Abstract
Mice that express the viral oncoprotein simian virus 40 (SV40) large T antigen (T-Ag) as a transgene provide useful models for the assessment of the state of the host immune response in the face of spontaneous tumor progression. Line SV11 (H2b) mice develop rapidly progressing choroid plexus tumors due to expression of full-length T-Ag from the SV40 promoter. In addition, T-Ag expression in the thymus of SV11 mice results in the deletion of CD8+ T cells specific for the three H2b-restricted immunodominant epitopes of T-Ag. Whether CD8+ T cells specific for the immunorecessive H2-Db-restricted epitope V of T-Ag survive negative selection in SV11 mice has not been determined. Immunization of SV11 mice with rVV-ES-V, a recombinant vaccinia virus expressing epitope V as a minigene, resulted in the induction of weak, but reproducible, epitope V-specific cytotoxic T-lymphocyte (CTL) responses. This weak lytic response corresponded with a decreased frequency of epitope V-specific CTL that could be recruited in SV11 mice. In addition, CTL lines derived from rVV-ES-V-immunized SV11 mice had reduced avidities compared to that seen with CTL derived from healthy mice. Despite this initial weak response, significant numbers of epitope V-specific CD8+ T cells were detected in SV11 mice ex vivo following a priming-boosting approach and these cells demonstrated high avidity for epitope V. The results suggest that low numbers of tumor-reactive CD8+ T cells with high avidity for epitope V survive negative selection in SV11 mice but can be expanded by specific boosting approaches in the tumor bearing host.
Active recruitment of the host's immune system for protection against spontaneous tumors remains an area of intense research for the development of new cancer therapies. In particular, studies targeting the recruitment of tumor-specific CD8+ cytotoxic T lymphocytes (CTL) have resulted in the characterization of a large number of CTL-recognized epitopes derived from a variety of cellular antigens (6, 7). CTL recognize peptide antigens bound to cell surface major histocompatibility complex (MHC) class I molecules (43). These peptides are typically liberated by proteolysis in the cytosol and then shuttled by the transporter associated with antigen processing into the endoplasmic reticulum, where they bind to newly formed MHC class I molecules that (upon proper folding) transit to the cell surface (58). Tumor-reactive T cells isolated from cancer patients have been shown to recognize epitopes derived from nonmutated regions of cellular proteins, suggesting that the induction of self-reactive T cells might be beneficial for the control of cancer (7, 44). Although many self-reactive T cells are deleted from the thymus during T-cell development (29, 31), some self-reactive T cells, including CD8+ T cells specific for tumor antigens (6, 17, 37, 67), survive negative selection and are detected in peripheral tissues (8, 10, 34, 45, 46, 49, 64, 71, 75). These residual self-reactive T cells typically have reduced levels of avidity for their antigen in the periphery, most likely due to the deletion of T cells bearing high-affinity T-cell receptors (TCRs) in the thymus (2, 27, 56). In addition, T cells specific for ligands that are not expressed or are present at low levels in the thymus are expected to survive negative selection more readily than T cells specific for antigens expressed at higher levels (1-3, 41, 56). Thus, immunotherapeutic strategies targeting tumor-associated self antigens should consider the nature of the epitopes that allow the survival of potentially self-reactive T cells. Such epitopes may be exemplified by some cryptic or subdominant T-cell epitopes that are generated at low levels by antigen processing or that form unstable complexes with MHC class I molecules (54), resulting in reduced antigen levels at the cell surface for presentation during T-cell development.
Cellular immunity to the simian virus 40 (SV40) oncoprotein large T antigen (T-Ag) in C57BL/6 mice is characterized by a hierarchical response to multiple CD8+ T-cell-recognized epitopes (38, 40). Four epitopes have been defined in T-Ag; these include epitope I (residues 206 to 215), epitope II/III (residues 223 to 231), epitope IV (residues 404 to 411), and epitope V (residues 489 to 497) (16, 33, 39, 61, 62). The CD8+ T-cell response to epitopes I, II/III, and V is H2-Db restricted, while the response to epitope IV is H2-Kb restricted. Immunization of C57BL/6 mice with wild-type (WT) T-Ag results in the induction of CD8+ T cells specific for the immunodominant epitopes I, II/III, and IV, which can be directly detected ex vivo by analysis with MHC tetramers (40). In contrast, epitope V-specific CD8+ T cells are not detected in WT T-Ag-immunized mice even following in vivo boosting or in vitro restimulation. Epitope V-specific CD8+ T cells are detected, however, following immunization of C57BL/6 mice with (a) a T-Ag variant in which epitopes I, II/III, and IV have been inactivated but which retains epitope V (61) or (b) recombinant vaccinia viruses (rVVs) expressing epitope V as a minigene or in the context of an unrelated protein (20). Thus, epitope V is stringently subdominant and has been designated immunorecessive. The basis for the subdominance of epitope V remains unknown but might be related to the relatively short half-life of H2-Db/epitope V complexes at the cell surface. Alternatively the subdominant phenotype might be due to the nature of the TCR repertoire (74).
Mice that express the SV40 T-Ag as a transgene provide a realistic model for the assessment of the recruitment of tumor-associated self antigen-specific T cells in the presence of developing tumors. Line SV11 (H2b) mice express SV40 T-Ag as a transgene from the virus early region promoter (42). T-Ag is expressed in the kidney and thymus of SV11 mice at low levels and in the choroid plexus tissues at higher levels, resulting in the development of choroid plexus papillomas (68, 69). T-Ag expression in the choroid plexus is initially detected at 14 days of age and leads to the development of small neoplastic clusters of cells by days 36 to 41 (69). Tumors reproducibly progress to carcinomas until mice succumb to tumor burden at a mean age of 104 days (68). Expression of T-Ag in the thymus results in the deletion of CD8+ T-cell precursors specific for the immunodominant epitopes I, II/III, and IV, as determined by the absence (even after in vitro restimulation) of detectable CTL following immunization of SV11 mice with rVVs encoding individual T-Ag epitopes (52). The previous investigation of this model by Schell et al. demonstrated that the growth of both early and advanced choroid plexus tumors was dramatically inhibited following the adoptive transfer of lymphocytes from naive C57BL/6 mice (52, 53). Control of tumor progression was associated with the priming of donor CD8+ T cells against the endogenous T-Ag that was specific for the immunodominant H2-Kb-restricted T-Ag epitope IV.
Although our previous investigations using an adoptive immunotherapy approach demonstrated that T-Ag-specific immunity was readily induced in SV11 mice, the question remains whether tumor antigen-specific CTL can be recruited from within the host T-cell repertoire. Thus, in this report we determined whether CTL specific for the immunorecessive epitope V could survive negative selection in SV11 mice. The results indicate that a residual population of epitope V-specific CTL exists in the periphery of SV11 mice, whose CTL have reduced avidity for epitope V compared to CTL derived from healthy C57BL/6 mice. Importantly, both quantitative and qualitative enhancement of the epitope V-specific CTL response was observed in SV11 mice following sequential boosting in vivo. These results suggest that by specific immunization, higher-quality tumor-specific CTL can be expanded in vivo from the residual host T-cell repertoire.
MATERIALS AND METHODS
Mice.
C57BL/6 (H2b) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and maintained at the animal facility of the Milton S. Hershey Medical Center. Mice between the ages of 6 to 12 weeks were routinely used. SV11 mice were generated by Palmiter et al. (42); the kinetics of tumor progression have been described previously (69). SV11 (H2b) mice express the SV40 T-Ag transgene under the control of its own enhancer/promoter. The SV11 line has been maintained in the animal facility of the Milton S. Hershey Medical Center by backcrossing T-Ag transgene-positive males with C57BL/6 females. SV11 transgene-positive mice (SV11+) were identified by PCR amplification of the transgene as described previously (52). Line 459 TCR-V mice expressing the TCRα and TCRβ chains specific for T-Ag epitope V and on the C57BL/6 background were generously provided by Satvir S. Tevethia (The Pennsylvania State University College of Medicine, Hershey, Pa.; unpublished data). All experimental protocols were approved by the institutional animal care and use committee of the Pennsylvania State University College of Medicine and comply with federal guidelines.
Cell lines and viruses.
B6/WT-19 and B6/T5Aa are WT T-Ag-transformed cell lines derived from C57BL/6 mouse embryo fibroblasts (38, 47). B6/T116A1 cells express a T-Ag derivative from which epitopes I and II/III have been deleted and in which epitope IV has been inactivated by alanine replacement of critical MHC class I anchor residues (Y406A, F408A, and C411A) (40). B6/T11Bb cells express a T-Ag variant from which epitope V has been deleted (38). B6/T122B1 cells express a T-Ag derivative in which all four H2b-restricted CTL epitopes in T-Ag (I, II/III, IV, and V) have been inactivated by alanine replacements of critical MHC class I anchor residues (N210A, N227A, F408A, and N493A) (40). B6/K-1,4,5 cells express a T-Ag variant lacking residues 134 to 263 (which inactivate epitopes I and II/III) and the point mutations V405L and I491F (which inactivate epitopes IV and V, respectively) (32, 63). All T-Ag-transformed cell lines were maintained in Dulbecco's modified eagle medium (DMEM) supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 100 μg of kanamycin per ml, 2 mM l-glutamine, 10 mM HEPES buffer, 0.075% (wt/vol) NaHCO3, and 5 to 10% fetal bovine serum. RMA (H2b) cells (35) were maintained in suspension with RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, and 50 uM 2-mercaptoethanol. rVV-ES-V and rVV-ES-gB498-505, which have been described previously (4, 20), encode T-Ag epitope V and herpes simplex virus glycoprotein B (gB) epitope 498-505, respectively, as minigenes preceded by an endoplasmic reticulum insertion sequence.
Synthetic peptides.
All peptides used were synthesized (using FMoc chemistry and an automated peptide synthesizer [9050 MilliGen PepSynthesizer]) at the Macromolecular Core Facility of the Milton S. Hershey Medical Center. Peptides were solubilized in dimethyl sulfoxide (Sigma, St. Louis, Mo.) and diluted to the appropriate concentration with RPMI 1640 medium. Peptides used in these experiments correspond to SV40 T-Ag epitopes IV/411L (VVYDFLKL) and V (QGINNLDNL), the control H2-Db binding peptide DbN5 (SMIKNLEYM), the H2-Db-restricted influenza virus nucleoprotein peptide 366-374 (ASNENMETM) (12), and the H2-Kb-restricted herpes simplex virus gB peptide 498-505 (SSIEFARL) (5).
