Abstract
Rationale
Pharmacological activation of GABAB receptors in the dorsal raphé nucleus (DRN) can escalate territorial aggression in male mice.
Objectives
We characterized this escalated aggression in terms of its behavioral and environmental determinants.
Methods
Aggressive behavior of resident male (CFW or ICR mouse) was assessed in confrontations with a group-housed intruder. Either baclofen (0.06 nmol/0.2 μl) or vehicle (saline) was microinjected into the DRN ten minutes before the confrontation. We examined baclofen-heightened aggression in five situations: aggression in a neutral arena and after social instigation (experiment 1), aggression during the light phase of the cycle (experiment 2), aggression without prior fighting experience (experiment 3), aggression toward a female (experiment 4), and aggression after defeat experiences (experiment 5). In addition, we examined the body targets towards which bites are directed and the duration of aggressive bursts after baclofen treatment.
Results
Regardless of the past social experience, baclofen escalated aggressive behaviors. Even in the neutral arena and after defeat experiences, where aggressive behaviors were inhibited, baclofen significantly increased aggression. Baclofen increased attack bites directed at vulnerable body areas of male intruders but not toward a female and only in the dark. Also, baclofen prolonged the duration of aggressive bursts.
Conclusions
For baclofen to escalate aggression, specific stimulation (male intruder) and tonic level of serotonin (dark cycle) are required. Once aggressive behavior is triggered, intra-DRN baclofen escalates the level of aggression to abnormal levels and renders it difficult to terminate. Also, baclofen counteracts the effects of novelty or past experiences of defeat.
Keywords: Dorsal raphé nucleus (DRN), GABAB receptor, serotonin (5-HT), escalated aggression, winning, defeat, light/dark period, female intruder, vulnerable targets, termination of aggressive behavior
Introduction
Serotonin (5-HT) has been implicated in the escalation of aggression in many species (Coccaro et al. 1997; Miczek et al. 2002, 2007; Kravitz and Huber 2003; de Boer and Koolhaas 2005). Most of the forebrain 5-HT is derived from rostral raphé nuclei, especially the dorsal and median raphé nuclei (DRN and MRN, respectively). The activity of 5-HT neurons in the raphé nuclei is regulated by several neurotransmitters and neuropeptides (Adell et al. 2002). GABA is one of the neurotransmitters that has a critical role in modulating 5-HT neural activity, and both GABAA and GABAB receptors are localized in the raphé nuclei (Bowery et al. 1987). Previously, we have shown that the activation of GABAB receptors in the DRN, but not MRN, enhanced territorial aggression in male mice (Takahashi et al. 2010a, 2010b). In vivo microdialysis showed that microinjection of an aggression-heightening dose of the GABAB receptor agonist, baclofen into the DRN increased the extracellular level of 5-HT in one of the key projection area, the medial prefrontal cortex (mPFC). This was possibly the result of the action of baclofen at presynaptic GABAB receptors located on non-serotonergic neurons within the DRN. The temporal pattern of increased 5-HT release in mPFC induced by intra-DRN baclofen corresponded to the aggression-heightening effect of baclofen, suggesting that increased 5-HT release in mPFC and escalated aggression are synchronized (Takahashi et al 2010b). In contrast, there was no interaction between escalated aggression induced by alcohol consumption and GABAB receptors in the DRN (Takahashi et al. 2010a). Therefore, it is likely that GABAB modulation in the DRN is involved in certain types of aggression but not others. In this study, we conducted further detailed characterizations to understand the determinants of escalated aggression induced by GABAB activation in the DRN.
The experience of aggressive encounters (either winning or defeat) can alter an animal’s subsequent behavior and also neural pathways in the brain (Miczek et al. 1982; Amorim and Almada 2005; Hsu et al. 2006; Haney et al. 1990; Coates and Herbert 2008; Oliveira et al. 2009; Fuxjager et al. 2010). It has been shown that repeated successful aggressive encounters can enhance the probability of winning in the next encounter, and also change the level of testosterone and the expression of androgen receptors in the brain reward system (Kudryavtseva et al. 2004; Caramaschi et al. 2008; Fuxjager et al. 2010). Previously, we observed that intra-DRN baclofen escalated aggression in male mice that had experienced more than ten victorious aggressive encounters (Takahashi et al. 2010a, 2010b). These repeated winning experiences in the past may have induced neural changes, and baclofen could thereby escalate aggression in those animals. On the other hand, repeated defeat experiences inhibit aggression in several animal species (Rutte et al. 2006) and eventually induce depressive-like behaviors (Miczek et al. 2008; Golden et al. 2011; Shimamoto et al. 2011). We sought to examine whether baclofen can escalate aggressive behaviors in animals without any past fighting experience, and also whether baclofen can restore aggression that has been suppressed by past defeat experiences.
In preclinical models for escalated and “pathological” aggression, there are changes in both quantity and quality of aggressive behaviors (Miczek et al. 2002; de Almeida et al. 2005; Haller and Kruk 2006). In contrast to the ritualistic species-typical aggressive behaviors, some models for abnormal aggression showed attacks directed at vulnerable body targets such as the face and abdominal areas (Haller et al. 2001). Other models of abnormal aggression in male rodents highlight aggressive behaviors against females (Sluyter et al. 2003). The form of the aggressive behaviors must be considered when evaluating whether aggression is to be considered species-typical or “pathological.”
In this study, we investigated the effect of intra-DRN baclofen treatment on aggression in several conditions to characterize whether (1) baclofen-heightened aggression is observed in novel surroundings which usually inhibit aggressive behavior, and after social instigation that escalates aggression, (2) it depends on the phase of the light-dark cycle, (3) it requires previous experience of victory, (4) it is inhibited by past defeat experience, and (5) it is categorized as “pathological” in terms of the pattern and target of the attack (i.e. males attacking females).
