Abstract
Objective
Some patients with rheumatoid arthritis (RA) who receive injectable biologics experience injection-site burning and stinging (ISBS); however, the prevalence of ISBS in the general RA population is unknown and may impact preference for an injectable biologic. This study assessed the prevalence of ISBS and associated comorbidities in patients with RA who receive injectable biologics.
Research Design and Methods
The physician and patient survey consisted of a retrospective chart review and a prospective assessment. In the former, each participating US rheumatologist reviewed the medical records of 5 randomly selected RA patients receiving an injectable biologic. In the prospective assessment, each rheumatologist was asked to report data based on interviews with up to 50 RA patients currently treated with an injectable biologic, who were asked whether they had ISBS during or after their most recent injection.
Results
Data were analyzed for 504 patients in the retrospective chart review, and 3326 patients in the prospective assessment, and were provided by 101 physicians. The overall prevalence of ISBS was 17% and 58% in the retrospective chart review and prospective analyses, respectively. Out of the 1939 prospectively-assessed patients who experienced at least some ISBS, 429 (22%) rated the level of ISBS as moderate to severe (13% of total). Increased risk of ISBS was associated with female gender, fibromyalgia, depression, and more severe RA.
Conclusions
The prevalence of ISBS is likely underestimated in many rheumatology practices. Specifically asking about it may identify patients who experience this side effect, provide a more accurate understanding of how significantly it affects them, and provide an opportunity for intervention in light of their preferences.
Keywords: arthritis, rheumatoid, injection-site reactions, tumor necrosis factor, patient-reported outcomes
INTRODUCTION
The introduction of tumor necrosis factor (TNF) inhibitors was a major advance in the treatment of rheumatoid arthritis (RA), resulting in significant reduction in pain, swelling, and radiographic damage, as well as improvements in physical function and health-related quality of life, compared with placebo or traditional disease-modifying antirheumatic drugs (DMARDs)1–3. Prior to 2009, and at the time of this study, 3 TNF inhibitors were approved by the Food and Drug Administration (FDA) for the treatment of patients with RA, and of these agents, 2 were administered via subcutaneous (SQ) injection: etanercept and adalimumab. Although these injectables may be more convenient for some patients than intravenous infusion administration of infliximab, the third TNF inhibitor, they may cause side effects that may affect treatment satisfaction for patients. One of these side effects is injection-site symptoms or reactions, which include any of the following: erythema, pain, itching, stinging, burning, or swelling. The prevalence and clinical importance of these injection-site reactions and symptoms for RA patients in clinical practice is unclear.
Data is limited even on the prevalence of injection-site symptoms and reactions in the general RA population. During clinical trials with adalimumab and etanercept, injection-site pain and reactions as high as 12% to 37% were reported,4,5 but the applicability of this observation to patients treated in routine clinical practice is uncertain. Moreover, rates of injection-site signs and symptoms, particularly those that are more subjective such as injection-site burning and stinging (ISBS), could be underestimated by physicians who may not routinely ask their patients about it. Additionally, patients may not complain about ISBS if they perceive it is a required trade-off in order to achieve the benefit of these medications; historically, injection-site symptoms and reactions have been a relatively common side effect of injectable TNF inhibitors3, 6–8. Etanercept and adalimumab are effective in reducing the signs and symptoms of RA, and inhibiting the progression of structural damage4, 5, 9. The overall treatment experience, which includes what the patient experiences at the time of injection (eg, ISBS), may contribute to a patient’s treatment preference between injectable TNF inhibitors.
Understanding the prevalence of ISBS in patients with RA is an important starting point in raising awareness of the potential impact of ISBS on outcomes (including satisfaction with therapy) for RA patients receiving long-term injectable biologic therapy. In 2008, the Rheumatoid Arthritis Patients Using Injectable Etanercept and Adalimumab (RACE) survey was conducted to assess the rates of key symptoms and side effects, including ISBS, experienced by patients being treated for RA with an injectable TNF inhibitor. Data were collected through both retrospective and prospective assessments. The survey focused on patients treated with the 2 injectable TNF inhibitors approved at the time, etanercept and adalimumab. The survey objectives were threefold: (1) to examine the prevalence of ISBS among RA patients receiving etanercept or adalimumab; (2) to compare the portion of patients with ISBS documented in medical records to rates reported by patients when specifically and prospectively asked about ISBS by their physicians; and (3) to identify factors independently associated with ISBS.
