Abstract
Heart valve disease is a significant and increasing global problem. In the developing world, the primary suffers are children and young adults, the critical engine of future economic growth. Up to 10 times the current number of known sufferers are undiagnosed in these countries. Among the most prevalent and neglected diseases are rheumatic heart disease and endomyocardial fibrosis. The etiologies of these diseases can be described in part as a dysregulation or reactivation of developmental biology pathways. Connecting mechanisms of valvulogenesis and disease etiology may therefore be an excellent strategy to identify therapeutic targets. These local diseases require local solutions tailored to local resources. Collaboration with experienced researchers should be an encouraged way forward to accelerate knowledge creation and clinical translation.
Keywords: Neglected diseases, Africa, rheumatic heart disease, endomyocardial fibrosis, disparity, socioeconomics, embryo, endothelial to mesenchymal transformation, fibrosis, congenital heart defects
Introduction
Cardiovascular disease (CVD) is the leading killer worldwide. 80% of all CVD cases occur in developing countries [1], causing more than 19% of deaths –more than war and malaria combined [2]. These rates are compounded by a lack of progress in applicable therapies because the cardiovascular needs of developing countries are inherently different from the degenerative pathologies of the Western world. Non-ischemic diseases characterized by fibrosis and inflammation XXX that affect young people are of serious concern in impoverished regions such as sub-Saharan Africa, requiring different treatment strategies than those that are effective in the ischemic afflictions of the elderly common in the developed world [3].
The pathological disparity arises from causal factors linked to economic conditions such as poor hygiene, inadequate nutrition, and lack of proper health care [3]. Without medical intervention, relatively benign childhood diseases quickly become degenerative, compounded by the effects of hunger and harsh environmental conditions on immune response. In consequence, CVD manifests at a much earlier age, creating a demographic in which young adults and children are the predominantly affected populations rather than the elderly [3–7]. This troubling trend has serious consequences for countries still progressing towards economic stability because it directly undermines both the foundational workforce and the ability of these societies to progress technologically and socially.