Method
Animals
Male CFW mice (Charles River Laboratories, Wilmington, MA) aged 4–5 weeks upon arrival were used as both residents and intruders for experiment 1 and 7. Male ICR mice (CLEA Japan, Inc, Tokyo, Japan) aged 5 weeks upon arrival were used for experiments 2 through 6. Resident males were housed in pairs with a female in a polycarbonate cage (28×17×14 cm for Experiment 1 and 22×32×13.5 cm for all other experiments) with wood chips as bedding material. Intruder males were group housed either seven to ten per cage (48×26×14 cm) with corn cob bedding (for experiment 1, 7) or five to seven per cage (22×32×13.5 cm) with wood chips (for experiments 2–6). Experiment 1and 7 were conducted at Tufts University in a vivarium with controlled humidity and temperature (35–40 %, 21±1 °C) on a reversed 12-h-light/dark cycle (lights off at 7:00 AM). Experiments 2–6 were conducted at the National Institute of Genetics (NIG), Japan with controlled humidity and temperature (50±10 %, 23±2 °C) on a 12-h-light/dark cycle (lights off at 8:00 PM). Food and water were freely available. All the behavioral experiments except experiment 2 were performed during the dark period. All procedures were approved either by the Institutional Animal Care and Use Committee (IACUC) of Tufts University or by the Institutional Committee for Animal Care and Use of NIG.
We used two different types of closed colony mouse (CFW and ICR, both albino) and tested them in two different laboratories (Tufts and NIG, respectively) in accordance with the move of the first author (AT). We compared aggressive behavior of CFW and ICR mice, that have been tested in this study and other studies (n=95 for CFW and n=85 for ICR), to assess the effects of genetic background and test environment on behavior and on the pharmacological effect of intra-DRN baclofen. There was no difference in the number of aggressive encounters to obtain stable levels of attack bites between CFW (X̄ = 10.0 encounters, Rang = 5–13) and ICR (X̄ = 9.8, Range = 6–13). In addition, the frequency of attack bites in animals with stable fighting level was not significantly different between CFW (X̄ = 31.0 bites, Range = 10–66) and ICR mice (X̄ = 28.4, Range = 10–53). Among these animals, n=61 CFW and n=30 ICR received the same dose of baclofen (0.06 nmol) into the DRN and were tested during the dark period of the light-dark cycle. Baclofen escalated attack bites equally in both CFW (185.1% increase) and ICR (189.9% increase) mice compared to vehicle treatment. Therefore, we decided to present the data from CFW and ICR mice together in this report.
Experiment 1: Aggression in the neutral arena and after social instigation
After being housed with a female for 3 weeks, the residents were tested for aggression toward an intruder male (Miczek and O’Donnell 1978). The female and pups were removed and an intruder male was introduced into the home cage of the resident male. Their behaviors were observed for 5 min after the first bite or the intruder was removed after 5 min if no attack bite occurred. Aggressive encounters were repeated every other day with the same intruder until the resident male showed a stable frequency of attack bites (<20 % variation). Once aggressive behavior in the home cage had stabilized, the residents were assessed more comprehensively for aggression in the home cage, in a neutral arena and also for heightened-aggression after social instigation. Each test was conducted once in a randomized sequence across animals.
Neutral arena tests
A novel and large polycarbonate cage (30×33×46 cm) with clean pine shavings on the floor was used for aggression tests. The resident and an intruder were placed into opposite corners of the neutral arena and their behaviors were observed for 5 min after the first bite, or the intruder was removed after 5 min if no attack bite occurred. Their behaviors were videotaped for later analysis. The novelty of this neutral arena reduces an animal’s aggression by ca. 50% compared to the home cage (Miczek and O’Donnell 1978).
Instigation procedure
The social instigation procedure was implemented as previously described (Fish et al. 1999). Briefly, a naïve male “instigator” was placed inside a protective shield (a clear, perforated, polycarbonate cylinder, 18×6 cm). The female and pups were removed from the home cage of the resident male, and the shielded instigator was placed in the middle of the cage. Immediately after the five-minute exposure to the instigator, the resident male was tested for aggression in the neutral arena using the same test procedure as before.
After the characterization of their aggression in these three situations, a guide cannula aimed at the DRN was implanted in the resident males (see Surgery and Cannulation). One week after surgery, residents were assessed for fighting 3 to 4 times in their home cage depending on their stability of fighting, and once in a neutral cage before starting microinjections. There was no difference in the levels of aggressive behaviors before and after the cannulation surgery (on average, 34.3 ± 7.8 bites and 33.2 ± 8.0 bites, before and after surgery, respectively).
Subsequently, we microinjected either saline or baclofen into the DRN of resident males in a counterbalanced order and assessed their aggression in the home cage, in a neutral arena, and after social instigation. Each resident male received a total of six microinjections, including three vehicle injections and three baclofen injections, again in counterbalanced order.
Experiment 2: Aggression during the light period
After 3 weeks of being housed with a female, the residents were tested for their aggression toward the same intruder male every other day during the dark period (10:00–12:00PM) until they showed stable levels of attack bites (<20 % variation). Once the home cage aggressive behavior had stabilized, they were tested for aggression in the daylight phase (1:00–3:00PM) until they showed stable aggression again (ranging from 1–2 encounters). There was no significant effect of the time of day on the frequency of attack bites.
Then all resident males were implanted with a cannula (see Surgery and Cannulation), and one week later they were assessed for fighting 3 times during the light phase before starting microinjections. There was no difference in the levels of aggressive behaviors before and after the cannulation surgery (on average, 27.6 ± 8.6 bites and 30.3 ± 14.0 bites, before and after surgery, respectively). Animals were then tested for aggression following intra-DRN microinjection of either saline or baclofen, administered 48 hours apart in counterbalanced order.
Experiment 3: Aggression without prior fighting experience
Resident males were pair-housed with females for 8 weeks so that they would be the same age as the experimental subjects in other experiments during aggression testing. A cannula was then implanted in each resident male (see Surgery and Cannulation), and ten days later its aggression toward a novel intruder male was assessed in the home cage. They were then tested for aggression after intra-DRN administration of saline and baclofen, as described in Experiment 2.
Experiment 4: Aggression against OVX female
The same animals that were used for experiment 3 were used for this study. Forty-eight hours after two encounters with a male intruder, baclofen was administered to all residents and a female intruder was then introduced into their home cage. Female intruders were ovariectomized ten days before the aggression test to ensure that all females were in a similar diestrus-like state.