PATIENTS AND METHODS
Overall Survey Design
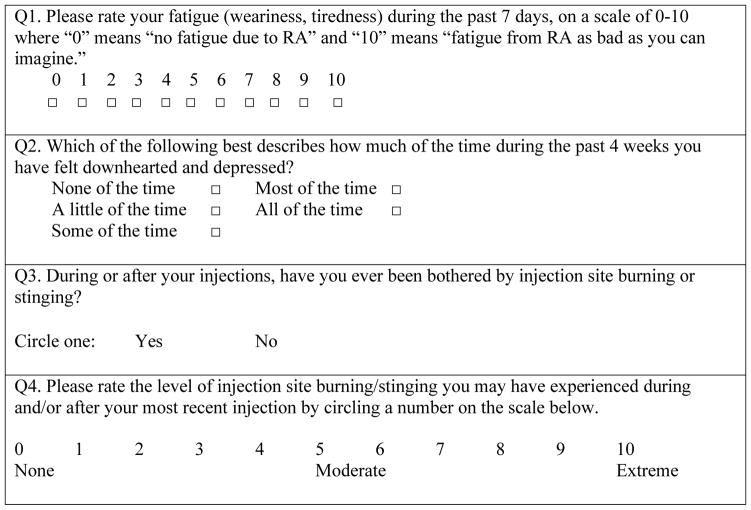
The RACE survey collected data retrospectively and prospectively on patients receiving etanercept or adalimumab. Data for the retrospective cohort of patients were collected via medical record review. Data for the prospective patient cohort were collected by patient assessments based on office visits. In the retrospective phase of the survey, rheumatologists were asked to complete 5 medical record reviews for RA patients who were currently being treated with either etanercept or adalimumab who had visited their offices in the preceding 6 months to assess whether they had recorded the occurrence of “injection-site reactions, pain, burning or stinging,” as well as additional common symptoms and comorbid conditions associated with RA. In the prospective phase, rheumatologists were asked to prospectively collect data and conduct brief interviews over a 3-week period with at least 1 and as many as 50 patients receiving SQ etanercept or adalimumab. Patients were asked direct questions specifically about ISBS by their physicians (see Table 1, which lists the questions asked in the prospective phase), as well as questions about additional common symptoms and comorbid conditions of RA.
Table 1.
Questions Asked of Patients in the Prospective Assessment
|
Physician Recruitment and Data Collection
International Medical Statistics (IMS) Health physician-level prescribing data were used to identify rheumatologists having considerable experience with SQ TNF inhibitors. Invitations to participate in the survey were distributed to 1720 rheumatologists who, based on the number of prescriptions written, represented the top 40% of rheumatologists with SQ TNF inhibitor experience between March 2007 and February 2008. Physicians identified in this way were recruited via e-mail or fax from either an opt-in list of physicians who agreed to do market research, or from the licensed American Medical Association (AMA) contact list. The targeted number of physician respondents was 100. Physicians responding to the request for participation were screened for eligibility. In order to qualify, physicians had to be board-certified or board-eligible rheumatologists, with at least 2 but no more than 30 years in practice. Additionally, each physician must have seen at least 5 patients who met the criteria described below.
In the first phase of the survey, rheumatologists completed 5 retrospective medical record reviews; data were collected online. Each physician was asked to randomly select 5 patient records for RA patients who were being treated with etanercept or adalimumab and had a follow-up visit for RA during the preceding 6 months; selected patients must have received at least 1 injection of their prescribed SQ TNF inhibitor. In addition to providing basic patient demographic information and a brief medical history, physicians were asked to check patients’ charts for any recorded mention of stinging, burning, or other injection-site reactions. Data were obtained only from the patients’ charts or records, rather than by physician recall.
