Abstract
Objective
Inflammatory activation of valve endothelium is an early phase of aortic valve disease pathogenesis, but subsequent mechanisms are poorly understood. Adult valve endothelial cells retain the developmental ability to undergo endothelial to mesenchymal transformation (EndMT), but a biological role has not been established. Here we test whether and how inflammatory cytokines (TNF-α and IL-6) regulate EndMT in embryonic and adult valve endothelium.
Methods and Results
Using in vitro 3D collagen gel culture assays with primary cells, we determined that IL-6 and TNF-α induce EndMT and cell invasion in dose dependent manners. Inflammatory-EndMT occurred through an Akt/NFκB-dependent pathway in both adult and embryonic stages. In embryonic valves, inflammatory-EndMT required canonical TGFβ signaling through Alk2/5 to drive EndMT. In adult valve endothelium, however, inflammatory-induced EndMT still occurred when Alk2/5 signaling was blocked. Inflammatory receptor gene expression was significantly upregulated in vivo during embryonic valve maturation. Endothelial-derived mesenchymal cells expressing activated NFκB were found distal to calcific lesions in diseased human aortic valves.
Conclusions
Inflammatory cytokine induced EndMT in valve endothelium is present in both embryonic and adult stages, acting through Akt/NFκB but differently utilizing TGFβ signaling. Molecular signatures of valve EndMT may be important diagnostic and therapeutic targets in early valve disease.
Keywords: invasion, calcification, transforming growth factor-beta, tumor necrosis factor-alpha, smooth muscle actin
Introduction
Degenerative heart valve disease (HVD) is a significant contributor to cardiovascular morbidity in the United States. Aortic valve dysfunction affects 2.5% of all Americans and 30% of the elderly1. HVD directly claims 20,000 lives annually in the US and sufferers have twice the risk of heart attacks, strokes, and heart failure1. In addition, congenital valve abnormalities affect 1%-2% of live births and lead to early structural deterioration2. Historically, aortic valve disease (AVD) was thought to be pathogenically similar to vascular atherosclerosis. Both diseases are characterized by loss of endothelial integrity, infiltration of inflammatory cells, accumulation of plasma lipoproteins, release of inflammatory cytokines, mesenchymal proliferation, extracellular matrix remodeling, and the growth of plaque lesions3. Unlike atherosclerosis, however, AVD leads to large, obstructive lesions containing significant matrix mineralization4. Randomized clinical trials using lipid lowering agents to halt AVD severity or progression have been disappointing5,6. These findings underscore the conclusion that mechanisms of aortic valve disease are distinctly different than vascular atherosclerosis. As a consequence, there are currently no established molecular biomarkers specific to AVD progression and no molecular targets for AVD therapy7.
We previously determined in mice that the degree of inflammation in aortic valves directly correlates with the degree of calcification8. Diseased human valve cusps become progressively thickened, with increases in the presence of macrophages and cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)9-11. Both IL-6 and TNF-α can activate arterial endothelial cells, which is a phenotypic change characterized by upregulation of adhesion molecules such as ICAM-1 and VCAM-1, recruitment of leukocytes, and increased monolayer permeability10,12,13. In atherosclerotic plaques, both IL-6 and TNF-α drive transcription through nuclear translocation of nuclear factor kappa b (NFκB), but their role in valve endothelium is unknown14,15.
Fetal human aortic valve endothelial cells also express ICAM-1 and VCAM-1, which suggests that endothelial activation also occurs during valve development16. TNF-α, IL-6, and NFκB expression levels are elevated in the myocardium of children with congenital heart defects affecting valve development17,18. NFκB inhibition during avian heart development leads to valvuloseptal defects and impaired outlet formation19. Leptin, a member of the IL-6 superfamily, was shown to induce EndMT in embryonic endocardium, in part through activating TGFβ20. Akt has also been shown to drive embryonic and adult EndMT21,22. Adult ovine aortic valve endothelium has been shown to undergo EndMT in vitro and in vivo23-25. Collectively, these findings motivate the hypothesis that inflammatory signaling toward EndMT may be a developmentally conserved signaling pathway in valve endothelium, but a biological rationale is unclear.
In this study, we determine that both TNF-α and IL-6 induce EndMT in embryonic and adult valve endothelium via an Akt/NFκB dependent pathway. In embryonic valve endocardium, NFκB acted upstream of TGFβ to induce EndMT through Alk2/5. We found increasing levels of TNF-α and IL-6 receptor gene expression in embryonic valve primordia during valve morphogenesis. Interestingly, while TGFβ could drive EndMT in adult valve endothelium through Alk2/5, TGFβ was not required for NFκB induced EndMT. Finally, we identify evidence of EndMT in calcified human aortic valves. Taken together, these findings support a developmentally conserved mechanism of valve remodeling via NFκB induced EndMT that may be a previously unrecognized molecular signature of early AVD.
Methods
We employed two well-characterized valve endothelial cell culture systems to test inflammatory signaling on EndMT in vitro: Porcine aortic valve endothelial cells (PAVEC) and quail embryonic endocardial explants (QEE)26-28. Additional details for each source are given in the supplement. PAVEC were isolated through collagenase digestion as previously described29, with purity confirmed via non-detection of the mesenchymal ACTA2 gene expression (> 37 cycles via RT-PCR). PAVEC (< passage 5) were cultured as confluent surface monolayers on the surface of type 1 collagen hydrogels (1.5 mg/ml, 50,000 cells/cm2). Pre-transformed (HH14− staged) quail endocardial explants (QEE) from the atrioventricular (AV) and outflow tract (OFT) valvulogenic regions were cultured as monolayer patches (without myocardium) on 1.5 mg/ml collagen gels as previously described27. EndMT was quantified via real-time PCR, immunohistochemistry, and Western blotting; while cell invasion was quantified at 60 μm depth with brightfield microscopy (Supplemental Figure I). The protein extraction and Western blot protocols are described in detail in the online supplement. NFκB protein nuclear co-localization was quantified for 100 cells for each treatment using Metamorph 7.1 software (Molecular Devices, Sunnyvale, CA). RNA extraction, purification, and RT-PCR were performed according to the manufacturer’s protocols. Primers used for RT-PCR are listed in Supplemental Table I. For NFκB transfection, PAVEC cells were trypsinized and electroporated with plasmid encoding the RelA subunit of NFκB (Addgene plasmid 23255) using Neon transfection system (Invitrogen, Carlsbad, CA) and further cultured in 5% serum, antibiotic-free DMEM for 24 hours30. The cells were then trypsinized, re-suspended in normal media (DMEM with 10% fetal bovine serum and 1% penicillin-streptomycin), and seeded onto gels. Invasion assay was performed as described above. Human diseased aortic valves were obtained from adults undergoing planned, non-elective valve replacement surgery at Robert Packer Hospital in Sayer, PA. All procedures were approved by Institutional Review Boards at Cornell and Robert Packer Hospital. Pediatric valves were obtained from Dr. Jonathan Chen, Cornell-Weill Medical School and NY Presbyterian Hospital. Results are expressed as mean ± S.E>M., n ≥ 3 independent cultures per treatment condition, with 5-6 explants pooled for each QEE sample. Data was analyzed with GraphPad Prism version 4 for Windows (GraphPad Software, San Diego, CA). Treatment effects were compared using Analysis of Variance (ANOVA) with Tukey’s post hoc paired tests, and data was transformed when necessary to obtain equal sample variances. Differences between means were considered significant at p ≤ 0.05. Expanded methods are provided in the Supplemental Information.
