Abstract
To improve delirium recognition and care, numerous serum biomarkers have been investigated as potential tools for risk stratification, diagnosis, monitoring, and prognostication of delirium. The literature was reviewed, and no evidence was found to support the clinical use of any delirium biomarker, although certain biomarkers such as S-100 beta and insulin-like growth factor-1 and inflammatory markers have shown some promising results that need to be evaluated in future studies with appropriate sample size, prospective designs, and in a more-generalizable population.
Keywords: delirium, biomarkers, serum
Although delirium is an independent predictor of morbidity, mortality, and healthcare costs,1,2 it is under-recognized in clinical practice.2 To improve delirium recognition and care, investigators have identified possible biomarkers that may help in diagnosing individuals with delirium, assessing the severity of delirium, defining endpoints for the resolution of delirium, developing new therapies, and monitoring response to treatment. The traditional view of delirium being a transient cognitive manifestation of acute illness is changing,1 and the science of biomarkers may provide insights into understanding the molecular mechanisms of the connection between delirium and neurodegenerative dementing illnesses.
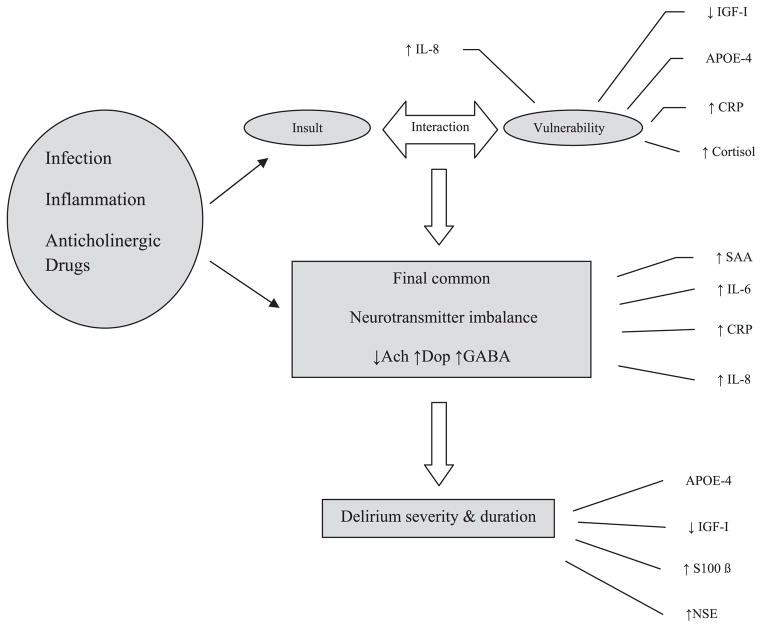
Delirium pathophysiology has not been clearly defined and may represent brain responses to local or systemic stress with interaction between central and peripheral biological pathways leading to the ultimate clinical scenario of delirium. There are two important hypotheses to explain the mechanisms at work: the neurotransmitter hypothesis and the inflammatory hypothesis. The neurotransmitter hypothesis describes the abundance or deficiency of certain neurotransmitters resulting in the symptoms associated with delirium. It has been proposed that delirium results from a complex interaction between various predisposing and precipitating factors.3 Figure 1 describes this interaction and suggests that neurotransmitter imbalance may direct the onset and severity of episodes of delirium. The final common pathway of neurotransmitter imbalance in delirious states is thought to result in an excessive release of dopamine, a deficiency in acetylcholine synthesis, and high or low levels of serotonin and gamma-amino-butyric acid (GABA).
Figure 1.
Complex interplay between inflammatory mediators and cholinergic system in delirium pathogenesis. IGF = insoluble growth factor; APOE = apolipoprotein E; CRP = C-reactive protein; SAA = Serum anticholinergic activity; IL = interleukin; NSE = neuro-specific enolase.
The inflammatory hypothesis emphasizes the role of stress-induced cytokines in delirium, including interleukin (IL)-1, IL-6, interferon, and tumor necrosis factor-alpha (TNF-α).4,5 This theory describes the similarities between sickness behavior induced by cytokines and delirium and explains the varying neurobehavioral manifestations of delirium through a cytokine lens. Based on animal literature, it is probable that these two hypotheses do not function as separate and distinct entities but rather as a complex interplay between the inflammatory milieu and disturbances in certain neurotransmitter systems.6
This review was performed as an update of the progress in the field of delirium-specific biomarkers and to provide the reader with a theoretical framework for exploring promising biomarkers for delirium as future avenues for research.
