Abstract
Context
Whole-slide imaging technology offers promise for rapid, Internet-based telepathology consultations between institutions. Before implementation, technical issues, pathologist adaptability, and morphologic pitfalls must be well characterized.
Objective
To determine whether interpretation of whole-slide images differed from glass-slide interpretation in difficult surgical pathology cases.
Design
Diagnostically challenging pathology slides from a variety of anatomic sites from an outside laboratory were scanned into whole digital format. Digital and glass slides were independently diagnosed by 2 subspecialty pathologists. Reference, digital, and glass-slide interpretations were compared. Operator comments on technical issues were gathered.
Results
Fifty-three case pairs were analyzed. There was agreement among digital, glass, and reference diagnoses in 45 cases (85%) and between digital and glass diagnoses in 48 (91%) cases. There were 5 digital cases (9%) discordant with both reference and glass diagnoses. Further review of each of these cases indicated an incorrect digital whole-slide interpretation. Neoplastic cases showed better correlation (93%) than did cases of nonneoplastic disease (88%). Comments on discordant cases related to digital whole technology focused on issues such as fine resolution and navigating ability at high magnification.
Conclusions
Overall concordance between digital whole-slide and standard glass-slide interpretations was good at 91%. Adjustments in technology, case selection, and technology familiarization should improve performance, making digital whole-slide review feasible for broader telepathology subspecialty consultation applications.
Whole-slide imaging at resolutions comparable to standard microscopic evaluation is now technologically feasible.1 A variety of commercial systems that perform technically simple and rapid image capture and viewing are available on the market.2 Accordingly, entire pathologic glass slides can now be converted into whole-slide digital files. As scan resolutions have increased and viewers have become more facile, these digital whole-slide images (WSI) can simulate the microscopic image viewed at any of the magnifications traditionally used to make light microscopic clinical interpretations. The uses of such virtual slides are many and include Internet- and other digital media-based continuing medical education and performance/validation testing methods3–5; digital-slide archiving, obviating the need to retain large, glass-slide–based files, particularly of rare or nonretained consultative materials6; quality assurance reviews7; and use of the digital files to make remote interpretations (telepathology) via Internet or internal network connections.8,9 Initial investigations have shown very good correlation of results between standard glass slide and digital whole-slide interpretations in breast,1,10 gastrointestinal,11 pulmonary,12 prostate,13 and mixed-specimen biopsies.8,9
The present study investigates the use of WSI technology as a platform for telepathology expert consultation. Use of this type of format should allow a pathologist anywhere in the world to send the virtual slide from his/her laboratory to a consultant in any location, via a high-speed Internet connection. Such an exchange would reduce the turnaround time of consultations and eliminate the selection bias of the sending pathologist in choosing static images as in formerly available telepathology systems. To perform a preliminary test of a WSI teleconsultation system, in a format mimicking the real-life experience of challenging cases that might be sent for consultative opinions, whole-slide images were made from glass surgical pathology slides, derived from an array of organ systems from a laboratory on one continent, and viewed by expert subspecialty consultants from an institution on another continent. Diagnostically difficult cases, requiring extensive review, were used to identify the potential pitfalls of this technology.
