Abstract
Isothiocyanates, a class of anti-cancer agents, are derived from cruciferous vegetables such as broccoli, cabbage and watercress, and have demonstrated chemopreventive activity in a number of cancer models and epidemiologic studies. Due to public interest in cancer prevention and alternative therapies in cancer, the consumption of herbal supplements and vegetables containing these compounds is widespread and increasing. Isothiocyanates interact with ATP-binding cassette (ABC) efflux transporters such as P-glycoprotein, MRP1, MRP2 and BCRP, and may influence the pharmacokinetics of substrates of these transporters. This review discusses the pharmacokinetic properties of isothiocyanates, their interactions with ABC transporters, and presents some data describing the potential for isothiocyanate-mediated diet–drug interactions.
Keywords: isothiocyanates, ABC transporters, pharmacokinetics, diet-drug interactions
Introduction
Isothiocyanates (ITCs) form a class of compounds known to have cancer preventive properties which occur widely as conjugates in Brassica and other vegetables of the family Cruciferae (e.g. cabbage, cauliflower, brussels sprouts, watercress, broccoli, kale) and the genus Raphanus (radishes and daikons). Organic ITCs (R-N=C=S) occur in plants as thioglycoside conjugates known as glucosinolates. Damage to plant cells, such as from cutting and chewing, releases myrosinase (β-thioglucoside glucohydrolase) that catalyses the hydrolysis of glucosinolates and the formation of ITCs by a Lossen rearrangement [1]. Intestinal microflora can also hydrolyse glucosinolates to ITCs in humans [2]. Allyl isothiocyanate, benzyl isothiocyanate (BITC), phenethyl isothiocyanate (PEITC) and sulforaphane are examples of naturally occurring isothiocyanates. Figure 1 lists the structures of several isothiocyanates.
Figure 1.
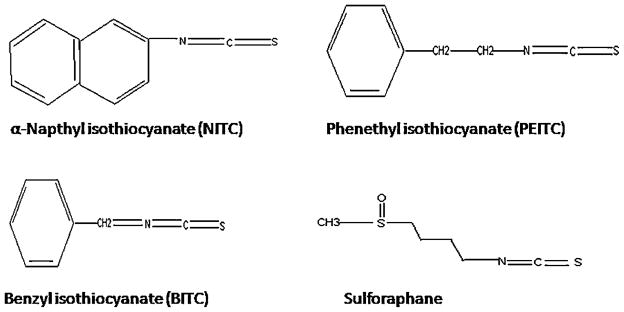
Structures of some naturally occurring isothiocyanates studied for interactions with ABC transporters
Isothiocyanates have been shown to reduce cancer risk in a number of epidemiologic studies as well as in animal models of cancer [3,4]. Early studies in this area reported that the intake of broccoli and watercress (which are sources of isothiocyanates) may reduce the risk for lung cancer by inhibition of CYP450 enzymes responsible for activation of procarcinogens [5,6]. Since then, isothiocyanates have been shown to have a variety of effects. In addition to CYP450 inhibition, isothiocyanates were reported to be inducers of Phase II enzymes such as UDP-glucuronosyltransferases (UGTs), sulfotransferases (SULTs) and quinone reductases (QRs), all of which play a role in the detoxification of activated carcinogens [7,8]. Induction of apoptosis is an important mechanism by which the isothiocyanates exert their anti-cancer effects [9,10]. Most recently, isothiocyanates have also been shown to exhibit anti-angiogenic activity, which may represent another mechanism of action [11]. Mechanisms of anti-cancer effects of isothiocyanates have been reviewed previously elsewhere [12].
Several studies carried out in this decade have revealed another property of isothiocyanates: they are substrates and inhibitors of a number of ABC transporters. ABC transporters transport a wide variety of substrates across cellular membranes, against a concentration gradient. These transporters are also over-expressed in a number of solid tumors contributing to multi-drug resistance observed in cancer, and their inhibition by isothiocyanates may represent another anti-cancer action of these compounds [13,14]. However, ABC transporters are also expressed at basal levels in several tissues involved in the absorption and elimination of exogenous compounds, and their inhibition by isothiocyanates may lead to diet–drug interactions. This article describes the interactions between isothiocyanates and ABC transporters and how they may affect the pharmacokinetics of co-administered drugs.
Pharmacokinetics of Isothiocyanates
Isothiocyanates are not only ubiquitous in the diet, but because they have shown anti-cancer activities, they are also marketed in herbal supplements as dried vegetable extracts, for example Cruciferous Vegetable Blend® (Nature’s Way) [15]. As a result, exposure to isothiocyanates through the diet and ingestion of dietary supplements is significant. The pharmacokinetic properties of several isothiocyanates have been investigated in rats and humans. Isothiocyanates have high oral bioavailability and do not exhibit adverse reactions at the doses administered. Isothiocyanates undergo metabolism mediated by glutathione-S-transferases and as discussed in detail here, are substrates for ABC transporters. Isothiocyanates exhibit nonlinear elimination profiles which could be a result of enzyme or transporter saturation, or both.
