Abstract
Background:
Owing to the limited validity of clinical data on the treatment of prostate cancer (PCa) and bone metastases, biochemical markers are a promising tool for predicting survival, disease progression and skeletal-related events (SREs) in these patients. The aim of this study was to evaluate the predictive capacity of biochemical markers of bone turnover for mortality risk, disease progression and SREs in patients with PCa and bone metastases undergoing treatment with zoledronic acid (ZA).
Methods:
This was an observational, prospective and multicenter study in which ninety-eight patients were included. Patients were treated with ZA (4 mg every 4 weeks for 18 months). Data were collected at baseline and 3, 6, 9, 12, 15 and 18 months after the beginning of treatment. Serum levels of bone alkaline phosphtase (BALP), aminoterminal propeptide of procollagen type I (P1NP) and beta-isomer of carboxiterminal telopeptide of collagen I (β-CTX) were analysed at all points in the study. Data on disease progression, SREs development and survival were recorded.
Results:
Cox regression models with clinical data and bone markers showed that the levels of the three markers studied were predictive of survival time, with β-CTX being especially powerful, in which a lack of normalisation in visit 1 (3 months after the beginning of treatment) showed a 6.3-times more risk for death than in normalised patients. Levels of these markers were also predictive for SREs, although in this case BALP and P1NP proved to be better predictors. We did not find any relationship between bone markers and disease progression.
Conclusion:
In patients with PCa and bone metastases treated with ZA, β-CTX and P1NP can be considered suitable predictors for mortality risk, while BALP and P1NP are appropriate for SREs. The levels of these biomarkers 3 months after the beginning of treatment are especially important.
Keywords: prostate cancer, bone metastases, bone markers, overall survival, disease progression, skeletal-related events
Metastases are the most deadly events in the progression of cancer and are commonly associated with poor prognosis. The skeleton is one of the most common organs to be affected by metastatic cancers. One such cancer is prostate cancer (PCa). Patients with bone metastases have a high degree of morbidity and present with severe pain and high incidence of hypercalcemia, spinal cord compression and pathological fractures. These skeletal-related events (SREs) lead to a reduction in the quality of life and survival rate and constitute an important public-health problem (Coleman, 1997; Mundy, 2002).
Depending on the primary tumour, bone metastases can be either lytic or blastic with respect to bone. Tumoral cells release factors that activate osteoclasts or osteoblasts. PCa bone metastases are blastic in nature. Our body compensates for the increase of bone resorption or formation by increasing bone formation or resorption, respectively, by means of the coupling phenomenon. Owing to this fact, patients with bone metastases present a higher degree of bone remodelling than healthy individuals, thereby making bone remodelling a useful indicator when diagnosing bone metastases.
Biochemical markers of bone turnover (formation or resorption) are substances present in serum or urine that allow us to measure the activity of osteoblasts and osteoclasts. These characteristics could make these markers useful tools in quantifying the degree of alteration of bone remodelling in the presence of bone metastases. Bisphosphonates, compounds that directly produce osteoclastic inhibition, have been used in the treatment of patients with bone metastases because they reduce the increase in osteoclastic activity produced by the presence of such metastases. Bisphosphonates are useful in the case of blastic or lytic metastases, due to the coupling phenomenon. The decrease in bone resorption and the concomitant decrease in bone formation lead to a decrease in the release of factors that also decrease metastatic growth (Buijs and Pluijm, 2009; Coleman et al, 2010).
All commercial bisphosphonates have been used to treat bone metastases, but zoledronic acid (ZA) is currently the preferred option due to its great potency and its ability to be administered less frequently (Polascik and Mouraviev, 2008). Zoledronic acid administration produces a significant decrease in bone remodelling in patients with bone metastases.
Bone scintigraphy is commonly used to assess the extent of bone metastases; its use is limited in monitoring of treatment efficacy because it is an expensive, time-consuming technique. Moreover, scintigraphy presents sensitivity but lacks specificity, primarily due to slow detectable changes, and the confounding appearance of lesions containing mixed and/or osteosclerotic areas (Cook and Fogelman, 2000). For this reason, it would be of great interest to have biochemical markers that could accurately predict bone metastases and allow for effective follow-up of the progress of disease. Biochemical markers of bone turnover may meet this need.
Previous studies suggest that the levels of bone markers are correlated with the presence, extent and progression of bone metastases, including SREs, disease progression and death (Leeming et al, 2006; Lipton et al, 2007; Lein et al, 2007). However, more studies are necessary in order to obtain more definitive conclusions.
The aim of this work was to study the levels of two biochemical markers of bone formation, bone alkaline phosphatase (BALP) and aminoterminal propeptide of procollagen I (PINP), and one marker of bone resorption, beta-isomer of carboxiterminal telopeptide of collagen I (beta-CTX), in a population of patients with PCa and bone metastases. Markers were studied in basal conditions and 3, 6, 9, 12, 15 and 18 months after the beginning of treatment with ZA, and their relationship with mortality, disease progression and SREs was analysed. Prostatic-specific antigen (PSA) levels and their correlation with bone markers were also analysed.
