Abstract
Rationale
Memantine is a potential treatment for alcoholic patients, yet few studies investigate the effect of concurrent treatment with memantine and ethanol on aggression. We evaluated aggressive behavior following ethanol consumption and treatment with glutamatergic drugs to characterize interactions between these compounds.
Objective
To use rodent models of aggression to examine interactions between glutamatergic compounds and ethanol.
Materials and Methods
Once male CFW mice reliably self-administered 1 g/kg ethanol or water, they were assessed for aggression in resident-intruder confrontations. Alternatively, aggression was evaluated following a social-instigation procedure. Animals were then injected with memantine, ketamine, neramexane, MTEP or LY379268 before aggressive confrontations. Effects of the pharmacological manipulations on salient aggressive and non-aggressive behaviors were analyzed.
Results
Moderate doses of memantine, neramexane and MTEP interacted with ethanol to increase the frequency of attack bites while ketamine did not. The highest dose of LY379268, an mGluR2/3 agonist, reduced both aggressive and non-aggressive behaviors after water and ethanol self-administration. Attack bites increased with social instigation and decreased with administration of high doses of MTEP and LY379268. Memantine and MTEP both reduced attack bite frequency in the instigation condition without reducing locomotor behavior.
Conclusions
Memantine and neramexane interacted with ethanol to heighten aggression. The binding characteristics of these compounds allow for ‘partial trapping’ by which some NMDARs are unblocked between depolarizations. We propose that this feature may contribute to the differential aggression-heightening interactions between these compounds and ethanol. MTEP also interacted with ethanol to escalate aggression, possibly through inhibition of mGluR5 modulation of NMDARs.
Keywords: Aggression, Glutamate Receptor, NMDA, mGluR, Dorsal Raphe, Ventral Tegmental Area
Introduction
Memantine, a clinically well-tolerated uncompetitive N-Methyl-D-aspartate receptor (NMDAR) channel blocker, has been suggested for the treatment of alcoholic individuals (Krystal et al. 1998; Rammes et al. 2001; Petrakis et al. 2004; Krupitsky et al. 2007a; Krupitsky et al. 2007b). In laboratory animals, this compound reduces cue-induced cravings and produces some interoceptive ethanol-like effects in the absence of detectable cognitive or behavioral impairments (Grant et al. 1991; Bienkowski et al. 2001). Likewise, other uncompetitive NMDAR channel blockers including phencyclidine (PCP), dizocilpine ((+)MK-801) and ketamine reduce ethanol-seeking behavior and substitute for alcohol in drug discrimination tasks (Grant et al. 1991; Krystal et al. 1998; Vivian et al. 2002). Yet, these compounds can produce schizophrenia-like symptoms and may possess substantial abuse liability (Moghaddam & Adams 1998; Mohn et al. 1999; Krystal et al. 2003). These seemingly paradoxical findings suggest that, while some NMDAR antagonists may be suitable for the treatment of neuropathologies, others cause severe cognitive and behavioral disturbances. The present investigation focuses on aggression, a behavior that is highly regulated by glutamatergic neurotransmission (Musty & Consroe 1982; Wilmot et al. 1987; Shaikh et al. 1994; Belozertseva & Bespalov 1999; Siegel et al. 1999; Sukhotina & Bespalov 2000; Lumley et al. 2004; Audet et al. 2009; Duncan et al. 2009). Specifically, we use pharmacological tools to examine the interaction between ethanol and glutamatergic compounds, targeting the NMDA receptor or metabotropic glutamate receptor (mGluR) subtypes, in mouse models of aggression.
In the human population, alcohol consumption is linked to approximately three million violent crimes committed annually in the United States (Greenfeld 1998). To address the biological basis of this correlation, investigations have aimed to identify the neural pathways that mediate ethanol-escalated aggression. Results from such studies have shown that alcohol potentiates defensive rage behavior in cats via activation of neurons in the medial hypothalamus (MH; Schubert et al. 1996; Siegel et al. 1997). This may reflect a lapse in the regulation of inhibitory neurotransmission. Because glutamate neurons play a major role in the maintenance of species-typical levels of aggression, we hypothesize that this dysregulation is caused in part by NMDAR antagonism by ethanol (Gruol et al. 1997).
To regulate aggressive behavior, the medial prefrontal cortex (mPFC) most likely extends modulatory glutamatergic input to the dorsal raphe nucleus (DRN; Celada et al. 2001; Lee et al. 2003). Consequently, serotonergic projections from the DRN can activate 5-HT1 receptors in the periaqueductal grey (PAG) and MH. This activation of inhibitory serotonin receptors is critical for the inhibition of defensive and aggressive behaviors in cats and rodents (Shaikh et al. 1997; Hassanain et al. 2003; Bannai et al. 2007). Thus, exposure to ethanol may antagonize NMDA receptors to reduce 5-HT1 activity in the MH and PAG, disinhibiting aggression (Lovinger et al. 1989; Rabe & Tabakoff 1990; Takadera et al. 1990; Peoples et al. 1997). Preclinical studies strengthen this hypothesis by showing that ethanol administration can heighten aggressive behaviors in some mice. These behaviors can be selectively reduced by 5-HT1A and 5-HT1B receptor agonism (Miczek et al. 1998; Fish et al. 1999; Miczek & de Almeida 2001). Likewise, evidence from 5-HT1A and 5-HT1B knockout mice points to the importance of these receptors in the expression of aggressive behavior (Saudou et al. 1994; Bouwknecht et al. 2001).
Glutamate neurons projecting from the PFC also excite striatal GABAergic neurons via NMDA heteroreceptors (Lammers & Van Rossum 1968; Gogos et al. 1998; Carlsson et al. 1997; Fernandes et al. 2004). Activation of these NMDA receptors increases GABAergic neurotransmission between the striatum and the ventral tegmental area (VTA; Taber & Fibiger 1995; Carlsson et al. 1997; Yonezawa et al. 1998; Jentsch & Roth 1999; Rahman & McBride 2002). In turn, this inhibitory signaling reduces dopamine neurotransmission from the VTA to the striatum, creating a feedback mechanism (Carlsson et al. 1997; Melis et al. 2002). Ethanol antagonism of cortical NMDA receptors may diminish inhibitory signaling from the striatum to the VTA to increase dopamine neurotransmission (Rabe & Tabakoff 1990; Takadera et al. 1990; Carlsson et al. 1997). Such increases in dopamine can also be associated with aggressive outbursts (Lammers & Van Rossum 1968; Gogos et al. 1998; Fernandes et al. 2004).