METHOD
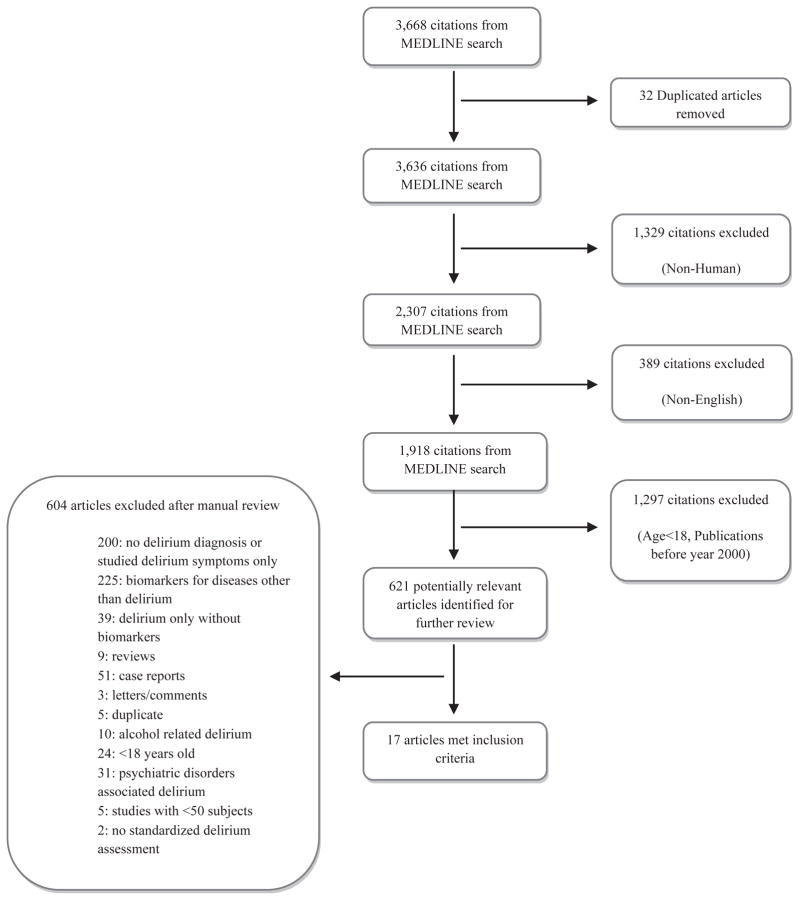
A comprehensive literature search of Medline was performed using the terms delirium, confusion, psychomotor agitation, encephalopathy, biological markers, biomarkers, serum products, serum break down products, anticholinergic, S-100 β, neuron-specific enolase (NSE), cytokines, acute-phase proteins, somatomedins, interleukin, inter-feron, tumor necrosis factor, C-reactive protein (CRP), insulin-like growth factor, leukemia inhibitory factor, nitric oxide, melatonin, and apolipoprotein E (ApoE). The search was limited to articles published in English during or after 2000. To ensure quality, only studies that met the following criteria were included: sample size of 50 or greater, used standardized methods for delirium diagnosis, and focused on biomarkers. Studies of alcohol-related delirium and Axis I psychiatric disorders were excluded. This search strategy produced 621 relevant studies. A manual review by two reviewers (BK, MZ) of the 621 citations using the criteria resulted in 17 articles (Figure 2). The findings of these studies formed the basis of the review. The findings were organized into three categories of delirium-specific biomarkers: risk factors, diagnostic and disease activity biomarkers, and markers for delirium severity and monitoring response to therapy (Table 1).
Figure 2.
Search strategy.
Table 1.