Rheumatologists who completed the retrospective medical record review were invited to participate in the prospective patient interview phase of the survey. Rheumatologists who agreed to participate were asked to complete at least 1 and as many as 50 patient interviews based on office visits within the subsequent 3 weeks and track patients’ experiences with etanercept or adalimumab. Physicians could report on their own patients and on those seen by other rheumatologists in their practice, as long as a rheumatologist or a designated allied health professional (eg, nurse practitioner or rheumatology nurse) asked the patient the survey questions, 2 of which had to do with ISBS (see Table 1, Questions 3 and 4). Patient data were reported anonymously and within Health Insurance Portability and Accountability Act (HIPAA) guidelines.
Data Assessment
The proportion of patients experiencing ISBS was assessed in the retrospective and prospective phases of the study and evaluated using descriptive statistics. In the prospective phase of the survey, multivariable ordinal logistic regression analysis was conducted to assess the risk of a patient experiencing ISBS, evaluating a number of factors, including cumulative duration of use of etanercept or adalimumab, gender, and injector type (autoinjector, pre-filled syringe, and vial/syringe [etanercept only]). The logistic regression analyses modeled an ordinal outcome as the probability of experiencing higher levels of ISBS, rated on a 10-point scale (see Table 1, Question 4); this was facilitated by regrouping the 10-point ISBS ratings into 3 groups: 0–5 (reference group), 6–8, and 9–10. The proportional odds assumption was checked by evaluating these categories as nominal groups. Generalized estimating equations accounted for patient clustering within physician practices, because patients treated by the same physician could have more similar outcomes than those treated by different physicians.
Physician participant characteristics were compared with those of other US rheumatologists using data obtained from several other large studies and surveys10–12. Similarly, patient characteristics were compared with data for other RA patients, and stratified by injector type, as well as time on therapy. Data for the comparator RA patient population was obtained from IMS PharMetrics, with qualified patients representing those who (1) were diagnosed with RA with at least 6 months continuous prior enrollment in the same health plan, (2) were on a SQ biologic for RA during the period, August – September 2008, and (3) were continuously enrolled in the same health plan at least 6 months prior to and 3 months after the first treatment with a SQ biologic.
RESULTS
Physician Characteristics
One hundred thirteen rheumatologists responded to the survey and underwent screening (7% of the total invited). Of these, 101 (89%) met the entry criteria and completed the retrospective phase of the survey; 2 respondents did not qualify, and 10 did not complete this part of the survey. Subsequently, 87 physicians (86% of the 101 completing the previous section) provided records for the prospective phase of the survey. Of these, 37 (43%) respondents provided 50 or more patient records; all records were included in the event that a physician provided more than 50 patient records. The demographic profile of physician participants (Table 2a) was similar to that of comparator, treating US-rheumatologist physician samples in terms of years in practice, number of patients managed,10 and patients’ RA severity12. Participants had, on average, 15 years in practice; each physician managed an average of 524 patients with RA.
Table 2a.
Physician Demographics and Survey Responses From Physicians Participating in the RACE Survey
| Characteristic | Survey Population (N = 101) |
|---|---|
| Years in practice, mean | 15 |
| Location, % | |
| East Coast | 50 |
| Midwest | 21 |
| Gulf Coast | 12 |
| Rocky Mountains | 1 |
| West Coast | 17 |
| Number of RA Patients in practice*, mean | 524 |
| Disease severity of patients in practice†, % | |
| Mild | 23 |
| Moderate | 49 |
| Severe | 28 |
Estimated number of unique patients currently being managed for rheumatoid arthritis (RA); patients managed by others in the same practice were not counted.
Physicians were asked to classify the RA patients that they currently manage into each category of disease severity.
Patient Characteristics
The mean patient age was 52 years in each section of the RACE survey, and the male:female ratio was approximately 1:2. In total, 3830 patients were included in the retrospective or prospective portions of the survey (504 and 3326 patients, respectively). The comparator RA patient population consisted of 3239 patients. Age and gender profiles were similar between the retrospective and prospective phases of the survey, as were the proportions of patients using etanercept versus adalimumab (Table 2b). The mean age and the gender profile of the comparator RA patient sample were similar to that of the retrospective and prospective populations (Table 2b). One notable difference between the survey population and the comparator RA population was the inclusion of a slightly higher percentage of patients younger than 45 years in RACE (Table 2b). According to patient records and patient surveys, on average, 79% and 71% of patients in the retrospective and prospective phases of the survey, respectively, had moderate to severe disease (Table 2b).