MATERIALS AND METHODS
Glass slides were selected from the files of a large anatomic pathology laboratory in South America, under a protocol approved by the institution's human subject review board. Slides were selected as being representative of challenging cases that might have been sent for expert consultation. Cases from a representative variety of organ systems were included to test a group of subspecialist consultant pathologists. For the purposes of this study, the submitted diagnosis from the originating laboratory was considered the reference interpretation for each case. Each glass slide was converted to a WSI (virtual slide) using a Zeiss Mirax Desk scanning device (Zeiss, Oberkochen, Germany; Figure 1). The WSIs were stored on a hard drive, which was sent to a large referral center in the United States, where glass-slide–based subspecialty consultative interpretation services are routinely rendered. Although the images were not sent directly through the Internet, the appearances and manipulation features of the WSI in the viewer were identical to what would be available if the WSI had been accessed from a remote server via the Internet. Remote access was not directly performed because of logistic issues. The slides were accompanied by short histories, including anatomic site, age and sex of the patient, and pertinent clinical findings. The WSI slides were reviewed by subspecialty pathologists using the Mirax Desk viewer and a high-resolution 24-inch monitor (Figure 2). The viewer allows the pathologist to review the digital image at any magnification ordinarily used in a standard microscope with similar resolution capability (up to ×400 with added capability of reviewing the image at any magnification among those of microscope objectives). All consultant pathologists were masked to any earlier interpretations. The consultant pathologists were instructed to make an interpretation of the whole-slide image as if they had been given the actual glass slide for consultation (the whole-slide image interpretation [WSII]). Following WSI evaluation, the actual glass slides from each case were shipped to the reference institution, where they were also evaluated by a different subspecialty pathologist based on the stated site of the specimen (the glass-slide interpretation [GSI]). An identical history and instructions for interpretation to that given in the WSI arm were given to each consultant pathologist in the GSI arm. Following completion of both study arms, the results of WSII, GSI, and submitting reference interpretation were compared. When discorrelations occurred, re-review of cases with the subspecialist pathologists was performed until a consensus final diagnosis was achieved. General and specific comments were solicited from the WSI reviewers regarding the use of the technology.
Figure 1.
The Zeiss Mirax Desk Imaging device is shown. This device scans single slides into whole-slide digital images, which are viewed on the Mirax viewer.
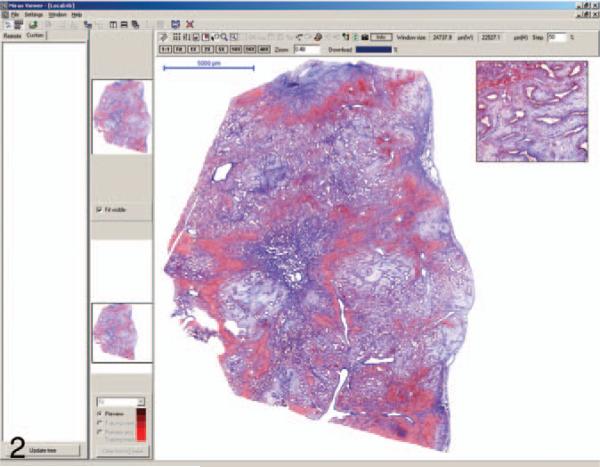
Figure 2.
The image shown in the Mirax viewer screen is a trichrome stain of one of the discrepant cases interpreted as biliary adenofibroma by the whole-slide reviewer and mesenchymal hamartoma by the glass-slide and reference reviewers. The main image can be magnified and moved about the screen by the use of a mouse and/or function buttons above the image.
RESULTS
The slide set was composed of 53 cases. The organ sites and submitting reference diagnoses (the reference interpretation) of the set are shown in Table 1 and represent a diverse variety of pathologic abnormalities likely to be submitted for expert consultation. The overall concordance rate for exact diagnosis between WSII, GSI, and reference interpretation was 85% (45 of 53). The correlation between the WSII and GSI was 91% (48 of 53), which indicates that in 3 cases, both consultants’ interpretations (WSII and GSI) did not agree with the submitted reference interpretation. Further review of these 3 cases by a third referee pathologist indicated that the consultants’ interpretations were more likely correct. Table 2 shows the correlation rate between WSII and GSI within each organ system examined. Errors were made in the WSII in lung, gastrointestinal, hematopathology, and dermatopathology subspecialties, but there was no evidence to suggest that there were specific interpretation difficulties inherent in any of these organ systems. It was determined to be more likely that the types of cases and the technology involved were responsible as the root cause of these errors. Table 3 shows the cases with discordant results between WSII and the concordant GSI and reference interpretation. In all 5 of these discordant cases (9%), the GSI was in agreement with the submitting reference diagnosis, and further review indicated an error in the WSII examination. Four of the 5 errors (80%) were in nonneoplastic entities, including emphysema, granulomatous colitis, hepatic mesenchymal hamartoma, and dermal vasculitis, with the remaining case being a mixed-cellularity Hodgkin lymphoma. Overall, therefore, neoplastic cases performed slightly better (93% concordance of WSII and GSI; 26 of 28 cases) than did nonneoplastic cases (88% concordance; 22 of 25 cases), although the difference was not significant (P > .5).