Two of the most prevalent CVDs in the young people of sub-Saharan Africa are endomyocardial fibrosis (EMF) and rheumatic heart disease (RHD) [8]. However, these pathologies represent an enormous burden of disease that is generally overlooked in the research priorities of developed nations; for example, less than 0.4% of global funding is allocated to rheumatic fever research despite the 68 million people currently affected by this disease [6,9–11]. Charitable initiatives for global health often skip over RHD, perhaps because of its non-infectious epidemiology, in their mission to eradicate infectious diseases such as malaria and HIV. Nor is it confronted in developed nations, which have succeeded in diminishing its influence through improved hygiene and access to antibiotics in the initial febrile stages. However, as long as poor sanitation and limited health care dominate the conditions of the third world, designing a therapeutic approach to diseases such as RHD that is amenable to the resources and environment of developing countries should be paramount.
The purpose of this review is to summarize the state of EMF and RHD in sub-Saharan Africa and to conclude with a discussion of future avenues for advancing both understanding and clinical effectiveness in treating these neglected diseases.
Rheumatic Heart Disease
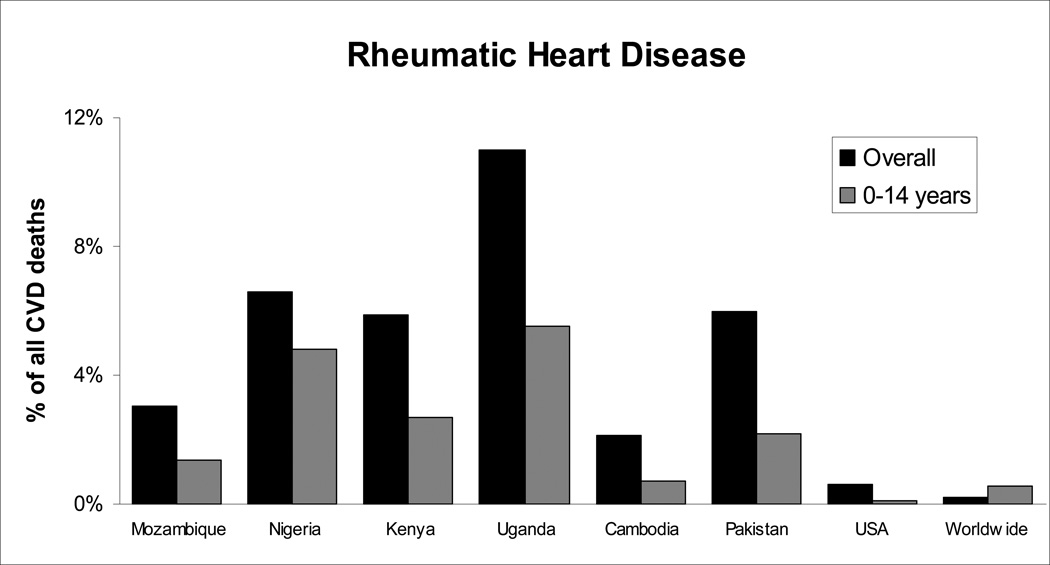
There are more than 68 million existing cases of RHD, causing 1.4 million deaths each year [12–15]. Case studies throughout Africa suggest that the majority of the disease burden is carried by children in developing countries, especially those in Sub-Saharan Africa (Figure 1). According to the American Heart Association, in most of the developing world RHD is the single largest cause of hospital admissions for children and young adults [23]. RHD arises from untreated complications of rheumatic fever (RF), associated with group-A streptococcal pharyngitis in children [11,23]. It is thought that antibodies against the initial infection cross-react with proteins in the heart creating an autoimmune reaction, which propagates inflammation and self-destruction of the valves and myocardium [24] [24]. Nine to thirty-nine percent of patients who contract acute rheumatic fever develop associated valve deficiency two to ten years after the initial febrile episode, creating a bimodal age distribution with peaks in late childhood and early adulthood. Twenty percent of patients with RHD will die before the age of five, and eighty percent before they reach 25 years old [25]. One of the most common manifestations of RHD is valve deformity, occurring in twenty-five to forty percent of chronic RHD patients [5,26] (Figure 2). Currently, the most common treatment for advanced stages of RHD is valve replacement, but both mechanical and bioprosthetic valves fail prematurely in these patients due to their young age and limited ability to access and manage anti-coagulation treatments [29,30]. The expense of replacement valves and long-term pharmaceutical strategies suggest that the methods now used to confront RHD valvular degeneration, imported from the first world, should be reconsidered within the African context.
Figure 1.
Significantly high mortality due to RHD in developing countries compared to Western nations. The majority of sufferers in these countries are less than 15 years old [16–22].
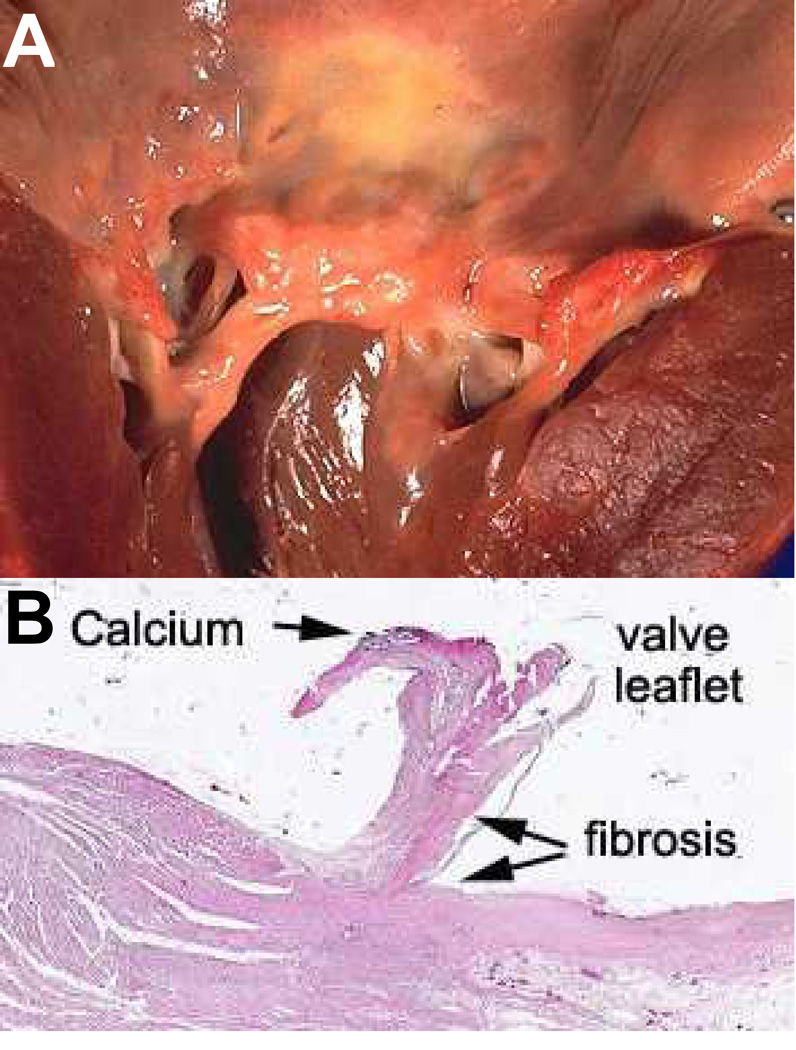
Figure 2.
