Graphical abstract
Keywords: Malaria transmission, Transgenic parasite, Translational repression, Crystalloid
Highlights
-
•
We have GFP-tagged the LCCL proteins PbLAP4, PbLAP5 and PbLAP6 in Plasmodium berghei.
-
•
PbLAP4, PbLAP5 and PbLAP6 associate with the crystalloid organelle in ookinetes.
-
•
Translational repression controls expression of the LCCL protein repertoire in gametocytes.
Abstract
Plasmodium LCCL proteins comprise a family of six proteins that function as a protein complex and have essential roles in sporozoite transmission. In Plasmodium berghei, family members PbLAP1, PbLAP2 and PbLAP3 have been shown to be expressed in gametocytes and, following gametogenesis and fertilization, to be targeted to distinctive multivesicular organelles termed crystalloids that form in the ookinete. Here, we show by GFP-tagging that PbLAP4, PbLAP5 and PbLAP6, like their family members, are associated with the crystalloids. However, in contrast to their family members, protein expression of PbLAP4, PbLAP5 and PbLAP6 was not detected in gametocytes, even though transcription of the corresponding genes is most prominent in the sexual blood stage parasites. These results suggest that translational repression controls expression of the LCCL protein repertoire and, consequently, the temporal function of the protein complex during P. berghei development in the mosquito.
Crystalloids are unique parasite structures implicated in malaria transmission by virtue of their restricted presence in ookinetes and young oocyst stages that develop in the mosquito vector (reviewed in [1]). Crystalloids appear in transmission electron microscopy (TEM) as large clusters of small spherical particles. Plasmodium falciparum crystalloids are virtually indistinguishable from those of P. berghei and high resolution electron micrographs show their subunit particles to be individually bound by a lipid bilayer [2]. Hence, Plasmodium crystalloids appear to be multivesicular organelles rather than particulate cytoplasmic inclusions. To date, three parasite proteins have been found associated with the crystalloids: PbLAP1 (also known as PbSR), PbLAP2 and PbLAP3, all members of a gametocyte-expressed family of LCCL-lectin adhesive-like domain proteins (LAPs) otherwise known as Plasmodium LCCL proteins or PCCp proteins [3–7]. This link is based not only on the presence of these three PbLAP family members in the crystalloids, but also by the failure of PbLAP1 knockout and deletion mutants to form crystalloids [4,5]. The LAP family is composed of six highly conserved and structurally related proteins with a modular architecture consisting of multiple domains implicated in protein, lipid and carbohydrate binding. Family members are both typified by, and named after, the LCCL domain that was initially identified in the horse shoe crab Limulus clotting factor C, the cochlear protein Coch-5b2, and the late gestation lung protein Lgl1 [8]. The LCCL domain is present either in single or multiple copies in all but one family member. LCCL proteins also possess a canonical amino-terminal endoplasmic reticulum (ER) signal peptide and are translocated into the ER [4].
With the exception of PbLAP3, all PbLAP family members have been studied by targeted gene disruption and show very similar loss-of-function phenotypes epitomized by a failure to form sporozoites in the oocysts (Table 1). This is not a foolproof genetic block as PbLAP knockout parasites occasionally produce sporulating oocysts, however sporozoite transmission has not been achieved with any of the PbLAP null mutant lines [4,6,9,10]. The apparent oocyst/sporozoite-associated function of the LCCL proteins is poorly compatible with their reported expression in gametocytes as these life cycle stages are several days and developmental transitions apart, and it has been proposed that the crystalloids provide a protein trafficking mechanism to deliver the LCCL proteins, and possibly other molecules, from the gametocyte to the oocyst [4,11]. The gametocyte-specific expression profiles of all the PbLAPs as ascertained by fluorescent protein tagging, GFP reporter studies and RT-PCR, combined with their very similar loss-of-function phenotypes, points to a functional co-dependence and indicates that these molecules are involved in a similar cell biological process and operate in concert, probably as a protein complex [1]. This is further supported by observations that simultaneous knockout of two family members gives the same phenotype as the single knockouts, showing a lack of functional redundancy [12]. Indeed, molecular interactions between LCCL protein family members have been shown in P. falciparum using co-immunoprecipitation experiments [13]. Moreover, a mechanism promoting interaction between family members based on conformational interdependence was recently reported [14].