Summary of Recent Evidence on the Role of Biomarkers in Delirium
| Study | Service | Sample Size | Age, Mean±SD | Category | Delirium Assessment Tool | Biomarker | Main Findings |
|---|---|---|---|---|---|---|---|
| Wilson16 | Medicine | 100 | 84.5 ± 4.2 | Risk* | CAM, DSM-III | IGF-1 | Low IGF-1 increases the risk of delirium |
| Macdonald15 | Medicine | 86 | 82.7 ± 6.6 | Risk | CAM | CRP | High levels predict the incidence of delirium |
| de Rooij4 | Medicine | 185 | 80 | Risk | CAM | Cytokines | Cytokines may contribute to pathogenesis of delirium |
| Tagarakis10 | Surgery | 154 | 70.1 ± 7.7–67.8 ± 7.4 | Risk | DRS | ApoE | No correlation between ApoE and postoperative delirium |
| Leung7 | Surgery | 190 | 72.5 ± 6 | Risk | CAM | ApoE | ApoE carrier status was associated with greater risk of early postoperative delirium |
| Van Munster9 | Medicine | 264 | 81.4–76.7 | Risk | CAM | ApoE | No evidence that ApoE carriers have higher risk of delirium |
| Van Munster16 | Surgery | 98 | 84.6 ± 7.1–83.2 ± 6.7 | Risk | CAM, DOS, DRS-R-98 | Cytokines | Patients with postoperative delirium have higher levels of IL-6 and IL-8 |
| Lemstra22 | Surgery | 68 | 78.5–80 | Risk | DSM-IV, CAM, DRS-R-98, Digit Span Test | CRP, IL-6, IGF-1 | No correlation found between preoperative inflammatory markers levels and postoperative delirium |
| Van Munster8 | Medicine, surgery | 656 | 77.4 ± 7.8–82.3 ± 7.6 | Risk | CAM, DOS | ApoE | Delirium associated with presence of ApoE-4 allele |
| Van Munster13 | Surgery | 120 | 84.8 ± 6.9–82.9 ± 7.9 | Risk, diagnosis† | CAM, DOS | Cortisol, IL, S-100 β | Cortisol, IL-6, and S-100 β may have a role in the pathogenesis of delirium. S-100 β is the strongest independent marker |
| Adamis11 | Medicine | 164 | 84.6 ± 6.57 | Risk, prognosis‡ | CAM, DRS | ApoE, Cytokines | Relationship between ApoE, low IGF-1, and delirium |
| Ely12 | ICU | 53 | 53.2 ± 21.9–65.4 ± 13.4 | Risk, prognosis | CAM-ICU | ApoE | Presence of ApoE significantly associated with a longer duration of delirium |
| Adamis5 | Medicine | 67 | 84.2 ± 6.3 | Risk, prognosis | CAM, DRS | Cytokines, IGF-1, ApoE | Low levels of IGF-I associated with delirium |
| Thomas18 | Medicine | 61 | 86.2 ± 4.5 | Diagnosis | CAM, Delirium Index | SAA | SAA levels did not correlate with electroencephalographic parameters in diagnosing delirium |
| Van Munster23 | Medicine | 412 | 81.6 ± 7.9–76.6 ± 7.6 | Diagnosis | CAM, DOS, DRS-R-98 | S-100 β | Higher levels of S-100 β were found in patients with delirium than in those without |
| Van Munster24 | Surgery | 120 | 84.8 ± 6.9–82.9 ± 7.0 | Diagnosis | CAM, DOS, DRS-R-98 | S-100 β, NSE | Delirium associated with high levels of S-100 β but not with NSE |
| Plaschke21 | ICU | 114 | 73.3 ± 6.0–67.3 ± 9.3 | Diagnosis | CAM-ICU | Cortisol, IL-6 | Early postoperative delirium after cardiac surgery associated with higher cortisol and IL-6 levels |
Risk markers were collected before delirium development.
Diagnosis markers correlate with delirium development.
Prognosis markers pertain to delirium severity or duration.
CAM = Confusion Assessment Method; DSM = Diagnostic and Statistical Manual of Mental Disorders; IGF = insulinlike growth factor; CRP = C-reactive protein; DRS = Delirium Rating Scale; ApoE = apolipoprotein E; DOS = Delirium Observation Screening Scale; IL = interleukin; NSE = neuron-specific enolase; ICU = intensive care unit; SAA = serum anticholinergic activity.