Results
TNF-α and IL-6 induce EndMT in adult valve endothelial cells
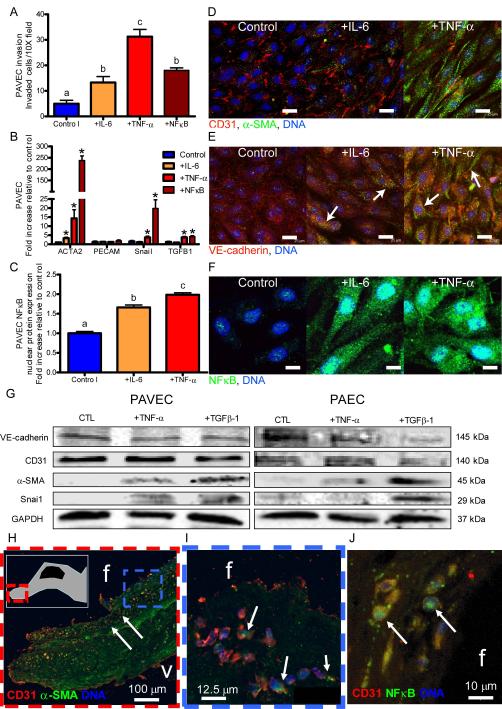
We first exposed 3D cultured adult PAVEC monolayers to doses of TNF-α or IL-6 and quantified changes in EndMT related gene expression, protein expression, and collagen matrix invasion. Both IL-6 and TNF-α in 3D culture induced loss of endothelial cell-cell contacts including PECAM-1 and VE-cadherin, acquisition of mesenchymal α-smooth muscle actin (α-SMA) and invasion into collagen matrix in a dose-dependent manner (Figure 1A,D,E,G; Supplemental Figures I-II). Genetic overexpression of NFκB alone was sufficient to cause 3D matrix invasion in PAVEC, supporting an NFκB-dependent EndMT mechanism (Figure 1A). Pro-EndMT related gene expression (ACTA2, Snail, TGFβ1) was significantly upregulated in response to NFκB overexpression (238±20.6, 15.0±1.1, and 4.2±0.5 fold respectively), TNF-α (14.3±4.8, 3.8±0.7, and 3.8±0.6 fold respectively, p<0.05), but not IL-6 (Figure 1B). EndMT-related protein expression (α-SMA, Snai1 increase and VE-cadherin decrease) was shown in PAVEC, but not porcine aortic endothelial cells (PAEC) in response to TNF-α (Figure 1G). TGFβ-1, however, induced EndMT protein expression in both PAVEC and PAEC (Figure 1G). Nuclear translocation of NFκB was significantly upregulated in response to either IL-6 or TNF-α (1.7±0.1 and 2.0±0.1 fold increase, p<0.05, Figure 1C,F; Supplemental Figure III). To control for cell number, we quantified PAVEC proliferation (via BrdU incorporation) and apoptosis (via TUNEL) in response to doses of TNF-α. PAVEC proliferation increased with TNF-α dose (up to 2.7±0.1 at 100 ng/mL, p<0.05, Supplemental Figure ID), while apoptosis remained unchanged (Supplemental Figure IE). Collectively, these results suggest that IL-6 and TNF-α induce EndMT and matrix invasion in adult PAVEC in vitro at least in part through NFκB, with TNF-α being a more potent EndMT inducer.
Figure 1.
Exposure to inflammatory cytokines induces mesenchymal transformation in adult endothelial cells. A.) Porcine aortic valve endothelial cell (PAVEC) mesenchymal transformation and invasion of the collagen matrix after 48 hour exposure to 100 ng/mL IL-6, 100 ng/mL TNF-α, or genetic overexpression of NFκB. B.) PAVEC EndMT-related gene expression after 48 hour exposure to 100 ng/mL IL-6 or TNF-α, or genetic overexpression of NFκB C.) PAVEC NFκB nuclear localization quantification. D.) Confocal images of control, +100 ng/mL IL-6, and +100 ng/mL TNF-α PAVEC at a 48 hour time point stained for CD31 (red), α-SMA (green), and DNA (blue). E.) Confocal images of control, +100 ng/mL IL-6, and +100 ng/mL TNF-α PAVEC at a 48 hour time point stained for VE-cadherin (red), α-SMA (green), and DNA (blue). F.) Confocal images of control, +100 ng/mL IL-6, and +100 ng/mL TNF-α PAVEC at a 48 hour time point stained for NFκB (green) and DNA (blue). G.) Western blots for PAVEC and porcine aortic valve endothelial cells (PAEC) exposed to 100 ng/mL TNF-α or TGFβ-1 for 48 hours. H.) Diseased human aortic valve endothelial cells co-expressing α-SMA and CD31 (on the fibrosa or outflow side and invaded, calcified nodule shown in inset, I.) Expanded frame of Figure 1H. J.) Nuclear translocation of NFκB in diseased human valve endothelial cells. Error bars show ±SEM, n ≥ 3 culture wells. Bars that do not share any letters are significantly different according to a one-way ANOVA with Tukey’s post test (p ≤ 0.05). For EndMT gene expression (Figure 1B), differences that are significantly different than the control according to an unpaired Student’s t-test are indicated with a *. Differences were considered significant at p ≤ 0.05. Scale bars = 25 μm (D, E) or 10 μm (F).