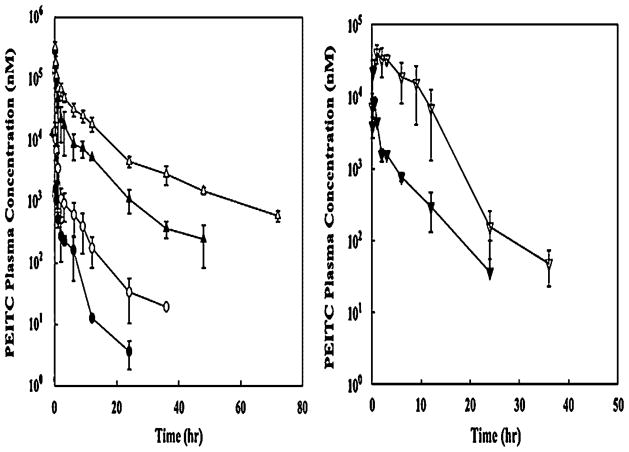
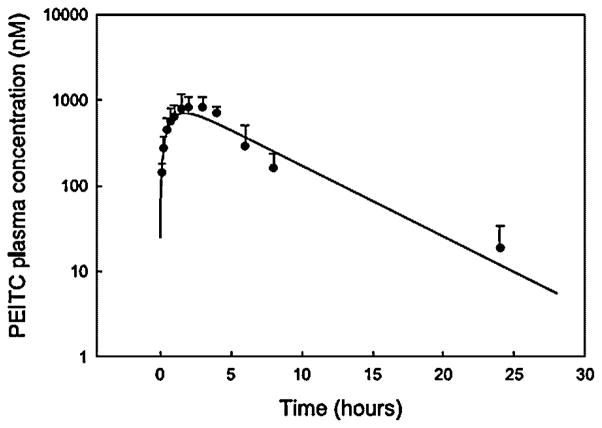
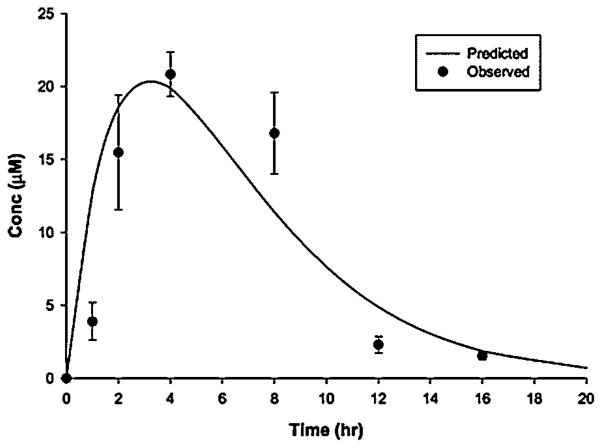
Phenethyl isothiocyanate and sulforaphane have been studied extensively to characterize their pharmacokinetic properties. Both have good bioavailability and are absorbed rapidly. The pharmacokinetics of PEITC both in rats and humans have been evaluated in our laboratory: PEITC has high oral bioavailability and low clearance (11.6 ml min−1 kg−1 at the lowest dose of 2 μmol/kg) in rats [16]. Nonlinear elimination and distribution are evident at high doses (Figure 2). In humans, following ingestion of the vegetable watercress at a 100 g dose, the mean Cmax value was 928 nM and the half-life was 4.9 h (Figure 3). The average oral clearance (clearance/availability) was 490 ml min−1, suggesting a low clearance [17]. We also investigated the pharmacokinetics of α-naphthyl isothiocyanate (NITC) [18]. NITC also exhibited a nonlinear pharmacokinetic profile with a plasma half-life of 6.1 h at a high dose of 75 mg/kg (Figure 4). While one study has reported that a high dose of sulforaphane (50 μmol) in rats resulted in high concentrations in plasma (Cmax of 20 μM) and a half-life of about 2.2 h (Figure 5), another study which measured all dithiocarbamates (that could represent sulforaphane, its metabolites and other isothiocyanates present) in the plasma reported a Cmax of 60 μM, and half-life of 6.7 h after a 150 μmol dose to rats [19,20]. Interestingly, while the Cmax from the two studies increased proportionally with dose, the elimination half-life decreased with the dose, indicating possible nonlinearities in sulforaphane disposition. These studies are summarized in Table 1.
Figure 2.
Pharmacokinetics of PEITC in rats. Left panel represents dose-normalized plasma concentrations of PEITC in rats dosed with 2 (●), 10 (○), 100 (▲) and 400 (△) μmol/kg of PEITC. The right panel represents plasma concentrations of PEITC after oral administration 10 (▼) and 100 (▽) μmol/kg doses. Data are expressed as mean±SD, n =3–4. The PEITC concentrations were determined by a specific LC/MS/MS assay. Reproduced from [16] with permission from Elsevier
Figure 3.