Materials and methods
Study group
The TUGAMO (‘Tumores UroGenitales Avanzados, Metástasis Óseas', advanced urogenital tumours, bone metastases) was an observational, prospective and national multicenter study (n=40 centres) performed in patients with prostate, renal cells and bladder cancer with bone metastases. This manuscript studies a subgroup of these patients with PCa. Male patients ⩾18 years old with histologically confirmed PCa with at least one diagnosed bone-lesion confirmed by bone scintigraphy were enrolled. Patients were excluded from the study if they presented with concomitant pathologies that could interfere in the evaluation of biochemical markers of bone turnover such as bone metabolic disorders, Paget's disease of bone, hyperparathyroidism, thyroid abnormalities, abnormal intestinal absorption or hepatic or renal alterations. Patients treated with bisphosphonates prior to their inclusion in the study and with dental infection and diagnosis of mandibular osteonecrosis were also excluded.
Ninety-eight patients with PCa and bone metastases were included in the study. Sixty had previously received hormonal treatment and 38 had received no prior treatment. Once ZA treatment was established, none of the patient received hormonal treatment. Table 1 shows the demographics and baseline disease characteristics of the patients studied.
Table 1. Patients demographics and baseline disease characteristics.
| Variable | |
|---|---|
| Mean age (years) |
71.9±8.4 |
| Weight (kg) |
77.5±15.1 |
|
Primary therapy n (%) | |
| None | 35 (37%) |
| Hormonal therapy |
60 (63%) |
|
Prior skeletal-related events n (%) | |
| No | 93 |
| Yes |
2 |
|
Soloway classification n (%) | |
| <6 Bone metastases | 42 (44.2%) |
| 6–20 Bone metastases | 30 (31.6%) |
| >20 Bone metastases | 11 (11.6%) |
| Super scan |
12 (12.1%) |
| Time from bone metastases diagnosis (months) |
1.8±2.1 |
| BALP (μg l−1) |
125±215 |
| P1NP (ng ml−1) |
393±766 |
| β-CTX (ng ml−1) | 0.930±1.113 |
Ninety-eight patients with PCa and bone metastases.
A follow-up of the patients was performed over 18 months. Baseline values (visit 0, v0) were those obtained when a patient initiated treatment with ZA (Zometa, Novartis Pharma, Basel, Switzerland), 4 mg every 4 weeks over 18 months. During the study, administration of medication and standard antineoplasic therapies, including cytotoxic chemotherapy agents were also allowed. Data were collected at v0, v1 (3 months), v2 (6 months), v3 (9 months), v4 (12 months), v5 (15 months) and v6 (18 months). The definition of SREs included pathological fractures, severe pain, hypercalcemia and spinal cord compression. Patients were also evaluated for disease progression by physical examination, including evaluation of ECOG performance and ECOG pain score.
This study was approved by the Ethics Committee of the Unitat d́Avaluació, Suport I Prevenció of Clinical Hospital of Barcelona (Spain). Informed consent was obtained from each patient before the study began.
Markers evaluation
Bone alkaline phosphatase was determined by ELISA (IDS, Boldon, UK). Inter- and intra-assay coefficients of variation of the method were <6.4% and <4.5%. Sensitivity was 0.7 μg l−1. The normal range for healthy adult men was ⩽15 μg/l. PINP was determined by electrochemiluminiscence (Elecsys, Roche, Mannheim, Germany). Inter- and intra-assay coefficients of variation of the method were 3.7% and 2.9%. Sensitivity was 5 ng ml−1. Normal range for healthy adult men was ⩽62 ng ml−1. Beta-isomer of carboxiterminal telopeptide of collagen I was determined by electrochemiluminiscence (Elecsys, Roche). Inter- and intra-assay coefficients of variation of the method were 4.7% and 4.6%. Sensitivity was 0.070 ng ml−1. The normal range for healthy adult men was ⩽0548 ng ml−1.
Prostatic-specific antigen was determined by electrochemiluminiscence (Elecsys, Roche). Inter- and intra-assay coefficients of variation of the method were <0.46% and <2.5%. Sensitivity was 0.03 ng ml−1. The normal range for healthy adult men was ⩽4 ng ml−1.
Statistical analyses
Bone markers were summarised in terms of mean, s.d., median and quartiles. Absolute and relative changes in bone markers as compared with baseline and previous visit were assessed at each visit. Absolute changes were summarised by mean and s.d. and their statistical significance was assessed by Wilcoxon test for paired samples. Relative changes were converted to logarithms and summarised by geometric mean and s.d. Their statistical significance was assessed using the Student's t-test for paired samples.
Receiver operating characteristic (ROC) analysis were performed to evaluate the predictive capability of bone markers levels for mortality, SREs and progression, obtaining the cutoff point that maximises sensitivity and specificity. Receiver operating characteristic curves were performed (1) with the baseline bone markers levels and (2) with changes in bone markers in v1 with respect to v0 (defined as marker in v1/marker in v0). Then, survival curves were constructed using the Kaplan–Meier method by taking into account four classifications, and comparisons were performed using the log-rank test:
Biomarker ‘higher than' or ‘equal to or lesser than' the upper limit of normality.
Basal biomarker ‘higher' or ‘equal to or lesser than' than the ‘cutoff' obtained from the ROC curves of basal values against death, SREs appearance or disease progression.
Biomarker value in v1/biomarker in v0 ‘higher' or ‘equal to or lesser than' the cutoff obtained from the corresponding ROC curves.
Normal or non-normal level of bone markers at v1.
Finally, Cox regression models were fitted to quantify the degree of association between potential predictors and mortality, the occurrence of SREs and progression. The models were summarised by hazard ratio estimate, s.e. and 95% confidence interval (CI) for hazard ratio. Also the P-value from hypothesis testing of the hazard ratio was shown.