Table 1.
Reference Interpretations of the Submitted Cases
| Lung |
| Bronchiectasis |
| Organizing pneumonia with Pneumocystis carinii |
| Pulmonary sarcoidosis with silicotic nodule |
| Pulmonary aspergilloma |
| Bullous emphysema |
| Centrilobular emphysema |
| Pulmonary adenocarcinoma |
| Pulmonary squamous cell carcinoma |
| Pulmonary small cell carcinoma |
| Upper gastrointestinal |
| Gastric adenocarcinoma (intestinal type) |
| Gastric adenocarcinoma (mucinous type) |
| Gastric stromal tumor |
| Intestinal ischemia |
| Cardiovascular |
| Cystic medial necrosis (aorta) |
| Aortic atherosclerosis |
| Coronary atherosclerosis (left-sided coronary) |
| Acute and chronic myocardial infarction |
| Bacterial endocarditis |
| Thyroid/salivary gland |
| Papillary carcinoma (thyroid) |
| Hashimoto thyroiditis |
| Follicular carcinoma (thyroid) |
| Pleomorphic adenoma (submaxillary) |
| Adenoid cystic carcinoma |
| Warthin tumor |
| Bone and soft tissue |
| Peritoneal leiomyosarcoma |
| Gouty tophus |
| Schwannoma |
| Prostate |
| Prostatic adenocarcinoma |
| Hematopathology |
| Hodgkin lymphoma, mixed-cellularity type |
| Castleman disease, plasma cell variant |
| Thymoma, spindle cell |
| Granulomatous lymphadenitis (Bacillus Calmette-Guerin) |
| Necrotizing granulomatous lymphadenitis (histoplasma) |
| Liver/gall bladder |
| Micronodular cirrhosis |
| Mesenchymal hamartoma cervix/uterus |
| Cervix/uterus |
| Squamous cell carcinoma (cervix, microinvasive) |
| Squamous cell carcinoma (cervix, advanced) |
| Endometrial adenocarcinoma |
| Hydatidiform mole (lower gastrointestinal) |
| Adenocarcinoma, mucinous (colon) |
| Lower gastrointestinal |
| Ulcerative colitis with pseudopolyps |
| Villous adenoma (right colon) |
| Ileocecal tuberculosis |
| Burkitt lymphoma of appendix |
| Kidney |
| Oncocytoma |
| Multicystic nephroma |
| Suppurative pyelonephritis |
| Wilm tumor |
| Lupus erythematosus |
| Renal infarction |
| Dermatology |
| Malignant melanoma |
| Cutaneous necrotizing vasculitis |
| Central nervous system |
| Astrocytoma |
Table 2.
Correlation Rate Between Whole-Slide and Glass-Slide Interpretations in Each Organ System
| Organ System, No. | Correlation Rate, % (No.) |
|---|---|
| Lung, 9 | 89 (8) |
| Liver/gastrointestinal tract, 11 | 82 (9) |
| Cardiovascular, 5 | 100 (5) |
| Hematopathology, 5 | 80 (4) |
| Thyroid/salivary, 6 | 100 (6) |
| Skin, 2 | 50 (1) |
| Kidney, 6 | 100 (6) |
| Prostate, 1 | 100 (1) |
| Gynecologic, 4 | 100 (4) |
| Bone/soft tissue, 3 | 100 (3) |
| Neuropathology, 1 | 100 (1) |
| Total neoplastic, 25 | 93 (23) |
| Total nonneoplastic, 28 | 88a (25) |
| Total, 53 | 91 (48) |
Difference nonsignificant, P > .5.