Pharmacokinetic profile of PEITC in humans after ingestion of 100 g of watercress. Data represented as mean±SD, n =3–4. Reproduced from [17] with permission from Elsevier
Figure 4.
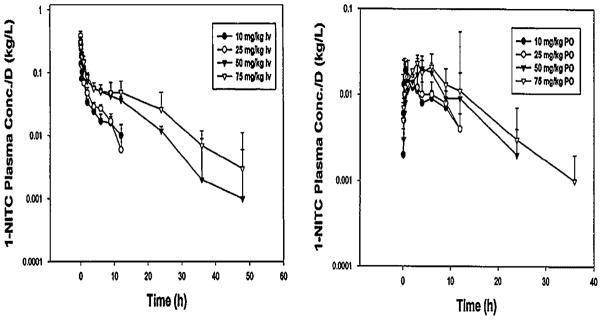
Pharmacokinetics of 1-NITC in rats. Left panel represents dose-normalized plasma concentrations of 1-NITC in rats dosed with indicated doses of 1-NITC. The right panel represents dose-normalized plasma concentrations after oral administration of 1-NITC. Data are expressed as mean±SD, n =4. Reproduced from [18] with permission from Wiley Interscience
Figure 5.
Plasma concentrations of sulforaphane versus time after a 50 μmol oral dose of sulforaphane in rats. Results are mean±SD, n =3. Reproduced from [19] with permission from ASPET publications
Table 1.
Pharmacokinetics of isothiocyanates
| ITC | Animal model/clinical study–dose | Half-life | Other parameters | Nonlinear pharmacokinetics | Reference |
|---|---|---|---|---|---|
| PEITC | Humans–100 g watercress | 4.9 h |
Cmax: 928 nM Tmax: 2.6 h CL/F: 29.5 l/h |
ND | [17] |
| PEITC | Rats 16.3 mg/kg–oral 66 mg/kg-i.v. |
13.1 h (i.v.) |
Cmax: 42.1 μM Tmax: 2.0 h |
Yes | [16] |
| Sulforaphane | Rats 265 mg oral |
4.9 h |
Cmax: 60 μM AUC: 491 μmol/l h |
ND | [20] |
| Sulforaphane | Rats 88.5 mg oral |
2.23 h |
Cmax: 20 μM Tmax: 4 h |
ND | [19] |
| NITC | Rats 75 mg/kg-i.v. |
8.2 h | Yes | [18] |
ND, not determined.
Isothiocyanates are distributed into various tissues, leading to the possibility that ITCs may affect transporter-mediated distribution of co-administered drug substrates. The tissue distribution of PEITC and phenhexyl isothiocyanate have been investigated, and these compounds distribute into the pancreas, spleen and nasal mucosa [21]. In humans, sulforaphane significantly distributed into the mammary gland [20].
The metabolism of isothiocyanates has mainly been studied in humans. The conjugation and excretion of ITCs is predominantly catalysed by glutathione S-transferase M1 (GSTM1) and GSTT1 [22], although a variety of other GSTs, including GST A1, P1, M2 and M4, are also involved to a minor extent. These conjugates are also transported by ABC transporters and are responsible for the intracellular glutathione depletion observed in vitro [23]. The nonlinearity observed in vivo might be a result of saturation of this efflux, or the decreased metabolism of the parent itself, with glutathione depletion contributing to the nonlinear pharmacokinetics observed in vivo.
Identification of Isothiocyanate-ABC Transporter Interactions
Using ATP-derived energy, ATP binding cassette (ABC) transporters operate at the cellular membrane to transport molecules against a concentration gradient. Like drug metabolizing enzymes, their primary function is likely the transport of endogenous molecules, but because of structural similarities between xenobiotics and endogenous substances, they also transport drugs across cell membranes.
These membrane proteins consist of trans-membrane domains, and a nucleotide binding domain [24]. They are present endogenously in tissues involved in absorption and elimination and may also affect the disposition of drugs processed by these tissues. Additionally, since transporters are widely present in other tissues, such as the biliary canaliculi of hepatocytes, the apical membranes of enterocytes and various blood–tissue barriers, they play an important role in drug distribution as well. The ABC transporters, that have been investigated for their interactions with isothiocyanates, include P-glycoprotein (P-gp, ABCB1), multidrug resistance protein 1 (MRP1, ABCC1), multidrug resistance protein 2 (MRP2, ABCC2) and breast cancer resistance protein (BCRP, ABCG2). All transporters except BCRP, which is termed a half transporter, consist of at least 12 transmembrane domains and a nucleotide binding domain with ATPase activity. BCRP consists of only six transmembrane domains and one nucleotide binding domain with ATPase activity. The tissues that express these transporters, as well as the compounds transported by them are listed in Table 2.
Table 2.
