Abstract
Background
Digitalis glycosides are known to improve the hemodynamic and neurohormonal perturbations that contribute to heart failure (HF) induced renal dysfunction (RD). The objective of this study was to determine if randomization to digoxin is associated with improvement in renal function (IRF) and to evaluate if patients with digoxin induced IRF have improved clinical outcomes.
Methods and Results
Patients in the Digitalis Investigation Group dataset with protocol driven 12 month serum creatinine levels (performed in a central laboratory, n=980) were studied. IRF was defined as a post randomization ≥ 20% increase in estimated glomerular filtration rate (eGFR). IRF occurred in 15.5% of the population (mean improvement in eGFR 34.5 ± 15.4%) and was more common in patients randomized to digoxin (adjusted OR=1.6, p=0.02). In patients without IRF, digoxin was not associated with reduced death or hospitalization (adjusted HR=0.96, 95% CI 0.8–1.2, p=0.67). However, in the group with IRF, digoxin was associated with substantially improved hospitalization free survival (adjusted HR=0.49, 95% CI 0.3–0.8, p=0.006, p interaction=0.026).
Conclusions
In this subset of the DIG trial, digoxin was associated with long term improvement in kidney function, and in patients demonstrating this favorable renal response, reduction in death or hospitalization. Additional research is necessary to confirm these hypothesis generating findings.
Keywords: Cardio-renal syndrome, improved renal function, digoxin, mortality
Introduction
Renal dysfunction (RD) has emerged as one of the most powerful risk factors for adverse outcomes in heart failure (HF) and is often a limiting factor in the utilization of evidence-based therapies and maintenance of euvolemia.(1) Notably, static renal dysfunction, a worsening in renal function, and even an improvement in renal function (IRF) have all been associated with worsened survival.(2–5) The high prevalence and prognostic importance of RD in HF has triggered substantial interest in the development of therapeutic strategies aimed at improving renal and clinical outcomes in these patients. Unfortunately, therapeutic agents with direct beneficial effects on renal function, such as the adenosine antagonists, synthetic natriuretic peptides, and vasopressin antagonists, have not meaningfully improved renal or clinical endpoints in relatively unselected HF populations.(6–8)
Some of the primary drivers of HF-induced RD are the hemodynamic and neurohormonal perturbations typical of severe HF, factors which also directly contribute to adverse outcomes. As such, it is possible that targeting cardio-renal dysfunction upstream to the kidneys (i.e., improving the hemodynamics and neurohormonal activation thought causal of the RD) could provide benefit to these high risk patients with respect to both renal and clinical outcomes. Digitalis glycosides are known to have beneficial hemodynamic and neurohormonal effects in patients with HF.(9) As a result, we hypothesized that HF patients randomized to digoxin would likely have a greater incidence of IRF compared to patients assigned to placebo. We additionally hypothesized that IRF in the setting of initiation of digoxin signifies a favorable therapeutic response to cardiac glycosides and, as such, serves to identify a group of patients that may derive a particularly large reduction in adverse clinical events with continued digoxin use. Thus, the primary objective of this proof of concept study was to evaluate the effects of digoxin on long term changes in kidney function and investigate if patients who experience IRF may have an exaggerated clinical benefit from digoxin therapy.
Methods
The Digitalis Investigation Group (DIG) trial was a National Heart, Lung and Blood Institute (NHLBI) sponsored, randomized, double-blind, placebo controlled trial of the effect of digoxin on clinical outcomes in patients with chronic heart failure. Methods and results have been previously published.(10,11) Briefly, 7,798 patients with chronic heart failure were randomized to digoxin or placebo at 302 clinical centers in the United States and Canada. Patients were eligible if they had a diagnosis of heart failure based on past clinical symptoms, signs, or radiologic evidence of pulmonary congestion. Patients were required to be on a stable dose of diuretic and an angiotensin converting enzyme inhibitor (if ejection fraction ≤45%) prior to entry into the trial. Exclusion criteria included atrial fibrillation, hypo or hyperkalemia, baseline creatinine level >3.0 mg/dL, listing for cardiac transplantation, or recent myocardial infarction/revascularization. A subset of the original study population underwent protocol driven assessment of laboratory values, such as serum creatinine and digoxin levels, in a central laboratory. Patients with data available to calculate estimated glomerular filtration rate (eGFR), both at the baseline and 12 month study visits, were included in the current analyses (n=980). There was no difference in the rate of randomization to digoxin in this subset compared to patients without this data available (p=0.14). Although differences in baseline characteristics of the patients in the current subset were present compared to patients missing this data, the effect of randomization appeared to be relatively preserved across these differences (Supplementary Table 1).
