate cancer. Int J Cancer. 2012;132 (12:2867–2875. doi: 10.1002/ijc.27973. [DOI] [PubMed] [Google Scholar]
- Law PT, Ching AK, Chan AW, Wong QW, Wong CK, To KF, Wong N. MiR-145 modulates multiple components of the insulin-like growth factor pathway in hepatocellular carcinoma. Carcinogenesis. 2012;33 (6:1–8. doi: 10.1093/carcin/bgs130. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25 (4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mavridis K, Stravodimos K, Scorilas A. Downregulation and prognostic performance of microRNA 224 expression in prostate cancer. Clin Chem. 2013;59 (1:261–269. doi: 10.1373/clinchem.2012.191502. [DOI] [PubMed] [Google Scholar]
- Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, Enke CA, George D, Horwitz EM, Huben RP, Kantoff P, Kawachi M, Kuettel M, Lange PH, Macvicar G, Plimack ER, Pow-Sang JM, Roach M, 3rd, Rohren E, Roth BJ, Shrieve DC, Smith MR, Srinivas S, Twardowski P, Walsh PC. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8 (2:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- Ostenfeld MS, Bramsen JB, Lamy P, Villadsen SB, Fristrup N, Sorensen KD, Ulhoi B, Borre M, Kjems J, Dyrskjot L, Orntoft TF. miR-145 induces caspase-dependent and -independent cell death in urothelial cancer cell lines with targeting of an expression signature present in Ta bladder tumors. Oncogene. 2010;29 (7:1073–1084. doi: 10.1038/onc.2009.395. [DOI] [PubMed] [Google Scholar]
- Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27 (12:1788–1793. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67 (13:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- Sachdeva M, Liu Q, Cao J, Lu Z, Mo YY. Negative regulation of miR-145 by C/EBP-beta through the Akt pathway in cancer cells. Nucleic Acids Res. 2012;40 (14:6683–6692. doi: 10.1093/nar/gks324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010a;70 (1:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva M, Mo YY. miR-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res. 2010b;2 (2:170–180. [PMC free article] [PubMed] [Google Scholar]
- Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA. 2009;106 (9:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardana G, Dowell B, Diamandis EP. Emerging biomarkers for the diagnosis and prognosis of prostate cancer. Clin Chem. 2008;54 (12:1951–1960. doi: 10.1373/clinchem.2008.110668. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Jung M, Mollenkopf HJ, Wagner I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G, Jung K. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010a;126 (5:1166–1176. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Stephan C, Busch J, Yousef GM, Jung K. Diagnostic, prognostic and therapeutic implications of microRNAs in urologic tumors. Nat Rev Urol. 2010b;7 (5:286–297. doi: 10.1038/nrurol.2010.45. [DOI] [PubMed] [Google Scholar]
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360 (13:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 2005;39 (4:519–525. doi: 10.2144/000112010. [DOI] [PubMed] [Google Scholar]
- Siegel R, Desantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62 (4:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- Spizzo R, Nicoloso MS, Lupini L, Lu Y, Fogarty J, Rossi S, Zagatti B, Fabbri M, Veronese A, Liu X, Davuluri R, Croce CM, Mills G, Negrini M, Calin GA. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-alpha in human breast cancer cells. Cell Death Differ. 2010;17 (2:246–254. doi: 10.1038/cdd.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, Tanaka Y, Dahiya R. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32 (5:772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460 (7254:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- Thomsen MK, Ambroisine L, Wynn S, Cheah KS, Foster CS, Fisher G, Berney DM, Moller H, Reuter VE, Scardino P, Cuzick J, Ragavan N, Singh PB, Martin FL, Butler CM, Cooper CS, Swain A. SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation. Cancer Res. 2010;70 (3:979–987. doi: 10.1158/0008-5472.CAN-09-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapasso JG, deKernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol. 1994;152 (5 Pt 2:1821–1825. doi: 10.1016/s0022-5347(17)32394-7. [DOI] [PubMed] [Google Scholar]
- Villadsen SB, Bramsen JB, Ostenfeld MS, Wiklund ED, Fristrup N, Gao S, Hansen TB, Jensen TI, Borre M, Orntoft TF, Dyrskjot L, Kjems J. The miR-143/-145 cluster regulates plasminogen activator inhibitor-1 in bladder cancer. Br J Cancer. 2012;106 (2:366–374. doi: 10.1038/bjc.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach S, Nolte E, Szczyrba J, Stohr R, Hartmann A, Orntoft T, Dyrskjot L, Eltze E, Wieland W, Keck B, Ekici AB, Grasser F, Wullich B. MicroRNA profiles of prostate carcinoma detected by multiplatform microRNA screening. Int J Cancer. 2012;130 (3:611–621. doi: 10.1002/ijc.26064. [DOI] [PubMed] [Google Scholar]
- Wafa LA, Cheng H, Plaa N, Ghaidi F, Fukumoto T, Fazli L, Gleave ME, Cox ME, Rennie PS. Carbidopa abrogates L-dopa decarboxylase coactivation of the androgen receptor and delays prostate tumor progression. Int J Cancer. 2012;130 (12:2835–2844. doi: 10.1002/ijc.26287. [DOI] [PubMed] [Google Scholar]
- Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng L, Zhou H, Zhao RC. miR-145 inhibits breast cancer cell growth through RTKN. Int J Oncol. 2009;34 (5:1461–1466. [PubMed] [Google Scholar]
- Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li D, Lai L, Jiang BH. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40 (2:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman MS, Chen Y, Deng G, Shahryari V, Suh SO, Saini S, Majid S, Liu J, Khatri G, Tanaka Y, Dahiya R. The functional significance of microRNA-145 in prostate cancer. Br J Cancer. 2010;103 (2:256–264. doi: 10.1038/sj.bjc.6605742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guo H, Qian G, Ge S, Ji H, Hu X, Chen W. MiR-145, a new regulator of the DNA fragmentation factor-45 (DFF45)-mediated apoptotic network. Mol Cancer. 2010;9:211. doi: 10.1186/1476-4598-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincke H, Oesterling JE, Blute ML, Bergstralh EJ, Myers RP, Barrett DM. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol. 1994;152 (5 Pt 2:1850–1857. doi: 10.1016/s0022-5347(17)32399-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.