These proposals regarding the neurocircuitry of ethanol-heightened aggression link dysregulation of glutamate receptors in the prefrontal cortex to maladaptive behavioral disinhibition. Preclinical studies have aimed to characterize the role of glutamatergic modulation in the inhibition of aggression using classical pharmacological tools. These investigations demonstrate that compounds acting as uncompetitive NMDAR open channel blockers such as phencyclidine (PCP) and ketamine can increase aggressive behavior in some laboratory animals (Musty & Consroe 1982; Takahashi et al. 1984; Wilmot et al. 1987; Audet et al. 2009). To date, however, evidence for the effects of neramexane and the clinically prescribed compound, memantine, on aggressive behavior is limited. The few relevant studies suggest that, in drug-naïve mice, memantine and neramexane reduce aggressive behaviors at doses that also induce sedation (Belozertseva & Bespalov 1999; Sukhotina & Bespalov 2000). To our knowledge, the present studies of aggression are the first to focus on the interaction between memantine-like drugs and ethanol. Likewise, these appear to be the first investigations of aggression describing the effect of concurrent treatment with drugs targeting mGluRs and acute ethanol. Thus, the present study employed experimental techniques with high translational validity to examine these issues. We used an ethanol self-administration procedure to investigate aggression following alcohol consumption. Additionally, we employed a non-pharmacological manipulation, social instigation, in an attempt to escalate mouse aggression by provoking resident animals with an inaccessible conspecific (Potegal & Tenbrink 1984; Fish et al. 1999). The social instigation procedure was selected to clarify how glutamatergic compounds may affect the perception of a threatening social stimulus. Using these procedures, the present investigation aimed to further define the role of NMDA and mGlu receptors in heightened aggression and behavioral disinhibition.
Materials and Methods
Subjects
Subjects were adult female and male Swiss Webster mice (CFW, Charles River Laboratories Wilmington, MA, USA), weighing 23–25 g on arrival. Male mice were assigned as residents, intruders, or instigators. Each resident was housed with a female in a 28x17x14 cm clear polycarbonate cage with a stainless steel wire lid, through which rodent chow and tap water could be accessed (Lab Diet 5001 Rodent Diet, PMI Nutrition International, Brentwood, MO, USA). Intruders and instigators were housed in groups of eight to eleven in 46x24x16 cm polycarbonate cages with stainless steel wire lids. Pine and cedar shavings were used as bedding for resident cages and corn cob shavings were used for animals housed in groups (Shepherd’s Specialty Blend Alpha-dri/Cob Blend, Shepherd’s Specialty Papers). Bedding in all cages was changed weekly at least 24 hours prior to behavioral testing. The vivarium was maintained on a 12-h light/dark photocycle (lights off at 0700 hours, lights on at 1900 hours). Behavioral assessment always occurred during the dark photoperiod when animals were most active. Mice were cared for according to the NIH Guide for the Care and Use of Laboratory Animals and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Tufts University.
Behavioral Measurements and Apparatus
Mouse behavior was recorded using a JVC Everio GZ-MG670 digital camera. All tests were video-taped and analyzed by a trained observer (intra-observer reliability: r >0.95) using The Observer XT software (Noldus, v.8.0.330 or v. 9.0.436; Wageningen, The Netherlands). The frequency and duration of each operationally defined behavior was coded by key presses on a custom-made keyboard. Aggressive behaviors included attack bites and sideways threats while non-aggressive behaviors consisted of walking and rearing (Mackintosh & Grant 1966; Miczek and O’Donnell 1978).
Drugs
95% ethyl alcohol was purchased from Pharmco-AAPER Products, Inc (Brookfield, Connecticut, USA) and was diluted with tap water to obtain a 6% ethanol solution (w/v). Memantine (1-amino-3,5-dimethyladamantane) was obtained from Forest Laboratories (Jersey City, New Jersey, USA) and purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Neramexane was a gift received from Merz Pharmaceuticals (Merz GmbH & Co. KGaA). Memantine and neramexane were diluted with dH2O. Ketamine was purchased from Bioniche Teoranta (Inverin Co, Galway, Ireland) and diluted in 0.9% saline. MTEP (3-((Methyl-1,3-thiazol-4-yl)ethynyl) pyridine hydrochloride) and LY379268 ((1R,4R,5S,6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid) were purchased from Tocris Bioscience (Ellisville, Missouri, USA) and dissolved 0.9% saline. Memantine, neramexane, ketamine, MTEP and LY379268 were injected intraperitoneally (i.p.) in a volume of 10 ml/kg of body weight.
Alcohol Self-Administration
Resident animals (n= 46) that were assigned to alcohol-heightened aggression experiments were limited to three hours of daily water access, Monday through Friday. During fluid self-administration sessions, females and pups were removed and an aluminum operant conditioning panel (16.5 × 15.9 cm) was secured in the cage (Miczek & de Almeida 2001). Each conditioning panel had two fluid troughs for the animal to insert its nose. Fluid was delivered by a pump, through a 30 ml syringe (Med Associates, St. Albans, VT, USA) connected to the trough by plastic tubing. Half of the animals received water or 6% ethanol (w/v) solution for right-side nose-poking and half for left-side nose-poking. A house light illuminated the cage and two cue lights were located above each trough. When animals interrupted the horizontal photobeam in the assigned trough, one poke was recorded. Simultaneously, an overhead stimulus light was illuminated and a click was heard.
During the initial 30 minute training session, one nose-poke in the appropriate trough resulted in the delivery of 40 μl of water (fixed ratio 1 schedule; FR1). In the following session, the ratio was increased to FR2. For the final stage of training and during testing sessions, animals received fluid on an FR5 schedule, resulting in a final dose of 1.0 g/kg ethanol or water according to body weight (Miczek et al. 1998). To ensure a consistent blood ethanol concentration, testing proceeded if animals consumed 1 g/kg of fluid within five minutes (Miczek et al. 1998; Miczek & de Almeida 2001). Session duration, and the number of fluid deliveries were recorded by an interfaced software package (MED-PC for Windows, v. 4.1; Med Associates).
Resident-Intruder Paradigm
The drug naïve resident male mice were assessed for aggressive behavior toward specific group-housed intruder animals. Following removal of the female and pups from a resident’s home cage, the intruder mouse was introduced. Bites were counted for 5 minutes following the initial attack (Miczek & O’Donnell 1978). If no attack bites occurred, the session was terminated at 5 minutes. In the rare cases when the intruder bit the resident, the intruder was removed immediately and replaced with another group-housed male. The latency to the first bite and the bite frequency generally began to stabilize after 6–8 sessions. Following behavioral stabilization (<20% variability in attack bites during the last 3 sessions), resident animals were habituated to intraperitoneal vehicle administration 10–30 minutes before the introduction of the intruder (Winslow & Miczek 1983). Once behavior stabilized with injections, experimental sessions began. To maintain high, stable levels of aggression, resident-intruder confrontations only occurred every other day. If baseline aggression decreased, the intruder animal was changed and the resident was required to achieve stability before experimental sessions began again.