We estimated the size of the hazard ratios in order that the study could present a statistical power of 80%. For this purpose, hazard ratios magnitudes, accumulated incidence of the study event (death, progression or SREs) and size of the compared groups were considered. According to these considerations, the hazard ratios that could detect results with a potency of 80% were >2.7 for death, >1.9 for progression and >2.5 for EREs appearance. The significance level was set at 0.05 for all tests.
Results
Bone markers and PSA levels at baseline and after treatment with ZA
The concentrations of bone markers did not differ significantly between the groups of patients with hormonal therapy and without previous hormonal treatment both in basal conditions and after treatment with ZA. Thus, all subsequent calculations were performed with the data obtained from all patients in the respective groups.
At baseline, patients with PCa and bone metastases exhibited elevated levels of bone markers, and a significant correlation was found between values obtained from markers and Soloway classification (Soloway et al, 1998), (Tables 1 and 2).
Table 2. Correlation between bone markers and Soloway classification.
| N | BALP μg l−1 | N | P1NP ng ml−1 | N | β-CTX ng ml−1 | |
|---|---|---|---|---|---|---|
| <6 Bone metastases |
37 |
29±34 |
38 |
78±138 |
38 |
0.439±0.500 |
| 6–20 Bone metastases |
28 |
122±177 |
28 |
299±347 |
27 |
0.925±1.068 |
| >20 Bone metastases |
8 |
287±271 |
10 |
790±347 |
10 |
1.544±1.364 |
| Super scan |
10 |
362±378 |
11 |
1361±1653 |
11 |
2.083±1.485 |
| Pa | — | <0.001 | — | <0.001 | — | 0.0007 |
Patients with PCa and bone metastases.
Kruskal–Wallis.
Table 3 shows the percentages of patients with pathological levels of bone markers throughout the study. Zoledronic acid treatment produced a gradual increase of the percentage of patients with normal values of biomarkers. Beta-isomer of carboxiterminal telopeptide of collagen I is the marker that experienced the highest degree of normalisation, with 93.3% of patients exhibiting normal levels after 18 months of ZA treatment.
Table 3. Percentages of patients with pathological levels of biochemical markers of bone turnover along the study.
| Visit | V0 | V1 | V2 | V3 | V4 | V5 | V6 |
|---|---|---|---|---|---|---|---|
| Numbers of patients |
98 |
95 |
87 |
77 |
66 |
58 |
57 |
| Marker BALP: elevated |
74.7 |
58.6 |
54.4 |
40.9 |
50.0 |
40.0 |
43.3 |
| Marker P1NP: elevated |
58.9 |
39.4 |
31.6 |
15.9 |
17.1 |
18.4 |
20.0 |
| Marker β-CTX: elevated | 47.9 | 31.0 | 17.5 | 11.4 | 10.0 | 7.9 | 6.7 |
Abbreviations: β-CTX=β-isomer of carboxiterminal telopeptide of collagen I; BALP=bone alkaline phosphatase; P1NP=aminoterminal propeptide of procollagen I.
Ninety-eight patients with PCa and bone metastases treated with zoledronic acid (4 mg every 4 weeks) during 18 months. V0: basal visit; V1, V2, V3, V4, V5 and V6: visits after 3, 6, 9, 12, 15 and 18 months, respectively, following the beginning of the treatment. Values are expressed as percentages with respect to the total numbers of patients in each visit.
Table 4 shows the mean values of biochemical markers of bone turnover throughout the study. Levels of markers tended to decrease significantly until v2 visit. From this point, no significant change was observed. As expected, levels of PSA in patients with previous hormonal treatment were lower than in those without previous treatment (mean value: 5.9 ng ml−1, 95% CI: 3.0–11.6 vs 64.4 ng ml−1, 95% CI: 29.7–139.9, P<0.01). After ZA treatment, values of PSA of previously untreated patients decreased significantly to the levels of treated patients (mean value: 7.80 ng ml−1 vs 2.03 ng ml−1), without significant differences between the two groups (P<0.09) and without significant differences along the whole period of the study (P<0.47). Levels of bone markers correlated positively with PSA values at baseline (P<0.001 for the three studied markers) and at visits 1, 2, 3, 4 and 5 (P<0.001 for BALP, P<0.01 for P1NP and P<0.05 for β-CTX). However, at visits 6 and 7, only values of BALP correlated with PSA levels (P<0.01).
Table 4. Levels of biochemical markers of bone turnover throughout the study.
| Visit | Numbers of patients | BAP (μg l−1) | P1NP (ng ml−1) | β-CTX (ng ml−1) |
|---|---|---|---|---|
| V0 |
98 |
126.7±208.8 |
399±741 |
0.967±1.094a |
| V1 |
95 |
52.7±74.4a |
157±253a |
0.456±0.612a |
| V2 |
87 |
35.3±62.9a,b |
110±224a,b |
0.308±0.384a |
| V3 |
77 |
38.6±80.0a |
71±139a |
0.255±0.354a |
| V4 |
66 |
35.3±57.5a,c |
52±77.0a |
0.209±0.255a |
| V5 |
58 |
31.2±49.6a |
55±146a |
0.216±0.267a,c,e |
| V6 | 57 | 26.0±33.9a,c,d | 47±74a,c,f | 0.235±0.252a,b,c,d,e |
Abbreviations: β-CTX=β-isomer of carboxiterminal telopeptide of collagen I; BALP=bone alkaline phosphatase; P1NP=aminoterminal propeptide of procollagen I.