Immunizations.
Mice were immunized by intravenous injection with 107 PFU of rVV-ES-V or rVV-ES-gB498-505 in 0.2 ml of phosphate-buffered saline containing 0.1% bovine serum albumin or by intraperitoneal injection of 3 × 107 to 5 × 107 live tumor cells in Hank's balanced salt solution.
In vitro stimulation of bulk CTL and CTL lines.
Lymphocytes isolated from spleen were restimulated in vitro with gamma-irradiated B6/WT-19 cells as described previously (52). Briefly, 107 red blood cell-depleted spleen cells were mixed with 5 × 105 gamma-irradiated (10,000 rads) B6/WT-19 cells in 4 ml of complete RPMI 1640 medium (supplemented with 10% fetal bovine serum) per well of a 12-well tissue culture plate. To establish CTL lines, approximately 106 responding lymphocytes were restimulated every 7 days with 5 × 105 gamma-irradiated B6/WT-19 cells and 5 U of recombinant interleukin-2 (generously provided by Amgen Inc., Thousand Oaks, Calif.)/ml.
Preparation of MHC class I tetramers, staining of epitope-specific T cells, and flow cytometry.
The production and characterization of MHC tetramers was achieved as described previously (40). For staining of lymphocyte populations, red blood cell-depleted lymphocytes were incubated with rat anti-mouse CD16/CD32 (BD-Pharmingen, San Diego, Calif.) and 50 μg of streptavidin (Molecular Probes)/ml for 30 min on ice to block Fc receptors and nonspecific binding of streptavidin-conjugated tetramers, respectively. After a single wash, spleen cells were incubated with phycoerythrin-labeled tetramers at a 1:200 dilution or as indicated in the figure legend (see Fig. 2 legend) and fluorescein isothiocyanate-labeled rat anti-mouse CD8a (clone 53-6.7; Pharmingen) for 1 h on ice. Cells were fixed with 2% paraformaldehyde and analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.), and the data were analyzed and prepared using FlowJo software (Tree Star, Inc.). The percentage of CD8+ cells that stained with epitope-specific MHC tetramers was determined by subtracting the percentage of CD8+ cells that stained with an irrelevant MHC tetramer in the same cell population. For MHC tetramer stability assays, CTL lines were incubated with a 1:25 dilution of the indicated tetramers and fluorescein isothiocyanate-labeled anti-CD8a for 1 h on ice. Cells were then washed three times with phosphate-buffered saline containing 2% fetal bovine serum and 0.1% sodium azide. Cells were placed at room temperature for the indicated times, after which aliquots of cells were collected, fixed immediately with 2% paraformaldehyde, and kept at 4°C prior to flow cytometric analysis. Determination of TCR variable β chain (TCRVβ) usage by CTL lines was accomplished by staining with a panel of Vβ-specific antibodies (BD-Pharmingen). Intracellular cytokine staining was performed as described previously (53).
FIG. 2.
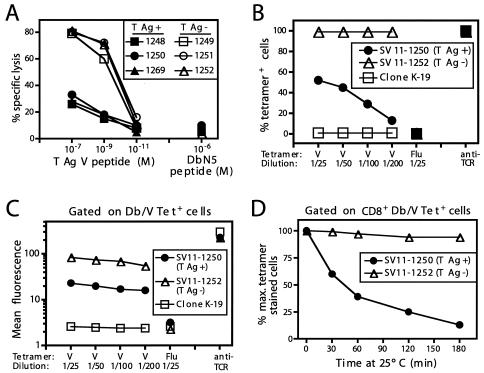
Epitope V-specific CTL derived from SV11 mice have low levels of avidity for the target epitope. (A) CTL lines derived from the cultures described for Fig. 1 by five in vitro passages were tested for lytic activity against B6/K-1,4,5 (lacking expression of T-Ag epitopes I, II/III, IV, and V) target cells pulsed with the indicated concentrations of epitope V or control DbN5 peptide in a 5-h 51Cr release assay. Effector-to-target cell ratio (E:T), 5:1. M, molar. (B and C) The epitope V-specific CTL lines SV11-1250 (T-Ag+) and SV11-1252 (T-Ag−) and the T-Ag epitope II/III-specific CTL clone K-19 were incubated with various dilutions of the Db/epitope V tetramer (V), the Db/Flu NP 366-374 epitope tetramer (Flu), or anti-TCRβ chain antibody in combination with anti-CD8a monoclonal antibody for 1 h on ice. Cells were washed and analyzed by flow cytometry. Data are presented as the percentage of tetramer or TCRβ chain-positive cells detected at each dilution (B) or the mean fluorescence intensity of tetramer or TCRβ chain staining among CD8+ T cells (C). (D) CTL lines 1250 and 1252 were incubated with a 1:25 dilution of Db/epitope V tetramer and a 1:50 dilution of anit-CD8a antibody for 1 h on ice followed by three washes to remove excess tetramer. Cells were then incubated at room temperature for the indicated times, after which aliquots of cells were removed and fixed prior to flow cytometric analysis. The data are plotted as percentages of the maximum numbers of tetramer-stained CD8+ T cells detected at time 0. The Db/V tetramer-positive gate was established using cells stained with the control Db/Flu tetramer. T-Ag+, SV11 mice; T-Ag−, transgene-negative littermates.
Cytotoxicity assays and limiting dilution analysis.
Assays for CTL lysis were performed on day 6 after in vitro restimulation as previously described (20). For peptide titration experiments, B6/K-1,4,5 target cells were pulsed with the indicated concentrations of peptides followed by three washes and then plated in 96-well V-bottom plates followed by the addition of CTL at the indicated effector-to-target T-cell ratios. Cultures were incubated at 37°C in 5% CO2 for 5 h, after which the supernatants were collected and counted on a Packard Instruments Cobra II γ-counter. Limiting dilution analysis was performed essentially as described previously (28). Briefly, splenocytes were obtained from groups of three to four naïve C57BL/6 mice or three to four SV11 or C57BL/6 mice that had been immunized 9 days earlier with rVV-ES-V. Pooled splenocytes from each group were serially diluted twofold and added (per dilution; range, 250,000 to 31,250 cells per well) to 48 wells of a 96-well microtiter plate in a total of 200 μl of complete RPMI medium including 2 × 103 γ-irradiated (10,000 rads) B6/WT-19 cells, 1 × 105 γ-irradiated (3,000 rads) naïve C57BL/6 splenocytes, 5% (vol/vol) T-STIM culture supplement (Becton Dickinson Biosciences), 0.1 M methyl-α-d-mannopyranoside (Sigma), and 0.25 U of recombinant interleukin-2. Control wells lacked addition of immune splenocytes. Cultures were incubated at 37°C in 5% CO2 for 7 days, after which equal proportions of each microculture were transferred to duplicate sets of V-bottom 96-well microtiter plates to test for lytic activity. B6/K-1,4,5 target cells grown in complete DMEM were labeled as a monolayer with 750 μCi of sodium 51chromate in T-75 flasks. Labeled cells were harvested by trypsinization, and 3 × 106 cells were pulsed with 1 μM T-Ag epitope V peptide or the control influenza virus nucleoprotein (Flu NP) 366-374 peptide in 6 ml of complete DMEM for 1.5 h at 37°C on a rocking platform. Target cells were washed three times to remove excess peptide, resuspended in complete RPMI medium, and added at 3 × 103 cells per well to wells containing lymphocytes aliquoted from the limiting dilution cultures to achieve a total volume of 200 μl. Cultures were incubated at 37°C in 5% CO2 for 5 h, after which cells were pelleted by centrifugation and 100 μl of supernatant was harvested and counted on a gamma counter. For calculation of positive responses, nonspecific lysis was first deducted from each culture by subtracting the counts obtained using Flu NP 366-374-pulsed target cells from the counts obtained using epitope V peptide-pulsed target cells. Samples were considered positive when the corrected counts were higher than the mean counts per minute (+ 3 standard deviations of the mean) obtained using naïve C57BL/6 spleen cells. Frequencies were estimated using the minimal χ2 method (65) as described previously (28).
RESULTS
A residual population of epitope V-specific CTL is detected in SV11 mice.