ABC transporters investigated for isothiocyanate-transport interactions
| Transporter | Tissue expression | Endogenous substrates | Drug substrates | References |
|---|---|---|---|---|
| PgP | Apical surfaces of epithelial cells e.g. intestine, biliary tubules, kidney, blood–brain barrier | Opioid peptides, e.g. β-endorphin | Morphine, Verapamil, Daunomycin | [25–27] |
| MRP1 | Canalicular membranes of hepatocytes | Bile salts, Estradiol-3-sulfate Estradiol-17-glucuronide |
Mitoxantrone Saquinavir Erythromycin |
[28] |
| MRP2 | Apical membranes of intestine, hepatocytes and kidney epithelial cells | Estradiol-17-glucuronide Estradiol-3-glucuronide |
Glucuronide/sulfate conjugates, Gluthathione conjugates of drugs, Methotrexate, Mitoxantrone. | [29–31] |
| BCRP | Hepatocytes, intestine, mammary gland, placenta | Estradiol-3-sulfate Estradiol-17-glucuronide, Dehydroepiandrosterone | Glucuronide/Sulfate conjugates of drugs, Estradiol, Methotrexate, Topotecan | [32,33] |
MRP1 (ABCC1) and MRP2 (ABCC2) are members of the multidrug resistance associated protein family that comprise nine members [34]. The two transporters have similar substrate specificity, transporting negatively charged compounds such as glutathione, glucuronide and sulfate conjugates of many compounds such as the glutathione conjugates of isothiocyanates and glucuronide- and sulfate-conjugated estrogens, in addition to drug substrates [29,30,35,36]. MRP1 is expressed in the basolateral membrane of epithelial cells in the lung, testis and kidney. MRP2 is expressed in apical membranes in the biliary canaliculi of hepatocytes, proximal kidney tubules, small intestine, colon, gall bladder and placenta [37–39].
Early studies investigating the transport of isothiocyanates, reported the export of glutathione conjugates of isothiocyanates by MRP2. Polarized MRP2-transfected MDCK II cells in Transwell plates, incubated with 5 μM NITC at the apical side for 24 h, caused an increased secretion of glutathione into the apical medium (4-fold increase) compared with control MDCK II cells [23]. This phenomenon occurred because the metabolites that were exported were hydrolysed in the apical extracellular space and the parent circulated back into the cell for another cycle. However, the glutathione could not re-enter and was concentrated in the apical extracellular space. In the same investigation, a protective effect against NITC-induced cholestasis was observed in MRP2-deficient rats indicating that MRP2 might be the major transporter responsible for the glutathione depletion via isothiocyanates. Using 14C-labeled PEITC, it was shown that PEITC and/or its metabolites are substrates for MRP2 [40]. At PEITC concentrations of 1, 5, 10 and 50 μM, intracellular concentrations of 14C were 55%, 14%, 12% and 61% lower in MDCK/MRP2 cells over-expressing MRP2, compared with wild type MDCKII cells that do not express MRP2. Treatment with 50 μM MK571, an MRP inhibitor, increased intracellular 14C accumulation by 2.5-, 3.5-, 4.5- and 1.8-fold at PEITC concentrations 1, 5, 10 and 50 μM. In the same study, it was also shown that MRP2-mediated transport of isothiocyanates was glutathione-dependent: a 1 h treatment with 25, 50 and 100 μM buthionine sulfoximine, an agent which inhibits the synthesis of glutathione, increased the intracellular accumulation of the radiolabeled tracer by 48%, 77% and 116%, respectively, at a concentration of 5 μM PEITC. All results were statistically significant at p<0.01.
Similar results were observed with sulforaphane in MRP1 and P-gp over-expressing cells [30,36]. The accumulation of intracellular sulforaphane was increased by inhibitors of P-gp and MRP1 in overexpressing cell lines, indicating that sulforaphane and/or sulforaphane glutathione is a substrate for these transporters. When the interaction between MRP1 and PEITC in MRP1 over-expressing PANC-1 cells was investigated, it was observed that a 2 h co-incubation with 100 μM MK571 increased the accumulation of 14C tracer by 6.8-, 5.9- and 1.9-fold at PEITC concentrations of 1, 5 and 20 μM, respectively [41]. Accumulation did not change at higher PEITC concentrations, suggesting a saturation of MRP1-mediated transport. The accumulation of 14C-PEITC was not changed in Caco-2, human breast cancer MCF-7/ADR, MDA435/LCC6 and MDA435/LCC6MDR1 (P-gp overexpression) cells in the absence and presence of the P-gp inhibitor verapamil or PSC833 [40,41], suggesting that PEITC is not a P-gp substrate.