Ninety-eight patients with Pca and bone metastasis treated with zoledronic acid (4 mg every 4 weeks) during 18 months. V0: basal visit; V1, V2, V3, V4, V5 and V6: visits after 3, 6, 9, 12, 15 and 18 months, respectively, following the beginning of the treatment. Values are expressed as mean±s.d. Statistical significance, P-values of Student's t-test: a <0.001 vs V0; b < 0.005 vs V1; c < 0.05 vs V2; d < 0.05 vs V3; e < 0.01 vs V4; f < 0.01 vs V5.
Global survival, defined as time between the beginning of treatment and death during the 18 months of treatment showed a mean value of 16.3 months. Ninety-eight patients started the study and 57 completed it. Losses of participants were due to death (21), failure to provide informed consent (4), loss to follow-up (7), clinical criteria (5) and others (changes of address, renal insufficiency, etc. (4)).
Capability of bone biomarkers to predict mortality
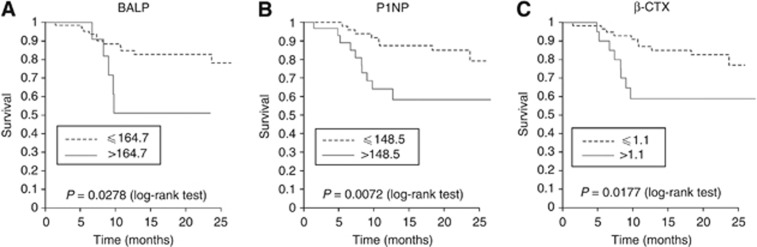
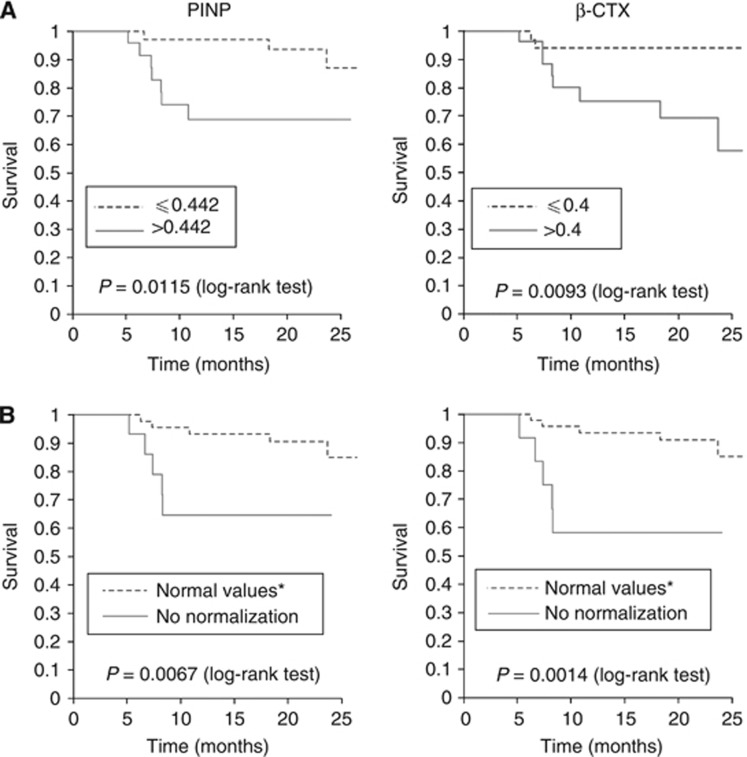
With respect to mortality, using the first categorisation explained in Materials and Methods (biomarker ‘higher than' or ‘equal to or lesser than' the upper limit of normality), no significant differences were obtained in the Kaplan–Meier curves plotting survival time (data not shown). However, Kaplan–Meier analysis distinctly demonstrated different survival curves for the three bone markers studied, according to the second (basal biomarker ‘higher' or ‘equal to or lesser than' than the ‘cutoff' obtained from the ROC curves of basal values against death) (Figure 1), the third (biomarker value in v1/biomarker in v0 ‘higher' or ‘equal to or lesser than' the cutoff obtained from the corresponding ROC curves) (Figure 2A) and the fourth (normal or non-normal level of bone markers at v1) classifications (Figure 2B). Tables 5 and 6 show the univariate Cox regression analysis derived from the above mentioned Kaplan–Meier curves. The hazard ratio indicates how many times greater the risk of death was in the given group relative to the other group. The three markers studied could be useful as predictors of survival, although β-CTX levels in v1 were especially significant in this regard. Our results show that values of β-CTX were especially relevant for survival during the 18 months of ZA treatment. A value of v1/v0>0.4 (a decrease of β-CTX in v1 of <40%) and/or lack of normality in levels of β-CTX, indicates a six-fold increase in risk of mortality along the study. Similar behaviour, although with minor statistical potency presents P1NP values. A decrease of P1NP in v1 of <44% and/or lack of normality, indicates a five-fold increase in the risk of mortality, and no changes in levels of BALP between v0 and v1 indicates an increase of three-fold in the risk of mortality.
Figure 1.
Kaplan–Meier analysis of overall survival after the completion of treatment with zoledronic acid according to basal levels of biomarkers. Stratification was performed using the cutoff points obtained from the ROC curves of ‘basal values of biomarkers at the beginning of the study' against death: (A) BALP, bone alkaline phosphatase. The cutoff point obtained in the ROC curve was 164.7 μg l−1; (B) PINP, aminoterminal propeptide of procollagen I. The cutoff point obtained in the ROC curve was 148.5 hg/ml; (C) β-CTX, β-isomer of carboxiterminal telopeptide of collagen. The cut-off point obtained in the ROC curve was 1.1 ng ml−1.