A) A rheumatic mitral valve showing severe thickening of the leaflet and chordae tendonae [27]. B) Hematoxylin and Eosin staining of a rheumatic mitral valve leaflet showing calcification, fibrosis, and leaflet thickening [28].
Endomyocardial Fibrosis
In the equatorial region of Africa, endomyocardial fibrosis has been found to be the second most frequent cause of acquired cardiovascular disease in young adults and children [16]. Recent studies have also found that EMF accounts for about twenty percent of all heart failure deaths in Uganda and Mozambique [7,16,31,32], with similar results from hospital-based studies in ten other African countries [3]. EMF is a restrictive cardiomyopathy generally characterized as a disorder of the tropical regions of the world. It is thought to begin as an initial feverish illness which is usually not of a severity to warrant medical presentation, especially in light of the high cost and inaccessibility of health care in developing countries. A latent phase follows, in which the fever subsides and there are no symptoms over a period of two to ten years. The disease asymptomatically advances, leading to degeneration of the heart as ventricular and endocardial thrombi accumulate and impede the normal function of the valves. Concurrently, unknown mechanisms cause the tissues of the heart to remodel, forming fibrous scars which further impede the ability of the heart to supply the body with adequate blood flow [33] (Figure 3).
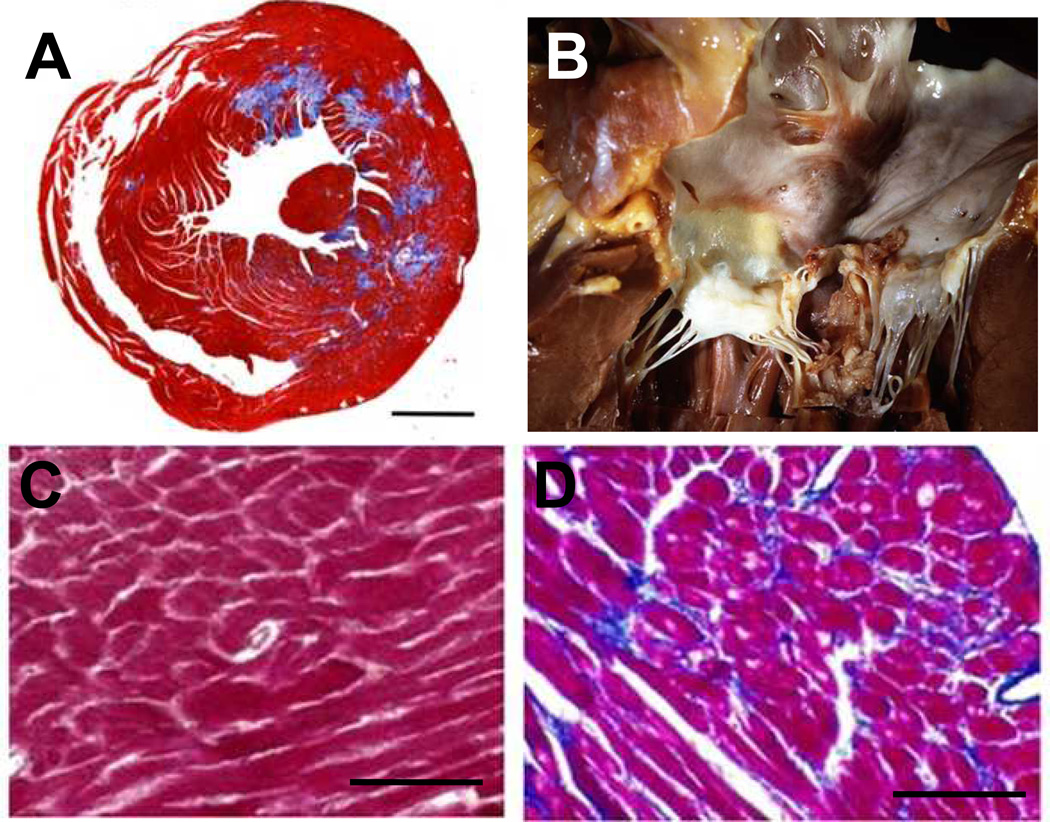
Figure 3.