Experiment 5: Aggression by mice that have been defeated
Males that had been used as intruders during repeated aggressive encounters before surgery were the subjects in this experiment. These males were housed in groups of five to seven per cage during the sequence of defeats. The test animals first had nine to eleven defeat experiences at 48-h intervals, receiving ca. 10 to 41 attack bites per session. Each was then implanted with a cannula, and they were housed individually for ten days, when their aggression toward a novel intruder male was assessed in their home cage. Microinjection and aggression testing then proceeded as in Experiment 3.
Experiment 6: Detailed attack behavior analysis
To further investigate whether the pattern of aggressive behavior after intra-DRN baclofen administration was abnormal or species-typical, we conducted an analysis of the attack targets. We used slow-motion video to re-analyze attack behavior following intra-DRN microinjection of baclofen in ICR mice during the dark phase (Takahashi et al. 2010b).
Experiment 7: Systemic baclofen treatment
To investigate the effect of systemically administered baclofen on aggressive behavior, we injected baclofen intraperitoneally and then examined changes in aggressive behavior. After 3 weeks of being housed with a female, the residents were tested for their aggressive behavior toward the same male intruder every other day during the dark period (10:00–12:00PM) until they showed stable levels of attack bites (<20 % variation). Intraperitoneal injections of saline were given every other day. Once the home cage aggressive behavior had stabilized, we combined saline i.p. injection and fighting three times to let animals habituate to test conditions. Animals were then tested for aggression following systemic administration of either saline or baclofen. All animals received in total two saline injections and four doses of baclofen injections (1, 1.78, 3, 5.8 mg/kg), administered 48 hours apart in counterbalanced order. The drug was injected 30 minutes before the aggression test.
Surgery and cannulation
Resident males were anesthetized by i.p. injection of a mixture of 100 mg/kg ketamine HCl and 10 mg/kg xylazine, and then were stereotaxically implanted with a 26-gauge guide cannula (Plastics One Inc., Roanoke, VA) aimed 2 mm above the dorsal raphé nucleus (DRN: AP, −4.2 mm; ML, ±1.5 mm; DV, −1.9 mm to bregma; angled 26° to the vertical) as calculated from a mouse brain atlas (Paxinos and Franklin 2001).A 33-gauge obdurator (Plastics One Inc., Roanoke, VA) that extended 0.5 mm beneath the tip of the guide cannula was inserted after surgery. The obdurator was moved daily to prevent blockage and scarring and also to habituate the animals to handling. After surgery animals were housed individually for 5 days to recover and were then pair-housed with the same female and pups. To prevent gnawing by the female, the obdurator and head mount were coated with a quinine preparation.
Microinjection and aggression test
The obdurator was removed and a 33-gauge microinjector (Plastics One Inc., Roanoke, VA) attached to PE 50 tubing was inserted into the guide cannula. The microinjector extended 2 mm below the end of the guide to reach the DRN. The other end of the tubing was connected to a 1 μL Hamilton syringe placed into an infusion pump (CMA Microdialysis, North Chelmsford, MA). The drug was infused in a volume of 0.2 μl over 2 min, and the microinjector was left in place for 1 min after the infusion to allow the drug to diffuse completely. Ten minutes after the microinjection, behavior was recorded for 5 min after the first attack bite. The test conditions and the number of animals for each experiment are summarized in Table 1.
Table 1.
Number of animals used in this study.
| Experiment | Animals included in analysis | Strain | Placements outside DRN | Did not finish |
|---|---|---|---|---|
| 1 Neutral arena and social instigation | 13 | CFW | 4 | 4* |
| 2 Aggression during the light period | 9 | ICR | 2 | |
| 3 No prior fighting experience | 10 | ICR | 1 | |
| 4 Aggression against OVX females | ||||
| 5 Mice that have been defeated | 9 | ICR | 3 | |
| 6 Detailed attack behavior analysis | 10+ | ICR | ||
| 7 Systemic baclofen effect | 11 | CFW |
Three animals died after surgery, and 1 animal lost its head mount.
Re-analyzed data from Takahashi et al. (2010b)
Histology
At the end of the experiment, mice were deeply anesthetized with ketamine and xylazine mixture and then were intracardially perfused with 0.9% saline followed by 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). After post-fixation in 4% PFA for at least 24 h, brains were placed into 30% sucrose solution. A freezing microtome was used to slice the brains into 60 μm sections which were stained with cresyl violet, and the placements of the cannulae were then verified. The injection site for each animal is depicted in Supplemental Fig. S1. The criterion for the correct placement was that the tip of the cannula resided within the DRN. If the estimated tip of the cannula was outside of the DRN, we categorized them as incorrect placements.
Drug
Baclofen ((±)-β-(aminomethyl)-4-chlorobenzenepropanoic acid) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was dissolved in 0.9% saline with ca. one minute of sonication. Baclofen was dissolved in the concentration of 15 mM and then aliquot and stored at −20 °C. Right before the test, this solution was sonicated again for one minute and then diluted to the final concentration of 0.3 mM (pH 6.2).
Behavioral analysis and statistics
Analysis of videotaped resident-intruder encounters was performed by a trained observer using software established in our laboratory (TanaMove0.07, NIG, Mishima, Japan) to quantify the frequency and duration of aggressive behaviors (attack bites, sideways threats, pursuit, tail rattles) and non-aggressive behaviors (walking, rearing, auto-grooming, contacts) (Grant and Mackintosh 1963; Miczek and O’Donnell 1978). The frequency of attack bites and sideways threats was analyzed, while duration was used for the other behaviors.
The targets of each bite were classified into two categories: vulnerable targets (head, throat, and abdomen) and non-vulnerable targets (neck, back, flanks; Haller et al. 2001). Video recordings were played in slow motion to allow precise identification of the targets. In addition, we calculated the attack/threat ratio (attack frequency/sideways threat frequency) and the duration of aggressive bursts, defined as a rapid sequence of attack bites and sideways threats. When either attack bites or sideways threats occurred less than one second apart, they were considered as part of a continuous burst. Then, the frequency distribution was calculated based on the duration of each continuous burst. Pearson’s Chi-square test was used to compare the frequencies of saline and baclofen in each class.