Figure 2.
(A) Kaplan–Meier analysis of overall survival after the completion of treatment with zoledronic acid according to the ratio ‘level of biomarker in v1/ level of biomarker in v0', for P1NP and β-CTX. The stratification was performed using the cutoff points obtained from the ROC curves of ‘changes in bone markers in v1 with respect to v0' against death. The cutoff point obtained in these ROC curves were 0.442 for PINP and 0.4 for β-CTX. (B) Kaplan–Meier analysis of overall survival after the completion of treatment with zoledronic acid according to the dichotomous criterion of achievement or not of a normal level of bone markers in the v1 for P1NP and β-CTX. *Values of biomarkers into the normal range are indicated in Materials and Methods. Kaplan–Meier curve of BALP is not presented because differences between groups according to these criteria were not statistically significant.
Table 5. Univariate Cox regression analysis of bone markers for survival prediction after 18 months of ZA treatment.
| Marker | Hazard ratio | s.d. | 95% CI | P-value |
|---|---|---|---|---|
|
BALP | ||||
| V0 elevateda | 1.469 | 0.9423 | 0.418–5.164 | 0.5485 |
| V0>164.7b | 3.157 | 1.7406 | 1.071–9.302 | 0.0371 |
| V1/V0>1.006c |
3.048 |
2.2604 |
0.713–13.039 |
0.1328 |
|
P1NP | ||||
| V0 elevateda | 1.564 | 0.7847 | 0.585–4.181 | 0.3722 |
| V0>148.5b | 3.372 | 1.6162 | 1.318–8.627 | 0.0112 |
| V1/V0>0.442c |
4.969 |
3.4755 |
1.261–19.573 |
0.0219 |
|
β-CTX | ||||
| V0 elevateda | 2.318 | 1.1262 | 0.894–6.007 | 0.0837 |
| V0>1.1b | 2.954 | 1.4138 | 1.156–7.547 | 0.0237 |
| V1/V0>0.4c | 6.100 | 4.8328 | 1.291–28.820 | 0.0224 |
Abbreviations: β-CTX=β-isomer of carboxiterminal telopeptide of collagen I; BALP=bone alkaline phosphatase; P1NP=aminoterminal propeptide of procollagen I.
Ninety-eight patients with PCa and bone metastases treated with zoledronic acid (4 mg every 4 weeks) during 18 months. V0: basal visit; V1: visit after 3 months following the beginning of the treatment.
Biomarker ‘higher than' the upper limit of normality.
Biomarker ‘higher than' the cut-off obtained from the ROC curves of basal values against death.
Biomarker value in v1/biomarker in v0 ‘higher than the cut-off obtained from the corresponding ROC curves.
Table 6. Univariate Cox regression analysis of bone markers for survival prediction according to normalisation in V1.
| Marker | Hazard ratio | s.d. | 95% CI | P-value |
|---|---|---|---|---|
| BALP | ||||
| No normalisationa |
2.029 |
1.4867 |
0.483–8.530 |
0.3341 |
| P1NP | ||||
| No normalisationa |
5.053 |
3.3285 |
1.389–18.376 |
0.0139 |
|
β-CTX | ||||
| No normalisationa | 6.271 | 4.0944 | 1.744–22.548 | 0.0049 |
Abbreviations: β-CTX=β-isomer of carboxiterminal telopeptide of collagen I; BALP=bone alkaline phosphatase; P1NP=aminoterminal propeptide of procollagen I.
Ninety-eight patients with PCa and bone metastases treated with zoledronic acid (4 mg every 4 weeks). V1: visit after 3 months following the beginning of the treatment.
Value of biomarker in the V1 higher than the upper limit of normality.
Bone biomarkers and disease progression
Distinctly than in the case of mortality, we did not find any association between bone marker levels and disease progression. Basal levels of bone biomarkers did not show any correlation with disease progression through Kaplan–Meier curves analysis. Normalisation of bone marker levels after ZA treatment or v1/v0 marker levels ratio also failed to show any statistically significant association with disease progression. As we did not find any significant differences in Kaplan–Meier curves, Cox regression analysis was not performed.
Capability of bone biomarkers to predict SREs appearance
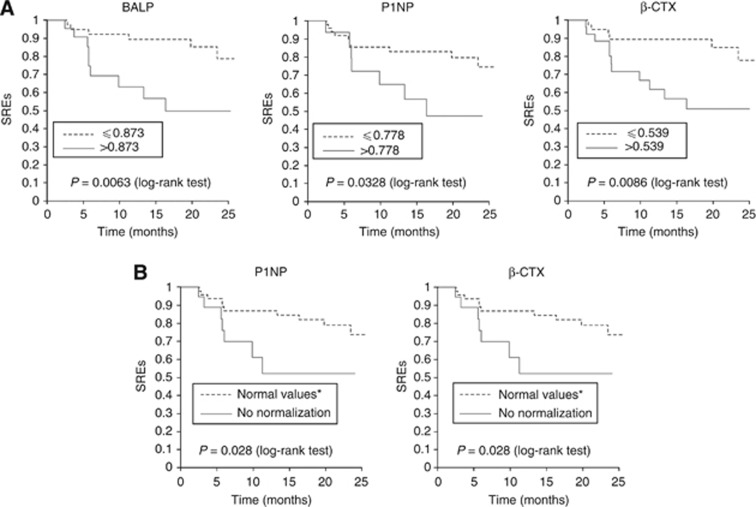
In the present work, two patients presented with SREs before the beginning of the study. Seven, 7, 5, 3, 2 and 2 new SREs were observed in v1, v2, v3, v4, v5 and v6 respectively. With respect to SREs appearance, Figure 3A and B show the relevant results. Tables 7 and 8 shows the univariate Cox regression analysis of Kaplan–Meier curves related to SREs development. In this case, values of the three markers were closely related to the SREs, and specially BALP, in which a rate in the values of v1/v0 of BALP that was higher than 0.873 was associated with a probability of SREs development 4.07-times higher, while a lack of normalisation in P1NP after treatment increased the probably of SREs appearance 3.8 times.