EndMT is present in calcified human aortic valves
In explanted human aortic valves with calcific lesions (Figure 1G-I) we identified a population of sub-endothelial cells co-expressing α-SMA and PECAM1, but not CD45 (Supplemental Figure IV). This suggests the cells were EndMT-derived, but not from an immune origin31. The number of invaded fibrosal endothelial cells was 11.13 ± 1.88 cells/40X field (SEM, n = 3 calcified adult valves). Endothelial cells located near the calcified nodule and on the fibrosa side showed decreased VE-cadherin expression (Supplemental Figure IVD). EndMT and potential EndMT-derived cells were only found in the fibrosa layer, distal to sites of calcified lesions. Furthermore, we found that many of these transformed and invaded cells also co-expressed nuclear NFκB (Figure 1H). Healthy pediatric human valves did not co-express α-SMA and VE-cadherin or nuclear NFκB (Supplemental Figures V and VI). These findings support that NFκB-mediated EndMT occurs in human calcific aortic valve disease, but is likely not involved in the mineralization process.
TNFα and IL-6 induce EndMT in embryonic valve endocardium
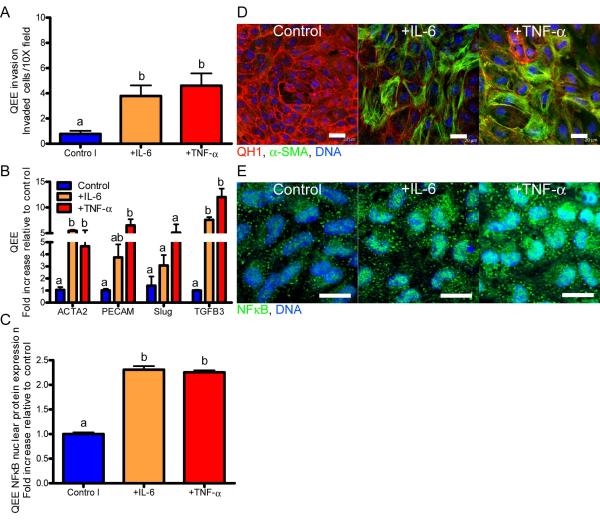
Similarly, we quantified the EndMT response characteristics of 3D cultured HH14− pre-transformed QEE monolayers from OFT or AV regions to doses of TNF-α and IL-6. As in adult valve endothelium, both IL-6 and TNF-α induced loss of QH1, acquisition of α-SMA, and collagen matrix invasion (Figure 2A, D). Embryonic endocardial EndMT-related genes (ACTA2, Slug, and TGFβ3) were significantly upregulated in valvulogenic endocardium by both IL-6 and TNF-α (Figure 2B). PECAM1 gene expression was significantly increased in response to inflammatory cytokine (Figure 2A). PECAM1 has been shown to be an early marker of cardiovascular development, and the increase in CD31 in culture is likely related to embryonic endocardial differentiation32. Downstream NFκB protein nuclear translocation also occurred in response to either IL-6 or TNF-α (Figure 2C, E; Supplemental Figure VII). There was no difference in explant area (a control for cell number) with IL-6 or TNF-α stimulation in comparison to control (Supplemental Figure VIIIC,D). Collectively, these results suggest that both TNF-α and IL-6 induce EndMT in embryonic valve endocardial cells in vitro with similar potency. We further quantified the expression of inflammatory signaling related genes in the AV and OFT valve forming regions of embryonic hearts across the period of valve morphogenesis (Day 2-Day 10). We determined that ICAM1, NFKB1, IL6RA (coding for IL-6Rα), and TNFRSF1A (coding for TNFR1) gene expression were significantly upregulated in both valve regions as EndMT and valve remodeling progressed (Supplemental Figure IX). These results suggest that inflammatory signaling is active in valves during morphogenesis in vivo and directly cause EndMT in valve endocardium in vitro.
Figure 2.
Exposure to inflammatory cytokines induces mesenchymal transformation in embryonic endocardial cells. A.) Quail endocardial explant (QEE) mesenchymal transformation and invasion of the collagen matrix after 48 hour exposure to 100 ng/mL IL-6 or TNF-α. B.) QEE EndMT-related gene expression after 48 hour exposure to 100 ng/mL IL-6 or TNF-α. C.) QEE NFκB nuclear localization quantification. D.) Confocal images of control, +100 ng/mL IL-6, and +100 ng/mL TNF-α QEE at a 48 hour time point stained for QH1 (red), α-SMA (green), and DNA (blue). E.) Confocal images of control, +100 ng/mL IL-6, and +100 ng/mL TNF-α QEE at a 48 hour time point stained for NFκB (green) and DNA (blue). Error bars show ±SEM, n ≥ 3 culture wells with pooled explants. Bars that do not share any letters are significantly different according to a one-way ANOVA with Tukey’s post test (p ≤ 0.05). Scale bars = 20 μm.