Further studies demonstrated that a number of isothiocyanates can increase the accumulation of MRP1 and P-gp substrates in cells that over-express these transporters (MCF7/ADR cells over-expressing P-gp and Panc-1 cells over-expressing MRP1) [13,41]. Cells were incubated with substrates daunomycin or vinblastine for 2 h along with 100 μM of various isothiocyanates. NITC exhibited strong effects on the accumulation of vinblastine and daunomycin in these cells (4- and 40-fold for daunomycin and vinblastine in MCF7/ADR cells and 3- and 5.5-fold for daunomycin and vinblastine in Panc-1 cells). BITC and PEITC also caused significant increases in the accumulation of daunomycin in CaCo2 cell monolayers (1.75- and 1.85-fold, respectively) [41]. PEITC treatment resulted in a 2-fold increase in the accumulation of vinblastine in Panc-1 cells but not MCF7/ADR cells, suggesting it is not a P-gp inhibitor. Weak P-gp inhibition by isothiocyanates was also reported by Barecki-Roach et al. [42]. PEITC or BITC, but not 1-NITC, when examined at 100 μM concentrations, decreased cellular GSH levels after 2 h incubations. PEITC reduced GSH to 10±3.5% of control values in untreated cells, while BITC reduced GSH to 15.3±6.4% of control values.
BCRP (ABCG2) is also widely expressed in all tissues, but shows the highest amount of expression in the placenta, liver and the intestinal apical membranes. BCRP is responsible for the efflux of endogenous steroids conjugated with glucuronide or sulfate moieties. Some drugs that are substrates of this transporter are topotecan, mitoxantrone, methotrexate, nitrofurantoin and sulfasalazine [32].
Intracellular accumulation of radiolabeled PEITC in BCRP over-expressing NCI-H460/MX20 cells was increased upon treatment with 10 μM fumitremorgin C (FTC) to 6.8-, 4.6-, 4.1-, 2.7- and 1.5-fold at 5, 10, 25 and 50 μM concentrations of PEITC, suggesting that PEITC and/or its metabolites are BCRP substrates [43]. This was further confirmed by measuring its accumulation in inside-out vesicles isolated from BCRP over-expressing MCF7/MX100 cells [44]. Since these systems do not have metabolizing enzymes, accumulation of the 14C tracer, after incubation with FTC, could be attributed to PEITC itself, as opposed to products of enzymatic metabolism. FTC (10 μM) decreased the ATP-dependent accumulation of 25 μM PEITC in inside-out vesicles by 1.7-fold. While competitive inhibition of transport by isothiocyanates would be expected since PEITC is a substrate of BCRP, isothiocyanates may also inhibit BCRP ATPase resulting in accumulation of BCRP drug substrates. Interactions between ITCs and ABC transporters are listed in Table 3.
Table 3.
In vitro studies of isothiocyanates as substrates/inhibitors of ABC transporters
| Cell line | Transporter | Isothiocyanates investigated as substrate (S)/inhibitor (I) | Reference |
|---|---|---|---|
| MDCK II | MRP2 | 1-Napthyl isothiocyanate (S) | [23] |
| HL60/AR | MRP1 | Allyl isothiocyanate (S) | [13] |
| 8226/Dox | P-gp | Sulforaphane (S) | [13] |
| Phenethyl isothiocyanate (S) | [13] | ||
| MCF7/ADR | P-gp | 1-Naphthyl isothiocyanate(I) | [13] |
| NHI-H460/MX20 | BCRP (BCRP ATPase) | Isothiocyanates (I) | [32] |
| NHI-H460/MX20 | BCRP | Phenethyl isothiocyanate (S) | [32] |
| MCF7 MX-100 | BCRP | Isothiocyanate (S) | [43] |
In vivo Pharmacokinetic Interactions of Benzyl Isothiocyanate and a BCRP Substrate Topotecan
There is one in vivo study in the literature that reported that the MRP2-mediated transport of glutathione-NITC induces cholestasis [23]. However, the in vivo effects of isothiocyanates on the pharmacokinetics of drug substrates of ABC transporters have not been investigated. We recently investigated the in vivo effects of the isothiocyanate BITC on the pharmacokinetics of topotecan, a known substrate of BCRP (and a minor substrate of P-gp). All animal studies were approved by the Institutional Animal Care and Use Committee at the University at Buffalo. The plasma concentration-time profile is shown in Figure 6. After non-compartmental analysis of the profiles, it was observed that both AUC0–720 min and AUC0–∞ values showed a trend of increasing after BITC co-administration in a dose-dependent manner (Table 4). Rats treated with topotecan alone (2 mg/kg dose) had AUC0–720 min of 10.9± 1.39 μg ml−1 min−1, whereas the AUC0–720min increased to 11.5±3.35, 14.1±1.70 and 16.5± 9.41 μg ml−1 min−1 following BITC co-administration at doses of 10, 50 and 100 mg/kg, respectively, corresponding to 1.1-, 1.3- and 1.5-fold increases compared with the control. Similarly, the AUC0–∞ increased in a dose-dependent manner and was 14.0±2.46, 19.8±5.68 and 25.0± 12.7 μg ml−1 min−1 following BITC doses of 10, 50 and 100 mg/kg, respectively, representing 1.2-, 1.8-and 2.2-fold increases compared with the control rats. Using the AUC0–∞ of topotecan following i.v. administration of 1 mg/kg topotecan determined in four rats (42.4±3.26 μg ml−1 min−1), F was calculated and presented in Table 4. The t1/2 increased with increasing doses of BITC and was 1.7-, 1.9- and 2.4-fold higher than that of the control rats following BITC doses of 10, 50 and 100 mg/kg, respectively. At the highest dose level of 100 mg/kg, the prolongation of t1/2 was significant (p<0.05). The Cmax and tmax of plasma topotecan were variable, and failed to show statistically significant differences among all the treatment groups (Table 4). BCRP expression is highly expressed in the kidney in rodents, and the majority of topotecan is eliminated through renal excretion. Therefore, BITC may inhibit BCRP located in the rat renal proximal tubules, resulting in an increased AUC and a decreased half-life.