Table 2b.
Patient Demographics and Survey Responses From Patients Participating in RACE Survey and From Comparator RA Patient Population
| Characteristic | Retrospective Chart Review Population (N = 504) | Prospective Assessment Population (N = 3326) | Comparator RA Patient Population‡ (N = 3239) |
|---|---|---|---|
| Mean age, years | 52 | 52 | 51 |
| Gender: female, % | 67 | 70 | 65 |
| Disease severity, % | |||
| Mild | 21 | 29 | NA |
| Moderate | 65 | 59 | NA |
| Severe | 14 | 12 | NA |
| Current biologic treatment, % | |||
| Etanercept | 54 | 56 | 64 |
| Adalimumab | 46 | 44 | 36 |
| >6 months on biologic, % | |||
| Etanercept or adalimumab | 55 | 68 | 81 |
| Etanercept | 54 | 71 | 84 |
| Adalimumab | 55 | 65 | 77 |
| Administration form: autoinjector pen*, % | 68 | 64 | NA |
| Injection performed by: patient†, % | 90 | 88 | NA |
NA, data not available.
Other options in survey: vial/syringe; pre-filled syringe.
Other options in survey: physician or allied health professional; someone else (eg, family member, friend).
From IMS Pharmetrics database
In both the chart review and prospective patient-interview portions of the survey, most patients used the autoinjector pen and self-injected (Table 2b). The proportion of patients with more than 6 months on biologic therapy was lower in the retrospective section than in the prospective section (55% vs 68%; Table 2b).
Injection-Site Burning and Stinging Results
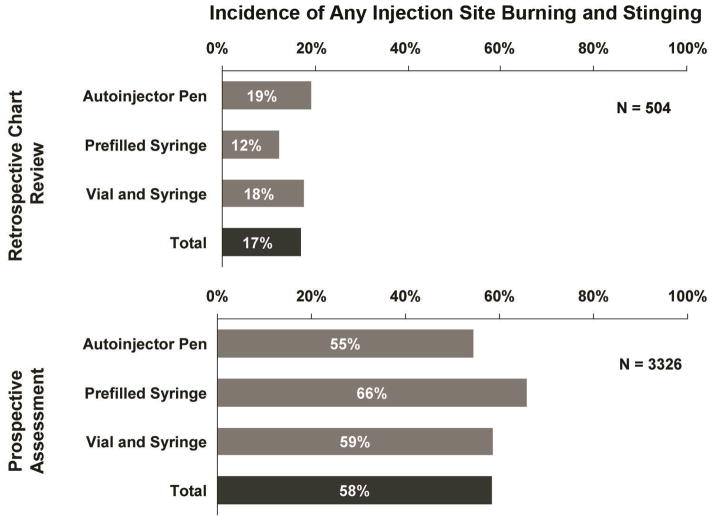
In the retrospective phase of the survey, ISBS was recorded in 86 out of 504 (17%) patient charts. In comparison, among 3326 patients explicitly asked about ISBS, 1939 (58%) reported experiencing at least some ISBS (Figure 1). Additionally, out of these 1939 patients who experienced at least some ISBS, 429 (22%, and 13% of the overall cohort) rated the level of burning/stinging as moderate to severe (ie, at least a 5 on a scale of 0 to 10) (Figure 2). In the prospective patient cohort, the proportion of patients receiving etanercept or adalimumab that experienced ISBS were similar (56% and 61%, respectively). In addition, the mean level of ISBS did not differ between the 2 agents.
Figure 1.
Injection-site burning and stinging (ISBS)—retrospective chart review versus prospective patient questioning. Incidence of ISBS was higher when physicians explicitly asked their patients about it compared to a review of medical records. Data are based on answers to the following questions: Retrospective chart review: “During the patient’s last visit, was there any explicit notation in the patient chart/record of any injection-site stinging/burning?” Prospective assessment: “Please rate the level of injection-site burning/stinging you experienced during and/or after your most recent injection using the scale below [10-point scale].”