Inflammatory EndMT acts through an Akt/NFκB pathway present in both adult and embryonic valve endothelium
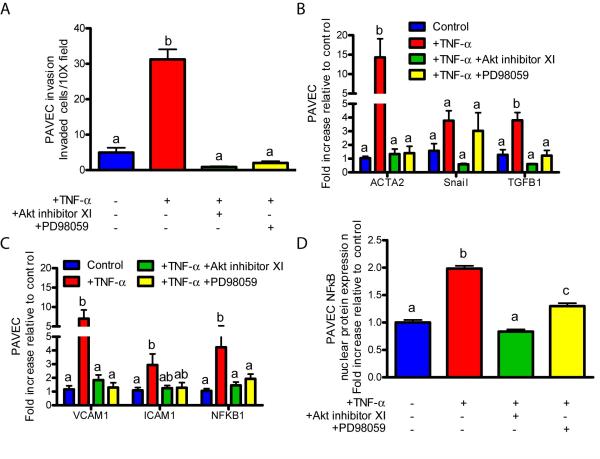
We next sought to identify the signaling pathway by which inflammatory cytokines induced EndMT through NFκB. Previous studies have shown that TNF-α activates NFκB-mediated transcription through Akt and/or MEK133-37. IL-6 activates STAT3 most potently, but there is also evidence of IL-6-induced NFκB activation through PI3K and Akt38-41. Utilizing our 3D culture system, molecular inhibition of either Akt (via 5 μm Akt inhibitor XI) or MEK1 (via 25 μm PD98059) significantly reduced TNF-α-induced NFκB expression and nuclear translocation in PAVEC, but only Akt inhibition resulted in complete knockdown (Figures 3C,D). Akt inhibition also completely blocked IL-6-induced NFκB gene expression and protein nuclear translocation, while STAT3 inhibition (via 5 μm PpYLKTK STAT3 inhibitor) had no effect (Supplemental Figures XC,D). Inhibition of Akt completely blocked TNF-α induced inflammatory receptor activation (VCAM1, ICAM1), EndMT associated gene expression (ACTA2, Snail, TGFβ1), and subsequent matrix invasion by PAVEC (Figure 3A-C). MEK1 blockade also inhibited TNF-α-induced inflammatory receptor activation, EndMT gene expression, and subsequent invasion, but to a lesser degree than Akt inhibition. In a similar fashion, Akt inhibition completely blocked IL-6-induced upregulation of inflammatory receptors, EndMT gene expression, and subsequent matrix invasion (Supplemental Figures XA-C). STAT3 inhibition, on the other hand, had no effect on any of these responses from IL-6.
Figure 3.
Inflammatory cytokines induce EndMT through Akt/MAPK/NFκB in PAVEC. A.) Cell invasion after a 48 hour to exposure to 100 ng/mL TNF-α or 100 ng/mL TNF-α with 5 μm Akt inhibitor XI or 25 μm PD98059 MEK1 inhibitor. B.) EndMT-related gene expression after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. C.) Inflammatory activation-related genes after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. D.) PAVEC NFκB nuclear localization quantification after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. Error bars show ±SEM, n ≥ 3 culture wells. Bars that do not share any letters are significantly different according to a one-way ANOVA with Tukey’s post test (p ≤ 0.05).
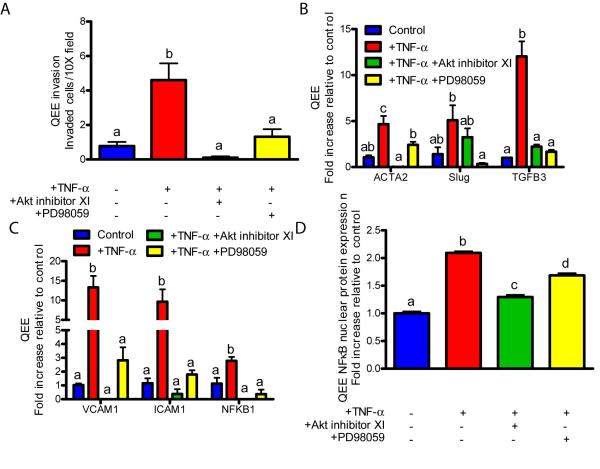
Identical experiments were conducted on avian pre-EndMT valvulogenic endocardial monolayers. As in adult valve endothelium, molecular inhibition of Akt or MEK1 both blocked TNF-α-induced NFκB gene expression and nuclear translocation. Akt inhibition completely blocked NFκB while MEK1 inhibition only partially blocked NFκB (Figure 4C,D). IL-6-induced NFκB gene expression and protein nuclear translocation was also blocked with Akt inhibition, but not STAT3 inhibition (Supplemental Figures XIC,D). Akt or MEK1 inhibition also blocked inflammatory receptor (VCAM1, ICAM1) upregulation, EndMT-related gene expression (ACTA2, Slug, TGFβ3), and collagen invasion by QEE cells (Supplemental Figures XIA-C). As a positive control, Akt1 gene expression was blocked by Akt inhibition in both PAVEC and QEE subjected to TNF-α (Supplemental Figures XII and XIII). Collectively, these results suggest that inflammatory cytokines initiate EndMT at the gene, protein, and function levels through an Akt/NFκB pathway that is conserved in both adult valve endothelial and embryonic valvulogenic endocardium. TNF-α was a more potent inducer of EndMT and resulted in more consistent in pathway responses in both cell types when compared with IL-6. IL-6 may also induce EndMT genes more strongly at an earlier or later time point.
Figure 4.
Inflammatory cytokines induce EndMT through Akt/MAPK/NFκB in QEE. A.) Cell invasion after a 48 hour exposure to 100 ng/mL TNF-α or 100 ng/mL TNF-α with 5 μm Akt inhibitor XI or 25 μm PD98059 MEK1 inhibitor. B.) EndMT-related gene expression after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. C.) Inflammatory activation-related genes after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. D.) QEE NFκB nuclear localization quantification after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. Error bars show ±SEM, n ≥ 3 culture wells with pooled explants. Bars that do not share any letters are significantly different according to a one-way ANOVA with Tukey’s post test (p ≤ 0.05).