Figure 6.
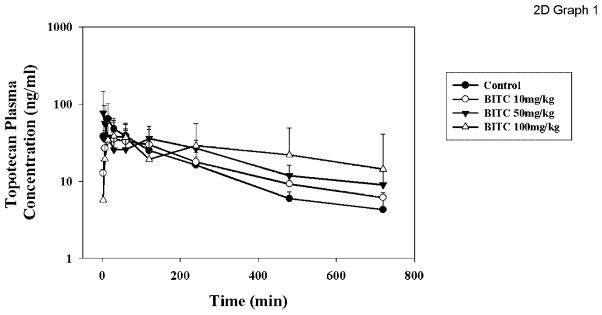
The plasma concentration-time profile of topotecan in rats with or without co-administration of BITC. Rats were administered the vehicle (tetraglycol) (●), 10 (○), 50 (▼), or 100 (△) mg/kg BITC orally by gavage. Topotecan was administered by gavage 2 min later. The blood samples were collected over time for 720 min after topotecan administration and plasma was separated and analysed for topotecan concentration by HPLC, as described in Materials and Methods. Data are expressed in mean±SD, n =4 or 5
Table 4.
Effect of BITC co-administration on topotecan pharmacokinetics in Sprague-Dawley rats
| Parameter | Control (n =5) | BITC
|
||
|---|---|---|---|---|
| 10 mg/kg (n =4) | 50 mg/kg (n =5) | 100 mg/kg (n =4) | ||
| AUC0–720 min (μg/ml · min) | 10.9±1.39 | 11.5±3.35 | 14.1±1.70 | 16.5±9.41 |
| AUC0–∞ (μg/ml · min) | 11.3±2.20 | 14.0±2.46 | 19.8±5.68 | 25.0±12.7 |
| Cmax (ng/ml) | 67.3±36.6 | 39.3±24.4 | 82.0±62.1 | 55.0±24.9 |
| tmax (min) | 27.0±19.6 | 48.8±48.0 | 28.2±51.6 | 93.8±99.8 |
| t1/2 (min) | 178±62.3 | 301±111 | 345±235 | 429±93.1a |
| F (%) | 12.8±1.64 | 16.5±2.89 | 23.3±6.69 | 29.4±14.9 |
p<0.05
In our in vivo investigation, BITC increased the mean oral bioavailability and systemic exposure and decreased the elimination of topotecan in rats in a dose-dependent manner. Based on in vitro studies, these observed effects may be a result of inhibition of the BCRP transporter at various sites affecting the disposition of topotecan. The results indicate that this interaction may be most important at the kidney level in the disposition of topotecan. Further studies using more specific probes for transporters and utilizing either isolated compounds, or mixtures of naturally occurring isothiocyanates may shed more light on the possible observable interactions.
While investigating pharmacokinetic transporter based diet–drug interactions, it is also important to consider the effects compounds may have on other transporters and enzymes that may be important in the disposition of a drug. Topotecan is known to be metabolized to N-desmethyl topotecan, a reaction mediated by CYP3A4. However, the percent of N-desmethyl topotecan formed is less than 0.7% in the plasma, and about 1–4% in the urine, indicating that inhibition of a CYP enzyme by BITC may not contribute significantly to its pharmacokinetics [45]. Uptake transporters may be involved in the passage of the ionized carboxylate form of topotecan across the cell membranes. ITCs may inhibit one or more intestinal uptake transporters that are responsible for the absorption of topotecan carboxylate. One such example could be organic anion-transporting polypeptides (OATPs), which are expressed on the apical membrane of the small intestine and mediate transmembrane transport of many amphipathic organic compounds including organic anions. It was recently reported that topotecan is a substrate of rat OATP3, which is involved in its renal secretion [46]. Interactions of ITCs with uptake transporters have not been investigated but could contribute to the discrepancies observed in the in vitro inhibition of ABC transporters and the in vivo effects of ITCs on drug disposition.