A positive response is represented in this bar graph for all patients who reported a score of ≥1 in response to this question.
Figure 2.
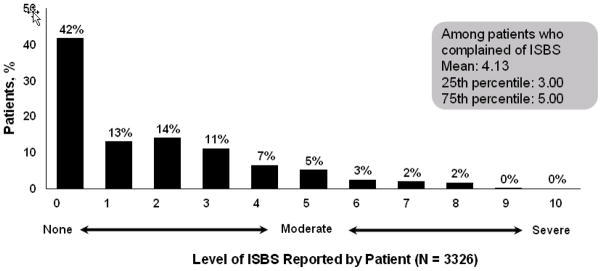
Distribution of ISBS severity. Data shown are from the prospective phase of the survey. Patients rated the level of ISBS experienced during and/or after the most recent injection using a 10-point scale. Among patients explicitly asked about ISBS, 1939/3326 (58%) reported experiencing at least some ISBS (summary of individual percentages/level of ISBS differs from actual percentage due to rounding (57% vs 58%, respectively); 429/1939 (22%) patients who experienced at least some ISBS (or 13% of the overall cohort [429/3326]) rated the level of burning/stinging as moderate to severe (ie, at least a 5 on a scale of 0 to 10).
A number of factors were significantly associated with the risk of greater ISBS (Table 3). Female gender, fibromyalgia, depression, and more severe RA were independently associated with a significantly increased likelihood of experiencing greater ISBS. The likelihood of ISBS was greatest for the etanercept vial and syringe, which had a roughly two-fold greater risk of ISBS compared with other injector mechanisms either for etanercept or adalimumab. Time on biologic therapy (≤6 months vs. >6 months, or <12 months vs. ≥12 months) and patient age did not significantly affect the likelihood of ISBS. Self-injection was associated with a decreased risk for ISBS compared with injection by a physician or an allied health care professional.
Table 3.
Multivariable-Adjusted Factors Evaluated For Their Risk of Greater Amounts of ISBS Among RA Patients Using Subcutaneously Injected Etanercept or Adalimumab
| Factor | Odds Ratio [95% CI] * |
|---|---|
| Female sex (referent to “Male”) | 1.5 [1.0, 2.3] |
| Fibromyalgia | 3.4 [1.0, 6.8] |
| Depression | 1.8 [1.1, 3.1] |
| Physician-assessed severity of RA | |
| Mild | 0.4 [0.2, 0.7] |
| Moderate | 0.4 [0.3, 0.8] |
| — Severe — | 1.0 (referent) |
| Administration form | |
| Etanercept vial and syringe | 2.4 [1.1, 5.1] |
| Etanercept prefilled syringe | 1.4 [0.9, 2.2] |
| Etanercept autoinjector | 1.0 [0.7, 1.4] |
| Adalimumab prefilled syringe | 1.1 [0.7, 1.7] |
| — Adalimumab autoinjector — | 1.0 (referent) |
| Person performing injection | |
| Patient | 0.3 [0.2, 0.6] |
| Someone else (eg, family member, friend) | 0.6 [0.3, 1.1] |
| Health care provider | 1.0 (referent) |
Factors included in this analysis, but which were not found to be associated with greater ISBS: time on biologic (≤6 months vs >6 months); patient age.
CI, confidence interval; OR, odds ratio.
This analysis models the probability of experiencing a greater amount of ISBS; patients were asked to rate the level of ISBS experienced during and/or after their most recent injection, on a scale of 0 (none) to 10 (extreme). Generalized Estimating Equations were used to adjust for clustering of patients within physician practices.