Neutral Cage Non-Instigation Confrontation
After bite frequencies stabilized in the home cage, neutral cage testing began for residents included in social instigation-heightened aggression experiments (n=27). The resident was placed in a large, clear polycarbonate cage (30x33x46 cm) with fresh pine shavings. Five seconds later, the assigned intruder was introduced to the cage (Fish et al. 1999). As in the home cage condition, sessions lasted five minutes following the first attack bite. Shavings were replaced following each session to eliminate scent cues (Fish et al. 1999). Neutral cage sessions were alternated with home cage confrontations. Animals were required to display stable levels of aggressive behavior in the neutral cage condition (<20% variability in attack bites during the last 3 sessions) before experimental sessions began.
Neutral Cage Instigation Confrontation
An experimentally naïve male instigator mouse was placed in a perforated, clear, polycarbonate protective cage (18x6 cm; Fish et al. 1999). After the female and pups were removed, the instigator cage was positioned in the middle of the resident home cage. This social instigation period lasted five minutes and was immediately followed by a neutral cage confrontation between the resident and the intruder. These sessions alternated with resident-intruder confrontations in the home cage. Social instigation generally induced significantly higher levels of aggression compared to trials that lacked the instigation procedure (Table 3; Miczek & O’Donnell 1978).
Table 3.
Aggressive and non-aggressive behaviors and social instigation
| Compound (mg/kg) | Attack Bite Frequency | Sideways Threat Frequency | Walking Duration (s) | Rearing Duration (s) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Memantine (n= 7) | NON INST | INST | NON INST | INST | NON INST | INST | NON INST | INST |
| 0.0 | 20.9(3.3) | 24.4(3.4) | 18.0(2.5) | 19(3.4) | 146.6(16.8) | 135.5(10.1) | 23.6(6.7) | 22.5(7.3) |
| 3.0 | 28.1(3.6) | 29.0(5.4) | 19.3(2.5) | 21.6(3.3) | 156.2(14.4) | 161.8(10.4) | 19.7(7.8) | 21.9(5.1) |
| 10.0 | 19.9(3.9) | 14.6(4.1) | 16.3(3.2) | 10.3(2.1)* | 173.4(11.5) | 176.1(20.7)* | 13.2(4.2) | 13.5(4.5) |
| 17.0 | 11.1(3.8) | 6.3(2.1)* | 10.9(3.1) | 4.7(1.6)* | 170.5(18.1) | 188.4(18.6)*# | 4.6(1.3)* | 8.4(2.1) |
| 30.0 | 1.7(.7)* | 0.14(.14)* | 2.4(1.6)* | 0.14(.14)* | 138.5(12.4) | 151.7(12.7) | 7.5(1.6)* | 7.1(2.6) |
| MTEP (n= 11) | ||||||||
| 0.0 | 19.3(2.8) | 27.1(3.0)# | 36.7(4.5) | 49.5(3.5)# | 147.9(7.6) | 132.7(8.2) | 23.3(2.3) | 22.9(2.7) |
| 1.0 | 19.1(3.5) | 25.3(2.4) | 33.1(5.7) | 44.5(3.0) | 126.5(11.2) | 136.0(6.6) | 22.5(5.3) | 25.6(2.1) |
| 3.0 | 18.4(2.4) | 23.0(4.3) | 32.8(4.4) | 37.0(6.7) | 120.4(10.0) | 135.0(11.4) | 17.9(3.9) | 22.1(5.2) |
| 5.6 | 15.2(2.8) | 17.7(2.9) | 25.2(4.2) | 31.5(4.4)* | 119.2(4.5) | 115.4(10.4) | 13.6(1.7) | 29.4(7.0)# |
| LY379268 (n=9) | ||||||||
| 0.0 | 13.9 (1.9) | 25.4 (3.1)# | 27.3 (1.8) | 43.1 (3.6)# | 106.9(6.1) | 111.7(3.0) | 20.0(2.7) | 23.1(1.9) |
| 0.3 | 10.3 (2.2) | 23.6 (5.4)# | 19.5 (2.7) | 35.1 (5.2)# | 102.3(9.5) | 103.2(7.0) | 15.8(2.5) | 21.1(2.5) |
| 1.0 | 7.7 (3.0) | 17.4 (5.8)# | 16.0 (5.1) | 25.9 (7.2)* | 66.5(15.7)* | 67.9(14.0)* | 10.3(3.4) | 9.3(2.6)* |
| 3.0 | 2.8 (1.2) | 5.8 (3.2)* | 4.6 (1.9)* | 11.2 (5.3)* | 25.7(5.0)* | 39.8(10.4)* | 3.7(1.3)* | 13.4(9.2) |
Mean shown with SEM in parentheses
p<0.05 statistical significance from vehicle administration
p<0.05 statistical significance from non-instigation condition
Systemic Injections and Aggression
On test days, animals assigned to alcohol and aggression studies consumed 1.0 g/kg ethanol or water. Promptly following fluid intake, animals were injected with various doses of MTEP (0.0, 1.0, 3.0, 5.6 mg/kg, n= 9; Klodzinska et al. 2004), LY379268 (0.0, 0.3,1.0, 3.0 mg/kg, n=8; Woolley et al. 2008), ketamine (0.0, 5.6, 10.0, 17.0, 30.0 mg/kg, n=8; Irifune et al. 1991), memantine (0.0, 1.0, 3.0, 10.0, 17.0 mg/kg, n=9; Belozertseva & Bespalov 1999) or neramexane (0.0, 0.3, 1.0, 10.0 mg/kg, n=12; Kotlinska et al. 2006). Animals in social-instigation experiments were injected with MTEP (0.0, 1.0, 3.0, 5.6 mg/kg, n=11), LY379268 (0.0, 0.3, 1.0, 3.0 mg/kg, n=9) or memantine (0.0, 3.0, 5.6, 10.0, 17.0, 30.0 mg/kg, n=7) preceding the instigation procedure. Aggression was evaluated 10–30 minutes following administration of the compound. Behavioral tests occurred in a randomized sequence according to a partial Latin square design, with the highest doses administered last. Each subject was tested at least three times in the vehicle condition (control) and once at every drug dose. Resident-intruder tests were conducted two to three times per week. The duration of each experiment was approximately three months. Our supply of neramexane could not be supplemented, preventing us from evaluating the behavioral effects of a higher dose.
Statistical Analysis
All nine series of experiments followed a repeated measures design, with statistical comparisons within subjects. For all experiments, interactions between ethanol and social instigation or glutamatergic compounds were examined using two-way repeated measures analysis of variance (dose x fluid/instigation; Sigmastat, version 3.11.0). In the case of a significant F value, post hoc comparisons were made using the Holm-Sidak test. In the presence of an interaction effect, main effects were not analyzed, as the effect of one factor depended on the level of the other. To simplify the report of our observations, only significant interactions were reported if present. If the 2-way ANOVA did not yield a significant interaction effect, main effects were reported. We analyzed aggressive and non-aggressive behaviors including attack bites and sideways threats along with walking and rearing. Under the present conditions, we observed alcohol-heightened aggression in an unusually small proportion of animals. Thus, these subjects were not analyzed separately from the mice that did not express ethanol-escalated aggressive behavior.