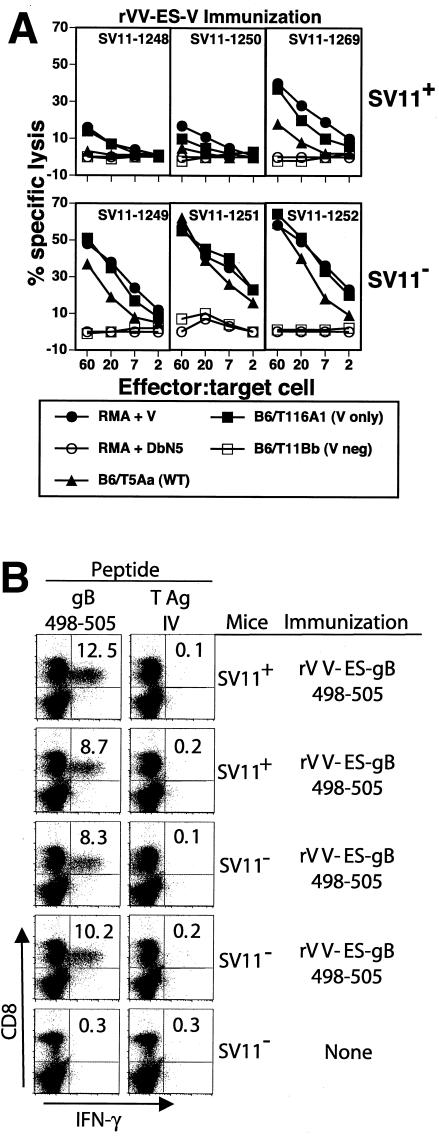
To determine whether SV11 mice are responsive to the immunorecessive epitope V of T-Ag, groups of 50-day-old SV11 mice or of their transgene-negative littermates were immunized with rVV-ES-V; after 4 weeks, spleen cells were isolated and restimulated in vitro with γ-irradiated B6/WT-19 T-Ag-transformed cells. After 6 days of growth, responder cells were tested for their ability to lyse 51Cr-labeled syngeneic RMA target cells that were pulsed with epitope V peptide or the unrelated H2-Db binding peptide DbN5 (Fig. 1A). Weak epitope V-specific CTL responses were consistently detected in cultures derived from SV11 mice, while much stronger CTL responses were detected in cultures derived from transgene-negative mice. Epitope V-specific CTL responses with an intermediate level of activity in SV11 mice were occasionally observed (SV11-1269; Fig. 1A). In total, 15 of 15 SV11 mice immunized with rVV-ES-V developed detectable epitope V-specific CTL responses (Fig. 1A and data not shown). CTL derived from transgene-negative mice, but not from SV11 mice, efficiently lysed the WT T-Ag-transformed cell line B6/T5Aa. In contrast, SV11-derived CTL lysed B6/T116A1 target cells (expressing a T-Ag variant in which the immunodominant epitopes I, II/III, and IV are inactivated) as efficiently as peptide V-pulsed RMA cells. Although the basis for this difference is not known, B6/T116A1 may present epitope V more efficiently than the WT T-Ag-transformed cells. Alternatively, the B5/T5Aa cell line may be less susceptible to CTL lysis than B6/T116A1 cells, as even CTL derived from transgene-negative mice lysed B6/T5Aa cells less efficiently than those from B6/T116A1 cells. Regardless of the explanation, these results indicate that the epitope V-specific CTL derived from SV11 mice are less efficient killers than CTL derived from transgene-negative mice. B6/T11Bb cells that express a T-Ag variant lacking only epitope V were not lysed.
FIG. 1.
Weak epitope V-specific CTL responses were detected in SV11 mice following immunization with rVV-ES-V. SV11 transgene-positive mice (SV11+) or their transgene-negative littermates (SV11−) were immunized at 50 days of age with 107 PFU of rVV-ES-V (A) or rVV-ES-gB498-505 (B). (A) After 3 weeks, spleen cells from individual mice were grown in vitro with gamma-irradiated B6/WT-19 cells for 5 days and then tested for their ability to lyse RMA (T-Ag-negative) target cells pulsed with 1 μM T-Ag 489-497 or the control peptide DbN5. In addition, lysis of transformed cell lines expressing WT T-Ag (B6/T5Aa) or T-Ag variants in which epitopes I, II/III, and IV were inactivated (B6/T116A1) or from which epitope V was deleted (B6/T11Bb) was determined in a standard 51Cr release assay. (B) Spleen cells were isolated on day 10 postimmunization and incubated in vitro with 1 μM peptide corresponding to the herpes simplex virus gB 498-505 epitope or T-Ag epitope IV in the presence of 1 μg of brefeldin A/ml for 6 h. Cells were then stained for detection of CD8+ T cells that had accumulated intracellular gamma interferon and analyzed by flow cytometry. neg, negative; V, epitope V.
To ensure that SV11 mice were not generally immunosuppressed, SV11 mice and their transgene-negative littermates were immunized with an rVV encoding the immunodominant H2-Kb-restricted epitope of herpes simplex virus gB (residues 498 to 505) as a minigene (rVV-ES-gB498-505). Spleen cells from immunized mice were subjected to a brief period of in vitro stimulation with either the gB498-505 peptide or the H2-Kb-restricted T-Ag epitope IV peptide prior to staining for the accumulation of intracellular gamma interferon. The results demonstrate that similar frequencies of gB498-505-specific CD8+ T cells were induced in SV11 mice and transgene-negative littermates (Fig. 1B). Thus, tolerance within the CD8+ T-cell compartment in SV11 mice is restricted to the T-Ag epitopes.
The weak CTL activity observed in cultures derived from SV11 mice immunized with rVV-ES-V could be explained by reduced numbers of epitope V-specific CTL, by compromised reactivity of these CTL, or by a combination of these mechanisms. Previous quantitation of the epitope V-specific CD8+ T-cell response in C57BL/6 mice following immunization with rVV-ES-V revealed that approximately 1% of splenic CD8+ T cells were specific for epitope V at 9 days postimmunization (40). A similar analysis of SV11 mice revealed that the frequency of epitope V-specific CD8+ T cells following immunization with rVV-ES-V was below the limit of detection by ex vivo analysis of splenocytes with MHC tetramers (data not shown). This finding is consistent with the low level of cytotoxicity detected after 6 days of in vitro restimulation (Fig. 1A). To estimate the frequency of epitope V-specific CTL in SV11 mice, limiting dilution analysis using SV11 and transgene-negative mice that were immunized with rVV-ES-V was performed. The data in Table 1 indicate that the levels of epitope V-specific CTL in rVV-ES-V-immunized SV11 mice were reduced approximately sevenfold compared to the levels seen with C57BL/6 mice. This result is consistent with the inability to detect epitope V-specific CTL from rVV-ES-V-immunized SV11 mice ex vivo.
TABLE 1.
Frequency analysis of epitope V-specific CTL in SV11 mice immunized with rVV-ES-Va
| Mouse strain | Reciprocal frequencyb | 95% confidence limit | Change from SV11− (fold) | χ2 |
|---|---|---|---|---|
| SV11− | 20,527 | 16,342-27,592 | 2.21 | |
| SV11+ | 140,451 | 115,689-178,699 | 6.8 | 3.20 |
SV11 transgene positive (SV11+) or transgene-negative (SV11−) mice were immunized with rVV-ES-V; after 9 days, splenocytes from groups of 3 to 4 mice were pooled, plated in serial dilutions, and stimulated with B6/WT-19 cells for limiting dilution analysis as described in Materials and Methods. After 7 days, cultures were tested for the ability to lyse 51Cr-labeled B6/K-1,4,5 target cells that had been pulsed with T-Ag epitope V.
Frequencies were estimated using the minimum chi-square test as described in Materials and Methods.
Epitope V-specific CTL lines derived from SV11 mice have a low avidity.
Although limiting dilution analysis indicated that epitope V-specific CTL are less abundant in SV11 mice than in C57BL/6 mice following specific immunization, reduced frequencies of epitope V-specific CTL might not completely explain the low level of epitope V-specific lytic activity observed following in vitro restimulation (Fig. 1A). Since reduced avidity of responding T cells might also contribute to the low level of CTL activity, we tested the sensitivity of CTL lines derived from rVV-ES-V-immunized SV11 mice for B6/K-1,4,5 target cells (lacking epitopes I, II/III, IV, and V) pulsed with decreasing concentrations of epitope V peptide (Fig. 2A). This analysis revealed that the CTL lines derived from SV11 mice had reduced lytic activity against epitope V-pulsed target cells and recognized epitope V peptide approximately 100-fold less efficiently than CTL lines derived from transgene-negative littermates. This result suggested that the weak immune response to epitope V observed in SV11 mice might additionally be due to the presence of CTL with a reduced avidity for epitope V.
To further characterize the avidity of the epitope V-specific CTL, we determined the efficiency of Db/epitope V tetramer binding to CTL lines derived from either SV11 mice or transgene-negative littermates. CTL lines were incubated with decreasing amounts of the Db/epitope V or control Db/Flu NP 366-374 epitope tetramers. All cells from CTL line 1252 (derived from a nontransgenic mouse) stained positively with the Db/epitope V tetramer at all dilutions (Fig. 2B). In contrast, only 50% of the cells from the SV11-derived CTL line 1250 stained positive with the Db/V tetramer at the lowest dilution of 1/25, suggesting that the majority of cells within line 1250 are weakly reactive with epitope V. In addition, the intensity of staining with the Db/epitope V tetramer was significantly reduced among CD8+, Db/epitope V tetramer+ cells from line 1250 compared to those from CTL line 1252 (Fig. 2C). Staining of CTL line 1250 at higher concentrations of Db/epitope V tetramer was not due to nonspecific binding of the tetramer, as evidenced by the lack of binding by the unrelated Db/Flu NP 366-374 tetramer at the highest concentration and the lack of nonspecific binding of the Db/epitope V tetramer to the H2-Db-restricted CTL clone K-19 (specific for T-Ag epitope II/III) (Fig. 2B and C). Inefficient staining of CTL line 1250 with the Db/epitope V tetramer was not due to decreased TCR expression, as staining with antibody to the TCRβ chain revealed high levels of surface expression on both epitope V-specific CTL lines (Fig. 2C). Expression levels of CD8 on the cell surface for the two CTL lines were also similar (data not shown). The intensity of T lymphocyte staining by MHC tetramers has been shown to correlate with the affinity of T-cell recognition in several antigen systems (9, 14). A recent report, however, suggested that the T-cell avidity correlates more precisely with the rate of tetramer dissociation than with binding of MHC tetramers under equilibrium conditions (19). Thus, we also performed experiments in which the CTL lines initially were stained with a high concentration of Db/epitope V tetramer followed by removal of excess tetramer and incubation at room temperature for increasing times to determine the relative stability of Db/epitope V tetramer binding to these CTL lines (Fig. 2D). The results reveal that while all cells from CTL line 1252 remained associated with Db/epitope V tetramer for the entire 3-h period, only 40% of cells from CTL line 1250 were associated with the Db/epitope V tetramer after 1 h and this number was reduced to 13% by the end of the 3-h incubation. Thus, taken together, these results indicate that the epitope V-specific CD8+ T cells derived from SV11 mice have a reduced avidity for epitope V compared to T cells derived from nontransgenic mice.
Variable TCR usage among epitope V-specific CTL lines.