DISCUSSION
In the retrospective portion of this survey, the prevalence of ISBS was in line with what was previously reported for injection-site pain and reactions in clinical trials4, 5. In the prospective portion, when patients were specifically asked about ISBS associated with the use of SQ etanercept and adalimumab, the prevalence of ISBS was high (58%). Of these individuals, approximately one quarter experienced moderate to severe ISBS, which represented 13% of the overall cohort. The prevalence of ISBS when patients were specifically queried was more than three-fold greater than what was documented in the retrospective review of medical records, suggesting that physicians may not be aware of the high prevalence of ISBS. If patients experience ISBS, switching administration forms of the same medication, switching medications, or employing strategies to minimize ISBS, as is done for patients taking injectable biologic therapies for multiple sclerosis (MS),13 may improve some patients’ experiences with injectable biologic therapies.
Injection-site burning and stinging may become more clinically important as the number of biologic therapies for RA increases. Historically, the occurrence of ISBS associated with SQ administration of TNF inhibitors may not have been a significant factor in selecting therapies in light of the substantial efficacy observed with these agents and the relatively similar ISBS profile of etanercept and adalimumab. As additional options for TNF inhibitors become available, and assuming comparable efficacy and safety between injectable anti-TNF agents, characteristics beyond efficacy and safety should be considered because they may contribute to the patient experience. This survey has shown that the incidence of ISBS is likely underestimated in many rheumatology practices, and that explicit questioning may identify patients who are experiencing ISBS. Identifying patients at risk of side effects such as ISBS could provide physicians with the opportunity to individualize therapy and enhance the physician-patient dialogue. In particular, we found that ISBS was greater for patients with more severe RA and those with fibromyalgia and depression. This latter observation may reflect the interplay between depression and pain sensitivity14. For these patients, side effects such as ISBS may be a more important component of patient preference for certain biologic agents and administration mechanisms than for patients without these conditions.
Although the impact of ISBS on clinical outcomes for patients with RA is unknown, there is evidence from patients with MS that shows pain during and after injections has been found to affect adherence15. Multiple sclerosis is a reasonable model of chronic disease from which to extrapolate to RA because, as is the case with RA, patients with MS obtain a symptomatic benefit from their treatment. In contrast, patients with chronic, often asymptomatic diseases (eg, osteoporosis or hypertension) may not be as readily aware of the benefits of medication. Patients with MS and RA might be expected to share a general reticence to discontinue effective treatments; however, patients with MS are more likely to adhere to treatment when injection-site reactions such as pain are minimized15, 16. It remains to be seen if similar patterns will emerge among patients with RA receiving injectable TNF inhibitors; this current survey was not able to directly assess the impact of ISBS (including severity) on adherence to therapy.
The biological mechanisms that mediate ISBS upon injection of TNF inhibitors are not well understood. Preclinical studies have explored a variety of mechanisms that may contribute to the understanding of why some TNF inhibitors are associated with severe injection-site pain, including burning and stinging. ISBS may be related to inflammatory mediators that are released during non-immune-stimulated mast cell degranulation17. Another area of investigation worth consideration is to evaluate the differential composition of TNF-inhibitor formulations, which can vary from a complex composition with strong buffering capacity5 to a more simple composition with weak buffering capacity18. Research to date has been limited to preclinical studies, and further work is needed to better understand the relevance of these potential mechanisms of action underlying ISBS to humans.
Among the strengths of our survey, we evaluated a large sample of more than 3000 RA patients who were specifically asked about ISBS using a standardized instrument administered by almost 100 US rheumatologists in diverse practice settings. However, in interpreting the results of this survey, certain limitations should be noted. Direct comparison of the observed results in the retrospective and prospective portions of the survey is not possible because these were not the same patient populations. However, these patients were drawn from the same physician practices, which should limit heterogeneity. We recognize that retrospective medical record review is an imperfect standard with which to judge the content of a past patient-physician encounter19, 20. The retrospective portion of the study occurred at a single time point and may potentially not be reflective of the general population of patients with RA who are using injectable TNF inhibitors. Additionally, it is possible that physicians and patients participating in and contributing to our analyses may not be representative of the spectrum of RA patients and their physicians in the United States. For example, we specifically sampled rheumatologists who had considerable experience with SQ TNF inhibitors. Nonetheless, the physician and patient characteristics were compared to other external data sources and found to be broadly similar. There also is a theoretical possibility for bias by physicians in selecting those patients who they thought might have injection site reactions.