RESULTS
Assessment of Delirium Risk Through Biomarkers
Genetic Markers
Apo-E is a protein that repairs and regenerates myelin and neuronal membranes during development or after injury through cholesterol mobilization.7 Six studies evaluated its role in delirium.7–12 One study analyzed the deoxyribonucleic acid samples of 704 participants and found that carriage of the Apo-E4 allele is associated with greater risk of developing delirium (odds ratio (OR) = 1.7, 95% confidence interval (CI) = 1.1–2.6), after adjusting for age, cognitive impairment, and functional limitation.8 It then pooled data from four more studies7,9–11 and found a similar association between Apo-E4 carriers and vulnerability to developing delirium (OR = 1.6, 95% CI = 0.9–2.7). In a cognitively impaired population, presence of the Apo-E4 allele was associated with longer delirium duration (OR = 7.32), but the CI was extremely wide (1.82–29.51).12 The median duration of delirium was 4 days for patients with an Apo-E4 allele and 2 days for patients without.
Inflammatory Mediators
An analysis of 120 older adults with hip fracture patients compared cytokines and cortisol levels between participants with and without delirium.13 The sample detection limit was set below 45 nmol/L for cortisol and below 20 pg/mL for IL-8. A linear mixed model showed that the highest levels of IL-8 (27.1 pg/mL, 95% CI = 13.6–53.1 pg/mL) and cortisol (666 nmol/L, 95% CI = 475–859 nmol/L) were found before delirium onset, but a detailed account on predicting the time to delirium onset from a nondelirious state was not provided. Another study reached similar findings with regard to IL-8 levels.14 In a medical population, high CRP levels predicted delirium incidence after adjusting for other covariates (P = .02).15 These findings raise the possibility of using IL-8, CRP, and cortisol as predictive markers of delirium and incorporating them into a clinical tool to better predict delirium onset.
Insulinlike Growth Factor-1
IGF-1 promotes neuronal development, survival, proliferation, and enhanced synaptic transmission in the central nervous system.16 A prospective study of 100 consecutively admitted older medical patients (≥75) without delirium, based on a negative interview using the Confusion Assessment Method (CAM) instrument and significant medical illness characterized by an Acute Physiology and Chronic Health Evaluation II score greater than 8, showed that low baseline IGF-1 levels on admission were associated with greater risk of incident delirium.16
Diagnostic and Disease Activity Markers for Delirium
Serum Anticholinergic Activity
A serum anticholinergic activity (SAA) assay was developed to measure the anticholinergic burden of drugs in individuals with psychiatric illnesses.17 Based on the hypothesis that delirium represents a state of cholinergic deficiency, SAA has been evaluated as a disease activity marker and a diagnostic tool for delirium.18–20 The search criteria for the current review resulted in only one study fulfilling the requirements of adequate sample size and standardized delirium measurement.18 That prospective cross-sectional study of 61 hospitalized individuals aged 80 and older assessed the correlation between SAA and quantitative electroencephalography, delirium occurrence, and cognitive measures.18 No significant differences in SAA levels between individuals with and without delirium were found. SAA levels did not correlate with any electroencephalographic parameters considered to be criterion standards of delirium diagnosis. These findings conflict with the suggestion that SAA reflects central anticholinergic burden, which has long been a criticism of the SAA assay. SAA may reflect peripheral anticholinergic activity, which in turn does not translate into central cholinergic deficiency resulting in delirium.