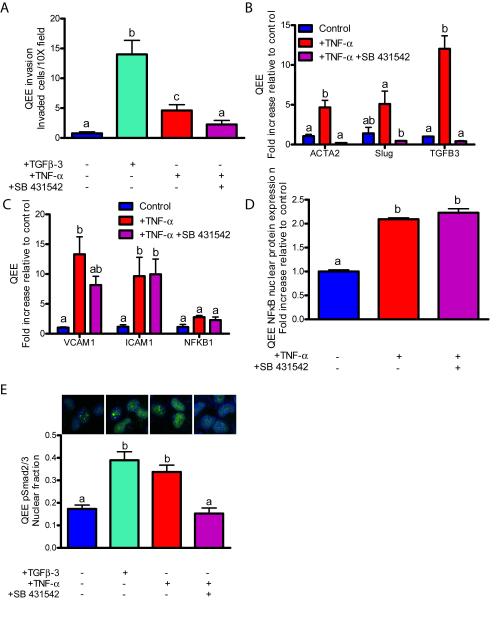
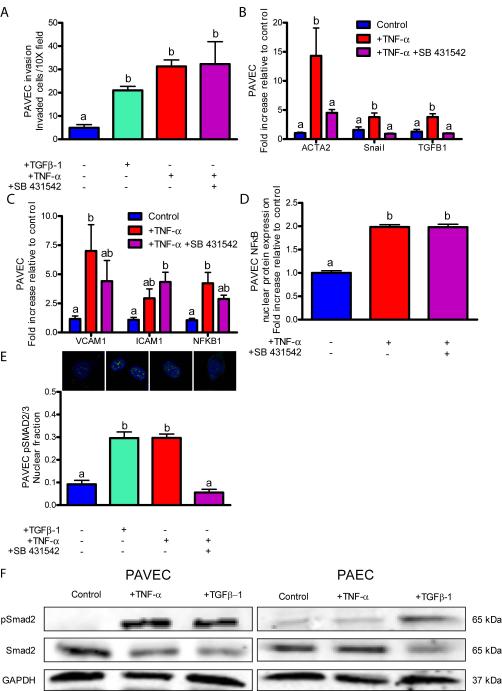
TGFβ signaling through Alk2/5 is required for inflammation-induced embryonic EndMT, but not for adult EndMT
Previous studies have identified a canonical TGFβ signaling pathway that induces EndMT and is isoform specific: the TGFβ1 isoform in adult VEC, while EndMT invasion is mediated by TGF–3 in avian embryonic valve endocardium23,42,43. We therefore tested whether TGFβ signaling was involved in inflammatory-induced EndMT. As a positive control, exogenous TGF–3 (100 ng/ml) induced EndMT matrix invasion in embryonic valve endocardium (Figure 5A), while exogenous TGF–1 induced EndMT in PAVEC (Figure 6A, Supplemental Figure XIV). TNF-α induced TGFB1 gene expression in PAVEC and TGFB3 in QEE (3.79±0.57 and 12.03±1.64, Figures 1B and 2B). IL-6 induced TGFB3 only in QEE (7.61±0.53, Figure 2B). Previous studies demonstrate that TGFβ type I activin receptor-like kinases 2 and 5 (Alk2/5) mediate TGFβ-induced EndMT44,45. Molecular inhibition of Alk2/5 signaling (via 10 μm SB 431542) in QEE reduced EndMT-related gene expression (Figure 5B, Supplemental Figure XVB), cell invasion induced by either TNF-α or IL-6 (Figure 5A, Supplemental Figure XVA), and Smad2/3 physphorylation and nuclear localization (Figure 5E), but did not affect inflammatory activation-related gene expression (Figure 5C, Supplemental Figure XVC) or NFκB protein nuclear localization (Figure 5D, Supplemental Figure XVD). Together, these results support that inflammatory cytokine-induced EndMT in embryonic valves acts at least in part by canonical TGFβ-Alk-Smad signaling that is downstream of NFκB.
Figure 5.
Embryonic endocardial monolayers co-opt TGFβ in inflammatory-EndMT signaling. A.) Cell invasion after a 48 hour exposure to 100 ng/mL TGFβ-3, 100 ng/mL TNF-α or 100 ng/mL TNF-α with 10 μm SB 431542 ALK5 inhibitor. B.) EndMT-related gene expression after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. C.) Inflammatory activation-related genes after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. D.) QEE NFκB nuclear localization quantification after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. E.) pSMAD2/3 nuclear localization following a 48 hour exposure to 100 ng/mL TGFβ-3, 100 ng/mL TNF-α, or 100 ng/mL TNF-α with 10 μm SB 431542 ALK5 inhibitor. Staining shows p-SMAD2/3 (green) or DNA (blue). Error bars show ±SEM, n ≥ 3 culture wells with pooled explants. Bars that do not share any letters are significantly different according to a one-way ANOVA with Tukey’s post test (p ≤ 0.05).
Figure 6.
Adult cells do not require TGFβ in inflammatory-EndMT signaling. A.) Cell invasion after a 48 hour exposure to 100 ng/mL TGFβ-1, 100 ng/mL TNF-α or 100 ng/mL TNF-α with 10 μm SB 431542 ALK5 inhibitor. B.) EndMT-related gene expression after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. C.) Inflammatory activation-related genes after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. D.) PAVEC NFκB nuclear localization quantification after a 48 hour exposure to 100 ng/mL TNF-α with inhibitors. E.) pSMAD2/3 nuclear localization following a 48 hour exposure to 1 ng/mL TGFβ-1, 100 ng/mL TNF-α, or 100 ng/mL TNF-α with 10 μm SB 431542 ALK5 inhibitor. Staining shows pSMAD2/3 (green) or DNA (blue). F.) Western blots for PAVEC or porcine aortic valve endothelial cells (PAEC) exposed to 100 ng/mL TNF-α or TGFβ-1 for 48 hours. Error bars show ±SEM, n ≥ 3 culture wells with monolayers. Bars that do not share any letters are significantly different according to a one-way ANOVA with Tukey’s post test (p ≤ 0.05).
In contrast to embryonic valve endocardium, Alk2/5 inhibition in adult valve endothelial cells (PAVEC) treated with TNF-α significantly decreased Smad2/3 phosphorylation and nuclear translocation (Figure 6E), but did not result in decreased cell invasion (Figure 6A), did not effect EndMT-related or inflammatory activation-related gene expression (Figures 6B,C), and did not inhibit NFκB nuclear localization (Figure 6D). Interestingly, endothelial cells from the porcine aorta show increased pSmad2 in response to TGFβ-1, but not to TNF-α treatment, suggesting a unique signaling behavior of PAVEC in response to TNF-α (Figure 6F). These results suggest that Alk2/5 dependent TGFβ signaling is not downstream of TNF-α-induced EndMT in adult valve endothelium. Therefore, while both embryonic and adult valve endothelium activate an EndMT program in response to TNF-α through Akt/NFκB, TGFβ signaling is required and downstream in embryonic, but not required in adult valve endothelium. Adult cells may bypass TGFβ signaling because activated NFκB has been shown to stabilize Snail, an EndMT-regulating transcription factor, in adult cells46. In mouse cardiac endothelial cells, treatment with SB431542, but not PD98059 completely blocked TGFβ-2-induced EndMT47.
Discussion
The molecular and cellular events that initiate and propagate aortic valve disease are poorly understood and understudied, particularly with the respect to endothelial dysfunction. While the occurrence of EndMT has been previously noted in adult valve endothelium, a biological role remains unclear. EndMT studies in embryonic valves have identified over 100 different regulatory genes, including members of the TGFβ, BMP, and VEGF signaling pathways and matrix proteins such as periostin and versican48-51. Misexpression and mutation of many of these genes have been identified in diseased aortic valves3. Our results identify a novel, inflammatory cytokine-induced EndMT pathway in aortic valve endothelium through Akt/NFκB, and further support that this pathway is conserved during embryonic valve development. These findings collectively suggest that an inflammatory reactivation of embryonic-like EndMT may be a mechanism of early aortic valve dysfunction.