Results
Experiment I: Resident-intruder aggression after ethanol self-administration and memantine, neramexane or ketamine treatment
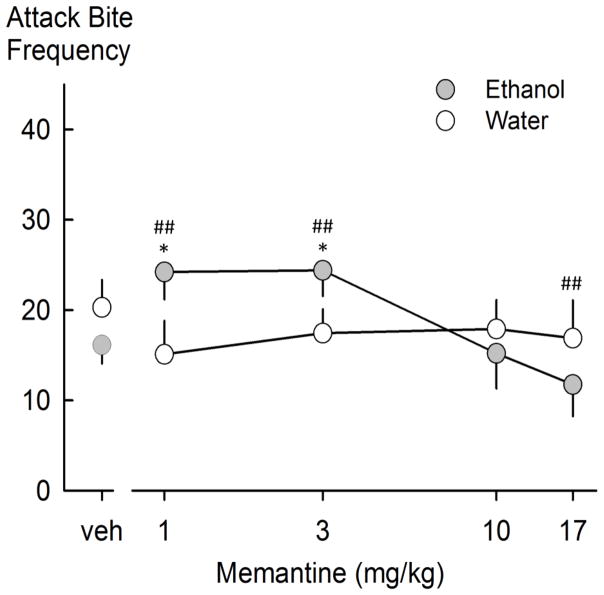
Memantine interacted with ethanol to increase the frequency of aggressive behaviors, including attack bites, and sideways threats [F(4,36)=7.128 and 7.889, respectively, p<0.001]. The Holm-Sidak post-hoc test revealed significant increases in attack bite frequencies at the 1.0 and 3.0 mg/kg doses in the ethanol condition (Figure 1). The absence of a sedative effect on walking or rearing suggests that memantine selectively interacted with ethanol (1 g/kg, 6% w/v) to enhance aggressive behaviors (Table 1).
Fig. 1.
Attack bite frequencies following water or ethanol self-administration and memantine drug treatment. Values are means ± SEM; *p<0.05 compared to vehicle, ##p<0.001 ethanol compared to water.
Table 1.
Non-aggressive behaviors and ethanol
| Compound (mg/kg) | Walking Duration (s) | Rearing Duration (s) | ||
|---|---|---|---|---|
|
| ||||
| Memantine (n=9) | H2O | EtOH | H2O | EtOH |
| 0.0 | 101.6 (14.6) | 86.2 (2.5) | 18.3 (4.2) | 15.3 (5.2) |
| 1.0 | 84.4 (10.0) | 95.4 (15.3) | 12.1 (2.9) | 19.1 (5.1) |
| 3.0 | 80.9 (10.5) | 98.5 (15.2) | 18.0 (5.7) | 19.9 (8.9) |
| 10.0 | 91.8 (11.4) | 79.7 (14.3) | 18.5 (5.2) | 19.0 (5.3) |
| 17.0 | 104.8 (17.8) | 96 (16.3) | 16.3 (4.0) | 26.0 (8.9) |
| Neramexane (n=12) | ||||
| 0.0 | 102.3 (8.7) | 104.9 (4.9) | 24.3 (6.4) | 17.4 (5.7) |
| 0.3 | 105.8 (5.2) | 88.2 (8.6)# | 29.4 (8.2) | 16.3 (5.6) |
| 1.0 | 106.2 (6.5) | 114.7 (7.2) | 24.7 (7.7) | 22.4 (6.2) |
| 3.0 | 99.4 (8.4) | 97.2 (10.4) | 21.7 (6.4) | 16.4 (4.6) |
| 10.0 | 126.7 (5.5)* | 115.3 (9.6) | 20.4 (5.2) | 15.4 (2.8) |
| Ketamine (n=8) | ||||
| 0.0 | 100.6 (5.5) | 108.4 (4.5) | 19.1 (5.5) | 15.5 (5.4) |
| 5.6 | 128.5 (8.5)* | 119.7 (9.4) | 16.5 (4.1) | 16.6 (4.3) |
| 10.0 | 151.0 (6.4)* | 119.5 (7.3)# | 17.8 (7.2) | 13.2 (3.8) |
| 17.0 | 141.6 (7.9)* | 138.2 (14.2)* | 12.2 (3.0) | 11.8 (3.9) |
| 30.0 | 127.4 (13.4)* | 123.4 (9.7) | 25.0 (6.6) | 13.2 (2.6)# |
| MTEP (n=9) | ||||
| 0.0 | 75.4 (12.5) | 84.8 (10.7) | 33.1 (4.4) | 23.7 (4.2) |
| 1.0 | 84.3 (12.1) | 99.4 (14.1) | 27.3 (4.9) | 16.4 (3.3) |
| 3.0 | 74.6 (10.0) | 73.7 (6.3) | 26.4 (4.7) | 23.2 (5.4) |
| 5.6 | 55.0 (8.6) | 53.8 (6.0) | 15.3 (4.4)* | 11.6 (3.8) |
| LY379268 (n=8) | ||||
| 0.0 | 71.9 (5.2) | 69.4 (5.8) | 32.0 (7.3) | 25.5 (7.9) |
| 0.3 | 78.9 (4.9) | 53.3 (7.1) | 22.4 (7.3) | 14.4 (4.1)# |
| 1.0 | 58.2 (6.7) | 48.9 (6.8) | 15.5 (4.0)* | 18.0 (8.6) |
| 3.0 | 34.1 (10.9)* | 13.9 (4.0)* | 9.4 (3.9)* | 1.1 (0.5) *# |
Mean shown with SEM in parentheses
p<0.05 statistical significance from vehicle administration
p<0.05 statistical significance from water condition
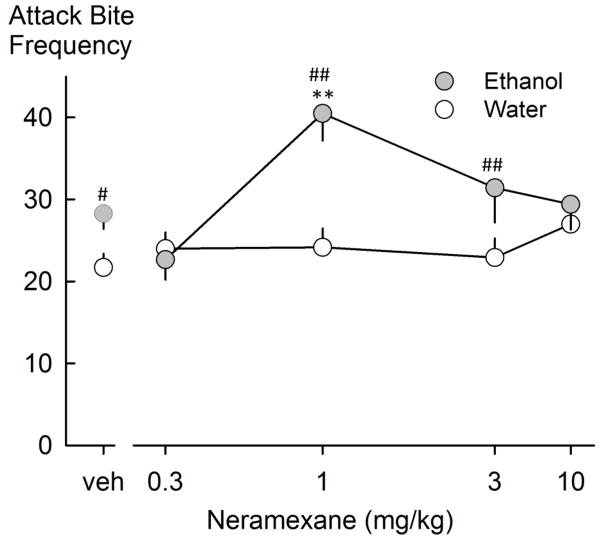
Two-way repeated measures ANOVA identified an interaction effect between neramexane and ethanol on attack bite frequency ([F(4,44)=5.372, p=0.001]; Figure 2). Post hoc analysis indicated that only ethanol-consuming mice that received 1.0 mg/kg of neramexane showed an increase in attack bites (Figure 2). Likewise, there was a significant interaction between neramexane and ethanol to increase the frequency of sideways threats [F(4,44)=3.599, p=0.013]. The Holm-Sidak test revealed that, following ethanol self-administration, animals that treated with1.0 mg/kg neramexane displayed significantly more threats. There was also a neramexane dose-dependent increase in the duration of walking behavior ([F(4,44)=4.84, p=0.002]; Table 1).