Table 1.
Loss-of-function phenotypes of five LCCL protein family members of Plasmodium berghei in Anopheles stephensi mosquitoes.
| PBANKA_000000a | 103520 | 130070 | 131950 | 131530 | 041760 |
| Name of gene product | PbLAP1 | PbLAP2 | PbLAP4 | PbLAP5 | PbLAP6 |
| Alternative name(s) | PbCCp3 PbSR | PbCCp1 | PbCCp2 | PbPNFA | PbCCp4 |
| References | [4,6] | [10] | [10] | [9] | [10] |
| Crystalloid formation | Absent | n/ab | n/a | n/a | n/a |
| Gametogenesis | Normal | ||||
| Ookinete development | Normal | ||||
| Oocyst transition | Normal | ||||
| Sporogenesisc | Highly reduced | ||||
| Transmission | Not achieved | ||||
Loss-of-function phenotype of PBANKA_020450 (PbLAP3) has not been published.
Not assessed.
Proportion of sporulating oocysts, rather than the level of sporulation per oocyst.
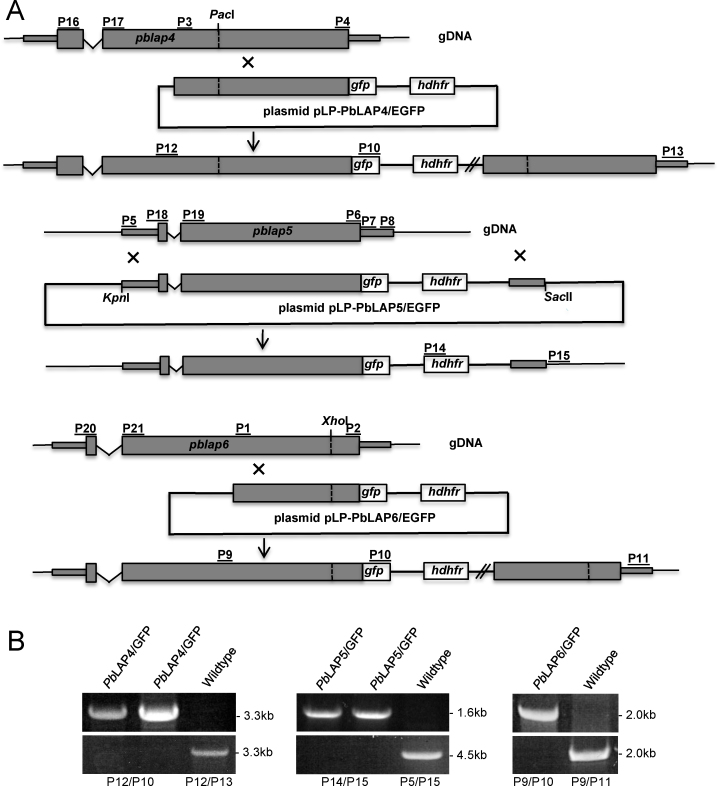
In this study we investigated whether the LCCL proteins PbLAP4 (PBANKA_131950), PbLAP5 (PBANKA_131530) and PbLAP6 (PBANKA_041760) displayed similar expression profiles as their family members, as well as an association with the crystalloids. Our strategy to investigate this was to generate genetically modified parasite lines in which the native proteins were fused at their carboxy-terminus with enhanced GFP, a strategy used successfully for PbLAP1, PbLAP2 and PbLAP3 [4,5]. This allows PbLAP::GFP fusions to be expressed from the endogenous promoter, but with a 3′UTR derived from the pbdhfr gene [4,5]. To achieve GFP-tagging of PbLAP6 we adopted a strategy of single crossover homologous recombination. A ca. 1.9 kb fragment of pblap6 corresponding to the 3′-part of the coding sequence was PCR amplified from genomic DNA with primers P1 (ACGAAGTTATCAGTCGACAGCCCCAGTTCAGACATAAAC) and P2 (ATGAGGGCCCCTAAGCTTTCTTTATGAGGAATAAATAAAATGTTTTTAAAC) (Fig. 1A) and introduced into SalI/HindIII-digested pDNR-EGFP [4] via in-fusion cloning (Takara Biotech) to give plasmid pDNR-PbLAP6/EGFP. The pblap6/egfp specific sequence was then transferred to pLP-hDHFR [5] via cre-loxp recombination to give plasmid pLP-PbLAP6/EGFP (Fig. 1A). The same strategy was used to tag PbLAP4 at its carboxy terminus. A ca. 3 kb fragment of pblap4 corresponding to the 3′-part of the coding sequence was PCR amplified from genomic DNA with primers P3 (ACGAAGTTATCAGTCGACAAGATGTCGAAAATATTTGTGCAT) and P4 (ATGAGGGCCCCTAAGCTTTCACATTCTGATATACACTGATTTATCA) and introduced into SalI/HindIII-digested pLP-PbLAP6/EGFP to give pLP-PbLAP4/EGFP (Fig. 1A). To achieve GFP-tagging of PbLAP5 we used a strategy of double crossover homologous recombination. The entire pblap5 coding sequence plus ca. 0.6 kb of upstream sequence was PCR amplified from genomic DNA with primers P5 (ACGAAGTTATCAGTCGAAGCTTCATACTGTTATATATTGCACATATAGCC) and P6 (ATGAGGGCCCCTAAGCTATTGTGGAGAAATATAATTTGTATAGATTG) (Fig. 1A) and cloned into SalI/HindIII-digested pDNR-EGFP to give plasmid pDNR-PbLAP5/EGFP. The 3′UTR of pblap5 was amplified with primers P7 (CCTTCAATTTCGACATAGAGGCATTTGACAAACAAAC) and P8 (GCGGCCGCTCTAGCATAATGTTTTATTTTTTCCATTTTCAGC) (Fig. 1A) and the resulting ca. 0.7 kb fragment cloned into NdeI-digested pLP-hDHFR by in-fusion cloning to give plasmid pLP-hDHFR/PbLAP5. The pblap5/egfp-specific sequence from pDNR-PbLAP5/EGFP was transferred to pLP-hDHFR/PbLAP5 by cre/loxp recombination to give the final construct pLP-PbLAP5/EGFP (Fig. 1A). Plasmid pLP-PbLAP5/EGFP was linearized with HindIII and SacII to remove the vector backbone prior to transfection, whereas plasmids pLP-PbLAP6/EGFP and pLP-PbLAP4/EGFP were linearized with XhoI and PacI, respectively. After transfection pyrimethamine-resistant parasites were selected and cloned, as described [15], to give parasite lines PbLAP4/GFP, PbLAP5/GFP and PbLAP6/GFP, respectively.
Fig. 1.
Generation and molecular analysis of genetically modified pblap parasite lines. (A) Targeting strategy for the GFP tagging of PbLAP4, PbLAP5 and PbLAP6 via crossover homologous recombination. The pblap genes are indicated with coding sequence (wide bars) and untranslated regions (narrow bars). Also indicated are the enhanced GFP module (gfp); the hDHFR selectable marker gene cassette (hdhfr); introns (v-shaped line); key restriction sites (PacI, XhoI, KpnI, SacII); and primers used for PCR amplification (P1-P21). (B) PCR diagnostic for the presence of modified GFP-tagged pblap alleles (top panels) and the absence of wildtype pblap alleles (bottom panels) from clonal parasite populations of PbLAP4/GFP (left panel), PbLAP5/GFP (middle panel) and PbLAP6/GFP (right panel). Wildtype parasites are included as negative and positive controls, respectively. Approximate sizes (in kb) of PCR products are indicated.