Pathology of endomyocardial fibrosis. (A) A fibrosed mouse heart, in which Masson’s trichrome staining illustrates excessive collagen fiber deposition (Bar = 1mm) [34]. (B) Regions of fibrosis accumulate on the myocardium, often mingling with the valve leaflets and chordae [35]. Masson’s trichrome staining of normal (C) and fibrotic (D) myocardium reveals increased collagen deposition (blue) and fiber disorganization in diseased regions (Bar = 200 um) [36].
Despite the high incidence, little is known about the mechanisms or pathology of EMF. Most patients do not present until the onset of the severe symptoms caused by increasing fibrosis and inadequate blood flow such as venous hypertension, valvular stenosis, thromboembolism or arrhythmia [37]. In such advanced stages of the disease, costly surgical intervention becomes the sole viable treatment strategy, which is both unavailable and expensive in the regions where EMF is most prevalent [38]. Despite the need for new therapeutic approaches, the number of publications concerning EMF has decreased since the 1980s [31], indicating that both a revival in interest and a novel approach to the disease are necessary.
Developmental Biology Perspectives
Large-scale studies conducted throughout Africa have emphasized the need for a new approach to understanding neglected conditions such as endomyocardial fibrosis and rheumatic heart disease. Both diseases degrade the normal function of valves in the heart, impairing cardiac output and effectively disabling the patient from contributing to their community. Developmental biology offers a promising new avenue towards understanding the neglected cardiovascular diseases of the developing world. As researchers unravel the embryonic blueprint of native valvulogenesis, a deeper understanding of the mechanisms of disease follows and fundamentally improved tissue engineering strategies may become possible.
The early heart is a single tube of endocardial cells surrounded by an outer layer of myocardium. In the first phases of development, the myocardium secretes a jelly-like matrix, forming protrusions which swell into the lumen of the endocardial tube. The endocardial cells then begin to shed cell-to-cell contacts and migrate from the surface down into the matrix in a process called the endothelial to mesenchymal transition (EMT). Concurrently, new mesenchymal cells proliferate and begin to digest the jelly-like matrix and secrete a denser material in its place, causing the protrusions to swell further into the lumen. These larger protrusions are known as “cushions” due to their soft and rounded morphology.
The next phase of development differs between atrioventricular and semilunar valves. For atrioventricular valves, fenestrations between the cushion and myocardial wall begin to coalesce in a process called delamination, allowing the cushion to separate from the wall as it is remodeled into a dense leaflet connected only at the caudal edge and the chordae tendonae. In the semilunar valve, migration of the neural crest cells, retraction of the myocardial casing, myocardial apoptosis, and endocardial differentiation occur concurrently, creating a depression which deepens gradually on the superior face, progressively excavating or sculpting the leaflet cusps [39].
Embryonic development is 99% successful in creating perfectly functional hearts. Understanding why the 1% of malfunctions occur can elucidate the steps of valvulogenesis which are integral to the development of a healthy heart. Normal developmental mechanisms provide the blueprint for outlining how the heart and valves malfunction in disease states and suggest therapeutic avenues which capitalize on the innate mechanisms and potential of the native heart. Both the molecular signaling pathways and the remodeling transitions of valvulogenesis have been implicated in various pathologies such as EMF and RHD [40–43], and are discussed below.
EMF and developmental biology
The endothelial to mesenchymal transition is an important phase in valvulogenesis during which quiescent endocardial cells migrate, change phenotype, and take on a new role. This same process has been shown in connection with fibrosis, most notably by Zeisberg et al. in 2007. Zeisberg showed that fibrosis arises from endothelial cells undergoing a transition to a mesenchymal then fibrotic phenotype in diseased tissue, establishing a direct connection between EMF-like pathology and processes governing developing heart valves [44].
EMT is regulated in both processes by transforming growth factor beta (TGF-β), shown to be necessary in development for migration, matrix digestion, cell separation, and hyperplasia [45–47] and in pathology for fibrosis to occur [44]. TGF-β modulation of EMT has also been implicated in cardiac fibroblast to collagen metabolism [48], derivation of interstitial fibroblasts [49] [49], and myocardial fibrosis [50]. These studies point towards a direct link between the roles of TGF-β controlled EMT during development and endomyocardial fibrosis pathogenesis.