Table 3.
Cases With Discrepancies Between Whole-Slide Image Interpretation (WSII) and Glass-Slide Interpretations (GSI)
| Organ System | WSII | GSI | Submitting Diagnosis |
|---|---|---|---|
| Lung | Honeycomb fibrosis, rule out usual interstitial pneumonitis | Bullous emphysema with hemorrhage | Bullous emphysema |
| Liver/gall bladder | Biliary adenofibroma | Mesenchymal hamartoma | Mesenchymal hamartoma |
| Hematopathology | Viral lymphadenitis versus peripheral T-cell lymphoma | Hodgkin lymphoma, mixed cellularity | Hodgkin lymphoma, mixed cellularity |
| Lower GI tract | Atypical vascular proliferation, rule out angiosarcoma | Acute granulomatous colitis | Ileocecal tuberculosis |
| Dermatology | Systemic hypersensitivity reaction | Superficial and deep perivascular and/or perineural granulomatous infiltrate with necrosis | Cutaneous necrotizing vasculitis |
Abbreviation: GI, gastrointestinal.
Negative comment from WSI reviewers related to virtual slide-viewing technical issues, such as fine resolution and ease and speed of navigation, especially at high magnifications. Comments also indicated initial unease or lack of confidence in arriving at a precise diagnosis when using this technology. Positive comments included the ability to make a confident diagnosis and that the ease of use of the instrumentation was acceptable in comparison to glass-slide review.
COMMENT
Based on the results of this study, WSI interpretation of consultative material is feasible. The correlation between WSI and glass-slide interpretation was good at 91% of cases (48 of 53 cases concurred). There is room for improvement, however, as the WSII was incorrect in the 5 noncorrelative cases (9%). There was no case in which the WSII “trumped” the GSI. Most of the misinterpreted WSI cases involved nonneoplastic entities; most notably difficult were pulmonary interstitial disease, dermal vasculitis, and unusual, benign hamartoma interpretations. However, WSI evaluation misclassified a mixed-cellularity Hodgkin lymphoma case, a process in which inflammatory entities are often in the differential diagnosis. This case was interpreted as either viral lymphadenitis or peripheral T-cell lymphoma in the WSI reviews. It would appear, therefore, that one of the findings of this study is that inflammatory conditions, particularly those requiring meticulous searching at high magnification, may be more difficult in the WSI format. This hypothesis is further corroborated by technology comments related to difficulty of navigation and resolving power at WSI high magnifications.
Despite the above limitations of this study, the results are not dissimilar from the results noted in prior WSI and glass-slide evaluation comparison studies. Weinstein and colleagues1 reported a 98% concordance in interpretation of breast cases but noted that when equivocal interpretations were included as miscorrelations to definitive diagnoses in more challenging cases, the concordance rate dropped to 89%. Costello et al,10 using WSI of breast core biopsies, showed that the correct diagnosis could be made in 90% of cases (9 of 10) but noted that individual pathologist's results varied substantially. Molnar and colleagues11 showed concordance between WSI and glass-slide interpretation in 92% of “routine” gastrointestinal pathology cases, with higher concordances noted in each modality, when compared with the reference diagnosis in each case (up to 96% for WSI) in which a “clinically important concordance” was considered correct. Interestingly, in their study,11 just as in the present report, GSI were always slightly ahead in concordance with the reference diagnosis when compared with the WSI interpretations (by about 2%). Using the model of lung tumors, Slodkowski et al12 showed 85% concordance between WSI and GSI. Again, low image quality was cited as a reason for discordant results. Fine et al,13 using immunohistochemical stains on difficult prostate needle cores as the testing platform for comparison of WSI and glass-slide interpretation, showed that the same pathologist examining both types of immunohistochemistry specimens, at times 6 months apart, showed that one pathologist achieved “almost perfect” results as measured by κ statistics, whereas 3 pathologists achieved “substantial” concordance, and 1 pathologist showed “moderate” concordance. The authors concluded that WSI“. . . can currently permit accurate interpretation of immunohistochemistry (IHC) stains in the setting of diagnostically difficult prostate biopsies for adequately trained pathologists.” 