Conclusions
Isothiocyanates are widely present in the human diet and possess the potential to affect the disposition of substrates for ABC transporters through drug transporter interactions. Currently little is known about the in vivo effects of these compounds on the pharmacokinetics of xenobiotics whose clearance and/or tissue distribution is determined by active transport; additional investigations of ITC effects on both uptake and efflux transporters may prove helpful in understanding these interactions.
Acknowledgments
Funding was from the Susan G. Komen Foundation and US Army Breast Cancer Research Program Contract DAMD 17-00-1-0376.
References
- 1.Shapiro TA, Fahey JW, Wade KL, Stephenson K, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 2.Getahun SM, Chung FL. Conversion of glucosi-nolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev. 1999;8:447–451. [PubMed] [Google Scholar]
- 3.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen Hvan den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 4.Hecht SS, Kenney PM, Wang M, Trushin N, Upadhyaya P. Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 2000;150:49–56. doi: 10.1016/s0304-3835(99)00373-0. [DOI] [PubMed] [Google Scholar]
- 5.Carmella SG, Borukhova A, Akerkar SA, Hecht SS. Analysis of human urine for pyridine-N-oxide metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific lung carcinogen. Cancer Epidemiol Biomarkers Prev. 1997;6:113–120. [PubMed] [Google Scholar]
- 6.Guo Z, Smith TJ, Wang E, et al. Effects of phenethyl isothiocyanate, a carcinogenesis inhibitor, on xenobiotic-metabolizing enzymes and nitrosamine metabolism in rats. Carcinogenesis. 1992;13:2205–2210. doi: 10.1093/carcin/13.12.2205. [DOI] [PubMed] [Google Scholar]
- 7.Yoxall V, Kentish P, Coldham N, Kuhnert N, Sauer MJ, Ioannides C. Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for its chemopreventive activity. Int J Cancer. 2005;117:356–362. doi: 10.1002/ijc.21191. [DOI] [PubMed] [Google Scholar]
- 8.Svehlikova V, Wang S, Jakubikova J, Williamson G, Mithen R, Bao Y. Interactions between sulforaphane and apigenin in the induction of UGT1A1 and GSTA1 in CaCo-2 cells. Carcinogenesis. 2004;25:1629–1637. doi: 10.1093/carcin/bgh169. [DOI] [PubMed] [Google Scholar]
- 9.Yi-Rong Chem WW, Tony Kong A-N, Tse-Hua T. Molecular mechanisms of c-Jun N-terminal kinase-mediated apoptosis induced by anticarcinogenic isothiocyanates. J Biol Chem. 1998;273:1769–1775. doi: 10.1074/jbc.273.3.1769. [DOI] [PubMed] [Google Scholar]
- 10.Singh A, Xiao D. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 11.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007;67:2239–2246. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 12.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng E, Kamath A, Morris ME. Effect of organic isothiocyanates on the P-glycoprotein- and MRP1-mediated transport of daunomycin and vinblastine. Pharm Res. 2002;19:1509–1515. doi: 10.1023/a:1020460700877. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Tang L, Gonzalez V. Selected isothio-cyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–1052. [PubMed] [Google Scholar]
- 15.iHerb.com Nature’s Way Cruciferous Vegetable Blend with Sulforaphane.
- 16.Ji Y, Kuo Y, Morris ME. Pharmacokinetics of dietary phenethyl isothiocyanate in rats. Pharm Res. 2005;22:1658–1666. doi: 10.1007/s11095-005-7097-z. [DOI] [PubMed] [Google Scholar]
- 17.Ji Y, Morris ME. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal Biochem. 2003;323:39–47. doi: 10.1016/j.ab.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Hu K, Morris ME. Pharmacokinetics of alpha-naphthyl isothiocyanate in rats. J Pharm Sci. 2005;94 :2441–2451. doi: 10.1002/jps.20460. [DOI] [PubMed] [Google Scholar]
- 19.Hu R, Hebbar V, Kim BR, et al. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther. 2004;310:263–271. doi: 10.1124/jpet.103.064261. Epub 2004 Feb 26. [DOI] [PubMed] [Google Scholar]
- 20.Cornblatt BS, Ye L, Dinkova-Kostova AT, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 21.Conaway CC, Jiao D, Kohri T, Liebes L, Chung FL. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in F344 rats. Drug Metab Dispos. 1999;27:13–20. [PubMed] [Google Scholar]
- 22.Ketterer B. Dietary isothiocyanates as confounding factors in the molecular epidemiology of colon cancer [comment] Cancer Epidemiol Biomarkers Prevent. 1998;7:645–646. [PubMed] [Google Scholar]
- 23.Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167:73–81. doi: 10.1016/s0300-483x(01)00459-0. [DOI] [PubMed] [Google Scholar]
- 24.Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Oude Elferink RP, Zadina J. MDR1 P-glycoprotein transports endogenous opioid peptides. Peptides. 2001;22:2015–2020. doi: 10.1016/s0196-9781(01)00564-2. [DOI] [PubMed] [Google Scholar]