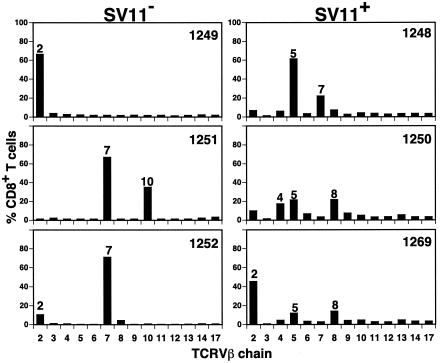
T-Ag expression in the thymus leads to the deletion of all detectable CTL precursors specific for the immunodominant T-Ag epitopes I, II/III, and IV in SV11 mice (52). Since negative selection might also alter the repertoire of epitope V-specific CD8+ T cells by the deletion of T cells expressing particular TCRs with high avidity for epitope V, we analyzed TCRVβ usage by CTL lines derived from rVV-ES-V-immunized SV11 mice or transgene-negative littermates. CTL lines derived from transgene-negative littermates preferentially utilized TCRVβ chains 2, 7, and 10 (Fig. 3). This result is similar to the results of previous analysis which demonstrated the use of TCRVβ chains 2, 7, and 9 among epitope V-specific CTL clones (40). In contrast, CTL lines derived from SV11 mice utilized Vβ chains 2, 4, 5, 7, and 8 (Fig. 3). Only CTL derived from SV11-1269 mice predominantly utilized a TCRVβ chain common to one of the transgene-negative CTL lines (Vβ2). Among the SV11-derived CTL, interestingly, CTL from this mouse initially had the strongest reactivity with epitope V (Fig. 1A). Whether all of the identified TCRVβ chains expressed by the T-cell population within a particular CTL line were specific for T-Ag epitope V was not determined. The differences observed between SV11 and transgene-negative mice in TCRVβ chain usage, however, suggest that the epitope V-specific CD8+ T-cell repertoire is altered by negative selection in SV11 mice such that T cells bearing certain TCRs either are not available or are present at reduced levels.
FIG. 3.
TCRVβ chain usage by epitope V-specific CTL lines. CTL lines derived from rVV-ES-V immunized SV11 (SV11+) and transgene-negative (SV11−) littermates were costained with anti-CD8 monoclonal antibody and a panel of anti-TCRVβ chain-specific antibodies. The percentage of CD8+ T cells expressing a particular TCRVβ chain was quantitated by flow cytometric analysis.
Skewed development of high-avidity TCR-transgenic epitope V-specific T cells in SV11 mice.
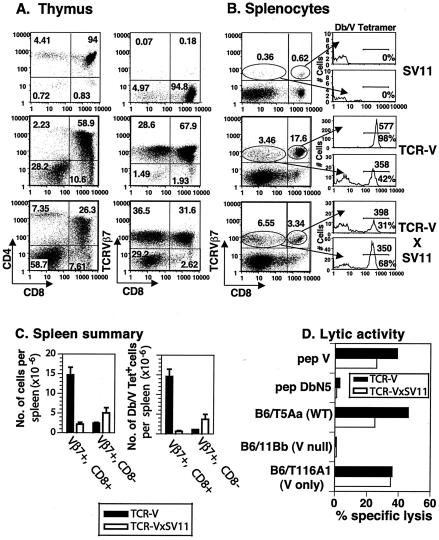
To monitor the fate of self-reactive epitope V-specific T cells during T-cell development, SV11 mice were bred with an epitope V-specific TCR-transgenic mouse line (designated TCR-V). TCR-V mice express the α and β TCR chains from the epitope V-specific CTL clone Y-5 (61) and will be described in greater detail elsewhere (S. S. Tevethia, personal communication). Staining of thymocytes from a representative TCR-V mouse revealed skewing toward CD4−CD8+ T cells compared to the results seen with thymocytes derived from a singly transgenic SV11 mouse (Fig. 4A). In addition, approximately 97% (67.9% CD8+ cells and 28.6% CD8− cells) of thymocytes from TCR-V mice expressed the transgenic TCRVβ7 receptor, revealing high penetrance of the transgene. Indicative of expression of both transgenic TCR α and β chains, approximately 97% of thymocytes from TCR-V mice also stained positive with Db/epitope V tetramer (data not shown). High levels of epitope V-specific CD8+ T cells were found in the peripheral lymphoid organs of TCR-V mice, as 17.6% of total spleen cells were CD8+ TCRVβ7+ (Fig. 4B [dot plot]). Approximately 98% of these CD8+ TCRVβ7+ T cells also stained positive with the Db/V tetramer (Fig. 4B [histogram]). Thus, approximately 81% of splenic CD8+ T cells in the TCR-V mice were epitope V specific.
FIG. 4.
Development of TCR-V-transgenic T cells is skewed in SV11 mice. The surface phenotype of lymphocytes isolated from thymus (A) and spleen (B) of singly transgenic SV11 and TCR-V or double-transgenic TCR-VxSV11 mice was analyzed by flow cytometry using the indicated reagents. In panel A, the percentage of total cells within each quadrant of the dot plot is indicated. In panel B, the percentage of cells within each gated region of the dot plot is indicated; histograms show Db/V tetramer staining of cells within each gate. Numbers on the histogram plots below the marker indicate the percentages of gated cells that stain positive with Db/V tetramer; numbers above the marker indicate the mean fluorescent intensity of staining with Db/V tetramer. (C) Summary of the data obtained using three naïve mice each for strains TCR-V and TCR-VxSV11 in experiments in which splenocytes were triple stained with anti-TCRVβ7, anti-CD8, and Db/epitope V tetramer. The data are presented as total cells per spleen (left panel) and total Db/epitope V tetramer+ cells per spleen (right panel). Error bars indicate standard errors from the means. (D) Lytic response of bulk splenocyte cultures following 4 days of in vitro stimulation with γ-irradiated B6/T116A1 cells. The responding cells were tested in a 5-h 51Cr release assay at an E:T of 50:1 for lytic activity against peptide-pulsed B6/K-1,4,5 cells, which lack expression of epitopes I, II/III, IV, and V, or the indicated T-Ag-transformed cell lines. V, epitope V; pep, peptide.
Analysis of thymocytes from double-transgenic TCR-VxSV11 mice revealed a twofold decrease in the frequency of CD4+ CD8+ double-positive cells (Fig. 4A, left panels) compared to the results seen with TCR-VxSV11 mice, with a corresponding twofold increase in the percentage of CD4− CD8− double-negative cells. Thymus cellularity in TCR-VxSV11 mice was also decreased to 1.5 × 107 ± 0.3 × 107 cells compared to 2.9 × 107 ± 0.7 × 107 cells in TCR-V mice (data not shown). Consistent with the decreased level of thymus cellularity, the percentage of CD8+ thymocytes that expressed the transgenic TCRVβ7 chain decreased from 67.9% (1.5 × 107 total cells) in TCR-V mice to 31.6% (3.8 × 106 total cells) in TCR-VxSV11 mice (Fig. 4A, right panels). Staining with Db/epitope V tetramer revealed that only 64% of TCR-VxSV11 thymocytes were specific for epitope V compared to 97% in TCR-V singly transgenic mice (data not shown). These results indicate that fewer TCR-V T cells survive negative selection in SV11 mice than in singly transgenic TCR-V mice.
Consistent with observations of the thymus, TCR-VxSV11 mice had fewer epitope V-specific T cells in the peripheral lymphoid organs than TCR-V mice. Although there were no significant differences in spleen cellularity (data not shown), TCR-VxSV11 mice showed a fivefold decrease in the percentage of splenic CD8+ T cells that expressed the transgenic TCRVβ7 chain (3.34 versus 17.6% in TCR-V mice) (Fig. 4B, left panels). To insure that this difference was significant, three mice from each line were analyzed and the total number of CD8+ TCRVβ7+ T cells per spleen was calculated. This analysis revealed an even larger discrepancy, as the CD8+ TCRVβ7+ fraction represented only 2.1 × 106 ± 0.5 × 106 total cells in TCR-VxSV11 mice compared to 14.7× 106 ± 1.9 × 106 total cells in TCR-V mice (a sevenfold reduction in total cell numbers) (Fig. 4C, left panel). Additionally, triple staining of the CD8+ TCRVβ7+ T cells revealed that only 30.9% of these cells (0.6 × 106 ± 0.2 × 106 total cells) bind to the Db/epitope V tetramer in TCR-VxSV11 mice versus 98.1% (14.5 × 106 ± 1.9 × 106) in TCR-V mice (Fig. 4B [histograms] and 4C [right panel]). Thus, a large proportion of the CD8+ TCRVβ7+ T cells detected in TCR-VxSV11 mice either are not specific for epitope V, perhaps due to pairing with nontransgenic TCRα chains, or have significantly reduced levels of avidity for binding the Db/epitope V tetramer.
A twofold increase in the percentage of CD8− TCRVβ7+ T cells was observed in TCR-VxSV11 splenocytes versus TCR-V mice (Fig. 4B, left panels; Fig. 4C, left panel). Staining of this cell population with Db/epitope V tetramer revealed that approximately 68% of the cells (3.7 × 106 ± 1.2 × 106 total cells) in TCR-VxSV11 mice are specific for epitope V compared to 42% (1 ± 0.02 × 106 total cells) in TCR-V mice, indicating a fourfold increase in this population of splenocytes in TCR-VxSV11 mice (Fig. 4B [histogram]; Fig. 4C [right panel]). None of the CD8− cells that stained with Db/epitope V tetramer expressed CD4 (data not shown). Thus, development of epitope V TCR-transgenic T cells in SV11 mice results in a 20-fold reduction in the total number of epitope V-specific CD8+ T cells in the spleen (consistent with increased negative selection of high-avidity epitope V-specific T cells). There was a corresponding fourfold increase, however, in the total number of CD8−, epitope V-specific T cells that were detected in the spleens of TCR-VxSV11 mice, perhaps due to down regulation of CD8 expression in the thymus or periphery. Down regulation of CD8 on TCR-transgenic T cells has been observed previously on T cells which develop in the presence of self antigen and might represent a mechanism to reduce the avidity of self-reactive T cells during T-cell development (3, 66).