13(p571) The concept of WSI telepathology has significant practical value in this particular application because immunohistochemistry stains may be performed in sites remote from the ordering laboratory. In a study of multiple types of specimens, mostly from dermal and genitourinary sites, Gilbertson and colleagues8 showed that, in 25 cases, there were no discordances between the reference and WSI diagnoses, but when complete “final” reports were compared between the 2 methods, there were discrepancies in 32% (8 cases). These discrepancies related to issues of grading, invasion, and other minor classification issues. The authors note that focal image quality was a major factor in the discrepancies but state that WSI is in evolution and shows “great promise for pathology.” Li and colleagues,9 in a large set of surgical pathology specimens from a diverse group of organ systems (400 cases, 20% were rated “diagnostically challenging”) showed high correlation of WSI and GSI as read by 2 pathologists. Their results again showed that GSIs were slightly more accurate, but overall, diagnostic accuracy was excellent for both methods (GSI, 96%–97%; WSI, 94%–95%). The overall findings of the present study are, therefore, similar to what has been shown in the past and, by extension, indicate that the process of telepathology consultation for more challenging cases via WSI technology is feasible.
The current study has limitations, however, because the pathologist interpreting both the WSI and glass slide did not have access to gross assessments or real-time conversations with the referring physician, both of which would be expected to improve performance, particularly with specific category evaluation. Consultants are often provided with the originating pathologist's differential diagnosis and are, therefore, “primed” to look for specific features allowing differentiation based on their expertise and experience. WSI and GSI evaluations were, therefore, in this study, all morphology-only evaluations. The key parameters of difference between glass-slide microscopic and WSI evaluations relate to the method in which the tissue is viewed. Glass-slide interpretations are made via standard microscope viewing, whereas WSI interpretations are made using video screens with manipulation of images via a computer-based viewer using specific mouse-driven buttons that allow movement about the digitally rendered histologic section and changes in magnification. Although inherently different methods, the pathologists using the WSI system appeared to be easily trained in its operation and, based on the results of this study, were able to arrive at accurate interpretations in most cases.
This study is to be considered only a preliminary result demonstrating feasibility. To fully validate a new, WSI-based system of telepathology consultation interpretation, performance of a much larger series of cases in each organ system must be compared with conventional microscope-based interpretations to ensure accuracy and patient safety. One preliminary study designed to evaluate WSI technology for frozen sections was recently reported14 on a large series of consecutive ovarian specimens. This report14 targeted a specific organ in a rigorous manner and did show specific issues of WSI interpretation related to this organ system. Additional study specifically targeting other organ systems will need to be undertaken to fully vet the clinical use of remote interpretation by WSI methods because anatomic site–specific interpretation issues may arise. Based on the present results, it would appear that one such entity-specific caveat may be that nonneoplastic conditions (inflammatory/infectious) are more difficult to interpret by the WSI method, specifically when careful examination at highest magnification is necessary. This latter mode of evaluation was specifically commented on by WSI participants as a particularly difficult aspect of the procedure.
Whole-slide imaging is an important new technology that can be used for remote interpretation and consultation. Expert subspecialty-based teleconsultation is important for optimizing patient care via second opinions on difficult or clinically imperative cases. Further study, using a larger series of cases, and with technologic improvements in the systems to provide higher resolution scanning and image display are required before this technology can be implemented in routine daily practice. In addition, technology will need to improve regarding image size and compression modalities, which could ultimately lead to more rapid transmission of high-resolution images through the Internet and for archival storage and access.