Repeated-measures one-way ANOVA was performed to examine the effect of the drug treatment on aggressive behaviors, non-aggressive behaviors and latency to the first bite. For experiment 1, repeated-measures two-way ANOVA was performed to examine the main effects and interaction of drug and experimental conditions (neutral arena and social instigation). In case of significant F value, Tukey-Kramer post-hoc tests were conducted (α=0.05). For experiment7, Dunnett’s t test was used as post-hoc tests to compare vehicle control with each dose of baclofen (α=0.05). One-way ANOVA was used to compare the mean duration of aggressive bursts between saline and baclofen treatments (experiment 6).
Results
Experiment 1: Aggression in the neutral arena and after social instigation
Aggressive behaviors of resident males in their home cage, in the neutral arena, and after social instigation were characterized before the surgery (Figure 1A). There was a significant effect of test condition on attack bites (F(2,24)=8.563, p=0.002), sideways threats (F(2,24)=4.137, p=0.029), tail rattles (F(2,24)=3.622, p=0.042) and walking (F(2,24)=22.367, p<0.001). Post-hoc analysis revealed a significant reduction in attack bites and a significant increase in walking in the neutral arena compared to home cage. Social instigation significantly increased attack bites and sideways threats compared to the neutral arena condition, almost returning them to home cage levels (p<0.05). There was a significant trend in attack latency (F(2,24)=3.269, p=0.055), and the latency to the first bite was shorter in home cage (7.3±1.5 sec) compared to neutral arena condition (34.5±12.1 sec) but not after social instigation (18.2±5.9 sec).
Figure 1.
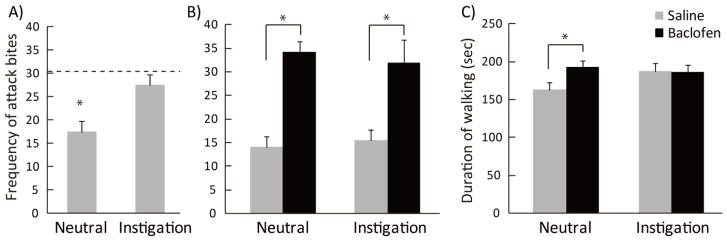
A) Pre-surgery characterization of attack bites in neutral arena (Neutral) and after social instigation (Instigation). Dotted line indicates the level of attack bites in the home cage. Effects of intra-DRN baclofen on B) frequency of attack bites and C) duration of walking in the neutral arena and instigation conditions. *p<0.05 compared to saline controls.
After animals were implanted with guide cannulae into the DRN, however, we found that the social instigation effect was no longer reliable. Two-way repeated-measures ANOVA showed neither a main effect of condition nor any drug×condition interaction on any aggressive behaviors and on attack latency. In contrast, a significant main effect of drug was observed on attack bites (F(1,24)=22.62, p<0.001), sideways threats (F(1,24)=19.19, p<0.001), pursuit (F(1,24)=19.79, p<0.001) walking (F(1,24)=5.48, p=0.028), contact (F(1,24)=9.70, p=0.005), and rearing (F(1,24)=7.18, p=0.013). Post-hoc tests showed that baclofen significantly increased aggressive behaviors including attack bites, sideways threats, and pursuit compared to saline treatment (Figure 1B, Table 2). In addition, baclofen significantly increased walking and reduced contact and rearing. A significant drug×condition interaction was observed only on walking, and baclofen significantly increased walking only in the neutral arena, not after social instigation (Figure 1C).
Table 2.
Aggressive and non-aggressive behaviors after intra-DRN baclofen or saline microinjection in several test conditions.
| Experiment 1
|
Experiment 2
|
Experiment 3
|
Experiment 5
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral cage | Social instigation | Light period | Without previous fight experiences | Repeated defeat experiences | ||||||
| Vehicle | Baclofen | Vehicle | Baclofen | Vehicle | Baclofen | Vehicle | Baclofen | Vehicle | Baclofen | |
| Sideways threats (f) | 18.4±2.5 | 36.2±5.4* | 19.3±2.6 | 33.8±4.6* | 31.0±6.7 | 28.0±2.1 | 27.6±3.8 | 43.9±6.4* | 4.8±1.9 | 18.7±5.0** |
| Attack bites (f) | 14.1±1.9 | 34.2±5.8* | 15.5±2.3 | 32.0±4.8* | 31.6±9.2 | 42.4±5.1 | 27.2±5.0 | 52.6±7.2* | 6.1±3.6 | 33.9±13.5* |
| Tail rattle (s) | 6.5±3.0 | 5.1±1.2 | 10.2±3.8 | 4.5±1.5 | 16.4±4.6 | 8.3±2.1 | 20.8±3.7 | 6.5±1.8** | 9.2±4.0 | 18.0±8.0 |
| Pursuit (s) | 0.3±0.1 | 1.4±0.3 | 0.5±0.2 | 1.1±0.3 | 0.1±0.1 | 0.1±0.1 | 0.3±0.2 | 1.8±0.7 | 0±0 | 0.2±0.2 |
| Grooming (s) | 10.7±1.7 | 14.8±4.0 | 16.6±2.7 | 18.4±2.8 | 23.3±6.3 | 43.3±12.1 | 20.4±8.4 | 5.0±1.8 | 8.0±2.7 | 7.7±2.5 |
| Rearing (s) | 40.5±7.6 | 22.5±3.0* | 31.2±4.9 | 26.1±3.5 | 41.3±10.3 | 53.1±6.1 | 23.0±7.1 | 22.7±4.0 | 93.5±8.4 | 85.9±7.3 |
| Walking (s) | 162.7±9.7 | 192.9±11.3* | 187.2±8.8 | 185.9±9.6 | 52.4±5.4 | 41.6±4.4* | 64.1±9.0 | 86.7±13.2 | 54.4±107 | 39.5±7.1 |
| Contact (s) | 3.4±0.9 | 1.1±0.2* | 1.5±0.3 | 0.9±0.3 | 17.1±9.9 | 9.5±5.4 | 13.9±6.5 | 16.8±5.2 | 105.2±16.3 | 83.5±14.3 |
| Mounting (s) | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 2.6±2.6 | 6.7±5.0 |
| Latency to first bite (s) | 24.2±5.6 | 31.5±14.4 | 19.8±3.3 | 12.3±4.6 | 13.1±9.5 | 3.1±1.2 | 65.4±28.0 | 15.2±4.4 | 237.3±30.1 | 134.4±38.7* |
p < 0.05,
p < 0.01 between vehicle control and baclofen within condition. (f) frequency, (s) duration (sec)
Experiment 2: Aggression during the light period
Repeated-measures one-way ANOVA showed no significant effect of intra-DRN baclofen on aggressive behaviors during the light phase (Figure 2, Table 3). There was a slight increase of attack bites after baclofen treatment, however this change was not significant (F(1,8)=2.34, p=0.165). A significant effect of drug was observed only on walking (F(1,8)=6.98, p=0.030), which was reduced by baclofen treatment. There was no significant difference in their attack latency.