Our survey was cross-sectional, which may account for the observation that the etanercept vial and syringe was associated with a higher risk of ISBS. It seems possible that many patients who had previously experienced significant ISBS with other administration forms of etanercept were subsequently switched to the etanercept vial and syringe prior to our survey; thus, our finding likely represents channeling of more sensitive etanercept patients to the vial and syringe. Finally, although our analysis showed no association between ISBS and the duration of therapy with etanercept or adalimumab, this conclusion is limited by the fact that patients were required to be current users of these medications, and we did not know patients’ prior experience patients had other SQ medications. For example, if a patient had initiated etanercept or adalimumab therapy, then experienced significant injection-site pain and discontinued therapy, this patient would not have been eligible to participate in the survey. Thus, the prevalence of ISBS reported here would be conservative, and the actual prevalence of ISBS may in fact be higher.
At the time this study was conducted, there were 2 FDA-approved injectable TNF inhibitors (etanercept and adalimumab) for the treatment of patients with RA. Since then, 2 additional injectable TNF inhibitors (golimumab and certolizumab pegol) have become available; thus, the applicability of the study results and conclusions to these newer agents is unknown at this time and warrants further investigation. Rates of injection site reactions and injection site pain with certolizumab pegol (200 mg Q2W plus methotrexate) were similar to placebo (plus methotrexate) groups in RAPID 121 (injection site reactions: 2.3% versus 0, respectively; injection site pain [including burning and stinging]: 2% versus 0, respectively) and Rapid 222 (injection site reactions: 1.2% versus 0, respectively; injection site pain [including burning and stinging]: 0 versus 0, respectively) placebo-controlled studies. Based on data from controlled phase 3 studies of golimumab in RA,23–25 2.4% to 11% of patients treated with golimumab (50 mg and 100 mg SQ, respectively) had injection site reactions compared with 2% to 3% of patients in the control-treated group; the most frequent manifestation was injection site erythema. occurring in 3% of patients treated with golimumab compared with 1% of patients in control-treated group.
In conclusion, data from this survey suggest that the prevalence of ISBS is likely to be underestimated in many rheumatology practices. When discussing treatment options with patients, efficacy, safety, and frequency of administration are often considered; given the high prevalence we observed, it seems reasonable to also discuss ISBS. Specifically asking about ISBS among patients recently starting or continuing SQ TNF inhibitor therapy may help to identify patients with ISBS who may benefit from forms of administration or medications with a lower prevalence of ISBS. Further studies are warranted to gauge the clinical importance of ISBS to patients and to evaluate more directly the comparative risk for ISBS between injectable TNF inhibitors.
CONCLUSIONS
In conclusion, findings from this study suggest that the incidence of ISBS among patients with RA may be higher than is generally documented or recognized by rheumatologists. When discussing TNF inhibitor treatment options with patients, injection-site burning, stinging, and reactions may be important factors to patients, in addition to efficacy, safety, and frequency of administration. Assuming comparable efficacy and safety between injectable anti-TNF agents, discussion of these considerations in light of the various treatment options may assist in the selection of the most appropriate therapeutic options based upon individual patient preferences.
Acknowledgments
Declaration of Funding
This study was sponsored by UCB Pharma Inc.
Writing and editorial assistance was provided by Jim Loss, PhD, CMPP, and David Schiffmann, PhD (ETHOS Health Communications) in compliance with the international guidelines for Good Publication Practice. Data analyses were performed by Ozge Uluscu, PhD, and Rob Sederman, MBA (C1 Consulting). UCB Pharma, Inc provided financial support for writing/editorial assistance and data analyses.
Footnotes
Declaration of Financial/Other Relationships
JRC is a consultant for Roche/Genentech, UCB, Amgen, Centocor, and CORRONA; has received research funding from Amgen, Roche/Genentech, UCB, Centocor, BMS, and CORRONA. CH and KH are employees of UCB.