Figure 3.
(A) Kaplan–Meier analysis for the appearance of SREs after the completion of treatment with zoledronic acid according to the ratio ‘level of biomarker in v1/ level of biomarker in v0', for BALP, P1NP and β-CTX. The stratification was performed using the cutoff points obtained from the ROC curves of ‘changes in bone markers in v1 with respect to v0' against SREs appearance. The cutoff points obtained in the ROC curves were 0.873 for BALP, 0.778 for P1NP and 0.539 for β-CTX. (B) Kaplan–Meier analysis for the appearance of SREs after the completion of treatment (18 months) with ZA according to the dichotomous criterion of achievement or not a normal level of bone markers in the v1 for P1NP and β-CTX. *Values of biomarkers into the normal range are indicated in Materials and Methods. Kaplan–Meier curve of BALP is not presented because differences between groups according to these criteria were not statistically significant.
Table 7. Univariate Cox regressing analysis of bone markers for SRES prediction after completion of ZA treatment.
| Marker | Hazard ratio | s.d. | 95% CI | P-value |
|---|---|---|---|---|
| BALP | ||||
| V0 elevateda | 0.796 | 0.3649 | 0.324–1.955 | 0.6192 |
| V0>78.4b | 2.052 | 0.8987 | 0.870–4.841 | 0.1008 |
| V1/V0>0.873c |
4.068 |
2.1641 |
1.434–11.540 |
0.0083 |
| P1NP | ||||
| V0 elevateda | 1.469 | 0.6441 | 0.622–3.469 | 0.3801 |
| V0>135.6b | 1.993 | 0.8395 | 0.873–4.551 | 0.1016 |
| V1/V0 >0.778c |
2.905 |
1.4521 |
1.0917.738 |
0.0329 |
|
β-CTX | ||||
| V0 elevateda | 2.303 | 0.9881 | 0.993–5.340 | 0.0519 |
| V0>0.584b | 2.006 | 0.8468 | 0.877–4.588 | 0.0991 |
| V1/V0>0.539c | 3.435 | 1.7529 | 1.263–9.339 | 0.0156 |
Abbreviations: β-CTX=β-isomer of carboxiterminal telopeptide of collagen I; BALP=bone alkaline phosphatase; P1NP=aminoterminal propeptide of procollagen I.
Ninety-eight patients with PCa and bone metastases treated with zoledronic acid (4 mg every 4 weeks). V0: basal visit; V1: visit after 3 months following the beginning of the treatment.
Biomarker ‘higher than' the upper limit of normality.
Biomarker ‘higher than' the cut-off obtained from the ROC curves of basal values against SREs appearance.
Biomarker value in v1/biomarker in v0 ‘higher than the cut-off obtained from the corresponding ROC curves.
Table 8. Univariate Cox regression analysis of bone markers for SRES prediction according to normalisation of values of bone markers at V1.
| Marker | Hazard ratio | s.d. | 95% CI | P-value |
|---|---|---|---|---|
| BALP | ||||
| No normalisationa |
0.972 |
0.5056 |
0.351–2.694 |
0.9571 |
| P1NP | ||||
| No normalisationa |
2.900 |
1.4720 |
1.073–7.843 |
0.0359 |
|
β-CTX | ||||
| No normalisationa | 3.847 | 1.9443 | 1.42810.359 | 0.0077 |
Abbreviations: β-CTX=β-isomer of carboxiterminal telopeptide of collagen I; BALP=bone alkaline phosphatase; P1NP=aminoterminal propeptide of procollagen I.
Ninety-eight patients with PCa and bone metastases treated with zoledronic acid (4 mg every 4 weeks) during 18 months. V1: visit after 3 months following the beginning of the treatment.
Value of biomarker in the V1 higher than the upper limit of normality.
It is important to point that all the values of hazard ratios that presented a statistical significance in the study, presented values higher than those considered to have a minimum of 80% of statistical power (see Materials and Methods).
Discussion
As higher levels of PSA are found with more advanced stages of PCa and with worse outcomes, PSA has been historically used as a staging and prognostic tool (Shariat et al, 2004). However, PSA is now commonly regarded as an indicator of prostate volume and is not independently diagnostic or prognostic in PCa. Owing to these limitations, there is an urgent need for new prognostic biomarkers to enhance the clinical management of PCa. This could be denominated the post-PSA era (Bickers et al, 2009).