Figure 2.

Attack bites and sideways threats after intra-DRN saline (gray bar) or baclofen (black bar) microinjection during the light phase. The inserted figure shows the effects of saline and baclofen in the same strain of mice during the dark phase. * p<0.05 compared to saline controls.
Table 3.
Systemic administration (i.p.) of baclofen and aggressive behaviors (Experiment 7)
| Control | 1 mg/kg | 1.78 mg/kg | 3 mg/kg | 5.6 mg/kg | |
|---|---|---|---|---|---|
| Sideways threat (f) | 20.8±3.4 | 23.3±2.3 | 14.7±1.1 | 26.5±4.4 | 13.5±2.5 |
| Attack bite (f) | 18.7±3.0 | 25.2±3.8 | 21.6±1.5 | 28.0±4.1* | 11.5±2.1 |
| Tail rattle (s) | 35.3±5.8 | 40.4±4.8 | 32.4±3.9 | 37.1±5.6 | 11.1±2.9* |
| Pursuit (s) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Grooming (s) | 26.8±3.4 | 18.4±3.6 | 20.3±4.9 | 16.1±3.0 | 7.3±4.3* |
| Rearing (s) | 30.7±5.1 | 34.5±9.4 | 44.7±10.9 | 24.8±7.9 | 5.5±3.1* |
| Walking (s) | 43.5±6.0 | 33.7±4.3 | 49.1±7.3 | 33.6±5.6 | 18.1±6.5* |
| Contact (s) | 3.5±2.0 | 0.1±0.1 | 0.5±0.4 | 1.5±0.8 | 9.5±3.9 |
p<.05 versus vehicle control. (f) frequency, (s) duration (sec).
Experiment 3: Aggression without prior fighting experience
Repeated-measures one-way ANOVA showed a significant effect of baclofen on attack bites (F(1,8)=9.04, p=0.017), sideways threats (F(1,8)=9.60, p=0.015), and tail-rattling (F(1,8)=17.71, p=0.003). Post-hoc tests showed that intra-DRN baclofen heightened attack bites and sideways threats, and reduced tail-rattling compared to saline treatment (Figure 3A, Table 4). We excluded one animal from the analysis because it did not show any aggressive behavior after either saline or baclofen treatment. There were no differences in pursuit and non-aggressive behaviors as a result of baclofen and saline. The latency to the first bite was shorter after baclofen than saline (Table 4), but this difference was not statistically significant (F(1,8)=2.699, p=0.139). Attack bite frequencies after saline and baclofen treatments were similar to those seen in mice that had repeated attack experience (Takahashi et al. 2010b, Figure 2 insert).
Figure 3.

Baclofen effects on attack bites and sideways threats after intra-DRN saline (gray bars) or baclofen (black bars) microinjection in mice without past fighting experience (A) and in mice that have been defeated (B). * p<0.05 compared to saline vehicle.
Experiment 4: Aggression against OVX female
None of the 11 males tested (ten with correct guide cannula placement, and one with the placement outside of DRN) showed aggressive behavior toward an ovariectomized female intruder after baclofen treatment.
Experiment 5: Aggression by mice that have been defeated
While 5 out of 10 previously defeated animals attacked an intruder after saline microinjection, 7 such animals attacked after intra-DRN baclofen treatment. Repeated-measures one-way ANOVA showed a significant effect of baclofen on attack bites (F(1,9)=5.61, p=0.042), sideways threat (F(1,9)=11.65, p=0.008) and attack latency (F(1,9)=5.27, p=0.047). Post-hoc tests showed that baclofen significantly increased the frequency of attack bites and sideways threat and shorten the latency to the first bite compared to saline control (Figure 3B, Table 5). When compared with the animals that did not have any fighting experience (Experiment 3; Figure 3A), the level of aggressive behavior was very low in previously defeated animals after both saline (6.1±3.6 and 27.2±5.3 bites) and baclofen treatment (33.9±13.5 and 52.6±7.6 bites in defeated and non-defeated animals, respectively). However, baclofen treatment increased the level of aggression of defeated animals to the baseline level of non-defeated animals.
Experiment 6: Body targets directed by attack
We used slow-motion video to re-analyze attack behavior following intra-DRN microinjection of baclofen in ICR mice during the dark phase (Takahashi et al., 2010b). On average, mice showed 65.8 ± 10.3 bites after baclofen treatment, compared to 29.0 ± 5.5 bites in control (F(1,9)=28.334, p<0.001). The frequencies of attack bites were higher than in the previously published study because the detailed slow-motion analysis counted small nips that were not included in the initial real-time analysis. Body targets bitten during attack were categorized into six regions (head, throat, abdomen, neck, back, and flanks). Repeated ANOVA showed significant effects of baclofen on attacks directed at abdomen (F(1,9)=18.608, p=0.002) and flanks (F(1,9)=51.311, p<0.001), and baclofen significantly increased attack bites toward the abdomen and flanks compared to controls (Figure 4). The percentage of attacks directed at vulnerable targets was significantly higher in baclofen-treated animals (22.9%) than controls (7.7%, F(1,9)=10.401, p=0.010).
Figure 4.
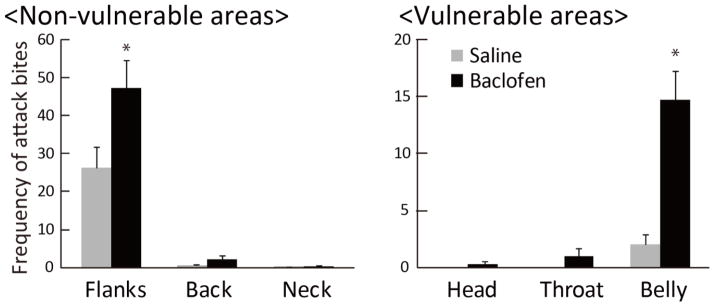
Baclofen effects on attack targets. *p<0.05 compared to saline.