Inflammatory Markers
Given the greater prevalence of delirium in disease states with a higher inflammatory burden such as sepsis, cancer, and the postoperative period, inflammatory markers may be useful for monitoring delirium disease activity. The relationship between various cytokines and delirium has been examined in medical and surgical inpatient populations.4,13,14 All studies documented significant associations between IL-6 and IL-8 and delirium. In one of the studies,4 using detection limits of 10 pg/mL for IL-6 and 20 pg/mL for IL-8, the authors reported that, in participants with IL-6 levels below the detection limit, there were more without delirium (69%) than with (47%, P = .04). Similarly, participants without delirium were more likely to have IL-8 levels below the detection limit (78% vs 55%; P = .001). After adjusting for age, cognitive impairment, and infection, having IL-6 and IL-8 levels above the detection limits was significantly associated with delirium (IL-6, OR = 2.4, 95% CI = 1.0–5.6; IL-8, OR = 2.6, 95% CI = 1.1–6.3). Similar results were demonstrated regarding IL-6 after open heart surgery,21 although two other studies failed to show such associations.11,22
Insulin-Like Growth Factor-1
Low IGF-1 levels in older medical patients were shown to be associated with delirium (P = .02).11 This study not only confirmed findings of a previous study that low levels of IGF-1 can predispose to delirium,16 but also extended the association with delirium at any point during the hospitalization. Conversely, high IGF-1 levels were associated with low Delirium Rating Scale (DRS) scores. The authors concluded that IGF-1 may be neuroprotective, and thus low levels can predispose to delirium and may increase its severity.5 Another study11 in 164 older adults admitted to a medical unit showed that prevalent delirium was significantly associated with a previous history of dementia, older age, greater illness severity, disability, and low IGF-1 levels. Based on these studies, it is plausible that an intervention aimed at increasing levels of IGF-1 may prevent or treat delirium.
Markers of Delirium Severity
S-100 β
S-100 β, a biomarker that indicates direct neuronal injury such as traumatic brain injury and cerebrovascular accidents has shown promise as a marker of disease severity in delirium.15 Astrocytes mainly express S-100 β, and high levels may indicate indirect injury to glial cells in delirium. A strong association between S-100 β and delirium has been demonstrated in three studies (two on older adults undergoing hip fracture repair and one in an inpatient medical population)15,23,24 after adjusting for confounders including cognitive impairment (OR = 4.01, 95% CI = 1.87–8.58).15 These studies demonstrated consistently high S-100 β levels in individuals with delirium. One of the studies24 also documented a correlation between S-100 β and the proinflammatory cytokines IL-6 and IL-8, hinting at the complex relationship between inflammation and possible cerebral damage.
Neuron-Specific Enolase
NSE is an enzyme present in neurons, and its release may indicate direct neuronal injury. There are limited data on the role of this biomarker in delirium. In individuals with hip fracture and delirium, no relationship was found between delirium and NSE levels.24 The relationship between delirium and NSE has not been firmly established. A major injury to the central nervous system (CNS) such as stroke, severe traumatic brain injury, or surgery on the CNS is required for NSE release and may not be evident in other causes of delirium.
DISCUSSION
Biomarkers have the potential to unlock the codes to the pathophysiology of delirium, diagnose delirium, and define the prognosis and long-term effect of delirium on cognition. Biomarkers can be useful in developing new therapies for delirium, can be applied to drug development, and can serve as indirect markers for assessing delirium severity. Small sample sizes of studies, cross-sectional designs, and lack of control over the baseline characteristics of the participant population limit the current evidence supporting the use of biomarkers in delirium. Additionally, difficulties in addressing the multiple etiologies and disease processes leading to delirium complicate results from the existing literature.
This review briefly describes the available literature over the last decade in the field of delirium biomarkers. The best available studies were included in this review to promote understanding of the complex pathophysiological processes responsible for delirium symptoms. This review builds upon a prior review25 that classified the biomarkers associated with delirium as risk and disease markers. The current literature establishes a role of the Apo-E4 allele as a risk factor for incident delirium and longer delirium duration.7–12 Higher IL-8,13,14 cortisol,13 and CRP15 levels may predict delirium development, along with low levels of IGF-1.16 The use of SAA to reflect central cholinergic activity has not been proven based on the study included in this review.17 High IL-6, IL-8, and S-100 β levels and low IGF-1 levels showed the strongest correlation with delirium.5,13,14,21 S-100 β has shown the most consistent association with delirium, after adjustment of confounders including baseline cognitive impairment.13
There is overlap between the role of inflammatory markers in delirium and the cholinergic system of the patient (Figure 1). Acetylcholine may inhibit the release of proinflammatory cytokine IL-6 and thereby help in controlling brain inflammation. Therefore, processes leading to cholinergic failure with depleted acetylcholine stores may lead to inadequate control over the inflammatory cascade and thus predispose to delirium. Delirium can also be viewed as development of sickness behavior with a significant cytokine contribution. The cytokines, along with producing symptoms of fever, weakness, and lethargy, cause impaired concentration, sleep disturbances, and agitation,26 some of the cardinal symptoms of delirium. These cytokines may induce a reduction in cholinergic activity,6 especially in older adults with an underlying neurodegenerative process such as Alzheimer’s disease. This results in a repetitive cycle of inadequate regulation of inflammation due to cholinergic depletion. An inadequately regulated system may partially explain the complex interaction between the inflammatory and neurotransmitter hypotheses.