Other promising connections between valvulogenic pathways and EMF include the role of growth-stimulating angiotensin-II in conjunction with TGF-β [51–53], tenascin-C regulation of cell behavior in embryonic and pathological remodeling [54], and stimulation of EMT by endothelin-1 [55]. The etiology of fibrosis as regulated by these developmental signaling pathways offers a fresh perspective on the progression of EMF and possibly novel therapeutic approaches.
RHD and Developmental Biology
Various studies have connected RHD valvulitis and developmental pathways, specifically with regards to TGF-β modulation, chondromodulin-1, and structural changes [40,41,56,57]. Kim et al. showed a correlation between elevated levels of TGF-β1 expression in rheumatic mitral valve leaflets and myofibroblast proliferation, valvular fibrosis, inflammation, and calcification [56]. This suggests that the pathological mechanisms of RHD may be similar to embryonic processes by which progenitor cells activate, proliferate, and migrate under the influence of TGF-β.
Healthy cardiac valves abundantly express chondromodulin-1, an antiangiogenic molecule, whereas rheumatic valves exhibit increased vascularization and calcification in areas of downregulated chondromodulin-1 [41]. This correlation points to a link between normal valvular function and proper maintenance of chondromodulin pathways, marking a potential therapeutic strategy in exogenous administration of antiangiogenic drugs. One option is candesartan, which blocks angiotensin-II receptor type 1 and has been shown to increase left ventricular hypertrophy regression after valve replacement surgery [58].
In many RHD valvular pathologies and similar degenerative conditions such as myxomatous valve disease, the trilaminar, structured healthy valve reverts to a disorganized embryonic-like phenotype [42,59]. Healthy quiescent valvular interstitial cells become activated osteoblasts, driving calcification near sites of inflammation [56]. This is a complementary process to valvulogenesis, in which the mesenchymal cells associated with condensation and organization of the leaflets move towards an inactive phenotype. Current studies have shown that reactivation of embryonic signaling pathways in diseased valves promotes regression of degenerative characteristics such as stiffening and inflammation [59]. Controlling valve remodeling and phenotypic changes using embryonic pathways could have exciting ramifications for RHD therapy.
The importance of the microenvironment in contributing to healthy cardiovascular development presents an opportunity for engineering to contribute to understanding of these clinical pathologies. Quantifying changes in micromechanical properties of the valves such as stiffness and extracellular matrix organization promises to elucidate both development and disease. Fluid dynamics, soft tissue biomechanics, and methodical quantitative analysis of contributing factors will complement the biological expertise of natural scientists in leading to a deeper understanding of these conditions and intervention strategies.
Paths Forward
Tissue engineered heart valves (TEHV) are the most promising therapy for the young patients of developing countries, offering both long-term viability and significant amelioration of the life-threatening aspects of valve pathologies. However, the field faces significant challenges in understanding the biomechanics and biology of growing valves in the pediatric applications which are of greatest importance in the young patient populations of developing countries, problems which can only be solved by referencing the natural processes by which the body develops. These issues are neglected in the West because more than 90% of valve replacement surgeries are performed in patients over 50 years of age [60], making the economic case for developing a pediatric TEHV a much lower priority.
The potential for tissue engineered valves for pediatric applications has been demonstrated in several studies, most recently with Weber et al.’s successful design and implantation of a marrow stromal cell-based autologous valve in non-human primates [61]. Creative rethinking may make the opportunities in this field particularly suited to the challenges and resources of Sub-Saharan Africa.
Tissue engineering can mean not only the comprehensive development of a fully functional valve, but also the experimental approach which uses biosynthetic platforms to recapitulate the native valvular environment in a tunable in vitro model. Principles from developmental biology such as those described above can be applied to these models in order to understand the parallels between valvulogenesis and RHD/EMF pathology. For example, cells could be isolated from a rheumatic valve, grown on a three-dimensional tissue engineered scaffold, treated with a TGF-β inhibitor and assessed for amelioration of inflammation. Researchers who are daily confronted with the realities of RHD and EMF-associated valvular pathology are uniquely suited to design low-cost experimental investigations which combine insights from developmental biology with the principles of tissue engineering in working towards a therapeutic solution for the young cardiac patients of the developing world.