- 26.King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci. 2001;4:268–274. doi: 10.1038/85115. [DOI] [PubMed] [Google Scholar]
- 27.Hsiao P, Sasongko L, Link JM, Mankoff DA, Muzi M, Collier AC, Unadkat JD. Verapamil P-glycoprotein transport across the rat blood–brain barrier: cyclosporine, a concentration inhibition analysis, and comparison with human data. J Pharmacol Exp Ther. 2006;317:704–710. doi: 10.1124/jpet.105.097931. [DOI] [PubMed] [Google Scholar]
- 28.Williams GC, Liu A, Knipp G, Sinko PJ. Direct evidence that saquinavir is transported by multi-drug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2) Antimicrob Agents Chemother. 2002;46:3456–3462. doi: 10.1128/AAC.46.11.3456-3462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vlaming ML, Mohrmann K, Wagenaar E, et al. Carcinogen and anticancer drug transport by Mrp2 in vivo: studies using Mrp2 (Abcc2) knockout mice. J Pharmacol Exp Ther. 2006;318:319–327. doi: 10.1124/jpet.106.101774. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Callaway EC. High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J. 2002;364:301–307. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mottino AD, Hoffman T, Jennes L, Vore M. Expression and localization of multidrug resistant protein mrp2 in rat small intestine. J Pharmacol Exp Ther. 2000;293:717–723. [PubMed] [Google Scholar]
- 32.Mao Q, Unadkat JD. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005;7:E118–E133. doi: 10.1208/aapsj070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61:14–25. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 35.Terashi K, Oka M, Soda H, et al. Interactions of ofloxacin and erythromycin with the multidrug resistance protein (MRP) in MRP-overexpressing human leukemia cells. Antimicrob Agents Chemother. 2000;44:1697–1700. doi: 10.1128/aac.44.6.1697-1700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer R, Kartenbeck J, Buchler M, Jedlitschky G, Leier I, Keppler D. Expression of the MRP gene-encoded conjugate export pump in liver and its selective absence from the canalicular membrane in transport-deficient mutant hepatocytes. J Cell Biol. 1995;131:137–150. doi: 10.1083/jcb.131.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer zu Schwabedissen HE, Jedlitschky G, Gratz M, et al. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metab Dispos. 2005;33:896–904. doi: 10.1124/dmd.104.003335. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann C, Gutmann H, Hruz P, Gutzwiller JP, Beglinger C, Drewe J. Mapping of multidrug resistance gene 1 and multidrug resistance-associated protein isoform 1 to 5 mRNA expression along the human intestinal tract. Drug Metab Dispos. 2005;33:219–224. doi: 10.1124/dmd.104.001354. [DOI] [PubMed] [Google Scholar]
- 39.Hazai E, Bikadi Z. Homology modeling of breast cancer resistance protein (ABCG2) J Struct Biol. 2008;162:63–74. doi: 10.1016/j.jsb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Ji Y, Morris ME. Transport of dietary phenethyl isothiocyanate is mediated by multidrug resistance protein 2 but not P-glycoprotein. Biochem Pharmacol. 2005;70:640–647. doi: 10.1016/j.bcp.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 41.Hu K, Morris ME. Effects of benzyl-, phenethyl-, and alpha-naphthyl isothiocyanates on P-glyco-protein- and MRP1-mediated transport. J Pharm Sci. 2004;93:1901–1911. doi: 10.1002/jps.20101. [DOI] [PubMed] [Google Scholar]
- 42.Barecki-Roach M, Wang EJ, Johnson WW. Quantitative evaluation of isothiocyanates as substrates and inhibitors of P-glycoprotein. J Pharm Pharmacol. 2003;55:1251–1257. doi: 10.1211/0022357021666. [DOI] [PubMed] [Google Scholar]
- 43.Ji Y, Morris ME. Effect of organic isothiocyanates on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21:2261–2269. doi: 10.1007/s11095-004-7679-1. [DOI] [PubMed] [Google Scholar]
- 44.Ji Y, Morris ME. Membrane transport of dietary phenethyl isothiocyanate by ABCG2 (breast cancer resistance protein) Mol Pharm. 2005;2:414–419. doi: 10.1021/mp050029f. [DOI] [PubMed] [Google Scholar]
- 45.Rosing H, Herben VM, van Gortelvan Zomeren DM, et al. Isolation and structural confirmation of N-desmethyl topotecan, a metabolite of topotecan. Cancer Chemother Pharmacol. 1997;39:498–504. doi: 10.1007/s002800050605. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto S, Yoshida K, Ishiguro N, Maeda T, Tamai I. Involvement of rat and human organic anion transporter 3 in the renal tubular secretion of topotecan [(S)-9-dimethylaminomethyl-10-hy-droxy-camptothecin hydrochloride] J Pharmacol Exp Ther. 2007;322:1246–1252. doi: 10.1124/jpet.107.123323. [DOI] [PubMed] [Google Scholar]