Diagnostic PCR on genomic DNA extracted from clonal lines of the transgenic parasites was used to confirm the correct integration of the modified alleles into their target loci, as well as the absence of the wildtype alleles. Primers P9 (CACAATTGGTATAACACCG) and P10 (GTGCCCATTAACATCACC) (Fig. 1A) amplified a unique ca. 2.0 kb fragment from parasite line PbLAP6/GFP (Fig. 1B), confirming correct integration of the gfp sequence downstream of the pblap6 allele. Conversely, primers P9 and P11 (CCTTTTATATTTTGTACCCATTTAATCG) (Fig. 1A) amplified a predicted fragment of ca. 2.0 kb from WT parasites, but not from PbLAP6/GFP parasites (Fig. 1B), demonstrating the absence of the wildtype pblap6 allele in the transgenic lines. Similarly, diagnostic PCR using primers P12 (GCCTAGTTCTCCTTCTGG) and P10 (Fig. 1A) amplified a unique ca. 3.3 kb fragment from parasite line PbLAP4/GFP (Fig. 1B), confirming correct integration of the gfp sequence downstream of the pblap4 allele. Conversely, primers P12 and P13 (TTTGATAGCACTCTTTCAAATGC) (Fig. 1A) amplified a predicted fragment of ca. 3.3 kb from WT parasites only (Fig. 1B). Diagnostic PCR using primers P14 (ACAAAGAATTCATGGTTGGTTCGCTAAACT) and P15 (CTCTTCCAATTGCTCATTTA) (Fig. 1A) amplified a unique ca. 1.6 kb fragment from parasite line PbLAP5/EGFP (Fig. 1B), confirming correct integration of the hdhfr selectable marker gene cassette into the pblap5 locus. Moreover, the absence of WT parasites was confirmed using primers P5 and P13 that amplified a ca. 4.5 kb fragment specific to the WT pblap5 allele (Fig. 1B). Validated PbLAP4/GFP, PbLAP5/GFP and PbLAP6/GFP clonal parasite lines exhibited normal asexual and sexual blood stage development.
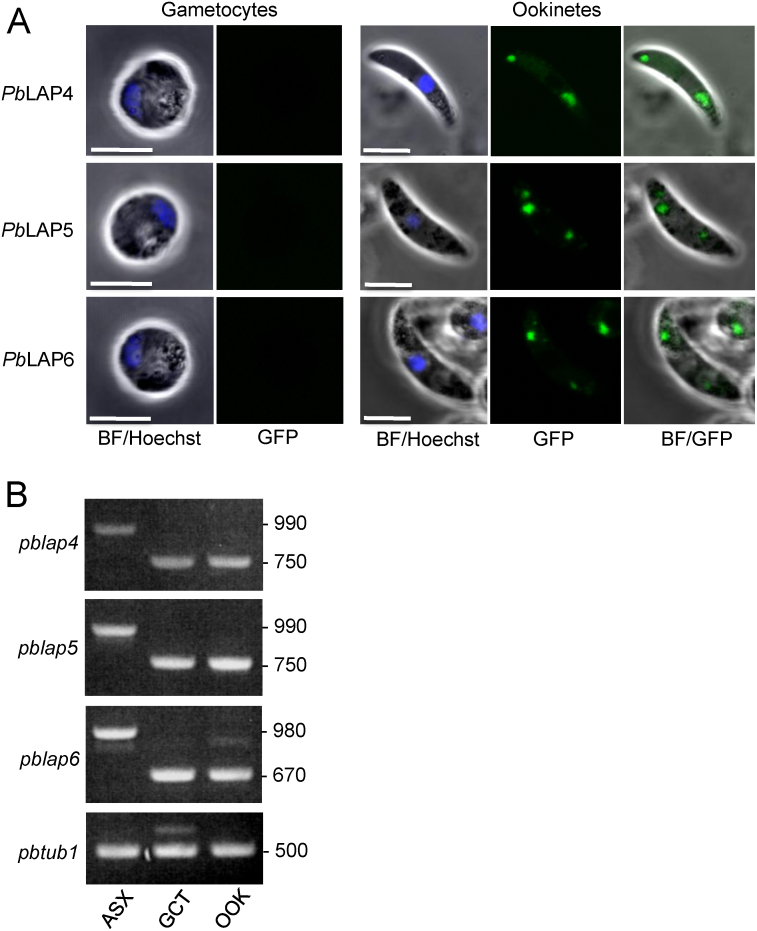
GFP fluorescence was not detected in mature oocysts of parasite lines PbLAP4/GFP, PbLAP5/GFP and PbLAP6/GFP, consistent with the demonstrated lack of discernible transcription of these pblap genes in oocysts and sporozoites by GFP reporter and RT-PCR studies [12]. To assess PbLAP protein expression and localization in ookinetes, ookinete cultures were set up from gametocytemic mouse blood. Mature ookinetes of parasite lines PbLAP4/GFP, PbLAP5/GFP and PbLAP6/GFP exhibited GFP-based fluorescence that typically distributed to 2–3 regions visibly associated with clusters of malaria pigment, characteristic of crystalloid targeting (Fig. 2A). This localization pattern is very similar to that observed for PbLAP1, PbLAP2 and PbLAP3 [4,5] indicating that – like their family members – PbLAP4, PbLAP5 and PbLAP6 associate with the crystalloids. This adds further strong support to the concept that all the Plasmodium LCCL proteins are involved in a common molecular process and act as a protein complex, consistent with their common loss-of-function phenotypes and lack of functional redundancy. Furthermore, the targeting of PbLAP5 to crystalloids demonstrates that the LCCL domain is not required to be present in individual family members in order for the protein to be sorted to this organelle. This argues against the concept that the LCCL domain constitutes an organellar-targeting signal for crystalloids.
Fig. 2.
Gene expression and subcellular distribution of pblap gene products. (A) Confocal bright field and GFP images of gametocytes and ookinetes of parasite lines PbLAP4/GFP, PbLAP5/GFP and PbLAP6/GFP. Both a longitudinal and transverse cross section of PbLAP6/GFP ookinetes are shown. Hoechst DNA stain (blue) marks position of nucleus. Scale bar = 5 μm. (B) PCR on genomic DNA (gDNA) and cDNA from parasite samples enriched for asexual blood stages (ASX), gametocytes (GCT) and ookinetes (OOK), using primers specific for pblap4 (P16/P17), pblap5 (P18/P19), pblap6 (P20/P21) and the control gene pbtub1 (encoding tubulin 1). The relative positions of these primers are indicated in Fig. 1A. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