To perform this work, we selected two biochemical markers of bone formation – BALP and P1NP – and one of bone resorption – β-CTX. Of these, BALP has high specificity, as it is a product only released by the active osteoblast and has demonstrated a great sensitivity for detecting increases in bone turnover (Garnero and Delmas, 1993). Its weakness, however, is that monoclonal antibodies against BALP exhibit a cross-reactivity of 15% with hepatic isoenzyme (Garnero and Delmas, 1993). As a result, values can be abnormally elevated in the presence of hepatic diseases. However, in our work, none of the PCa patients with bone metastases presented simultaneously with hepatic metastases. Thus, no interference of hepatic on bone isoenzyme was present. Aminoterminal propeptide of procollagen type I and β-CTX have been recently recommended by the International Osteoporosis Foundation and the International Federation of Clinical Chemistry and Laboratory Medicine as reference markers in all clinical studies (Vasikaran et al, 2011).
In a first stage, biomarkers of bone formation and bone resorption were evaluated for their ability to act as useful tools for the diagnosis of bone metastases (Coleman, 1997; Leeming et al, 2006; Huang and Ouyang, 2012). All biomarkers were indicative of skeletal involvement, albeit with different sensitivity. In previous works, one group of authors of the present work found a sensitivity of 87.5% and a specificity of 100% for BALP (>30 ng ml−1) in the detection of bone metastases in PCa (Lorente et al, 1996), the values of BALP being more efficient than PSA levels in the prediction of positive bone scans. De la Piedra et al (2003) found a specificity and sensitivity of 100% in the levels of P1NP and β-CTX. This high sensitivity and specificity was due to the fact that this is the only work in the literature in which all PCa patients were studied before any treatment was initiated. In the present work, the patients showed elevated baseline levels of BALP, P1NP and β-CTX. Brasso et al (2006) also found an elevation in these markers. The increase in the levels of bone formation at the onset of skeletal metastases reflects the dominant osteoblastic element of the bone remodelling process in these patients, although due to the coupling remodelling phenomena, markers of bone resorption are also elevated. This fact produces a high correlation between markers, as we found in the present work. The fact that bone metastases produce an increase in remodelling is corroborated by the correlation between bone markers levels and Soloway classification, as described in the present and previous works (Coleman, 1997; Leeming et al, 2006).
Although hormonal treatment tends to produce an increase in the levels of biochemical markers of bone turnover (Smith et al, 2004) (a tendency to higher values was observed in treated patients), in basal conditions, the concentration of bone markers did not differ significantly between patients with and without hormonal treatment. After ZA treatment, values of all markers decreased and values of bone markers of treated and untreated groups of patients were quite similar. This is the reason for which we performed all the subsequent calculations with the data obtained from all patients in the study group.
Owing to the antiosteoclastic action of ZA, the first effect observed after ZA treatment was the significant decrease in bone markers. The highest degree of normalisation was observed in β-CTX, presenting a 93.3% of the patients normal values of this marker after 18 months of treatment.
Our results show that values of β-CTX were especially relevant for the prediction of survival during the 18 months of ZA treatment. A decrease of β-CTX in v1 of <40% and/or lack of normality in levels of β-CTX, indicates a six-fold increase in the risk of mortality along the study. Similarly, a decrease of P1NP in v1 of <44% and/or lack of normality, indicates a five-fold increase in the risk of mortality, and no changes in levels of BALP between v0 and v1 indicates an increase of three-fold in the risk of mortality. In agreement with our results, Jung et al (2011) found that P1NP and cross-linked carboxiterminal telopeptides of collagen I (ICTP) could be considered suitable predictors for survival in patients with PCa and bone metastases. Brasso et al (2006) found that patients with elevated serum P1NP, BAP and CTX at the time of diagnosis of metastatic PCa had significantly lower rates of survival than patients with normal levels of these biomarkers.
In the present work, we did not find any association between bone markers and disease progression. This result is consistent with that of Mountzios et al (2010), who found that none of the bone markers were able to predict clinical outcomes. Santini et al (2006) also found a very limited impact of β-CTX levels on the detection of disease progression.
With respect to survival prediction, the three bone markers were suitable predictors for SREs appearance, with BALP and P1NP being the most potent. In this case, a value of v1/v0>0.873 in BALP (a decrease of BALP in v1 of <87%) indicates a four-fold increase in the risk of SREs. The specific osteoblastic – and in consequence osseous – nature of BALP and P1NP could be the reason behind the close association between high bone formation levels and SREs, as compared with β-CTX. Lein et al (2009) found that in PCa patients treated with ZA, a decrease of P1NP below 25% was predictive for SREs development.
Conclusions
In conclusion, use of bone markers and especially of β-CTX, in the follow-up of patients with PCa and bone metastases improved prediction of the disease development, complementing predictions based exclusively on clinical data. The decrease in β-CTX following the first administration of ZA is closely correlated with survival, while the decrease in BALP and P1NP is a strong predictor of the appearance of SREs. Use of these three biomarkers provides an interesting advantage, not only for clinicians regarding clinical decisions, but also for patients regarding their own decisions on treatment planning.
Acknowledgments
This study was supported by Novartis Oncology Spain. As this was a multicenter study, Adknoma Health Research (Barcelona, Spain) collected and ordered data throughout the study. We especially thank Margarida Garcia from Adknoma who followed carefully the development of the study. M. Martín-Fernández was a fellow of the Conchita Rábago Foundation.