In addition to the finding of a decrease in the number of epitope V-specific CD8+ T cells detected in TCR-VxSV11 mice, CD8+ TCRVβ7+ spleen cells from TCR-VxSV11 mice stained less intensely (mean fluorescence, 398) with Db/epitope V tetramer than CD8+ TCRVβ7+ cells from TCR-V mice (mean fluorescence, 577) (Fig. 4B [histograms]). This represents a 31% decrease in staining intensity. Since decreased tetramer staining could be explained by a decrease in the TCR expression levels, the intensity of anti-TCRVβ7 staining was calculated for the data presented in Fig. 4B. This analysis revealed a 20% decrease in the expression level of TCRVβ7 on CD8+ T cells from TCR-VxSV11 mice versus that seen with TCR-V mice (mean fluorescence, 79 versus 93, respectively; data not shown). Thus, decreased tetramer staining is associated with a decrease in TCR expression by epitope V-specific CD8+ T cells from TCR-VxSV11 mice. Decreased TCR expression also has been associated with the survival of self-reactive TCR-transgenic T cells (48, 55). Interestingly, Db/epitope V tetramer staining levels were comparable on CD8− TCRVβ7+ spleen cells from TCR-V and TCR-VxSV11 mice (Fig. 4B, histograms); this corresponded to the finding of similar levels of TCRVβ7 expression (mean fluorescence, 79 and 73, respectively). This finding might be explained by studies that revealed that the binding of MHC tetramers to specific TCRs is reduced in the absence of CD8 expression (15).
The remaining epitope V-specific T cells detected in TCR-VxSV11 mice were capable of differentiating into effector CTL, as evidenced by the lytic activity of splenocyte cultures following 4 days of in vitro stimulation with T-Ag-transformed C57BL/6 cells. TCR-VxSV11-derived CTL lysed both epitope V peptide-pulsed target cells (Fig. 4D) and target cells expressing WT T-Ag or a T-Ag variant in which the dominant epitopes I, II/III, and IV were inactivated (V only). Target cells expressing a T-Ag variant that lacks epitope V (V loss) were not lysed. The efficiency of lysis by TCR-VxSV11 CTL was reduced on peptide V-pulsed and WT T-Ag-transformed target cells compared to that seen with CTL derived from TCR-V mice (consistent with reduced numbers of epitope V-specific T cells in the double-transgenic mice) (Fig. 4B and C). These results suggested that relatively few high-avidity TCR-transgenic T cells survived negative selection in SV11 mice. Those epitope V-specific T cells that survived apparently did so by down regulation of the TCR or CD8 molecule. These results obtained using TCR-V-transgenic mice support our findings that higher-avidity epitope V-specific T cells either are absent from the T-cell repertoire of SV11 mice or are present at greatly reduced levels.
In vivo expansion levels of residual epitope V-specific CD8+ T cells determined using a heterologous priming and boosting protocol.
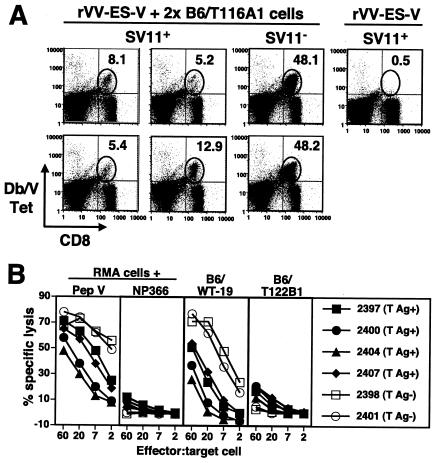
Mylin et al. have shown previously that epitope V-specific memory CD8+ T-cell frequency can be dramatically expanded in C57BL/6 mice by secondary immunization with B6/T116A1 cells, which express a T-Ag variant that lacks expression of the three dominant CTL epitopes I, II/III, and IV (40). Thus, we determined whether booster injections might expand the limited epitope V-specific CD8+ T-cell response detected in SV11 mice following primary immunization with rVV-ES-V. Groups of SV11 mice and their nontransgenic littermates at 50 days of age were immunized with rVV-ES-V followed by booster injections with live B6/T116A1 cells at 4 and 6 weeks postimmunization. Spleen cells were analyzed by ex vivo staining with MHC tetramers 7 days following the last injection (Fig. 5A). Epitope V-specific T cells were detected in SV11 mice at frequencies of between 5 and 13% of CD8+ T cells. This expansion was significant compared to that seen with mice which received only rVV-ES-V immunization 10 days prior to analysis; for these mice, Db/epitope V tetramer+ cells could not be detected above background levels (Fig. 5A, upper right panel). By contrast, epitope V-specific T cells represented 48% of CD8+ T cells in transgene-negative littermates (Fig. 5A, middle right column), confirming that recruitment of epitope V-specific CD8+ T cells in SV11 mice was compromised. CTL derived from SV11 mice administered booster injections, however, displayed significant lytic activity against both epitope V peptide-pulsed and T-Ag-transformed target cells, including WT T-Ag-transformed cells (compare Fig. 5B and Fig. 1). CTL activity detected in transgene-negative littermates was more efficient (Fig. 5B), in consistency with the increased numbers of epitope V-specific CD8+ T cells detected ex vivo (Fig. 5A, middle right column). These results suggest that increased numbers of epitope V-specific CD8+ T cells can be recruited in SV11 mice following a priming and boosting regimen.
FIG. 5.
The residual epitope V-specific CD8+ T cells in SV11 mice are expanded by a priming-boosting regimen of specific immunization. (A) SV11 mice (SV11+) or their transgene-negative littermates (SV11−) were immunized with 1 × 107 PFU rVV-ES-V at 50 days of age and then boosted at weeks 4 and 6 with 3 × 107 B6/T116A1 cells. Splenocytes were harvested 7 days following the last booster injection, stained ex vivo with anti-CD8 and Db/V tetramer, and analyzed by flow cytometry. Some mice received only primary immunization with rVV-ES-V 10 days prior to analysis (fourth column). The gated regions indicate the locations of CD8+ Db/V tetramer+ cells; the percentages of CD8+ T cells which stained positive with the Db/V tetramer are indicated. Each plot represents the results for an individual mouse. Background staining with the control Db/Flu NP 366-374 tetramer was 0.5% of CD8+ T cells. (B) The lytic response of splenocyte cultures (derived from the mice described for panel A) following 6 days of in vitro stimulation with γ-irradiated B6/WT-19 cells was determined in a 5-h 51Cr release assay using RMA target cells pulsed with 1 μM epitope V peptide (pep V) or control NP 366-374 peptide (NP366) or the indicated T-Ag-transformed cell lines. Each plotted line represents CTL derived from an individual SV11 (T-Ag+) or transgene-negative (T-Ag-) mouse.
CTL derived from SV11 mice by immunization and boosting against epitope V have a high avidity for epitope V.
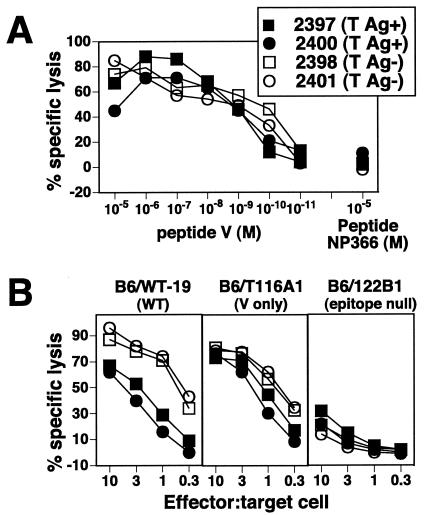
To determine whether the quantitative enhancement of the epitope V-specific CD8+ T-cell response in SV11 mice was also accompanied by increased quality, CTL lines were derived from both SV11 and transgene-negative mice by in vitro stimulation. The sensitivity of each CTL line was determined by lysis of B6/K-1,4,5 (T-Ag epitope-negative) target cells that had been pulsed with titrated amounts of the epitope V peptide (Fig. 6A). CTL lines derived from both SV11 and transgene-negative mice were highly sensitive to epitope V peptide, with no apparent difference in the levels of activity. This result is in contrast to the more than 100-fold difference in recognition levels observed for CTL lines derived from mice immunized with rVV-ES-V alone (Fig. 2A). Less-efficient lysis was observed using T-Ag-transformed target cells, with which 10-fold more SV11-derived CTL were required to lyse WT T-Ag-transformed B6/WT-19 cells compared to the required number of CTL derived from transgene-negative littermates (B6/WT-19) (Fig. 6B). This difference cannot be explained by a lower percentage of epitope V-specific T cells in the cultures, as all cells within each culture stained positively with anti-CD8 and Db/epitope V tetramer (data not shown).
FIG. 6.
CTL lines derived from SV11 mice given a priming-boosting regimen have increased avidity for epitope V. CTL lines (derived from the cultures described for Fig. 4 by 8 in vitro passages) were tested in a 5-h 51Cr release assay for their ability to lyse B6/K-1,4,5 (T-Ag epitope-negative) target cells that had been pulsed with titrated amounts of epitope V peptide (V) or the control peptide NP 366-374 (A) or T-Ag-transformed C57BL/6 cells (B). (A) CTL lines were used at an E:T of 10:1. M, molar. All CTL lines were derived from individual SV11 (T-Ag+) or transgene-negative (T-Ag−) mice.
Epitope V-specific CD8+ T cells derived from SV11 and transgene-negative mice that received a priming and boosting regimen use similar TCRβ chains.