Fig. 2.
Attack bite frequencies following water or ethanol self-administration and neramexane drug treatment. Values are means ± SEM; **p<0.001 compared to vehicle, #p<0.05, ##p<0.001 ethanol compared to water.
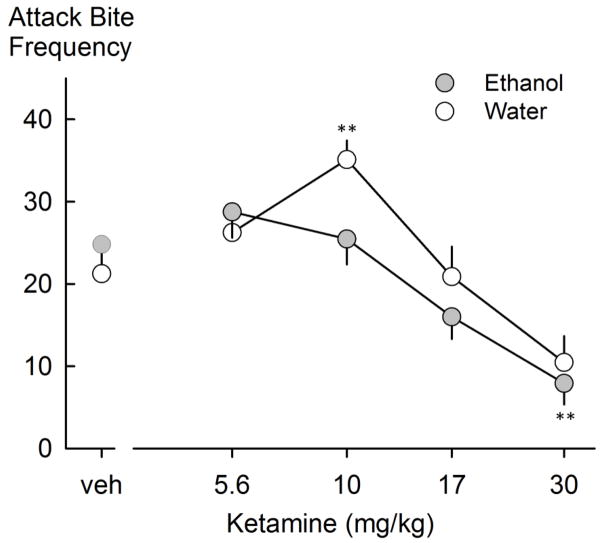
Following ketamine treatment, animals showed a main effect of reduced attack bites [F(4,28)=12.936, p<0.001]. However, there was a near-significant interaction between the dose of ketamine and ethanol (p=0.058). Post-hoc analysis of the main drug dose-effect on attack bites indicated an increase from vehicle at the 10.0 mg/kg dose following water intake and a decrease at the 30.0 mg/kg dose following ethanol drinking (Figure 3). There was also a main effect of reduced sideways threats following ethanol self-administration [F(1,7)=12.482, p=0.01]. Likewise, ethanol consumption reduced the duration of rearing behavior [F(1,7)=7.035, p=0.033]. As with neramexane, ketamine increased the duration of walking [F(4,28)=5.014, p=0.004]. The Holm-Sidak post hoc test showed that this elevation occurred primarily in the water condition, and most notably at the 10.0 mg/kg dose (Table 1).
Fig. 3.
Attack bite frequencies following water or ethanol self-administration and ketamine drug treatment. Values are means ± SEM; **p<0.001 compared to vehicle.
Experiment II: Resident-intruder aggression after ethanol self-administration and MTEP or LY379268 treatment
MTEP and ethanol interacted to produce aggression-heightening effects, as measured by attack bite and sideways threat frequencies [F(3,24)=3.68, p= 0.027; F(3,24)= 4.523, p=0.012]. The Holm-Sidak post-hoc test revealed that animals administered the 1.0 mg/kg dose of MTEP displayed heightened aggressive behavior, but only after ethanol intake (Table 2). Both MTEP and ethanol reduced the duration of rearing behavior ([F(3,24)=3.311, p=0.036, F(1,8)=5.385, p=0.048]; Table 1).
Table 2.
Aggressive behaviors and ethanol
| Compound (mg/kg) | Attack Bite Frequency | |
|---|---|---|
|
| ||
| MTEP (n=9) | H2O | EtOH |
| 0.0 | 20.3 (2.3) | 19.0 (1.6) |
| 1.0 | 14.6 (3.0)* | 23.9 (3.0)# |
| 3.0 | 12.0 (3.5)* | 15.7 (4.5) |
| 5.6 | 6.2 (1.4)* | 4.9 (1.9)* |
| LY379268 (n=8) | ||
| 0.0 | 16.9 (2.3) | 16.8 (1.8) |
| 0.3 | 19.5 (2.8) | 12.9 (3.5) |
| 1.0 | 14.1 (2.8) | 14.9 (4.4) |
| 3.0 | 6.0 (2.0)* | 2.8 (1.7)* |
Mean shown with SEM in parentheses
p<0.05 statistical significance from vehicle administration
p<0.05 statistical significance from water condition
LY379268 had non-specific effects on all social and locomotor behaviors. In particular, LY379268 reduced attack bites [F(3,21)=7.343, p=0.002], sideways threats [F(3,21)=9.189, p< 0.001], walking duration [F(3,21)=19.811, p<0.001] and rearing duration [F(3,21)=5.499, p=0.006] (Tables 1, 2). Regardless of the dose of LY379268, ethanol significantly reduced the duration of walking [F(1,7)=34.347, p<0.001] and rearing [F(1,7)=18.073, p=0.004] (Table 1).
Experiment III: Social instigation-heightened aggression and systemic administration of MTEP, LY379268 or memantine
MTEP treatment had no significant effect on aggressive or non-aggressive behaviors in this experimental protocol. In this experiment, instigation significantly increased the frequency of attack bites [F(1,10)=8.009, p=0.018] and sideways threats [F(1,10)=5.87, p=0.039] and the duration of rearing ([F(1,10)=5.528, p=0039]; Table 3).
LY379268 significantly reduced the frequency of attack bites [F(3,24)=6.938, p=0.002] and sideways threats [F(3,24)=11.19, p<0.001]. The Holm-Sidak post-hoc test revealed that the highest dose, 3.0 mg/kg, reduced the frequency of attack bites and threats as compared to vehicle. LY379268 also had behaviorally non-specific effects, reducing the duration of both walking [F(3,24)=23.165, p<0.001] and rearing [F(3,24)= 4.308, p=0.014]. Also, in this experiment, the instigation procedure consistently increased the frequency of attack bites [F(1,8)=10.328, p=0.012] and sideways threats ([F(1,8)=13.541, p=0.006]; Table 3).
Memantine treatment significantly reduced the frequency of attack bites [F(4,24)=25,585, p<0.001], sideways threats [F(4,24)=21.699, p<0.001], and the duration of rearing [F(4,24)=4.219, p=0.01]. Drug administration also increased the duration of walking [F(4,24)=3.856, p=0.015] in both the instigation and non-instigation conditions. In this experiment, the instigation procedure only had a non-significant trend to enhance aggressive behavior (Table 3).