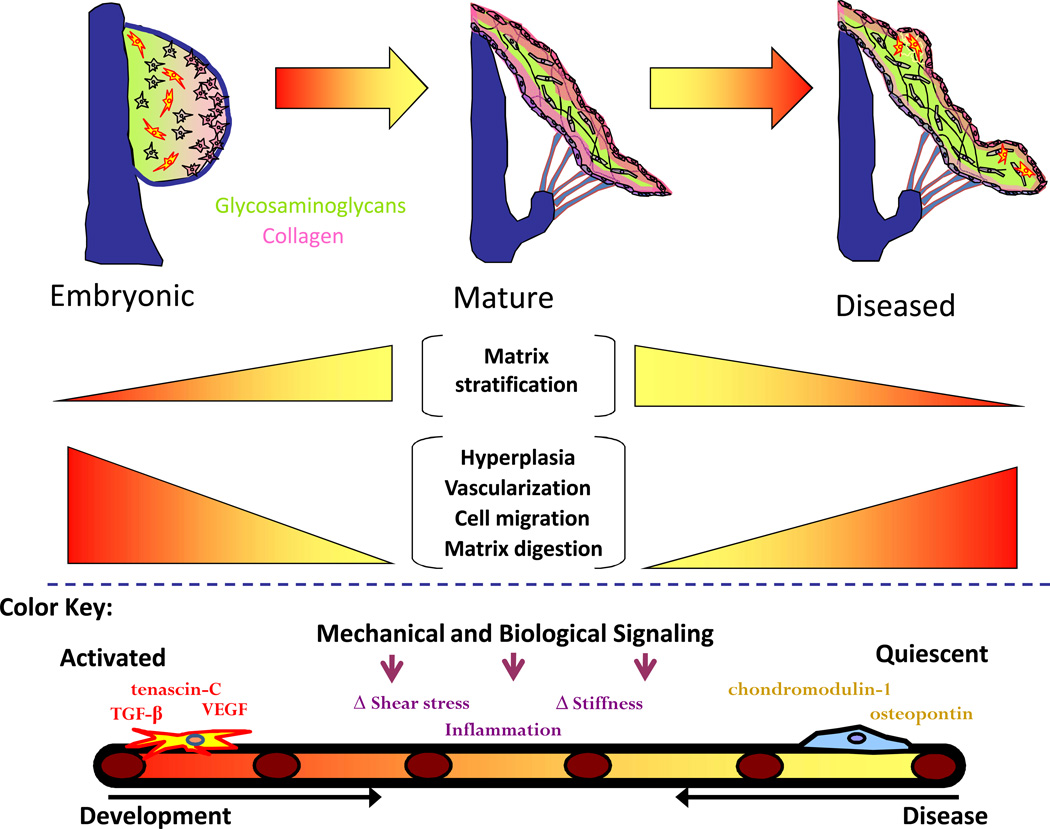
Figure 4.
Similar pathways in valve development and disease. Mechanisms such as vascularization, ECM organization, and cell migration are controlled by common molecules including, but not limited to, TGF-β, VEGF, chondromodulin-1, and tenascin-C.
References
- 1.Yusuf S, Vaz M, Pais P. Tackling the challenge of cardiovascular disease burden in developing countries. Am Heart J. 2004;148:1–4. doi: 10.1016/j.ahj.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Mathers C, Fat DM, Boerma J. The global burden of disease: 2004 update. WHO. 2008 [Google Scholar]
- 3.Mayosi BM. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart. 2007;93:1176–1183. doi: 10.1136/hrt.2007.127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutazind T. Survival after first presentation with endomyocardial fibrosis. Br Heart J. 1972;34:403–407. doi: 10.1136/hrt.34.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilherme L, Ramasawmy R, Kalil J. Rheumatic fever and rheumatic heart disease: Genetics and pathogenesis. Scand J Immunol. 2007;66:199–207. doi: 10.1111/j.1365-3083.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- 6.Mayosi B, Robertson K, Volmink J, et al. The Drakensberg declaration on the control of rheumatic fever and rheumatic heart disease in Africa. S Afr Med J. 2006;96:246. [PubMed] [Google Scholar]
- 7.Mocumbi AO, Ferreira MB, Sidi D, Yacoub MH. A population study of endomyocardial fibrosis in a rural area of Mozambique. N Eng J Med. 2008;359:43–49. doi: 10.1056/NEJMoa0708629. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Reddy S, Ounpuu S. Global burden of cardiovascular diseases: Part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circ. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 9.Moran M, Guzman J, Ropars AL, et al. Neglected Disease Research and Development: How Much Are We Really Spending? PLoS Med. 2009;6:0137–0146. doi: 10.1371/journal.pmed.1000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 11.Wayne AR, Schlant RC, Fuster V. Hurst’s The Heart, Arteries and Veins. New York, McGraw-Hill: Health Professions Division; 1998. [Google Scholar]
- 12.Drakensberg Declaration on the Control of Rheumatic Fever and Rheumatic Heart Disease in the World. 2011 [Google Scholar]