We previously showed by GFP tagging that PbLAP1, PbLAP2 and PbLAP3 are first expressed as protein in gametocytes [4,5]. It was therefore surprising that we could not observe green fluorescence in gametocytes of parasite lines PbLAP4/GFP, PbLAP5/GFP and PbLAP6/GFP (Fig. 2A). To shed more light on this we investigated gene transcription of pblap4, pblap5 and pblap6 by total RNA extraction from wildtype P. berghei ANKA parasites followed by reverse transcription and PCR. For each gene tested we used primer pairs that flanked an intron in order to distinguish between amplification from cDNA or contaminating gDNA (Fig. 1A). Primers P16 (GCACTTTCTTTACGTGAATGGAG) and P17 (GCCAACTACTACGCCCATC) amplified a pblap4 cDNA-specific predicted product of ca. 750 bp in samples enriched for gametocytes or ookinetes. A ca. 990 bp predicted product was amplified from gDNA owing to the presence of an intron situated in between the primer annealing sites (Fig. 2B). By contrast, pblap-specific cDNA was not amplified from asexual blood stages (derived from P. berghei ANKA clone 2.33 which does not produce gametocytes), indicating that the product in the gametocyte sample was not amplified from cDNA from contaminating asexual blood stages (Fig. 2B). Similarly, primers P18 (GATACATAAATGCTACAGTGAGAATTATGAC) and P19 (CCCATCGAACAGAAAAATGC) amplified a pblap5 cDNA-specific predicted product of ca. 750 bp from sexual but not asexual blood stages (Fig. 2B). The same primers gave rise to a ca. 990 bp predicted product from gDNA due to an intron between the primer annealing sites (Fig. 2B). Primers P20 (CGCATGTATGTGTGAATGTAGC) and P21 (ACATTAATGCACCATTCCCAT) amplified a pblap6 cDNA-specific predicted amplicon of ca. 670 bp from gametocytes. The amplification product with this primer pair from gDNA was ca. 980 bp, again because of an intron situated between the primer annealing sites (Fig. 2B). The pbtub1 (encoding tubulin 1) cDNA-specific primers TUB1cDNA-F (TAGGACAGGCTGGTATCCAAG) and TUB1cDNA-R (CTTGTGGTGATGGCCAGC) amplified a predicted cDNA product of ca. 500 bp (ca. 1200 bp from gDNA) confirming the presence of cDNA (Fig. 2B). Because gametocytes contaminate the ookinete sample, but not the other way around, these results indicate that mRNA corresponding to pblap4, pblap5 and pblap6 is in fact most abundant in the sexual blood stages, consistent with other mRNA expression studies of these three genes [12]. This inverse relationship between mRNA and protein expression in gametocytes and ookinetes fits well with a scenario of translational repression (TR). TR is a process of translational silencing of mRNA that in P. berghei is specific to female gametocytes and involved in development of the parasite post-fertilization [16]. Indeed pblap4, pblap5 and pblap6 are predicted to be subject of TR in studies using DOZI (development of zygote inhibited) null mutants, while pblap1, pblap2 and pblap3 are not [16]. This is fully consistent with our observations reported here and in other studies [4,5]. In further support of TR, neither PbLAP4, PbLAP5 and PbLAP6 were detected in the female gametocyte proteome, as opposed to PbLAP1, PbLAP2 and PbLAP3 [17].