Based on the current evidence, studies of biomarkers should focus on enrolling vulnerable medical and surgical patients before they develop delirium with assessment of risk markers through serial blood draws, comparing the levels during the delirious episode with delirium duration and severity, measured using standardized diagnostic tools and then following up with levels after delirium resolution. The important confounders, such as age, race, baseline cognitive impairment, and severity of illness, should be adjusted in the final analysis. This could serve multiple purposes, such as identifying the best marker for early risk assessment leading to prevention strategies, monitoring the disease, and developing a timeline for delirium resolution. Having standardized tools globally to analyze biomarkers with the same reference standards in multiple laboratories will help in the generalization of results. Establishment of institution-specific biorepositories with storage of blood and tissue samples will also help investigators answer the research questions retrospectively.
Strengths of this review include the incorporation of the best-available and most-recent studies, assessment of delirium with standardized diagnostic tools, and inclusion of medical and surgical literature. Limitations of this work are exclusion of studies before 2000, which may have eliminated some good studies with supportive or contradictory evidence; assessment of biomarkers in the serum with no correlation with cerebrospinal fluid levels; and the inability to calculate sensitivity, specificity, and positive and negative predictive values with the available literature.
In conclusion, proper use of biomarkers should provide a better definition of delirium and prediction of its natural history. Identification of relevant biomarkers in the field of delirium will not only help in better understanding the complex pathophysiology, but will also pave the way forward for development of newer disease-modifying agents. The paucity of well-designed, scientifically rigorous studies in the delirium biomarker field makes necessary a multiinstitutional collaborative effort to generate valid, reproducible, generalizable data to help answer some of the hypotheses generated by this review.
Acknowledgments
Sponsor’s Role: Manuscript design, methods, subject recruitment, data collections, analysis and preparation were funded by a grant from the National Institute on Aging (R01AG034205-01A1) and a grant from the National Institute on Mental Health (R24MH080827).
Footnotes
Conflict of Interest: There are no conflicts of interest for the authors of this manuscript.
Author Contributions: Babar A. Khan: Concept and design of the review, preliminary data gathering, and preparation of the manuscript. Mohammad Zawahiri: Design of the review, data gathering, and preparation of the manuscript. Noll L. Campbell: Literature search to identify appropriate studies, design of the review, and preparation of the manuscript. Malaz A. Boustani: Design of the review, literature appraisal, and significant contribution to the manuscript. All authors had a role in study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of the manuscript.