- 13.Watkins DA, Zuhlke LJ, Engel ME, Mayosi BM. Rheumatic fever: Neglected again. Science. 2009;324:37. doi: 10.1126/science.324.5923.37b. [DOI] [PubMed] [Google Scholar]
- 14.Paar J, Berrios NM, Rose JD, et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Cardiol. 2010;105:809–814. doi: 10.1016/j.amjcard.2010.01.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rheumatic Fever and Rheumatic Heart Disease: Report of a WHO Expert Consultation. Geneva: 2004. [Google Scholar]
- 16.Freers J, MayanjaKizza H, Ziegler JL, Rutakingirwa M. Echocardiographic diagnosis of heart disease in Uganda. Trop Doct. 1996;26:125–128. doi: 10.1177/004947559602600310. [DOI] [PubMed] [Google Scholar]
- 17.Anabwani GM, Bonhoeffer P. Prevalence of heart disease in school children in rural Kenya using colour-flow echocardiography. East Afr Med J. 1996;73:215–217. [PubMed] [Google Scholar]
- 18.Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. New Eng J Med. 2007;357:470–476. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 19.Paar JA, Berrios NM, Rose JD, et al. Prevalence of Rheumatic Heart Disease in Children and Young Adults in Nicaragua. Amer J Cardiol. 2010;105:1809–1814. doi: 10.1016/j.amjcard.2010.01.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadiq M, Islam K, Abid R, et al. Prevalence of rheumatic heart disease in school children of urban Lahore. Heart. 2009;95:353–357. doi: 10.1136/hrt.2008.143982. [DOI] [PubMed] [Google Scholar]
- 21.Ansa VO, Ekott JU, Bassey EO. Profile and outcome of cardiovascular admissions at the university of Uyo Teaching Hospital, Uyo - A five year review. Niger J Clin Pract. 2008;11:22–24. [PubMed] [Google Scholar]
- 22.American Heart Association. Heart Attack and Angina Statistics. 2011 [Google Scholar]
- 23.Dajani AS, Ayoub E, Bierman FZ, et al. Guidelines for the Diagnosis of Rheumatic Fever - Jones Criteria, 1992 Update. JAMA. 1992;268:2069–2073. [PubMed] [Google Scholar]
- 24.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oli K, Asmera J. Rheumatic Heart Disease in Ethiopia: Could it be more malignant? Ethiop Med J. 2004:421–428. [PubMed] [Google Scholar]
- 26.Chin TK, Chin EM, Siddiqui T, Sundell AK. Rheumatic Heart Disease [Internet] 2010 Available from: http://emedicine.medscape.com/article/891897-overview.
- 27.Rheumatic Fever [Internet] Available from: www.ourmed.org.