The similar loss-of function phenotypes of the pblap family members strongly imply that a complete PbLAP repertoire is needed to form a functional LCCL protein complex and allow normal sporozoite transmission. It is tempting to speculate that a functional LCCL protein complex would also be necessary for any gametocyte-specific function of these proteins. The TR of pblap4, pblap5 and pblap6 identified in this study reveals a potential mechanism to reduce the gametocyte-specific expression of select PbLAP family members and in doing so control the amount of functional LCCL protein complex that is present in gametocytes. Within the LCCL protein family PbLAP2 and PbLAP4 are structural paralogues, as are PbLAP3 and PbLAP5 [1]. It has been poorly understood why the parasite encodes such similar proteins from distinct genes. The notion that of each pair of the PbLAP paralogues only one is translationally repressed points to a biological need for their differential expression, which may have been the reason behind their ancestral gene duplication. The situation in P. berghei appears to be different from that in P. falciparum where all the LCCL protein family members have been shown to be expressed as proteins during gametocytogenesis and to form a ‘complete’ multi-protein complex in mature gametocytes [13,18]. Thus, TR of the LCCL proteins in P. falciparum appears to be either absent, or less effective than that of its orthologues in P. berghei. The subcellular distribution of the LCCL proteins in P. falciparum gametocytes includes a vesicular secretion to the parasitophorous vacuole, resulting in exposure of the protein complex to the extracellular environment upon gametogenesis, to which various putative functions have been attributed [7,13,18–20]. Notably, a similar secretion of PbLAPs to the parasitophorous vacuole of P. berghei gametocytes is not apparent, although it cannot be ruled out that this occurs at low levels that are difficult to detect [4,5]. Besides considerable dissimilarities in gametocytogenesis, the TR of select PbLAP family members could be a reason behind these species-specific differences.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Dessens J.T., Saeed S., Tremp A.Z., Carter V. Malaria crystalloids: specialized structures for parasite transmission? Trends in Parasitology. 2011;27(3):106–110. doi: 10.1016/j.pt.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meis J.F., Ponnudurai T. Ultrastructural studies on the interaction of Plasmodium falciparum ookinetes with the midgut epithelium of Anopheles stephensi mosquitoes. Parasitology Research. 1987;73(6):500–506. doi: 10.1007/BF00535323. [DOI] [PubMed] [Google Scholar]
- 3.Trueman H.E., Raine J.D., Florens L., Dessens J.T., Mendoza J., Johnson J. Functional characterization of an LCCL-lectin domain containing protein family in Plasmodium berghei. Journal of Parasitology. 2004;90(5):1062–1071. doi: 10.1645/GE-3368. [DOI] [PubMed] [Google Scholar]
- 4.Carter V., Shimizu S., Arai M., Dessens J.T. PbSR is synthesized in macrogametocytes and involved in formation of the malaria crystalloids. Molecular Microbiology. 2008;68(6):1560–1569. doi: 10.1111/j.1365-2958.2008.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeed S., Carter V., Tremp A.Z., Dessens J.T. Plasmodium berghei crystalloids contain multiple LCCL proteins. Molecular and Biochemical Parasitology. 2010;170(1):49–53. doi: 10.1016/j.molbiopara.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claudianos C., Dessens J.T., Trueman H.E., Arai M., Mendoza J., Butcher G.A. A malaria scavenger receptor-like protein essential for parasite development. Molecular Microbiology. 2002;45(6):1473–1484. doi: 10.1046/j.1365-2958.2002.03118.x. [DOI] [PubMed] [Google Scholar]