Identifying mechanisms conserved between embryonic and adult valve endothelium remains challenging because there is currently no species for which both embryonic and adult valve endothelial cell cultures have been obtained. Porcine aortic valve endothelial cells are the most characterized and studied adult valve endothelial population, and can be obtained from animals without disease29,52-54. Human aortic valve endothelial cells have been obtained from recipient hearts from cardiac transplantation surgeries or rejected valve donors. While no gross calcification may be present, the valve endothelium exhibits non-negligible inflammatory activation55-58 Paruchuri et al. identified clonally isolated sub-populations of human pulmonary VEC that were capable of EndMT in response to TGFβ, which supports our findings in pure whole VEC populations59. Holliday et al. recently showed that in vitro cultured human aortic VEC isolated from heart transplant surgeries express endothelial phenotype markers and elevated α-SMA, which is in contrast to in vivo expression patterns in non-diseased human and porcine aortic valves16,60-62. These co-expression findings suggest that human aortic valve endothelium from these patients may have an increased propensity or capacity for EndMT60. Furthermore, several studies demonstrate that inflammatory endothelial activation and calcific degeneration occurs preferentially on the fibrosa side of the valve4,63,64. Our analysis of calcified human aortic valves found invaded cells co-expressing α-SMA and CD31 on the fibrosa surface, suggesting that EndMT correlates with side-specific propagation of AVD65. In situ mRNA profiling of valve endothelial cells determined hundreds of genes that are differently expressed between sides, with many further modified in experimental hypercholesteremia52,53. The inflow or ventricularis surface is exposed to pulsatile unidirectional shear stress, while the outflow fibrosa surface experiences nearly oscillatory shear stress65. When either endothelial surface is exposed to opposite-sided flow profiles, they similarly upregulate inflammatory receptors66. Recently, Wu and colleagues demonstrated that both sides of valve endothelium have the same genetic origin67. Our results support and extend these findings to suggest that fibrosa-sided environmental conditions are more conducive to inflammatory activation and EndMT, but additional studies on side-specific endothelial populations are warranted.
Cellular and molecular analysis of embryonic valve formation is almost exclusively conducted with avian and mouse models. Though there are some differences in cardiac anatomy between avians and mammals, the morphogenesis, remodeling, and final anatomy of the clinically important left side of the heart is more similar between chicks and humans than in mice68-71. Biological assays with avian and mouse valve progenitors demonstrate identical molecular mechanisms and participants, though which isoforms used may differ72,73. Importantly, chick embryos are inexpensive, can be obtained without additional animal sacrifice, and are very tolerant of experimentation. Our findings of progressive upregulation of inflammatory receptors in valve forming in embryonic chicks is supported by observations in fetal human aortic valves16. Our result that EndMT and matrix invasion occurs at least through TGF–1 in adult porcine cells and TGF–3 in embryonic quail is supported by several studies23,43. Therefore, we have confidence that the general molecular regulation we identify is likely conserved across age, but we cannot definitively rule out species differences in the isoforms utilized.
The downstream fate of EndMT-derived mesenchyme in adult valves is of great interest, but was not a focus of this study. Bischoff and Aikawa recently proposed that EndMT creates a type of progenitor-like cell that renews the valve interstitial cell (VIC) population and maintains tissue homeostasis74. Both embryonic and adult valvular mesenchymal cells have the potential for osteogenic differentiation75,76, but whether these cells are directly EndMT-derived or from subsequent differentiation processes is not yet known. TNF-α and NFκB have been linked to the mesenchymal cell molecular and cellular mechanisms that mediate valvular calcification9,77. TNF-α expression and nuclear localization of NFκB is present in stenotic human aortic valves78, and TNF-α treatment of valve interstitial cells in 2D culture induces calcium nodule formation and expression of runx2 independent of osteogenic media9,79,80. We found EndMT derived subendothelial cells in calcified human aortic valves, but were always located distal to calcific lesions. This was not unexpected, as valve calcifications are associated with endothelial loss81. Taken together, these results support that inflammatory EndMT is a component of early stage AVD, but how these progeny contribute to and/or regulate downstream calcific progression is still unclear.
In conclusion, our results establish an inflammatory mechanism of early aortic valve pathogenesis that is, in part, developmentally conserved. Age dependent use of canonical TGFβ signaling may be an important mode of inflammatory disease pathogenesis distinct from potentially healthy tissue formation and remodeling. Selective molecular inhibition of the AKT/NFκB pathway in valve endothelium may therefore be a potential strategy for impacting early stage aortic valve disease.
Supplementary Material
Acknowledgements
We acknowledge Jennifer Richards and Phillip Buskohl for assistance with sample collection.
Sources of Funding Funding for this work was provided by The Hartwell Foundation, the National Institute of Health (HL110328), the National Science Foundation (CBET-0955712) the American Heart Association Scientist Development Grant (#0830384N), and the LeDucq Foundation (Project MITRAL).