Acknowledgments
Zeiss, Inc, provided support for whole-slide scanning and image review.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
Presented in part at the 97th Annual Meeting of the United States and Canadian International Academy of Pathology, Denver, Colorado, March 1–7, 2008.
References
- 1.Weinstein RS, Descour MR, Liang C, et al. An array microscope for ultra-rapid virtual slide processing and telepathology: design, fabrication, and validation study. Hum Pathol. 2004;35(11):1303–1314. doi: 10.1016/j.humpath.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Rojo MG, Garcia GB, Mateos CP, Garcia JG, Vicente MC. Critical comparison of 31 commercially available digital slide systems in pathology. Int J Surg Pathol. 2006;14(4):285–305. doi: 10.1177/1066896906292274. [DOI] [PubMed] [Google Scholar]
- 3.Neel JA, Grindem CB, Bristol DG. Introduction and evaluation of virtual microscopy in teaching veterinary cytopathology. J Vet Med Educ. 2007;34(4):437–444. doi: 10.3138/jvme.34.4.437. [DOI] [PubMed] [Google Scholar]
- 4.Glatz-Krieger K, Spornitz U, Spatz A, Mihatsch MJ, Glatz D. Factors to keep in mind when introducing virtual microscopy. Virchows Arch. 2006;448(3):248–255. doi: 10.1007/s00428-005-0112-2. [DOI] [PubMed] [Google Scholar]
- 5.Stewart J, III, Miyazaki K, Bevans-Wilkins K, Ye C, Kurtycz DF, Selvaggi SM. Virtual microscopy for cytology proficiency testing: are we there yet? Cancer. 2007;111(4):203–209. doi: 10.1002/cncr.22766. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien MJ, Sotnikov AV. Digital imaging in anatomic pathology. Am J Clin Pathol. 1996;106(4)(suppl 1):S25–S32. [PubMed] [Google Scholar]
- 7.Ho J, Parwani AV, Jukic DM, Yagi Y, Anthony L, Gilbertson JR. Use of whole slide imaging in surgical pathology quality assurance: design and pilot validation studies. Hum Pathol. 2006;37(3):322–331. doi: 10.1016/j.humpath.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson JR, Ho J, Anthony L, Jukic DM, Yagi Y, Parwani AV. Primary histologic diagnosis using automated whole slide imaging: a validation study. BMC Clin Pathol. 2006;6:4. doi: 10.1186/1472-6890-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Liu JL, Xu H, et al. A feasibility study of virtual slides in surgical pathology in China. Hum Pathol. 2007;38(12):1842–1848. doi: 10.1016/j.humpath.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Costello SSP, Johnston DJ, Dervai PA, O'Shea DG. Development and evaluation of the virtual pathology tool in telepathology. J Med Internet Res. 2003;5(2):e11. doi: 10.2196/jmir.5.2.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar B, Berczi L, Diczhazy C, et al. Digital slide and virtual microscopy based routine and telepathology evaluation of routine gastrointestinal biopsy specimens. J Clin Pathol. 2003;56(6):433–438. doi: 10.1136/jcp.56.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slodkowska J, Chyczewski L, Wojciechowski M. Virtual slides: application in pulmonary pathology consultations. Folia Histochem Cytobiol. 2008;46(1):121–124. doi: 10.2478/v10042-008-0018-3. [DOI] [PubMed] [Google Scholar]
- 13.Fine JL, Grzybicki DM, Silowash R, et al. Evaluation of whole slide image immunohistochemistry interpretation in challenging prostate needle biopsies. Hum Pathol. 2008;39(4):564–572. doi: 10.1016/j.humpath.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Fallon MA, Wilbur DC, Prasad ML. Ovarian frozen section diagnosis: use of whole slide digital imaging shows excellent correlation between virtual slide and original interpretations in a large series of cases [abstract 929]. Mod Pathol. 2008;21(suppl 1):203A. doi: 10.5858/2009-0320-OA.1. [DOI] [PubMed] [Google Scholar]