The duration of aggressive bursts was also examined using the same data set. The temporal pattern of aggressive behaviors during a 5 min test after either saline or baclofen treatment in two representative animals is represented in Figure 5A. It seemed that the duration of bursts became longer after baclofen treatment, and thus we analyzed the duration of each aggressive burst. In total, there were 166 and 206 aggressive bursts in saline and baclofen treated animals, respectively. Baclofen increased the frequencies of long bursts, but not short bursts, compared to saline (Figure 5B,C). Pearson’s chi-square test showed a significant effect of baclofen in the longest burst class (>10 sec; χ2(1) =7.14, p<0.05) and also slightly in an intermediate burst class (1.0<t<3.2 sec; χ2(1)=3.27, p<0.10). However, there was no difference in the frequencies of short bursts (<1.0 sec) between the treatment. Thus, baclofen-treated animals showed more prolonged aggressive bursts than saline-treated mice. The average duration of aggressive bursts was longer after baclofen treatment than after saline (F(1,370)=3.885, p=0.0495).
Figure 5.
(A) Temporal pattern of aggressive behaviors of resident mice after intra-DRN saline and baclofen treatments. (B) Scatter plot of the duration of each aggressive burst in saline (Sal) and baclofen (Bac) treated animals. (C) Frequency distribution of the duration of aggressive bursts in saline and baclofen treated animals. The interval of each class follows to half-log step. * p<0.05, + p<0.10 compared to saline. An aggressive burst consists of attack bites and sideways threats, and it was considered as a continuous burst event when the next bite or threat occurred within one second.
The attack/threat ratio was slightly high in baclofen treatment (1.90 ± 0.35) than saline (1.19 ± 0.16). However, this difference was not statistically significant (F(1,9)=3.052, p=0.1146).
Experiment 7: Systemic treatment baclofen
Repeated-measures one-way ANOVA showed a significant effect of baclofen on aggressive behaviors (F(4,40)≥4.201, p≤0.0062 for attack bites, sideways threats, and tail-rattle) and non-aggressive behaviors (F(4,40)≥4.683, p≤0.0034 for grooming, rearing, walking, and contact). Dunnett’s t test showed that intermediate dose of baclofen (3 mg/kg) significantly increased attack bites without affecting non-aggressive behaviors (Table 6). In contrast, the highest dose of baclofen (5.6 mg/kg) significantly reduced grooming, rearing, walking, and tail-rattling due to sedative effect.
Discussion
We have previously shown that pharmacological activation of GABAB receptors in the DRN increased territorial aggression in male mice, and this manipulation was accompanied by an increase in 5-HT release in the medial prefrontal cortex (Takahashi et al. 2010b). To understand which type of aggression is enhanced by this DRN-GABAB receptor modulation, this study aimed to obtain more detailed information on “baclofen-heightened aggression” using different test conditions. In the dark phase, baclofen escalated inter-male aggression in a context-independent manner. Baclofen escalated aggressive behaviors both in the home cage and in a neutral arena. In contrast, baclofen-heightened aggression was prevented by two conditions; (1) suppression during the light phase of the diurnal cycle and (2) inhibition by female intruder. Thus, for baclofen to escalate aggression, it must have a specific provocation from the intruder (e.g. male pheromone), and also the tonic level of 5-HT activity (during the dark phase of the diurnal cycle) in the brain (see below).
Aggressive behaviors in animals are adaptive, allowing them to protect territory, females and offspring, and food resources. To understand maladaptive or pathological levels of aggression, which translates to clinically significant levels of aggression (or violence) in humans, we need to dissect those escalated types of aggression from species-typical behaviors in animal models (Miczek et al. 2002, 2004; Haller and Kruk 2006; Nelson and Trainor 2007; de Boer et al., 2009). One important aspect is the nature of the attack behaviors, which can be examined by assessing the targets of attacks (Haller et al. 2002) or females (Sluyter et al. 2003). For example, optogenetic activation of a subdivision of the ventromedial hypothalamus (VMHvl) induced abnormal attack behavior toward females and even toward inanimate objects (Lin et al. 2011). On the other hand, alcohol self-administration escalates aggressive behaviors in a certain portion of animals, and those rats took longer to terminate the aggressive burst (Miczek et al. 1992). Lengthy bursts of aggressive behavior can be costly in terms of energy demands, and thus this may be also another aspect of maladaptive aggressive behavior. In the current study, we observed that the animals with intra-DRN baclofen treatment increased attack directed to the vulnerable body targets (especially to the abdomen) of male intruders. Also baclofen treatment prolonged aggressive bursts compared to controls. In contrast, baclofen-treated resident males did not attack female intruders. Similarly, we also observed that these resident males did not attack their cage-mate female or pups after the resident-intruder confrontations. Therefore, intra-DRN baclofen escalates aggressive behaviors with certain constraints. For example, baclofen did not disrupt the recognition of signals from intruder (e.g. visual and olfactory info of male intruder). Once the aggressive behavior was triggered by a certain input from the male intruder, DRN-GABAB modulation escalates aggression to abnormal levels and delayed the termination of aggressive burst, that could be interpret as maladaptive, or pathological, aggression.