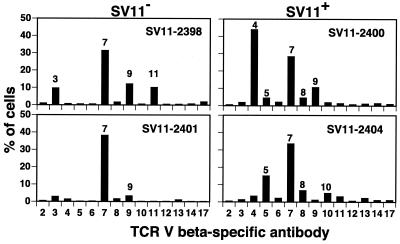
Since the priming and boosting approach resulted in both a quantitative and a qualitative increase in the responding epitope V-specific CD8+ T cells, we examined the TCR repertoire of epitope V-specific T cells which accumulate following booster injections. CTL lines from rVV-ES-V-immunized and B6/T116A1-boosted mice were stained with a panel of TCRVβ-specific antibodies. All cells were determined to be positive for CD8 and Db/epitope V tetramer staining (data not shown). Analysis of CTL derived from two different transgene-negative littermates revealed a polyclonal population predominantly expressing TCRVβ7, with lower numbers of T cells staining for TCRVβ3, TCRVβ9, and TCRVβ11 (Fig. 7, left panels). These results are similar to those of a previous study by Mylin et al. in which epitope V-specific CD8+ T cells derived from C57BL/6 mice immunized and boosted one time with B6/T116A1 (V only) cells predominantly expressed TCRVβ chains 2, 7, and 9 (40). In addition, two of three CTL lines derived from transgene-negative littermates following a single immunization with rVV-ES-V had predominantly TCRVβ7-expressing CD8+ T cells (Fig. 3). Analysis of CTL lines derived from SV11 mice given the priming and boosting regimen revealed prominent usage of TCRVβ7 by both lines tested (Fig. 7, right panels). Although CTL line 2400 predominantly (45%) utilized TCRVβ4, a significant proportion (28%) of the epitope V-specific T cells expressed TCRVβ7. Less-utilized receptors included TCRVβ5, TCRVβ8, TCRVβ9, and TCRVβ10. By contrast, only one of three CTL lines derived from SV11 mice that received just the initial immunization with rVV-ES-V contained TCRVβ7-expressing cells (Fig. 3, right panels). Thus, epitope V-specific CTL derived from both SV11 and transgene-negative mice following a specific priming and boosting regimen are polyclonal in nature, with prominent usage of TCRVβ7.
FIG. 7.
Epitope V-specific CTL lines derived from SV11 mice given a priming-boosting regimen are phenotypically similar to CTL lines derived from transgene-negative mice. CTL lines derived from the SV11 (SV11+) and transgene-negative (SV11−) mice described for Fig. 4 were stained with a panel of TCRVβ chain-specific antibodies; the percentages of cells that stained positive with each antibody are presented. All cells were Db/epitope V tetramer positive.
DISCUSSION
This study demonstrates that using an epitope-specific priming and boosting regimen for SV11 mice, a residual population of high-avidity, self-reactive, tumor antigen-specific CD8+ T cells can be significantly expanded. A limited population of CD8+ T-cell precursors specific for the immunorecessive epitope V of T-Ag survive negative selection in SV11 mice despite the fact that the CD8+ T cells specific for three immunodominant epitopes are undetectable (52). The epitope V-specific CD8+ T-cell repertoire of SV11 mice is limited by negative selection, as evidenced by the low numbers and activity of epitope V-specific CD8+ T cells in the primary immune response. These results suggest that while the majority of surviving epitope V-specific CD8+ T cells have a reduced avidity for epitope V, a residual population of higher-avidity epitope V-specific T cells can be expanded in vivo following specific boosting of the primary response.
We have shown previously that despite the loss of CTL specific for the immunodominant epitopes I, II/III, and IV, the CTL response to T-Ag epitope V persists in tumor-bearing line 501 T-Ag-transgenic mice (51). In contrast to the results presented in the present report, line 501 mice represent a model of peripheral tolerance, since T-Ag expression is not detected in 501 mice until they are approximately 3 months of age (36). However, this finding did provide a precedent, namely, that epitope V-specific CD8+ T cells are less susceptible to toleragenic mechanisms than T cells directed to the immunodominant T-Ag epitopes. Similar observations were made with TRAMP mice, which develop prostatic adenocarcinomas due to the expression of T-Ag as a transgene from the prostate-specific rat probasin promoter (22-24). Granziero et al. demonstrated that TRAMP mice were tolerant to the immunodominant CTL epitopes of T-Ag but retained responsiveness to a previously undefined subdominant epitope, T-391. The basis for the observed tolerance was unclear, since the original characterization of TRAMP mice indicated that T-Ag is not expressed in the thymus (22). A more recent study using an enhanced RT-PCR method, however, revealed low levels of T-Ag expression in the thymus of TRAMP mice (77). Grossman et al. (24) demonstrated that TRAMP mice develop T-Ag epitope V-specific, but not immunodominant epitope IV-specific, CTL following peptide immunization, although the quantitative and qualitative aspects of the CD8+ T-cell response to epitope V were not addressed. In the results obtained using several models of T-Ag-transgenic mice, therefore, epitope V-specific CD8+ T cells have been found to be less susceptible to tolerance than the CD8+ T cells specific for the immunodominant T-Ag epitopes. The present report indicates that only a subset of epitope V-specific T cells survive negative selection in the T-Ag-expressing thymus.
The survival of epitope V-specific CD8+ T cells in SV11 mice might be explained in part by the fact that epitope V forms relatively unstable complexes with H2-Db compared to the immunodominant T-Ag epitopes (20, 38). Thus, even if the T-Ag epitopes were generated at equivalent rates, fewer epitope V complexes would be expected to accumulate at the cell surface for presentation to developing T cells. This level may be below the critical threshold required for the deletion of all epitope V-specific precursor T cells in the thymus (2, 56). Low-affinity binding to MHC is often associated with subdominant epitopes (74) and may contribute to the immunorecessive phenotype of epitope V (20). This same property may allow the survival of some self-reactive T cells. In support of this idea, Sherman and colleagues demonstrated that T cells specific for the dominant p53 CD8+ T-cell epitope recognized in p53 knockout mice are deleted from WT mice (67). Consequently, the T-cell response to p53 in WT mice is focused on an epitope classified as subdominant in p53 knockout mice. Similar results have been shown for other self-tumor antigens (13, 26, 50) and for self-reactive CD4+ T cells (25). These results are consistent with the idea that in cases in which the antigen is expressed in the thymus, T cells specific for subdominant or cryptic epitopes are more likely to survive negative selection than T cells specific for dominant epitopes (57).
The endogenous repertoire of epitope V-specific CD8+ T cells in SV11 mice is limited in both quantity and quality. Previous studies which examined the characteristics of specific T cells derived from mice expressing transgenic antigens have shown that these T cells had reduced avidity compared to T cells derived from mice that lacked expression of the same antigens (10, 17, 34, 37, 45, 46, 56, 59, 71, 75). In addition, a number of recent studies have characterized T cells specific for tissue antigens that have low avidity for their target epitopes (8, 26, 49, 64, 67). In several cases, these peripheral T cells were unable to recognize the level of antigen presented in the peripheral tissues, maintaining a state of ignorance (8, 26, 49, 64). We also failed to detect spontaneous priming of epitope V-specific CD8+ T cells with the endogenous T-Ag in SV11 mice (data not shown), suggesting that epitope V-specific T cells maintain a state of ignorance of epitope V unless primed by exogenous immunization. These residual epitope V-specific T cells appear to be resistant to the effects of peripheral tolerance, as preliminary evidence indicates that SV11 mice remain responsive to immunization with rVV-ES-V even at 3 months of age, when significant tumor mass has accumulated (data not shown).
CD8+ T cells specific for the immunodominant T-Ag epitopes I, II/III, and IV are undetectable in SV11 mice (52), suggesting that the dominant T-Ag epitopes are expressed at significant levels in the thymus compared to epitope V. Of note, a similar priming and boosting approach was unable to recruit any residual CD8+ T cells specific for the immunodominant epitopes (data not shown). Alternatively, the thymic precursor T-cell repertoire might be devoid of lower-avidity TCRs specific for the immunodominant T-Ag epitopes, precluding the survival of epitope I-, II/III-, and IV-specific CD8+ T cells in SV11 mice. The finding that some TCR-V-transgenic CD8+ T cells survived negative selection in SV11xTCR-V mice supports the suggestion that negative selection of epitope V-specific T cells is inefficient or incomplete in SV11 mice. Survival of TCR-V T cells in SV11 mice, however, was associated with the loss of CD8 expression and the down regulation of the transgenic TCR. In contrast, Zheng et al. (77) demonstrated that TCR-transgenic T cells specific for the immunodominant H2-Kk-restricted T-Ag epitope are completely deleted from the thymus of TRAMP mice during T-cell development. Although results using these two T-Ag-transgenic mouse lines cannot be directly compared, it is tempting to speculate that similar mechanisms are involved in selection of their T-cell repertoires. Future studies will address the extent to which negative selection in SV11 mice can affect the development of TCR-transgenic T cells specific for the H2b-restricted immunodominant T-Ag epitopes.
Although several TCRVβ chains were shown to be utilized by epitope V-specific CTL lines, TCRVβ7-expressing cells represented a prominent population among epitope V-specific CTL derived from both singly primed or primed-boosted C57BL/6 mice (reference 40 and this study). TCRVβ7-expressing cells also were prominent among CTL lines derived from SV11 mice given the priming-boosting regimen specific for epitope V. In the absence of boosting the epitope V-specific T-cell response in vivo, only one of three CTL lines derived from SV11 mice contained TCRVβ7-expressing T cells. Thus, in healthy mice the TCRVβ7+ population might represent high-avidity epitope V-specific CD8+ T cells that predominate over lower avidity clones in vivo. In SV11 mice, however, a lack of significant numbers of epitope V-specific T cells bearing high-avidity TCRs might allow the initial expansion of lower-avidity clones. Previous studies which characterized the T-cell repertoire that expands from the memory T-cell pool following antigenic challenge revealed preferential expansion of higher-avidity CTL that resulted in avidity maturation of the T-cell population (9, 30, 60). This might explain the finding that subsequent boosting of SV11 mice resulted in the expansion of TCRVβ7-expressing epitope V-specific CD8+ T cells. Although the avidity of the TCRVβ7+ epitope V-specific T cells was not directly determined in the present study, their appearance in SV11 mice was associated with the detection of higher-avidity epitope V-specific CTL.
The quality of epitope V-specific CTL obtained in SV11 mice after a single immunization with rVV-ES-V is reminiscent of that of some CTL derived from human cancer patients. In these studies, CTL derived from peripheral blood lymphocytes or tumor-infiltrating lymphocytes by in vitro stimulation with autologous tumor cells or specific peptides had a relatively low avidity for their target epitopes compared to the results seen with high-avidity CTL specific for foreign antigens (11, 21, 72). Higher-avidity CD8+ T cells specific for tumor-associated antigens have recently been identified after in vitro growth in the presence of low concentrations of peptide or peptide-pulsed dendritic cells or by sorting MHC tetramer-stained T cells (18, 70, 73, 76), indicating that low numbers of higher-avidity T cells that are present in cancer patients can be expanded under the appropriate conditions. Defining approaches for the in vivo expansion of these residual high-avidity T cells will represent a major advance for tumor immunotherapy. The similarities observed between the results of studies of human cancer patients and the results presented here suggest that SV11 mice provide a realistic model for the assessment of approaches for the recruitment of residual high-avidity tumor-specific T cells in the tumor-bearing host.