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 3.Hakuno D, Kimura N, Yoshioka M, Fukuda K. Molecular mechanisms underlying the onset of degenerative aortic valve disease. J Mol Med. 2009;87:17–24. doi: 10.1007/s00109-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 4.Mohler ER, III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 5.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 6.Butcher JT, Mahler GJ, Hockaday LA. Aortic valve disease and treatment: The need for naturally engineered solutions. Adv Drug Deliver Rev. 2011;63:242–268. doi: 10.1016/j.addr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Beckmann E, Grau JB, Sainger R, Poggio P, Ferrari G. Insights into the use of biomarkers in calcific aortic valve disease. J Heart Valve Dis. 2010;19:441–452. [PMC free article] [PubMed] [Google Scholar]
- 8.Hjortnaes J, Butcher J, Figueiredo J-L, Riccio M, Kohler RH, Kozloff KM, Weissleder R, Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur Heart J. 2010;31:1975–1984. doi: 10.1093/eurheartj/ehq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaden JJ, Kilic R, Sarikoc A, Hagl S, Lang S, Hoffmann U, Brueckmann M, Borggrefe M. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int J Mol Med. 2005;16:869–872. [PubMed] [Google Scholar]
- 10.Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kilic R, Sarikoc A, Pinol R, Hagl S, Lang S, Brueckmann M, Borggrefe M. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–87. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Davutoglu V, Celik A, Aksoy M. Contribution of selected serum inflammatory mediators to the progression of chronic rheumatic valve disease, subsequent valve calcification and NYHA functional class. J Heart Valve Dis. 2005;14:251–256. [PubMed] [Google Scholar]
- 12.Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal. 2008;10:1089–1100. doi: 10.1089/ars.2007.2007. [DOI] [PubMed] [Google Scholar]
- 13.Von der Thüsen JH, Kuiper J, Van Berkel TJC, Biessen EAL. Interleukins in atherosclerosis: Molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SH, Best PJM, Edwards WD, Holmes DR, Carlson PJ, Celermajer DS, Lerman A. Nuclear factor-kappa B immunoreactivity is present in human coronary plaque and enhanced in patients with unstable angina pectoris. Atherosclerosis. 2002;160:147–153. doi: 10.1016/s0021-9150(01)00546-9. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: Implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 17.Mou SS, Haudek SB, Lequier L, Pena O, Leonard S, Nikaidoh H, Giroir BP, Stromberg D. Myocardial inflammatory activation in children with congenital heart disease. Crit Care Med. 2002;30:827–832. doi: 10.1097/00003246-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Qing M, Schumacher K, Heise R, Wöltje M, Vazquez-Jimenez JF, Richter T, Arranda-Carrero M, Hess J, von Bernuth G, Seghaye M-C. Intramyocardial synthesis of pro- and anti-inflammatory cytokines in infants with congenital cardiac defects. J Am Coll Cardiol. 2003;41:2266–2274. doi: 10.1016/s0735-1097(03)00477-7. [DOI] [PubMed] [Google Scholar]
- 19.Hernández-Gutierrez S, García-Peláez I, Zentella-Dehesa A, Ramos-Kuri M, Hernández-Franco P, Hernández-Sánchez F, Rojas E. NF-κB signaling blockade by Bay 11-7085 during early cardiac morphogenesis induces alterations of the outflow tract in chicken heart. Apoptosis. 2006;11:1101–1109. doi: 10.1007/s10495-006-6984-z. [DOI] [PubMed] [Google Scholar]
- 20.Nath AK, Brown RM, Michaud M, Sierra-Honigmann MR, Snyder M, Madri JA. Leptin affects endocardial cushion formation by modulating EMT and migration via Akt signaling cascades. J Cell Biol. 2008;181:367–380. doi: 10.1083/jcb.200708197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meadows K, Iyer S, Stevens M, Wang D, Shechter S, Perruzzi C, Camenisch T, Benjamin L. Akt promotes Endocardial-Mesenchyme Transition. J Angiogenes Res. 2009;1:1–9. doi: 10.1186/2040-2384-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park D-H, Thum T. Transforming Growth Factor-β–Induced Endothelial-to-Mesenchymal Transition Is Partly Mediated by MicroRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361–369. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- 23.Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-β-mediated and non-transforming growth factor-β-mediated transdifferentiation in vitro. Am J Pathol. 2001;159:1335–1343. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J-H, Wylie-Sears J, Bischoff J. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem Bioph Res Co. 2008;374:512–516. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wylie-Sears J, Aikawa E, Levine RA, Yang J-H, Bischoff J. Mitral Valve Endothelial Cells With Osteogenic Differentiation Potential. Arterioscler Thromb Vasc Biol. 2011;31:598–607. doi: 10.1161/ATVBAHA.110.216184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerraty M, Mohler ER. Models of aortic valve calcification. J Invest Med. 2007;55:278–283. doi: 10.2310/6650.2007.00012. [DOI] [PubMed] [Google Scholar]
- 27.Runyan RB, Markwald RR. Invasion of mesenchyme into three-dimensional collagen gels: A regional and temporal analysis of interaction in embryonic heart tissue. Dev Biol. 1983;95:108–114. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- 28.Bernanke DH, Markwald RR. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982;91:235–245. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- 29.Butcher JT, Penrod AM, Garcia AJ, Nerem RM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler Thromb Vasc Biol. 2004;24:1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 31.Hermiston ML, Xu Z, Weiss A. CD45: A Critical Regulator of Signaling Thresholds in Immune Cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- 33.Devin A, Lin Y, Liu ZG. The role of the death-domain kinase RIP in tumour necrosis factor-induced activation of mitogen-activated protein kinases. EMBO reports. 2003;4:623–627. doi: 10.1038/sj.embor.embor854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhawan P, Richmond A. A novel NF-κB-inducing kinase-MAPK signaling pathway up-regulates NF-κB activity in melanoma cells. J Biol Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanden Berghe W, Plaisance Sp, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein Kkinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 36.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 37.Pincheira R, Castro AF, Ozes ON, Idumalla PS, Donner DB. Type 1 TNF receptor forms a complex with and uses jak2 and c-Src to selectively engage signaling pathways that regulate transcription factor activity. J Immunol. 2008;181:1288–1298. doi: 10.4049/jimmunol.181.2.1288. [DOI] [PubMed] [Google Scholar]
- 38.Taga T, Kishimoto T. gp130 and the interleukin-6 family of cytokines. Annu Rev of Immunol. 2003;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 39.Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML. Interleukin-6 inhibits transforming growth factor-β-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J Biol Chem. 1999;274:23013–23019. doi: 10.1074/jbc.274.33.23013. [DOI] [PubMed] [Google Scholar]