Cristina Meseguer is an employee of Novartis Farmaceútica, Spain. The other authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Bickers B, Aukhim-Hasie C. New molecular biomarkers for the prognosis and management of prostate cancer. The post PSA era. Anticancer Res. 2009;29:3289–3298. [PubMed] [Google Scholar]
- Brasso K, Christensen IJ, Johansen JS, Teisner B, Garnero P, Price PA, Iversen P. Prognostic value of PINP, bone alkaline phosphatase, CTX-I, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66:503–513. doi: 10.1002/pros.20311. [DOI] [PubMed] [Google Scholar]
- Buijs JT, Pluijm G. Osteotropic cancers: from primary tumor to bone. Cancer Lett. 2009;273:177–193. doi: 10.1016/j.canlet.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Lipton A, Roodman GD, Guise TA, Boyce BF, Brufsky AM, Clézardin P, Croucher PI, Gralow JR, Hadji P, Holen I, Mundy GR, Smith MR, Suva LJ. Metastasis and bone loss: advancing treatment and prevention. Cancer Treat Rev. 2010;36:615–620. doi: 10.1016/j.ctrv.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook GJR, Fogelman I.2000Diagnosis and monitoring on bone metastases: scintigraphy Tumor bone diseases and osteporosis in cancer patients, Body JJ(ed)pp 131–154.Marcel Dekker: New York [Google Scholar]
- De la Piedra C, Castro-Errecaborde NA, Traba ML, Méndez-Dávila C, García Moreno C, Rodriguez de Acuña L, Rodriguez Molina J. Bone remodeling markers in the detection of bone metastases in prostate cancer. Clin Chim Acta. 2003;331:45–53. doi: 10.1016/s0009-8981(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Garnero P, Delmas PD. Assessment of the serum levels of bone alkaline phosphatase with a new immunoradiometric assay in patients with metabolic bone disease. J Clin Endocrin Metab. 1993;77:1046–1053. doi: 10.1210/jcem.77.4.8104954. [DOI] [PubMed] [Google Scholar]
- Huang Q, Ouyang X. Biochemicals-markers for the diagnosis of bone metastasis: A clinical review. Cancer Epidemiology. 2012;36:94–98. doi: 10.1016/j.canep.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Jung K, Miller K, Wirth M, Albrech M, Lein M. Bone turnover markers as predictors of mortality risk in prostate cancer patients with bone metastases following treatment with zoledronic acid. Eur Urol. 2011;59:604–612. doi: 10.1016/j.eururo.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Leeming DJ, Koizumi M, Byrjalsen I, Li B, Qvist P, Tankó LB. The relative use of eight collagenous and noncollagenous markers for diagnosis of skeletal metastases in breast, prostate or lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:32–28. doi: 10.1158/1055-9965.EPI-05-0492. [DOI] [PubMed] [Google Scholar]
- Lein M, Wirth M, Miller K, Eickenberg HU, Weissbach L, Schmidt K, Haus U, Stephan C, Meissner S, Loening SA, Jung K. Serial markers of bone turnover in men with metastatic prostate cancer treated with zoledronic acid for detection of bone metastases progression. Eur Urol. 2007;52:1381–1387. doi: 10.1016/j.eururo.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Lein M, Miller K, Wirth M, Weissbach L, May C, Schmidt K, Haus U, Schrader M, Jung K. Bone turnover markers as predictive tools for skeletal complications in men with metastatic prostate cancer treated with zoledronic acid. Prostate. 2009;69:624–632. doi: 10.1002/pros.20917. [DOI] [PubMed] [Google Scholar]
- Lipton A, Cook RJ, Major P, Smith MR, Coleman RE. Zoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activity. Oncologist. 2007;12:1035–1043. doi: 10.1634/theoncologist.12-9-1035. [DOI] [PubMed] [Google Scholar]
- Lorente JA, Morote J, Raventos C, Encabo G, Valenzuela H. Clinical efficacy of bone alkaline phosphtase and prostate specific antigen in the diagnosis of bone metastases in prostate cancer. J Urol. 1996;155:1348–1351. [PubMed] [Google Scholar]
- Mountzios G, Terpos E, Syrigos K, Papadimitriou C, Papadopoulos G, Bamias A, Mavrikakis M, Dimopoulos MA. Markers of bone remodeling and skeletal morbidity in patients with solid tumors metastatic to the skeleton receiving the bisphosphonates zoledronic acid. Transl Res. 2010;155:247–255. doi: 10.1016/j.trsl.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Metastases to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Polascik TJ, Mouraviev V. Zoledronic acid in the management of metastatic bone disease. Ther Clin Risk Manag. 2008;4:261–268. doi: 10.2147/tcrm.s2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini D, Vicenzi B, Hannon RA, Brown JE, Dicuonzo G, Angeletti S, La Cesa A, Coleman RE, Tonini G, Budillon A, Caraglia M, Holen I. Changes in bone resorption and vascular endothelial growth factor after a single zoledronic acid infusion in cancer patients with bone metastases from solid tumours. Oncol Rep. 2006;15:1351–1357. [PubMed] [Google Scholar]
- Shariat SF, Canto EI, Kattan MW, Slawin KM. Beyond prostate-specific antigen: new serologic biomarkers for improved diagnosis and management of prostate cancer. Rev Urol. 2004;6:58–72. [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Goode MJ, Zietman AL, McGovern FJ, Lee H, Finkelstein JS. Bicalutamide monothetapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol. 2004;22:2546–2553. doi: 10.1200/JCO.2004.01.174. [DOI] [PubMed] [Google Scholar]
- Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, Moinuddin M. Stratification of patients with prostate cancer based on extent of disease on initial bone scan. Cancer. 1998;61:195–202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA, IOF-IFCC Bone Marker Standards Working Group Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatmen: a need for internatoional reference standards. Osteoporosis Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]