Acknowledgments
This work was supported by a research grant from the Four Diamonds Foundation (T.D.S.) and research grant CA25000 from the National Cancer Institute, National Institutes of Health (to Satvir S. Tevethia).
I thank Melanie Epler, Andrew Gaydos, and Stacy Keller for quality technical assistance, Lawrence Mylin for helpful suggestions, and Satvir Tevethia for support, discussions, and critical review of the manuscript.
REFERENCES
- 1.Alam, S. M., P. J. Travers, J. L. Wung, W. Nasholds, S. Redpath, S. C. Jameson, and N. R. Gascoigne. 1996. T-cell-receptor affinity and thymocyte positive selection. Nature 381:616-620. [DOI] [PubMed] [Google Scholar]
- 2.Ashton-Rickardt, P. G., A. Bandeira, J. R. Delaney, L. Van Kaer, H. P. Pircher, R. M. Zinkernagel, and S. Tonegawa. 1994. Evidence for a differential avidity model of T cell selection in the thymus. Cell 76:651-663. [DOI] [PubMed] [Google Scholar]
- 3.Auphan, N., G. Schonrich, M. Malissen, M. Barad, G. Hammerling, B. Arnold, B. Malissen, and A. M. Schmitt-Verhulst. 1992. Influence of antigen density on degree of clonal deletion in T cell receptor transgenic mice. Int. Immunol. 4:541-547. [DOI] [PubMed] [Google Scholar]
- 4.Blaney, J. E., E. Nobusawa, M. A. Brehm, R. H. Bonneau, L. M. Mylin, T.-M. Fu, Y. Kawaoka, and S. S. Tevethia. 1998. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72:9567-9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonneau, R. H., L. H. Salvucci, D. C. Johnson, and S. S. Tevethia. 1993. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology 195:62-70. [DOI] [PubMed] [Google Scholar]
- 6.Boon, T., J.-C. Cerottini, B. Van den Eynde, P. van der Bruggen, and A. Van Pel. 1994. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 12:337-365. [DOI] [PubMed] [Google Scholar]
- 7.Boon, T., and P. van der Bruggen. 1996. Human tumor antigens recognized by T lymphocytes. J. Exp. Med. 183:725-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouneaud, C., P. Kourilsky, and P. Bousso. 2000. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity 13:829-840. [DOI] [PubMed] [Google Scholar]
- 9.Busch, D. H., and E. G. Pamer. 1999. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189:701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabaniols, J. P., R. Cibotti, P. Kourilsky, K. Kosmatopoulos, and J. M. Kanellopoulos. 1994. Dose-dependent T cell tolerance to an immunodominant self peptide. Eur. J. Immunol. 24:1743-1749. [DOI] [PubMed] [Google Scholar]
- 11.Celis, E., V. Tsai, C. Crimi, R. DeMars, P. A. Wentworth, R. W. Chesnut, H. M. Grey, A. Sette, and H. M. Serra. 1994. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc. Natl. Acad. Sci. USA 91:2105-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerundolo, V., T. Elliot, J. Elvin, J. Bastin, H.-G. Rammensee, and A. Townsend. 1991. The binding affinity and dissociation rates of peptides for class I major histocompatibility complex molecules. Eur. J. Immunol. 21:2069-2075. [DOI] [PubMed] [Google Scholar]
- 13.Colella, T. A., T. N. Bullock, L. B. Russell, D. W. Mullins, W. W. Overwijk, C. J. Luckey, R. A. Pierce, N. P. Restifo, and V. H. Engelhard. 2000. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J. Exp. Med. 191:1221-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford, F., H. Kozono, J. White, P. Marrack, and J. Kappler. 1998. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity 8:675-682. [DOI] [PubMed] [Google Scholar]
- 15.Daniels, M. A., and S. C. Jameson. 2000. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J. Exp. Med. 191:335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deckhut, A. M., J. D. Lippolis, and S. S. Tevethia. 1992. Comparative analysis of core amino acid residues of H-2Db-restricted cytotoxic T-lymphocyte recognition epitopes in simian virus 40 T antigen. J. Virol. 66:440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Visser, K. E., T. A. Cordaro, D. Kioussis, J. B. Haanen, T. N. Schumacher, and A. M. Kruisbeek. 2000. Tracing and characterization of the low-avidity self-specific T cell repertoire. Eur. J. Immunol. 30:1458-1468. [DOI] [PubMed] [Google Scholar]
- 18.Dunbar, P. R., J. L. Chen, D. Chao, N. Rust, H. Teisserenc, G. S. Ogg, P. Romero, P. Weynants, and V. Cerundolo. 1999. Cutting edge: rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J. Immunol. 162:6959-6962. [PubMed] [Google Scholar]
- 19.Dutoit, V., V. Rubio-Godoy, M. A. Doucey, P. Batard, D. Lienard, D. Rimoldi, D. Speiser, P. Guillaume, J. C. Cerottini, P. Romero, and D. Valmori. 2002. Functional avidity of tumor antigen-specific CTL recognition directly correlates with the stability of MHC/peptide multimer binding to TCR. J. Immunol. 168:1167-1171. [DOI] [PubMed] [Google Scholar]
- 20.Fu, T.-M., L. M. Mylin, T. D. Schell, I. Bacik, G. Russ, J. W. Yewdell, J. R. Bennink, and S. S. Tevethia. 1998. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J. Virol. 72:1469-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gervois, N., Y. Guilloux, E. Diez, and F. Jotereau. 1996. Suboptimal activation of melanoma infiltrating lymphocytes (TIL) due to low avidity of TCR/MHC-tumor peptide interactions. J. Exp. Med. 183:2403-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granziero, L., S. Krajewski, P. Farness, L. Yuan, M. K. Courtney, M. R. Jackson, P. A. Peterson, and A. Vitiello. 1999. Adoptive immunotherapy prevents prostate cancer in a transgenic animal model. Eur. J. Immunol. 29:1127-1138. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg, N. M., F. DeMayo, M. J. Finegold, D. Medina, W. D. Tilley, J. O. Aspinall, G. R. Cunha, A. A. Donjacour, R. J. Matusik, and J. M. Rosen. 1995. Prostate cancer in a transgenic mouse. Proc. Natl. Acad. Sci. USA 92:3439-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossmann, M. E., T. Davila, and E. Celis. 2001. Avoiding tolerance against prostatic antigens with subdominant peptide epitopes. J. Immunother. 24:237-241. [PubMed] [Google Scholar]
- 25.Harrington, C. J., A. Paez, T. Hunkapiller, V. Mannikko, T. Brabb, M. Ahearn, C. Beeson, and J. Goverman. 1998. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity 8:571-580. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez, J., F. Garcia-Pons, Y. C. Lone, H. Firat, J. D. Schmidt, P. Langlade-Demoyen, and M. Zanetti. 2002. Identification of a human telomerase reverse transcriptase peptide of low affinity for HLA A2.1 that induces cytotoxic T lymphocytes and mediates lysis of tumor cells. Proc. Natl. Acad. Sci. USA 99:12275-12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell 76:17-27. [DOI] [PubMed] [Google Scholar]
- 28.Jennings, S. R., K. L. Fresa, P. A. Lippe, J. E. Milici, and S. S. Tevethia. 1988. Frequency analysis of simian virus 40-specific cytotoxic T lymphocyte precursors in the high responder C57BL/6 mouse strain. J. Gen. Virol. 69:2493-2503. [DOI] [PubMed] [Google Scholar]
- 29.Kappler, J. W., N. Roehm, and P. Marrack. 1987. T cell tolerance by clonal elimination in the thymus. Cell 49:273-280. [DOI] [PubMed] [Google Scholar]
- 30.Kedl, R. M., W. A. Rees, D. A. Hildeman, B. Schaefer, T. Mitchell, J. Kappler, and P. Marrack. 2000. T cells compete for access to antigen-bearing antigen-presenting cells. J. Exp. Med. 192:1105-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kisielow, P., H. Blüthmann, U. D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333:742-746. [DOI] [PubMed] [Google Scholar]
- 32.Lill, N. L., M. J. Tevethia, W. G. Hendrickson, and S. S. Tevethia. 1992. Cytotoxic T lymphocytes (CTL) against a transforming gene product select for transformed cells with point mutations within sequences encoding CTL recognition epitopes. J. Exp. Med. 176:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippolis, J. D., L. M. Mylin, D. T. Simmons, and S. S. Tevethia. 1995. Functional analysis of amino acid residues encompassing and surrounding two neighboring H-2Db-restricted cytotoxic T lymphocyte epitopes in simian virus 40 tumor antigen. J. Virol. 69:3134-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, G. Y., P. J. Fairchild, R. M. Smith, J. R. Prowle, D. Kioussis, and D. C. Wraith. 1995. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 3:407-415. [DOI] [PubMed] [Google Scholar]
- 35.Ljunggren, H.-G., and K. Kärre. 1985. Host resistance directed selectively against H-2-deficient lymphoma variants. J. Exp. Med. 162:1745-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marton, I., S. E. Johnson, I. Damjanov, K. S. Currier, J. P. Sundberg, and B. B. Knowles. 2000. Expression and immune recognition of SV40 Tag in transgenic mice that develop metastatic osteosarcomas. Transgenic Res. 9:115-125. [DOI] [PubMed] [Google Scholar]
- 37.Morgan, D. J., H. T. Kreuwel, S. Fleck, H. I. Levitsky, D. M. Pardoll, and L. A. Sherman. 1998. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J. Immunol. 160:643-651. [PubMed] [Google Scholar]