Discussion
Low doses of memantine and neramexane heightened aggression in mice that self-administered ethanol (Figures 1, 2). At a moderate dose, neramexane interacted with ethanol to also increase locomotor activity, suggesting heightened general arousal (Figure 2, Table 1). Unlike these compounds, ketamine did not interact with ethanol to heighten aggressive behaviors (Figure 3). Rather, in the absence of ethanol, a moderate dose of ketamine increased both aggressive and non-aggressive behaviors. Manipulation of mGluRs revealed that mGluR5 receptor antagonism by MTEP increased the frequency of attack bites in the presence of ethanol while mGluR2/3 receptor agonism by LY379268 did not. MTEP treatment did not affect the duration of walking, indicating that this compound interacted with ethanol to selectively heighten aggression (Table 2). Conversely, LY379268 dose-dependently reduced the duration of walking and the frequency of rearing (Table 2).
The present study also investigated memantine, MTEP and LY379268 in a protocol that aimed to heighten aggression through social instigation. Animals administered MTEP or LY379268 demonstrated robust instigation effects in the vehicle condition (Table 3). Following instigation, memantine reduced the frequency of attack bites and sideways threats without reducing the duration of walking behavior (Table 3). Likewise, after instigation, MTEP selectively reduced the frequency of sideways threats (Table 3). There was no indication of a pro-aggressive effect when memantine or MTEP was administered in conjunction with ethanol. LY379268 reduced the frequency of attack bites and sideways threats in the instigation condition. However, this anti-aggression effect was accompanied by sedation. Results from the instigation experiments suggest that an ethanol interaction is necessary for select glutamatergic compounds to intensify aggressive behavior.
Our current data demonstrate that ethanol interacts with memantine-like compounds and MTEP to increase the frequency of attack bites. A number of behavioral studies relate impulsive and aggressive outbursts to disturbances in glutamatergic activity. Using a delay-discounting task in rats, one investigation demonstrated that ketamine induced higher rates of small reward lever responding (Floresco et al. 2008). Preference for an immediate, small reward may translate to the human condition of impulsivity, which has been linked to aggressive outbursts. Accordingly, in both mice and rats, treatment with PCP can increase the frequency of attack bites in the resident-intruder protocol (Burkhalter & Balster 1979; Audet et al. 2009, but Tyler & Miczek 1982). In isolated mice, however, memantine, MK-801, PCP or neramexane have shown anti-aggressive effects (Belozertseva & Bespalov 1999). These findings demonstrate that competitive NMDAR antagonists profoundly impact the regulation of impulsive behaviors.
In vivo and in vitro studies have helped characterize the pathways and mechanisms involved in behavioral regulation. According to these models, the PFC, which has prominent glutamatergic projections to the DRN and to the striatum, regulates behavioral inhibition (Celada et al. 2001; Lee et al. 2003). In the DRN, antagonism of NMDA receptors could reduce inhibitory 5-HT1 regulation of the PAG and the MH. These two structures are strongly associated with the expression of aggression and defense (Schubert et al. 1996; Shaikh et al. 1997; Miczek et al. 1998; Celada et al. 2001; Hassanain et al. 2003; Lee et al. 2003; Bannai et al. 2007). By a similar mechanism, glutamate neurons projecting from the PFC to the striatum activate NMDA heteroreceptors on GABAergic neurons (Taber & Fibiger 1995; Carlsson et al. 1997; Yonezawa et al. 1998; Jentsch & Roth 1999; Rahman & McBride 2002; Melis et al. 2002). Blockade of NMDA receptors in the striatum could reduce GABAA receptor-mediated inhibition of dopamine neurotransmission between the VTA and the striatum. Such increases in dopaminergic activity are also implicated in heightened aggressive behavior (Lammers & Van Rossum 1968; Miczek 1974; Gogos et al. 1998; Carlsson et al. 1997; Fernandes et al. 2004).
These proposed pathways and mechanisms of aggression suggest that glutamatergic antagonism may lead to behavioral disinhibtion. Thus, it is not unexpected that NMDAR antagonists could have pro-aggressive effects. However, our findings also illustrate a differentiation between two types of compounds that block the NMDAR ion channel, memantine and ketamine. We hypothesize that this differential interaction with alcohol derives from dissimilarities in mechanisms of cellular action. Memantine and neramexane are unique uncompetitive NMDAR antagonists with low receptor affinities, enabling ‘partial trapping’ – a tendency to unblock some receptors between depolarizations (Blanpied et al. 1997; Kotermanksi et al. 2009). Other uncompetitive NMDAR antagonists such as ketamine, PCP and MK-801 have high receptor affinities and slow on-off kinetics (Blanpied et al. 1997; Chen & Lipton 1997). These putative ‘trapping’ blockers require a depolarization for the termination of receptor blockade (Parsons et al. 1995; Blanpied et al. 1997; Chen & Lipton 1997). Consequently, administration of ‘trapping’ blockers disrupts normal excitatory activity and results in numerous aversive side effects (Lipton 1993). The unique kinetic characteristics of memantine and neramexane allow these drugs to selectively treat hyperglutamatergic states. However, these binding features may also contribute to an aggression-heightening interaction with acute ethanol self-administration.
Although the binding kinetics of ethanol are not adequately characterized, results indicate that acute ethanol treatment functionally antagonizes the NMDAR complex. In vitro analysis of recombinant NMDA receptors reveals reduced sensitivity to ethanol following elimination of the NR2A and NR2B subunits (Chu et al. 1995; Allgaier 2002). Additionally, glycine administration restores ethanol-inhibited Ca2+ influx, suggesting that ethanol blocks the glycine site on the NR1 subunit (Peoples et al. 1997; Takadera et al. 1990; Lovinger et al. 1989). In behavioral studies, the NR2 knockout induces locomotor incoordination and prevents mice from developing place preference following conditioning with ethanol injections (Boyce-Rustay & Holmes 2006). The NR2A knock-out animals also showed reduced sedation resulting from an interaction between ethanol and MK-801 (Palachick et al. 2008). Although there are strong implications for the necessity of the NR1, NR2A and NR2B subunits, these findings cannot conclusively link one NMDAR site to the effects of ethanol.
The second experiment reveals that ethanol intake in conjunction with MTEP administration results in escalated aggressive behavior. We propose that the interaction between MTEP and ethanol results from antagonism of Gq-protein-coupled, postsynaptic mGlu5 receptors, which may positively modulate the NMDAR complex (Conn & Pin 1997; Awad et al. 2000; Benquet et al. 2002). Without excitatory input from NMDA receptors, GABAergic neurons may reduce inhibition of dopaminergic neurons projecting from the VTA to the nucleus accumbens (Carlsson et al. 1997; Yonezawa et al. 1998; Jentsch & Roth 1999; Melis et al. 2002; Rahman & McBride 2002; Homayoun et al. 2004). In turn, heightened dopaminergic neurotransmission can lead to escalated aggressive behavior (Lammers & Van Rossum 1968; Gogos et al. 1998; Carlsson et al. 1997; Fernandes et al. 2004). Support for this hypothesis is further strengthened by evidence of high densities of mGlu5 receptors in the striatum, co-localized with NMDA receptors (Shigemoto et al. 1993; Lujan et al. 1996).