- 7.Pradel G., Hayton K., Aravind L., Iyer L.M., Abrahamsen M.S., Bonawitz A. A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. Journal of Experimental Medicine. 2004;199(11):1533–1544. doi: 10.1084/jem.20031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trexler M., Banyai L., Patthy L. The LCCL module. European Journal of Biochemistry. 2000;267(18):5751–5757. doi: 10.1046/j.1432-1327.2000.01641.x. [DOI] [PubMed] [Google Scholar]
- 9.Ecker A., Bushell E.S., Tewari R., Sinden R.E. Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Molecular Microbiology. 2008;70(1):209–220. doi: 10.1111/j.1365-2958.2008.06407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raine J.D., Ecker A., Mendoza J., Tewari R., Stanway R.R., Sinden R.E. Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathogens. 2007;3(3):e30. doi: 10.1371/journal.ppat.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnham P.C., Bird R.G., Baker J.R., Desser S.S., el-Nahal H.M. Electron microscope studies on motile stages of malaria parasites. VI. The ookinete of Plasmodium berghei yoelii and its transformation into the early oocyst. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1969;63(2):187–194. doi: 10.1016/0035-9203(69)90145-x. [DOI] [PubMed] [Google Scholar]
- 12.Lavazec C., Moreira C.K., Mair G.R., Waters A.P., Janse C.J., Templeton T.J. Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Molecular and Biochemical Parasitology. 2009;163(1):1–7. doi: 10.1016/j.molbiopara.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Simon N., Scholz S.M., Moreira C.K., Templeton T.J., Kuehn A., Dude M.A. Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. Journal of Biological Chemistry. 2009;284(21):14537–14546. doi: 10.1074/jbc.M808472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saeed S., Tremp A.Z., Dessens J.T. Conformational co-dependence between Plasmodium berghei LCCL proteins promotes complex formation and stability. Molecular and Biochemical Parasitology. 2012;185(2):170–173. doi: 10.1016/j.molbiopara.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janse C.J., Ramesar J., Waters A.P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nature Protocols. 2006;1(1):346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 16.Mair G.R., Braks J.A., Garver L.S., Wiegant J.C., Hall N., Dirks R.W. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313(5787):667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan S.M., Franke-Fayard B., Mair G.R., Lasonder E., Janse C.J., Mann M. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121(5):675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Scholz S.M., Simon N., Lavazec C., Dude M.A., Templeton T.J., Pradel G. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. International Journal for Parasitology. 2008;38(3–4):327–340. doi: 10.1016/j.ijpara.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Delrieu I., Waller C.C., Mota M.M., Grainger M., Langhorne J., Holder A.A. PSLAP: a protein with multiple adhesive motifs, is expressed in Plasmodium falciparum gametocytes. Molecular and Biochemical Parasitology. 2002;121(1):11–20. doi: 10.1016/s0166-6851(02)00016-6. [DOI] [PubMed] [Google Scholar]
- 20.Pradel G., Wagner C., Mejia C., Templeton T.J. Plasmodium falciparum: co-dependent expression and co-localization of the PfCCp multi-adhesion domain proteins. Experimental Parasitology. 2006;112(4):263–268. doi: 10.1016/j.exppara.2005.11.010. [DOI] [PubMed] [Google Scholar]