References
- 1.Witlox J, Nurslings LS, de Jungle JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 2.Boustani M, Baker MS, Campbell N, et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5:69–75. doi: 10.1002/jhm.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5:132–148. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- 4.De Rooij SE, van Munster BC, Korevaar JC, et al. Cytokines and acute phase response in delirium. J Psychosom Res. 2007;62:521–525. doi: 10.1016/j.jpsychores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Adamis D, Lunn M, Martin FC, et al. Cytokines and IGF-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing. 2009;38:326–332. doi: 10.1093/ageing/afp014. discussion 251. [DOI] [PubMed] [Google Scholar]
- 6.Eikelenboom P, Hoogendijk WJ, Jonker C, et al. Immunological mechanisms and the spectrum of psychiatric syndromes in Alzheimers disease. J Psychiatr Res. 2002;36:269–280. doi: 10.1016/s0022-3956(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 7.Leung JM, Sands LP, Wang Y, et al. Apolipoprotein E e4 allele increases the risk of early postoperative delirium in older patients undergoing non-cardiac surgery. Anesthesiology. 2007;107:406–411. doi: 10.1097/01.anes.0000278905.07899.df. [DOI] [PubMed] [Google Scholar]
- 8.Van Munster BC, Korevaar JC, Zwinderman AH, et al. The association between delirium and the apolipoprotein E epsilon 4 allele: New study results and a meta-analysis. Am J Geriatr Psychiatry. 2009;17:856–862. doi: 10.1097/JGP.0b013e3181ab8c84. [DOI] [PubMed] [Google Scholar]
- 9.Van Munster BC, Korevaar JC, de Rooij SE, et al. The association between delirium and the apolipoprotein E epsilon4 allele in the elderly. Psychiatr Genet. 2007;17:261–266. doi: 10.1097/YPG.0b013e3280c8efd4. [DOI] [PubMed] [Google Scholar]
- 10.Tagarakis GI, Tsolaki-Tagaraki F, Tsolaki M, et al. The role of apolipoprotein E in cognitive decline and delirium after bypass heart operations. Am J Alzheimers Dis Other Demen. 2007;22:223–228. doi: 10.1177/1533317507299415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamis D, Treloar A, Martin FC, et al. ApoE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry. 2007;22:688–694. doi: 10.1002/gps.1732. [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Girard TD, Shintani AK, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007;35:112–117. doi: 10.1097/01.CCM.0000251925.18961.CA. [DOI] [PubMed] [Google Scholar]
- 13.Van Munster BC, Bisschop PH, Zwinderman AH, et al. Cortisol, interleukins and S-100 β in delirium in the elderly. Brain Cogn. 2010;74:18–23. doi: 10.1016/j.bandc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Van Munster BC, Korevaar JC, Zwinderman AH, et al. Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald A, Adamis D, Treloar A, et al. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing. 2007;36:222–225. doi: 10.1093/ageing/afl121. [DOI] [PubMed] [Google Scholar]
- 16.Wilson K, Broadhurst C, Diver M, et al. Plasma insulin growth factor-1 and incident delirium in older people. Int J Geriatr Psychiatry. 2005;20:154–159. doi: 10.1002/gps.1265. [DOI] [PubMed] [Google Scholar]
- 17.Tune L, Coyle JT. Serum levels of Anticholinergic drugs in treatment of acute extrapyramidal side effects. Arch Gen Psychiatry. 1980;37:293–297. doi: 10.1001/archpsyc.1980.01780160063007. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C, Hestermann U, Kopitz J, et al. Serum anticholinergic activity and cerebral cholinergic dysfunction: An EEG study in frail elderly with and without delirium. BMC Neurosci. 2008;9:86. doi: 10.1186/1471-2202-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flacker JM, Cummings V, Mach JR, et al. The association of serum anticholinergic activity with delirium in elderly medical patients. Am J Geriatr Psychiatry. 1998;6:31–41. [PubMed] [Google Scholar]
- 20.Plaschke K, Hill H, Engelhardt R, et al. EEG changes and serum anticholinergic activity measured in patients with delirium in the intensive care unit. Anaesthesia. 2007;62:1217–1223. doi: 10.1111/j.1365-2044.2007.05255.x. [DOI] [PubMed] [Google Scholar]
- 21.Plaschke K, Fichtenkamm P, Schramm C, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010;36:2081–2089. doi: 10.1007/s00134-010-2004-4. [DOI] [PubMed] [Google Scholar]
- 22.Lemstra AW, Kalisvaart KJ, Vreeswijk R, et al. Pre-operative inflammatory markers and the risk of postoperative delirium in elderly patients. Int J Geriatr Psychiatry. 2008;23:943–948. doi: 10.1002/gps.2015. [DOI] [PubMed] [Google Scholar]
- 23.Van Munster BC, Korevaar JC, Korse CM, et al. Serum S-100 β in elderly patients with and without delirium. Int J Geriatr Psychiatry. 2010;25:234–239. doi: 10.1002/gps.2326. [DOI] [PubMed] [Google Scholar]
- 24.Van Munster BC, Korse CM, de Rooij SE, et al. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol. 2009 May 27;9:21. doi: 10.1186/1471-2377-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcantonio ER, Rudolph JL, Culley D, et al. Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci. 2006;61A:1281–1286. doi: 10.1093/gerona/61.12.1281. [DOI] [PubMed] [Google Scholar]
- 26.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]