The second important finding in this study was that baclofen-heightened aggression was dependent on the time of day. Circadian rhythmicity in the 5-HT system in the brain has an important role to modulate the sleep/wake cycle in animals (Jouvet 1999; Sun et al. 2002). Similarly, circadian variation in aggressive behaviors induced by electric foot-shock has been reported in mice (Sofia and Salama 1970). Previously, we found that an aggression-heightening dose of baclofen microinjected into the DRN increased the extracellular level of 5-HT in the medial prefrontal cortex in the dark-phase (Takahashi et al. 2010b). In the present study, we found that intra-DRN baclofen treatment escalated aggressive behaviors in the dark but not in the light phase. It is possible that this lack of baclofen-heightened aggression during the light phase is the result of specific modulation of DRN 5-HT neurons by GABAB receptors that depends on the time of day. Tao et al. (1996) showed that the intra-DRN baclofen reduced 5-HT level in the DRN and also in the nucleus accumbens of rats. However, Abellan et al. (2000) reported that microinfusion of the same dose of baclofen as Tao et al. enhanced 5-HT release in the DRN, while a higher concentration reduced 5-HT output in rats. These divergent effects of baclofen on 5-HT release seem to be due to the time of day when the studies were conducted, Tao et al. (1996) during the dark phase and Abellan et al. (2000) in the light (Serrats et al. 2003). We cannot simply compare these findings with our results because the currently used microinjected dose of baclofen in the DRN differed from those used in earlier studies which were administered via reverse microdialysis. Nonetheless, these results suggest that the diurnal difference in basal 5-HT activity can result in different effects of baclofen on aggressive behavior.
We found that baclofen could increase inter-male aggression in situations that usually inhibit aggressive behaviors: i) outside of the home territory and ii) after experiencing repeated defeat. Defeat stress had the strongest inhibitory effect among these conditions on the subsequent aggressive encounter, and only a few animals showed aggressive behaviors after several repeated defeat experiences. However, intra-DRN baclofen treatment restored aggression in most of those animals. The 5-HT system is one of the important candidates implicated in the defeat effect (Raab 1971; Miczek et al. 2008). SSRI treatment recovered the depressive-like phenotypes in defeated animals (Berton et al. 2006), and defeated animals also have enhanced 5-HT neuron activity and reduced 5-HT1A receptor expression in the DRN and other brain areas (McKittrick et al. 1995; Cooper et al. 2009). Activation of 5-HT1A receptors in the DRN successfully reduced the behavioral consequence of conditioned defeat in male hamsters (Cooper et al. 2008). It is possible that intra-DRN baclofen treatment changes 5-HT activity and this modulation somehow activates 5-HT1A receptors to inhibit the effect of defeat stress in subsequent encounters.
These results suggested that activation of GABAB receptors in the DRN can modulate the activity of 5-HT and increase inter-male aggression in several test conditions. However, the DRN also contains large number of other types of neurons that are not serotonergic, and many of those neurons also project to distant brain nuclei (Beitz et al. 1986; Ma et al. 1991; Petrov et al. 1992; Van Bockstaele et al. 1993; Kanno et al. 2008, Kirifides et al. 2001; Kim et al. 2004; Halberstadt and Balaban, 2006). Those neurons can also be affected by local baclofen treatment. Thus the possibility remains that these non-serotonergic neurons, but not 5-HT neurons, can be the activator of aggressive behavior. Whether the aggression-heightening effect of baclofen is the result of the change in 5-HT or due to the other neurotransmitter system has to be addressed in the future by using advanced genetic techniques such as conditional gene targeting and optogenetics.
Clinically, the role of GABAB receptors on aggressive behavior has not yet been fully examined. One study has shown that oral administration of baclofen inhibited provoked aggressive responses in subjects that had a history of childhood conduct disorder, while the same treatment escalated aggressive responses in control subjects in the laboratory setting (Cherek et al. 2002). In preclinical models, baclofen consistently reduces escalated aggressive behaviors induced by electric shock, isolation housing, and apomorphine treatment in rodents (Belozertseva and Andreev 1999; Rodgers and Depaulis 1982; Rudissaar et al. 2000). In contrast, we found that both systemic and intra-DRN administration of low to moderate doses of baclofen can increase aggressive behavior in male mice in our resident-intruder test procedure. These results are consistent with the finding of different effects of baclofen on human aggression in conduct disorder subjects vs. control subjects.
Supplementary Material
Schematic representation of injection sites for experiment 1–5 in mouse coronal brain section (Paxinos and Franklin 2011). White circles indicate injection sites within the DRN and black circles represent injection sites outside the DRN. In 4 cases in experiment 1, precise localization could not confirmed due to technical problems.
Acknowledgments
The authors would like to thank J Thomas Sopko for his outstanding technical assistance. This research was funded by NIAAA grant AA013983 (KAM) and by KAKENHI (22830130 & 23683021) and Research Foundation for Opto-Science and Technology (AT)
Footnotes
Potential conflict of interest: There is no conflict of interest in this paper.
References
- Abellán MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABAB receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Abellán MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Amorim M, Almada V. The outcome of male–male encounters affects subsequent sound production during courtship in the cichlid fish. Anim Behav. 2005;69:595–601. [Google Scholar]
- Beitz AJ, Clements JR, Mullett MA, Ecklund LJ. Differential origin of brainstem serotonergic projections to the midbrain periaqueductal gray and superior colliculus of the rat. J Comp Neurol. 1986;250:498–509. doi: 10.1002/cne.902500408. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Andreev BV. Regulation of the mouse aggressive behavior (pharmacologic analysis of the GABAergic mechanism) Zh Vyssh Nerv Deiat Im I P Pavlova. 1999;49:780–788. [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Caramaschi D, de Boer SF, Vries HD, Koolhaas JM. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav Brain Res. 2008;189:263–272. doi: 10.1016/j.bbr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SD, Pietras CJ, Steinberg JL. Effects of chronic paroxetine administration on measures of aggressive and impulsive responses of adult males with a history of conduct disorder. Psychopharmacology. 2002;159:266–274. doi: 10.1007/s002130100915. [DOI] [PubMed] [Google Scholar]