- 38.Mylin, L. M., R. H. Bonneau, J. D. Lippolis, and S. S. Tevethia. 1995. Hierarchy among multiple H-2b-restricted cytotoxic T lymphocyte epitopes within simian virus 40 T antigen. J. Virol. 69:6665-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mylin, L. M., A. M. Deckhut, R. H. Bonneau, T. D. Kierstead, M. J. Tevethia, D. T. Simmons, and S. S. Tevethia. 1995. Cytotoxic T lymphocyte escape variants, induced mutations, and synthetic peptides define a dominant H-2Kb-restricted determinant in simian virus 40 tumor antigen. Virology 208:159-172. [DOI] [PubMed] [Google Scholar]
- 40.Mylin, L. M., T. D. Schell, D. Roberts, M. Epler, A. Boesteanu, E. J. Collins, J. A. Frelinger, S. Joyce, and S. S. Tevethia. 2000. Quantitation of CD8+ T-lymphocyte responses to multiple epitopes from simian virus 40 (SV40) large T antigen in C57BL/6 mice immunized with SV40, SV40 T-antigen-transformed cells, or vaccinia virus recombinants expressing full-length T antigen or epitope minigenes. J. Virol. 74:6922-6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oehen, S. U., P. S. Ohashi, K. Burki, H. Hengartner, R. M. Zinkernagel, and P. Aichele. 1994. Escape of thymocytes and mature T cells from clonal deletion due to limiting tolerogen expression levels. Cell. Immunol. 158:342-352. [DOI] [PubMed] [Google Scholar]
- 42.Palmiter, R. D., H. Y. Chen, A. Messing, and R. L. Brinster. 1985. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumors in transgenic mice. Nature 316:457-460. [DOI] [PubMed] [Google Scholar]
- 43.Pamer, E., and P. Cresswell. 1998. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 16:323-358. [DOI] [PubMed] [Google Scholar]
- 44.Pardoll, D. M. 1999. Inducing autoimmune disease to treat cancer. Proc. Natl. Acad. Sci. USA 96:5340-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson, D. A., R. J. DiPaolo, O. Kanagawa, and E. R. Unanue. 1999. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity 11:453-462. [DOI] [PubMed] [Google Scholar]
- 46.Poindexter, N. J., C. Landon, P. J. Whiteley, and J. A. Kapp. 1992. Comparison of the T cell receptors on insulin-specific hybridomas from insulin transgenic and nontransgenic mice. Loss of a subpopulation of self-reactive clones. J. Immunol. 149:38-44. [PubMed] [Google Scholar]
- 47.Pretell, J., R. S. Greenfield, and S. S. Tevethia. 1979. Biology of simian virus 40 (SV40) transplantation rejection antigen (TrAg). In vitro demonstration of SV40 TrAg in SV40 infected non-permissive mouse cells by the lymphocyte-mediated cytotoxicity assay. Virology 97:32-41. [DOI] [PubMed] [Google Scholar]
- 48.Rocha, B., and H. von Boehmer. 1991. Peripheral selection of the T cell repertoire. Science 251:1225-1228. [DOI] [PubMed] [Google Scholar]
- 49.Sandberg, J. K., L. Franksson, J. Sundback, J. Michaelsson, M. Petersson, A. Achour, R. P. Wallin, N. E. Sherman, T. Bergman, H. Jornvall, D. F. Hunt, R. Kiessling, and K. Karre. 2000. T cell tolerance based on avidity thresholds rather than complete deletion allows maintenance of maximal repertoire diversity. J. Immunol. 165:25-33. [DOI] [PubMed] [Google Scholar]
- 50.Scardino, A., D. A. Gross, P. Alves, J. L. Schultze, S. Graff-Dubois, O. Faure, S. Tourdot, S. Chouaib, L. M. Nadler, F. A. Lemonnier, R. H. Vonderheide, A. A. Cardoso, and K. Kosmatopoulos. 2002. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J. Immunol. 168:5900-5906. [DOI] [PubMed] [Google Scholar]
- 51.Schell, T. D., B. B. Knowles, and S. S. Tevethia. 2000. Sequential loss of cytotoxic T lymphocyte responses to simian virus 40 large T antigen epitopes in T antigen transgenic mice developing osteosarcomas. Cancer Res. 60:3002-3012. [PubMed] [Google Scholar]
- 52.Schell, T. D., L. M. Mylin, I. Georgoff, A. K. Teresky, A. J. Levine, and S. S. Tevethia. 1999. Cytotoxic T-lymphocyte epitope immunodominance in the control of choroid plexus tumors in simian virus 40 large T antigen transgenic mice. J. Virol. 73:5981-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schell, T. D., and S. S. Tevethia. 2001. Control of advanced choroid plexus tumors in SV40 T antigen transgenic mice following priming of donor CD8+ T lymphocytes by the endogenous tumor antigen. J. Immunol. 167:6947-6956. [DOI] [PubMed] [Google Scholar]
- 54.Schoenberger, S. P., and E. E. Sercarz. 1996. Harnessing self-reactivity in cancer immunotherapy. Semin. Immunol. 8:303-309. [DOI] [PubMed] [Google Scholar]
- 55.Schonrich, G., U. Kalinke, F. Momburg, M. Malissen, A. M. Schmitt-Verhulst, B. Malissen, G. J. Hammerling, and B. Arnold. 1991. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell 65:293-304. [DOI] [PubMed] [Google Scholar]
- 56.Sebzda, E., V. A. Wallace, J. Mayer, R. S. Yeung, T. W. Mak, and P. S. Ohashi. 1994. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science 263:1615-1618. [DOI] [PubMed] [Google Scholar]
- 57.Sercarz, E. E., P. V. Lehmann, A. Ametani, G. Benichou, A. Miller, and K. Moudgil. 1993. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 11:729-766. [DOI] [PubMed] [Google Scholar]
- 58.Shastri, N., S. Schwab, and T. Serwold. 2002. Producing nature's gene-chips: the generation of peptides for display by MHC class I molecules. Annu. Rev. Immunol. 20:463-493. [DOI] [PubMed] [Google Scholar]
- 59.Slifka, M. K., J. N. Blattman, D. J. Sourdive, F. Liu, D. L. Huffman, T. Wolfe, A. Hughes, M. B. Oldstone, R. Ahmed, and M. G. Von Herrath. 2003. Preferential escape of subdominant CD8+ T cells during negative selection results in an altered antiviral T cell hierarchy. J. Immunol. 170:1231-1239. [DOI] [PubMed] [Google Scholar]
- 60.Slifka, M. K., and J. L. Whitton. 2001. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat. Immunol. 2:711-717. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka, Y., R. W. Anderson, W. L. Maloy, and S. S. Tevethia. 1989. Localization of an immunorecessive epitope on SV40 T antigen by H-2Db-restricted cytotoxic T-lymphocyte clones and a synthetic peptide. Virology 171:205-213. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka, Y., M. J. Tevethia, D. Kalderon, A. E. Smith, and S. S. Tevethia. 1988. Clustering of antigenic sites recognized by cytotoxic T lymphocyte clones in the amino terminal half of SV40 T antigen. Virology 162:427-436. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka, Y., and S. S. Tevethia. 1990. Loss of immunorecessive cytotoxic T lymphocyte determinant V on SV40 T antigen following cocultivation with site-specific cytotoxic T lymphocyte clone Y-5. Intervirology 31:197-202. [DOI] [PubMed] [Google Scholar]
- 64.Targoni, O. S., and P. V. Lehmann. 1998. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J. Exp. Med. 187:2055-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taswell, C. 1981. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 126:1614-1619. [PubMed] [Google Scholar]
- 66.Teh, H. S., H. Kishi, B. Scott, and H. Von Boehmer. 1989. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J. Exp. Med. 169:795-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theobald, M., J. Biggs, J. Hernandez, J. Lustgarten, C. Labadie, and L. A. Sherman. 1997. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J. Exp. Med. 185:833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Dyke, T., C. Finlay, and A. J. Levine. 1985. A comparison of several lines of transgenic mice containing the SV40 early genes. Cold Spring Harbor Symp. Quant. Biol. 50:671-678. [DOI] [PubMed] [Google Scholar]
- 69.Van Dyke, T. A., C. Finlay, D. Miller, J. Marks, G. Lozano, and A. J. Levine. 1987. Relationship between simian virus 40 large tumor antigen expression and tumor formation in transgenic mice. J. Virol. 61:2029-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Elsas, A., S. H. van der Burg, C. E. van der Minne, M. Borghi, J. S. Mourer, C. J. Melief, and P. I. Schrier. 1996. Peptide-pulsed dendritic cells induce tumoricidal cytotoxic T lymphocytes from healthy donors against stably HLA-A*0201-binding peptides from the Melan-A/MART-1 self antigen. Eur. J. Immunol. 26:1683-1689. [DOI] [PubMed] [Google Scholar]
- 71.von Herrath, M. G., J. Dockter, and M. B. A. Oldstone. 1994. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity 1:231-242. [DOI] [PubMed] [Google Scholar]
- 72.Wolfel, T., A. Van pel, V. Brichard, J. Schneider, B. Seliger, M. zum Buschenfelde, and T. Boon. 1994. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur. J. Immunol. 24:759-764. [DOI] [PubMed] [Google Scholar]
- 73.Yee, C., P. A. Savage, P. P. Lee, M. M. Davis, and P. D. Greenberg. 1999. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 162:2227-2234. [PubMed] [Google Scholar]
- 74.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]
- 75.Yule, T. D., A. Basten, and P. M. Allen. 1993. Hen egg-white lysozyme-specific T cells elicited in hen egg-white lysozyme-transgenic mice retain an imprint of self-tolerance. J. Immunol. 151:3057-3069. [PubMed] [Google Scholar]
- 76.Zeh, H. J., III, D. Perry-Lalley, M. E. Dudley, S. A. Rosenberg, and J. C. Yang. 1999. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 162:989-994. [PubMed] [Google Scholar]
- 77.Zheng, X., J. X. Gao, H. Zhang, T. L. Geiger, Y. Liu, and P. Zheng. 2002. Clonal deletion of simian virus 40 large T antigen-specific T cells in the transgenic adenocarcinoma of mouse prostate mice: an important role for clonal deletion in shaping the repertoire of T cells specific for antigens overexpressed in solid tumors. J. Immunol. 169:4761-4769. [DOI] [PubMed] [Google Scholar]