- 40.Middleton G, Hamanoue M, Enokido Y, Wyatt S, Pennica D, Jaffray E, Hay RT, Davies AM. Cytokine-induced nuclear factor kappa B activation promotes the survival of developing neurons. J Cell Biol. 2000;148:325–332. doi: 10.1083/jcb.148.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang JY, Li Y, Shen BF. PI3-K/Akt pathway contributes to IL-6-dependent growth of 7TD1 cells. Cancer Cell Int. 2003;3:1–4. doi: 10.1186/1475-2867-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: A role for transforming growth factor β3. Dev Dynam. 1997;209:296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 43.Ramsdell AF, Markwald RR. Induction of endocardial cushion tissue in the avian heart is regulated, in part, by TGFβ-3-mediated autocrine signaling. Dev Biol. 1997;188:64–74. doi: 10.1006/dbio.1997.8637. [DOI] [PubMed] [Google Scholar]
- 44.Mercado-Pimentel ME, Hubbard AD, Runyan RB. Endoglin and Alk5 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2007;304:420–432. doi: 10.1016/j.ydbio.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sridurongrit S, Larsson J, Schwartz R, Ruiz-Lozano P, Kaartinen V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev Biol. 2008;322:208–218. doi: 10.1016/j.ydbio.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh AK, Nagpal V, Covington JW, Michaels MA, Vaughan DE. Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): Differential expression of microRNAs during EndMT. Cell Signal. 2012;24:1031–1036. doi: 10.1016/j.cellsig.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Armstrong EJ, Bischoff J. Heart Valve Development: Endothelial Cell Signaling and Differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Person AD, Klewer SE, Runyan RB, Kwang WJ. International Review of Cytology. Vol. 243. Academic Press; 2005. p. 287. [DOI] [PubMed] [Google Scholar]
- 51.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guerraty MA, Grant GR, Karanian JW, Chiesa OA, Pritchard WF, Davies PF. Hypercholesterolemia induces side-specific phenotypic changes and peroxisome proliferator-activated receptor-γ pathway activation in swine aortic valve endothelium. Arterioscler Thromb Vasc Biol. 2010;30:225–231. doi: 10.1161/ATVBAHA.109.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farivar RS, Cohn LH, Soltesz EG, Mihaljevic T, Rawn JD, Byrne JG. Transcriptional profiling and growth kinetics of endothelium reveals differences between cells derived from porcine aorta versus aortic valve. Eur J Cardio-Thorac. 2003;24:527–534. doi: 10.1016/s1010-7940(03)00408-1. [DOI] [PubMed] [Google Scholar]
- 55.Ku C-H, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, Yacoub MH, Chester AH. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71:548–556. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 56.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I-547–552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 57.Hafizi S, Taylor PM, Chester AH, Allen SP, Yacoub MH. Mitogenic and secretory responses of human valve interstitial cells to vasoactive agents. J Heart Valve Dis. 2000;9:454–458. [PubMed] [Google Scholar]
- 58.Jian B, Narula N, Li Q-y, Mohler ER, III, Levy RJ. Progression of aortic valve stenosis: TGF-β1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 59.Paruchuri S, Yang J-H, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human Pulmonary Valve Progenitor Cells Exhibit Endothelial/Mesenchymal Plasticity in Response to Vascular Endothelial Growth Factor-A and Transforming Growth Factor-β2. Circ Res. 2006;99:861–869. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holliday CJ, Ankeny RF, Jo H, Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301:H856–867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, Nerem RM. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: Influence of shear stress. Arterioscler Thromb Vasc Biol. 2006;26:69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 62.Metzler S, Digesu C, Howard J, Filip To S, Warnock J. Live en face imaging of aortic valve leaflets under mechanical stress. Biomech Model Mechan. 2011:1–7. doi: 10.1007/s10237-011-0315-1. [DOI] [PubMed] [Google Scholar]
- 63.Mohler ER., 3rd Are atherosclerotic processes involved in aortic-valve calcification? Lancet. 2000;356:524–525. doi: 10.1016/S0140-6736(00)02572-1. [DOI] [PubMed] [Google Scholar]
- 64.Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. A1396. doi: 10.1016/j.amjcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 65.Kilner PJ, Yang G-Z, Wilkes AJ, Mohiaddin RH, Firmin DN, Yacoub MH. Asymmetric redirection of flow through the heart. Nature. 2000;404:759–761. doi: 10.1038/35008075. [DOI] [PubMed] [Google Scholar]
- 66.Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4 and TGF-β1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29:254–260. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B. Nfatc1 Coordinates Valve Endocardial Cell Lineage Development Required for Heart Valve Formation / Novelty and Significance. Circ Res. 2011;109:183–192. doi: 10.1161/CIRCRESAHA.111.245035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hinton RB, Jr., Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 69.Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiol Genomics. 2003;15:165–176. doi: 10.1152/physiolgenomics.00033.2003. [DOI] [PubMed] [Google Scholar]
- 70.Sedmera D, Cook AC, Shirali G, McQuinn TC. Current issues and perspectives in hypoplasia of the left heart. Cardiol Young. 2005;15:56–72. doi: 10.1017/S1047951105000132. [DOI] [PubMed] [Google Scholar]
- 71.Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230:239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- 72.Mercado-Pimentel ME, Runyan RB. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs. 2007;185:146–156. doi: 10.1159/000101315. [DOI] [PubMed] [Google Scholar]
- 73.Boyer AS, Ayerinskas, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208:530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- 74.Bischoff J, Aikawa E. Progenitor Cells Confer Plasticity to Cardiac Valve Endothelium. J Cardiovasc Transl Res. 2011;4:710–719. doi: 10.1007/s12265-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 75.Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol. 2010;338:127–135. doi: 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarιkoç A, Kιlιç R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle C-E, Borggrefe M. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 78.Toli K, Paraskevas KI, Poulakou MV, Agrogiannis G, Kavantzas N, Xanthopoulos V, Iliopoulos DG, Mantas I, Papachristodoulou A, Patsouris E, Mikhailidis DP, Perrea DN. Association between plasma levels and immunolocalization of cytokines in heart valve lesions: a possible target for treatment? Expert Opin Ther Targets. 2008;12:1209–1215. doi: 10.1517/14728222.12.10.1209. [DOI] [PubMed] [Google Scholar]
- 79.Kaden JJ, Dempfle CE, Kilic R, Sarikoc A, Hagl S, Lang S, Brueckmann M, Borggrefe M. Influence of receptor activator of nuclear factor kappa B on human aortic valve myofibroblasts. Exp Mol Pathol. 2005;78:36–40. doi: 10.1016/j.yexmp.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, Furukawa K-I. TNF-α accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther. 2011;337:16–23. doi: 10.1124/jpet.110.177915. [DOI] [PubMed] [Google Scholar]
- 81.Poggianti E, Venneri L, Chubuchny V, Jambrik Z, Baroncini LA, Picano E. Aortic valve sclerosis is associated with systemic endothelial dysfunction. J Am Coll Cardiol. 2003;41:136–141. doi: 10.1016/s0735-1097(02)02622-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.