- Coates JM, Herbert J. Endogenous steroids and financial risk taking on a London trading floor. Proc Nat Acad Sci U S A. 2008;105:6167–6172. doi: 10.1073/pnas.0704025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL. Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry. 1997;154:1430–1435. doi: 10.1176/ajp.154.10.1430. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida RMM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol. 2005;526:51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Caramaschi D, Natarajan D, Koolhaas JM. The vicious cycle towards violence: focus on the negative feedback mechanisms of brain serotonin neurotransmission. Front Behav Neurosci. 2009;3:52. doi: 10.3389/neuro.08.052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–39. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Nat Acad Sci U S A. 2010;107:12393–12398. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–259. [Google Scholar]
- Halberstadt AL, Balaban CD. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience. 2006;140:1067–1077. doi: 10.1016/j.neuroscience.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Rodriguiz RM, Caron MG, Wetsel WC, Liposits Z. Behavioral responses to social stress in noradrenaline transporter knockout mice: effects on social behavior and depression. Brain Res Bull. 2002;58:279–284. doi: 10.1016/s0361-9230(02)00789-x. [DOI] [PubMed] [Google Scholar]
- Haller J, Kruk MR. Normal and abnormal aggression: human disorders and novel laboratory models. Neurosci Biobehav Rev. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Haller J, van de Schraaf J, Kruk MR. Deviant forms of aggression in glucocorticoid hyporeactive rats: a model for “pathological” aggression? J Neuroendocrinol. 2001;13:102–107. doi: 10.1046/j.1365-2826.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- Haney M, Noda K, Kream R, Miczek KA. Regional serotonin and dopamine activity: Sensitivity to amphetamine and aggressive behavior in mice. Aggress Behav. 1990;16:259–270. [Google Scholar]
- Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Kanno K, Shima S, Ishida Y, Yamanouchi K. Ipsilateral and contralateral serotonergic projections from dorsal and median raphe nuclei to the forebrain in rats: immunofluorescence quantitative analysis. Neurosci Res. 2008;61:207–218. doi: 10.1016/j.neures.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Kirifides ML, Simpson KL, Lin RCS, Waterhouse BD. Topographic organization and neurochemical identity of dorsal raphe neurons that project to the trigeminal somatosensory pathway in the rat. J Comp Neurol. 2001;435:325–340. doi: 10.1002/cne.1033. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13:736–743. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bondar NP, Avgustinovich DF. Effects of repeated experience of aggression on the aggressive motivation and development of anxiety in male mice. Neurosci Behav Physiol. 2004;34:721–730. doi: 10.1023/b:neab.0000036013.11705.25. [DOI] [PubMed] [Google Scholar]
- Lee R, Chong B, Coccaro E. Growth hormone responses to GABAB receptor challenge with baclofen and impulsivity in healthy control and personality disorder subjects. Psychopharmacology. 2011;215:41–48. doi: 10.1007/s00213-010-2116-0. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Yin GF, Ai MK, Han JS. Serotonergic projections from the nucleus raphe dorsalis to the amygdala in the rat. Neurosci Lett. 1991;134:21–24. doi: 10.1016/0304-3940(91)90499-j. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo S, De Almeida RMM, Bannai M, Fish EW, DeBold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann NY Acad Sci. 2004;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Faccidomo SP, Fish EW, DeBold JF. Neurochemistry and molecular neurobiology of aggressive behavior. In: Blaustein J, editor. Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology. Springer; New York: 2007. pp. 285–336. [Google Scholar]
- Miczek KA, Fish EW, DeBold JF, de Almeida RMM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and γ-aminobutyric acid systems. Psychopharmacology. 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology. 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, Tornatzky W, DeBold JF, Vatne TM. Alcohol and “bursts” of aggressive behavior: ethological analysis of individual difference in rats. Psychopharmacology. 1992;107:551–563. doi: 10.1007/BF02245270. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Silva A, Canário AV. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc Biol Sci. 2009;276:2249–2256. doi: 10.1098/rspb.2009.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. The hypothalamic paraventricular and lateral parabrachial nerve nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J Comp Neurol. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- Raab A. Der Serotoninstoffwechsel in einzelnen Hirnteilen von Tupaia (Tupaia belangeri) bei soziopsychischem. Stress Z Vgl Physiol. 1971;72:54–66. [Google Scholar]
- Rodgers RJ, Depaulis A. Gabaergic influences on defensive fighting in rats. Pharmacol Biochem Behav. 1982;17:451–456. doi: 10.1016/0091-3057(82)90303-3. [DOI] [PubMed] [Google Scholar]
- Rudissaar R, Pruus K, Skrebuhhova-Malmros T, Allikmets L, Matto V. Involvement of GABAergic neurotransmission in the neurobiology of the apomorphine-induced aggressive behavior paradigm, a model of psychotic behavior in rats. Methods Find Exp Clin Pharmacol. 2000;22:637–640. doi: 10.1358/mf.2000.22.8.802276. [DOI] [PubMed] [Google Scholar]
- Rutte C, Taborsky M, Brinkhof MWG. What sets the odds of winning and losing? Trends Ecol Evol. 2006;21:16–21. doi: 10.1016/j.tree.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Serrats J, Artigas F, Mengod G, Corte R. GABAB receptor mRNA in the raphe nuclei: co-expression with serotonin transporter and glutamic acid decarboxylase. J Neurochem. 2003;84:743–752. doi: 10.1046/j.1471-4159.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Debold JF, Holly EN, Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology. 2011;218:271–279. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyter F, Arseneault L, Moffitt TE, Veenema AH, de Boer SF, Koolhaas JM. Toward an animal model for antisocial behavior: parallels between mice and humans. Behav Genet. 2003;33:563–574. doi: 10.1023/a:1025730901955. [DOI] [PubMed] [Google Scholar]
- Sofia RD, Salama AI. Circadian rhythm for experimentally-induced aggressive behavior in mice. Life Sci. 1970;9:331–338. doi: 10.1016/0024-3205(70)90221-3. [DOI] [PubMed] [Google Scholar]
- Sun X, Deng J, Liu T, Borjigin J. Circadian 5-HT production regulated by adrenergic signaling. Proc Natl Acad Sci U S A. 2002;99:4686–4691. doi: 10.1073/pnas.062585499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Kwa C, DeBold JF, Miczek KA. GABAA receptors in the dorsal raphé nucleus of mice: escalation of aggression after alcohol consumption. Psychopharmacology. 2010a;211:467–477. doi: 10.1007/s00213-010-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABAB receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci. 2010b;30:11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Ma Z, Auerbach SB. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Brit J Pharmacol. 1996;119:1375–1384. doi: 10.1111/j.1476-5381.1996.tb16049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic representation of injection sites for experiment 1–5 in mouse coronal brain section (Paxinos and Franklin 2011). White circles indicate injection sites within the DRN and black circles represent injection sites outside the DRN. In 4 cases in experiment 1, precise localization could not confirmed due to technical problems.