Evidence for mGluR2/3 regulation of NMDA receptors is less definite. In laboratory animals, mGluRu2/3 agonism inhibits the motor effects of PCP, suggesting some mGluR2/3 modulation of NMDA receptors (Cartmell et al. 1999). However, since mGlu2/3 receptors are primarily located on presynaptic terminals, this effect could reflect inhibition of glutamate release into the synapse rather than direct modulation of NMDAR activity. Additionally, there is sparse evidence that the essential NR1 subunit of the NMDAR is found presynaptically (Petralia et al. 1994). The lack of mGluR2/3 modulation of the NMDAR complex may explain the absence of an interaction between ethanol and the mGlu2/3 receptor agonist, LY379268. This finding strengthens our hypothesis that concurrent NMDAR antagonism by ethanol and memantine-like compounds is necessary to observe an aggression-heightening effect.
While memantine-like compounds are generally regarded as NMDAR antagonists, there is some evidence that this class of drugs can also function as α7 nAChR antagonists, D2 agonists, and 5-HT3 antagonists (Rammes et al. 2001, Aracava et al. 2004; Seeman et al. 2008). Ongoing investigations using pretreatments with relevant agonists and antagonists will help determine if our findings are influenced by interactions between memantine-like compounds and non-NMDA receptors. We are also presently studying the effects of memantine on animals that intermittently consume high doses of ethanol. Preliminary findings reveal that memantine escalates aggression in these animals during ethanol withdrawal (Hwa et al. 2011; Dodman, Hwa, Nathanson, Newman, Miczek; unpublished data). To identify subpopulations of serotonergic and dopaminergic neurons that are dysregulated by NMDAR antagonism, aggression will be evaluated following microinjections into the VTA and DRN. Using these procedures, we aim to further characterize the link between glutamate neurotransmission, aggression and behavioral disinhibition.
Acknowledgments
Funded by National Institute on Alcoholism and Alcohol Abuse AA013983
Special thanks to Keisha Dodman, Georgia Gunner, Nishani Hewage, Lara Hwa, Tom Sopko and Kate Woodard for their expert and dedicated assistance.
References
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Aracava Y, Pereira EFR, Maelicke A, Albuquerque EX. Memantine blocks α7* nicotinic acetylcholine receptors more potently than N-Methyl-D-aspartate receptors in rat hippocampal neurons. J Pharm Exp Ther. 2004;312(3):1195–1205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- Audet MC, Goulet S, Dore FY. Impaired social motivation and increased aggression in rats subchronically exposed to phencyclidine. Physiol Behav. 2009;96:394–398. doi: 10.1016/j.physbeh.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology. 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Bespalov AY. Effects of NMDA receptor channel blockade on aggression in isolated male mice. Aggress Behav. 1999;25:381–396. [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Krzascik P, Koros E, Kostowski W, Scinska A, Danysz W. Effects of a novel uncompetitive NMDA receptor antagonist, MRZ 2/579 on ethanol self-administration and ethanol withdrawal seizures in the rat. Eur J Pharmacol. 2001;413:81–89. doi: 10.1016/s0014-2999(01)00743-9. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol. 1997;77:309–323. doi: 10.1152/jn.1997.77.1.309. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der GJ, Maes RA, Hen R, Olivier B. Absence of 5-HT1B receptors is associated with impaired impulse control in male 5-HT1B knockout mice. Biol Psychiatry. 2001;49:557–568. doi: 10.1016/s0006-3223(00)01018-0. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology. 2006;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, Balster RL. Effects of phencyclidine on isolation-induced aggression in mice. Psych Reports. 1979;45:571–576. doi: 10.2466/pr0.1979.45.2.571. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Hansson LO, Waters N, Carlsson ML. Neurotransmitter aberrations in schizophrenia: New perspectives and therapeutic implications. Life Sci. 1997;61:75–94. doi: 10.1016/s0024-3205(97)00228-2. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HV, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 1997;499:27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Anantharam V, Treistman SN. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J Neurochem. 1995;65:140–148. doi: 10.1046/j.1471-4159.1995.65010140.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Inada K, Farrington JS, Koller BH, Moy SS. Neural activation deficits in a mouse genetic model of NMDA receptor hypofunction in tests of social aggression and swim stress. Brain Res. 2009;1265:186–195. doi: 10.1016/j.brainres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C, Paya-Cano JL, Sluyter F, D’Souza U, Plomin R, Schalkwyk LC. Hippocampal gene expression profiling across eight mouse inbred strains: towards understanding the molecular basis for behaviour. Eur J Neurosci. 2004;19:2576–2582. doi: 10.1111/j.0953-816X.2004.03358.x. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MTL, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharm. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Knisely JS, Tabakoff B, Barrett JE, Balster RL. Ethanol-like discriminative stimulus effects of non-competitive n-methyl-d-aspartate antagonists. Behav Pharmacol. 1991;2:87–95. [PubMed] [Google Scholar]
- Greenfeld LA. Alcohol and Crime: an analysis of national data on the prevalence of alcohol involvement in crime. US Department of Justice; Washington DC: 1998. pp. 1–33. [Google Scholar]
- Gruol DL, Parsons KL, DiJulio N. Acute ethanol alters calcium signals elicited by glutamate receptor agonists and K+ depolarization in cultured cerebellar Purkinje neurons. Brain Res. 1997;773:82–89. doi: 10.1016/s0006-8993(97)00912-8. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Bhatt S, Siegel A. Differential modulation of feline defensive rage behavior in the medial hypothalamus by 5-HT1A and 5-HT2 receptors. Brain Res. 2003;981:201–209. doi: 10.1016/s0006-8993(03)03036-1. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irifune M, Shimizu T, Nomoto M. Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav. 1991;40:399–407. doi: 10.1016/0091-3057(91)90571-i. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Tatarczynska E, Chojnacka-Wojcik E, Nowak G, Cosford NDP, Pilc A. Anxiolytic-like effects of MTEP, a potent and selective mGlu5 receptor agonist does not involve GABAA signaling. Neuropharmacology. 2004;47:342–350. doi: 10.1016/j.neuropharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Kotermanski SE, Wood JT, Johnson JW. Memantine binding to a superficial site on NMDA receptors contributes to partial trapping. J Physiol. 2009;587(19):4589–4603. doi: 10.1113/jphysiol.2009.176297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska JA, Bochenski MA, Danysz WB. N-methyl-D-aspartate and group 1 metabotropic glutamate receptors are involved in the expression of ethanol-induced sensitization in mice. Behav Pharmacol. 2006;17(1):1–8. doi: 10.1097/01.fbp.0000181600.95405.c7. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007a;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Rudenko AA, Burakov AM, Slavina TY, Grinenko AA, Pittman B, Gueorguieva R, Petrakis IL, Zvartau EE, Krystal JH. Antiglutamatergic strategies for ethanol detoxification: comparison with placebo and diazepam. Alcohol Clin Exp Res. 2007b;31:604–611. doi: 10.1111/j.1530-0277.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, Stetson P, Trevisan LA, Charney DS. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- Lammers AJJC, Van Rossum JM. Bizzare social behavior in rats induced by a combination of a peripheral decarboxylase inhibitor and DOPA. Eur J Pharmacol. 1968;5:103–106. doi: 10.1016/0014-2999(68)90163-5. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Prospects for clinically tolerated NMDA antagonists: open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci. 1993;16:527–532. doi: 10.1016/0166-2236(93)90198-u. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Lumley LA, Robison CL, Slusher BS, Wozniak K, Dawood M, Meyerhoff JL. Reduced isolation-induced aggressiveness in mice following NAALADase inhibition. Psychopharmacology. 2004;171:375–381. doi: 10.1007/s00213-003-1610-z. [DOI] [PubMed] [Google Scholar]
- Mackintosh JH, Grant EC. The effect of olfactory stimuli on the agonistic behaviour of laboratory mice. Z Tierpsychol. 1966;23:584–587. doi: 10.1111/j.1439-0310.1966.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, de Almeida RMM. Oral drug self-administration in the home cage of mice: alcohol- heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology. 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Hussain S, Faccidomo S. Alcohol-heightened aggression in mice: attenuation by 5-HT1A receptor agonists. Psychopharmacology. 1998;139:160–168. doi: 10.1007/s002130050701. [DOI] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: Effects of psychomotor stimulants and L-dopa. Psychopharmacology. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Intraspecies aggression in rats: effects of d-amphetamine and chlordiazepoxide. Psychopharmacologia. 1974;39:275–301. doi: 10.1007/BF00422968. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Musty RE, Consroe PF. Phencyclidine produces aggressive behavior in rapid eye movement sleep- deprived rats. Life Sci. 1982;30:1733–1738. doi: 10.1016/0024-3205(82)90307-1. [DOI] [PubMed] [Google Scholar]
- Palachick B, Chen Y, Enock AJ, Karlsson R, Mishina M, Holmes A. role of major NMDA or AMPA receptor subunits in MK-801 potentiation of ethanol intoxication. Alcohol Clin Exp Res. 2008;32(8):1479–1492. doi: 10.1111/j.1530-0277.2008.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J Pharmacol Exp Ther. 1995;274:1182–1191. [PubMed] [Google Scholar]
- Peoples RW, White G, Lovinger DM, Weight FF. Ethanol inhibition of N-methyl-D-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol. 1997;122:1035–1042. doi: 10.1038/sj.bjp.0701483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal M, Tenbrink L. Behavior of attack-primed and attack-satiated female golden hamsters (Mesocricetus auratus) J Comp Psychol. 1984;98:66–75. [Google Scholar]
- Rabe CS, Tabakoff B. Glycine site-directed agonists reverse the actions of ethanol at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1990;38:753–757. [PubMed] [Google Scholar]
- Rahman S, McBride WJ. Involvement of GABA and cholinergic receptors in the nucleus accumbens on feedback control of somatodendritic dopamine release in the ventral tegmental area. J Neurochem. 2002;80:646–654. doi: 10.1046/j.0022-3042.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- Rammes G, Mahal B, Putzke J, Parsons C, Spielmanns P, Pestel E, Spanagel R, Zieglgansberger W, Schadrack J. The anti-craving compound acamprosate acts as a weak NMDA-receptor antagonist, but modulates NMDA-receptor subunit expression similar to memantine and MK-801. Neuropharmacology. 2001;40:749–760. doi: 10.1016/s0028-3908(01)00008-9. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, Lemeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Schubert K, Shaikh MB, Han Y, Pohorecky L, Siegel A. Differential effects of ethanol on feline rage and predatory attack behavior: an underlying neural mechanism. Alcohol Clin Exp Res. 1996;20:882–889. doi: 10.1111/j.1530-0277.1996.tb05267.x. [DOI] [PubMed] [Google Scholar]
- Seeman P, Caruso C, Lasaga M. Memantine agonist action at dopamine D2High receptors. Synapse. 2008;62:149–153. doi: 10.1002/syn.20472. [DOI] [PubMed] [Google Scholar]
- Shaikh MB, De Lanerolle NC, Siegel A. Serotonin 5-HT1A and 5-HT2/1C receptors in the midbrain periaqueductal gray differentially modulate defensive rage behavior elicited from the medial hypothalamus of the cat. Brain Res. 1997;765:198–207. doi: 10.1016/s0006-8993(97)00433-2. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Siegel A, Schubert KL, Shaikh MB. Neurotransmitters regulating defensive rage behavior in the cat. Neurosci Biobehav Rev. 1997;21:733–742. doi: 10.1016/s0149-7634(96)00056-5. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TA, Gregg TR, Kruk MR. Neuropharmacology of brain-stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–389. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Sukhotina IA, Bespalov AY. Effects of the NMDA receptor channel blockers memantine and MRZ 2/579 on morphine withdrawal-facilitated aggression in mice. Psychopharmacology. 2000;149:345–350. doi: 10.1007/s002130000386. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neuroscience. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takadera T, Suzuki R, Mohri T. Protection by ethanol of cortical neurons from N-methyl-D-aspartate-induced neurotoxicity is associated with blocking calcium influx. Brain Res. 1990;537:109–114. doi: 10.1016/0006-8993(90)90346-d. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Morato GS, Montiero-de-Lima TC. Effects of ketamine on experimental animal models of aggression. Braz J Med Biol Res. 1984;17:171–178. [PubMed] [Google Scholar]
- Tyler CB, Miczek KA. Effects of phencyclidine on aggressive behavior in mice. Pharmacol Biochem Behav. 1982;17:503–510. doi: 10.1016/0091-3057(82)90311-2. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl- D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis) Psychopharmacology. 2002;162:273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- Wilmot CA, Vander Wende C, Spoerlein MT. The effects of phencyclidine on fighting in differentially housed mice. Pharmacol Biochem Behav. 1987;28:341–346. doi: 10.1016/0091-3057(87)90450-3. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Miczek KA. Habituation of aggression in mice: Pharmacological evidence of catecholaminergic and serotonergic mediation. Psychopharmacology. 1983;81:286–291. doi: 10.1007/BF00427564. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DNC. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist LY379268 in mouse models predictive of antipsychotic activity. Psychopharmacology. 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. Involvement of γ-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol. 1998;341:45–56. doi: 10.1016/s0014-2999(97)01435-0. [DOI] [PubMed] [Google Scholar]