- 28.Braun M. General and Systemic Histopathology: Rheumatic Valvulitis [Internet] Available from: medsci.indiana.edu. [Google Scholar]
- 29.Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian FL, Rahimtoola S. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158. doi: 10.1016/s0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 30.Zilla P, Brink J, Human P, Bezuidenhout D. Prosthetic heart valves: Catering for the few. Biomaterials. 2008;29:385–406. doi: 10.1016/j.biomaterials.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 31.Bukhman G, Ziegler J, Parry E. Endomyocardial Fibrosis: Still a Mystery after 60 Years. PLoS Negl Trop Dis. 2008;2:e97. doi: 10.1371/journal.pntd.0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor DH, Somers K, Hutt MSR, Manion WC, Darbela PG. Endomyocardial Fibrosis in Uganda (Davies Disease): an Epidemiologic Clinical and Pathologic Study. Amer Heart J. 1967;74:687–709. doi: 10.1016/0002-8703(67)90509-1. [DOI] [PubMed] [Google Scholar]
- 33.Mocumbi AO, Yacoub S, Yacoub MH. Neglected tropical cardiomyopathies: II. Endomyocardial fibrosis: myocardial disease. Heart. 2008;94:384–390. doi: 10.1136/hrt.2007.136101. [DOI] [PubMed] [Google Scholar]
- 34.Teekakirikul P, Eminaga S, Toka O, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires TGF-β. J Clin Invest. 2010;120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke AP. Infectious Endocarditis [Google Scholar]
- 36.Niu JL, Azfer A, Rogers LM, Wang XH, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies JNP, Ball JD. The pathology of endomyocardial fibrosis in Uganda. Brit Heart J. 1955;17:337–359. doi: 10.1136/hrt.17.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright IG, Walker Ia, Yacoub MH. Specialist surgery in the developing world: luxury or necessity? Anaesthesia. 2007;62(Suppl):184–189. doi: 10.1111/j.1365-2044.2007.05308.x. [DOI] [PubMed] [Google Scholar]
- 39.Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc London [Biol] 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakuno D, Kimura N, Yoshioka M, Fukuda K. Molecular mechanisms underlying the onset of degenerative aortic valve disease. J Mol Med. 2009;87:17–24. doi: 10.1007/s00109-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 41.Yoshioka M, Yuasa S, Matsumura K, Kimura K. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nature Med. 2006;12:1151–1159. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 42.Markwald RR, Norris RA, Moreno-Rodriguez R, Levine RA. Developmental basis of adult cardiovascular diseases: Valvular heart diseases. Ann NY Acad Sci. 2010;1188:177–183. doi: 10.1111/j.1749-6632.2009.05098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shworak NW. Angiogenic modulators in valve development and disease: does valvular disease recapitulate developmental signaling pathways? Curr Opin Cardiol. 2004;19:140–146. doi: 10.1097/00001573-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 45.Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGF beta 2 TGF beta 3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Bio. 1999;208:530–545. doi: 10.1006/dbio.1999.9211. [DOI] [PubMed] [Google Scholar]
- 46.Brown CB, Boyer AS, Runyan RB, Barnett JV. Antibodies to the type II TGF beta receptor block cell activation and migration during atrioventricular cushion transformation in the heart. Dev Bio. 1996;174:248–257. doi: 10.1006/dbio.1996.0070. [DOI] [PubMed] [Google Scholar]
- 47.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 48.Chen K, Mehta JL, Li DY, Joseph L, Joseph J. Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ Res. 2004;95:1167–1173. doi: 10.1161/01.RES.0000150369.68826.2f. [DOI] [PubMed] [Google Scholar]
- 49.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming Growth Factor beta-Induced Endothelial-to-Mesenchymal Transition: A Switch to Cardiac Fibrosis? Trends Cardiovasc Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med. 2009;19:247–252. doi: 10.1016/j.tcm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 52.Chu PY, Mariani J, Finch S, et al. Bone Marrow-Derived Cells Contribute to Fibrosis in the Chronically Failing Heart. Am J Pathol. 2010;176:1735–1742. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haudek SB, Cheng J, Du J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imanaka-Yoshida K, Hiroe M, Yoshida T. Interaction between cell and extracellular matrix in heart disease: Multiple roles of tenascin-C in tissue remodeling. Histol Histopathol. 2004;19:517–525. doi: 10.14670/HH-19.517. [DOI] [PubMed] [Google Scholar]
- 55.Widyantoro B, Emoto N, Nakayama K. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2379–2380. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 56.Kim L, Kim DK, Yang WI, et al. Overexpression of transforming growth factor-beta 1 in the valvular fibrosis of chronic rheumatic heart disease. J Korean Med Sci. 2008;23:41–48. doi: 10.3346/jkms.2008.23.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cunningham MW. Autoimmunity and molecular mimicry in the pathogenesis of post-streptococcal heart disease. Front Biosci. 2003;8:533–543. doi: 10.2741/1067. [DOI] [PubMed] [Google Scholar]
- 58.Dahl JS, Videbaek L, Poulsen MK, et al. Effect of candesartan treatment on left ventricular remodeling after aortic valve replacement for aortic stenosis. Am J Cardiol. 2010;106:713–719. doi: 10.1016/j.amjcard.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 59.Hinton RB, Lincoln J, Deutsch GH, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 60.Lewin M, Otto C. The bicuspid aortic valve - adverse outcomes from infancy to old age. Circ. 2005;111:832–834. doi: 10.1161/01.CIR.0000157137.59691.0B. [DOI] [PubMed] [Google Scholar]
- 61.Weber B, Scherman J, Emmert MY, et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr059. [DOI] [PubMed] [Google Scholar]