References
- 1.Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–39. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 2.van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–74. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 3.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 4.Enbrel [prescribing information] Thousand Oaks, CA: Immunex Corp; 2010. [Google Scholar]
- 5.Humira [prescribing information] North Chicago, IL: Abbott Laboratories; 2010. [Google Scholar]
- 6.Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–59. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 7.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol. 2009;36:898–906. doi: 10.3899/jrheum.080791. [DOI] [PubMed] [Google Scholar]
- 8.Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–86. doi: 10.7326/0003-4819-130-6-199903160-00004. [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Ruiz A, Pijoan JI, Ansuategui E, et al. Tumor necrosis factor alpha drugs in rheumatoid arthritis: systematic review and metaanalysis of efficacy and safety. BMC Musculoskelet Disord. 2008;9:52. doi: 10.1186/1471-2474-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cush JJ. Biological drug use: US perspectives on indications and monitoring. Ann Rheum Dis. 2005;64(suppl 4):iv18–iv23. doi: 10.1136/ard.2005.042549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furfaro N, Dewing K, Carlone J, Hansbrough K. Nurse and physician differences in perception of patient knowledge and approach to education in the treatment of rheumatoid arthritis. Abstract presented at: American College of Rheumatology 2008 Annual Scientific Meeting; October 24–29, 2008; San Francisco, CA. [Last accessed 13 May 2010]. Available at: http://acr.confex.com/acr/2008/webprogram/Paper2043.html. [Google Scholar]
- 12.New study highlights need for better interaction and education between rheumatoid arthritis patients and care providers [Reuters Web site] [Last accessed 13 May 2010];2008 Oct 28; Available at: http://www.reuters.com/article/pressRelease/idUS140818+28-Oct-2008+PRN20081028.
- 13.Jolly H, Simpson K, Bishop B, et al. Impact of warm compresses on local injection-site reactions with self-administered glatiramer acetate. J Neurosci Nurs. 2008;40:232–39. doi: 10.1097/01376517-200808000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Katona C, Peveler R, Dowrick C, et al. Pain symptoms in depression: definition and clinical significance. Clin Med. 2005;5:390–95. doi: 10.7861/clinmedicine.5-4-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen BA, Rieckmann P. Emerging oral therapies for multiple sclerosis. Int J Clin Pract. 2007;61:1922–30. doi: 10.1111/j.1742-1241.2007.01561..x. [DOI] [PubMed] [Google Scholar]
- 16.Ross AP. Tolerability, adherence, and patient outcomes. Neurology. 2008;71(suppl 3):S21–S23. doi: 10.1212/WNL.0b013e31818f3dcb. [DOI] [PubMed] [Google Scholar]
- 17.Lamour S, Bracher M, Nesbitt A. The PEG component of certolizumab pegol inhibits degranulation by stimulated mast cells. Abstract presented at: American College of Rheumatology 2009 Annual Scientific Meeting; October 17–21, 2009; Philadelphia, PA. [Last accessed 13 May 2010]. Available at: http://acr.confex.com/acr/2009/webprogram/Paper13684.html. [Google Scholar]
- 18.CIMZIA [prescribing information] Smyrna, GA: UCB, Inc; 2010. [Google Scholar]
- 19.Kimberlin CL, Winterstein AG. Validity and reliability of measurement instruments used in research. Am J Health-Syst Pharm. 2008;65:2276–84. doi: 10.2146/ajhp070364. [DOI] [PubMed] [Google Scholar]
- 20.Worster A, Haines T. Advanced statistics: understanding medical record review (MRR) studies. Acad Emerg Med. 2004;11:187–192. [PubMed] [Google Scholar]
- 21.Keystone E, van der Heijde D, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis. Arthritis Rhem. 2008;58:3319–29. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 22.Smolen J, Landewe RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomized controlled trial. Ann Rheum Dis. 2009;68:797–804. doi: 10.1136/ard.2008.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor α given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68:789–96. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor α inhibitors (GO-AFTER study): a multicentre, randomized, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210–21. doi: 10.1016/S0140-6736(09)60506-7. [DOI] [PubMed] [Google Scholar]
- 25.Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor α monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naïve patients with active rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–83. doi: 10.1002/art.24638. [DOI] [PubMed] [Google Scholar]