Estimated GFR was calculated using the four variable Modified Diet and Renal Disease (MDRD) equation.(12) Although the Chronic Kidney Disease Epidemiology Collaboration formula (CKD-EPI) has demonstrated a greater accuracy in the estimation of GFR in several populations, this formula has not been well validated in HF populations. Notably CKD-EPI gives relative estimates of chronic kidney disease prevalence that are discordant (higher) than that found when comparing CKD-EPI to MDRD in populations where the superior accuracy in estimation of GFR were determined (where chronic kidney disease estimates are lower with CKD-EPI than MDRD).(1) Thus MDRD, which has been extensively utilized in outcomes based research in HF, was employed in the primary analyses. The primary analyses evaluating the interaction between randomization to study drug, IRF, and death or hospitalization were repeated using the CKD-EPI formula to ensure similar results were obtained. IRF was defined as baseline to one year ≥20% improvement in eGFR in order to account for the non-linear relationship between serum creatinine and GFR and to maintain consistency with our prior studies of IRF.(3,5,13) The DIG trial was conducted and supported by the NHLBI in collaboration with the DIG study investigators. This analysis was conducted using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the DIG investigators or the NHLBI.
Statistical Methods
The two primary analyses in this proof of concept study focused on: 1) the relative incidence of IRF between patients randomized to digoxin vs. placebo and 2) the relative association between death or hospitalization and randomization to digoxin in strata of patients that did or did not experience IRF. As such, the primary endpoints were: 1) the association between IRF and randomization to digoxin and 2) the interaction between IRF, study drug assignment, and subsequent death or hospitalization. Given the limited number of patients with 1 year eGFR available, the combination of death or hospitalization was chosen as the primary endpoint to maximize power with planned subanalyses for the component endpoints. Given the previously reported importance of serum digoxin levels on the mortality benefit of digoxin therapy in this population, subanalysis incorporating digoxin levels at 12 months using previously identified clinically relevant levels was also undertaken.(14) Values reported are mean ± standard deviation, median (quartile 1 - quartile 4), and percentile. Independent Student’s t-test or the Mann-Whitney U test was used to compare continuous parameters. Pearson’s Chi Square was used to evaluate categorical variables. Proportional hazards modeling was used to evaluate time to event associations with death or hospitalization with time zero defined as the 12 month study visit (since by definition patients had to survive to this visit in order for serum creatinine to be determined). Candidate covariates for multivariable modeling were obtained by screening all baseline variables for a univariate association with either IRF (primary analysis 1) or death or hospitalization (primary analysis 2) for a p≤ 0.2. These covariates were removed using backwards elimination (likelihood ratio) and variables with a p≤ 0.2 were retained.(15) Adjusted survival curves for hospitalization or death were plotted for the four combinations of groups between patients that did or did not experience IRF and patients receiving placebo or digoxin. The same covariates used in the primary multivariable models were used to produce the adjusted survival curves. Significance was defined as 2-tailed p<0.05 for all analyses excluding tests of interaction where p values <0.1 were considered significant. Statistical analysis was performed with IBM SPSS Statistics version 19.0 (IBM Corp., Armonk, NY).
Results
Baseline characteristics and the effect of randomization to digoxin on death or rehospitalization in the DIG trial has been previously reported.(11) Baseline characteristics of the 980 patients in the current analysis and comparison of those with and without IRF at 12 months are presented in Table 1. In total, 152 patients (15.5%) experienced IRF with a mean improvement in eGFR of 34.5 ± 15.4%. Amongst patients with normal baseline renal function (eGFR >90 ml/min/1.73 m2) only 2.7% experienced IRF, whereas in those with significant baseline RD (i.e., eGFR <60 ml/min/1.73 m2), the incidence of IRF was higher (21.5%, Figure 1). Baseline characteristics were similar between patients that did or did not experience IRF (Table 1). Notable exceptions were a lower baseline eGFR, a lower prevalence of male gender, and a slightly younger age in the IRF group. In the overall sample, randomization to digoxin was associated with a greater incidence of IRF (18% vs. 12%, OR=1.5, p=0.024). This association remained significant after adjustment for baseline eGFR (OR=1.5, p=0.03), eGFR at 12 months (OR=1.5, p=0.026), and baseline characteristics associated with IRF (sex, race, age, cardio-thoracic ratio, NYHA class, baseline eGFR; OR= 1.6, p=0.018). Amongst patients that did experience IRF, the relative degree of improvement in eGFR was similar in both the digoxin and placebo groups. Furthermore, there were no significant differences in baseline characteristics between patients in the placebo and digoxin groups that experienced IRF (Table 1). In patients experiencing IRF, there were no differences in the use of diuretics, angiotensin converting enzyme inhibitors, nitrates, hydralazine, or potassium supplementation at one year between the placebo and digoxin groups (p ≥0.23 for all comparisons). Notably, in patients experiencing IRF, the rate of discontinuation of angiotensin converting enzyme inhibitor therapy was small and similar between the placebo group (2 patients) and the digoxin group (3 patients, p=0.67).
Table 1.
Baseline characteristics of the overall cohort and patients with and without improvement in renal function
| Characteristics | Overall Cohort (n=980) |
No IRF (n=828) |
Yes IRF (n=152) |
p | IRF and | p | |
|---|---|---|---|---|---|---|---|
| Placebo (n=60) |
Digoxin (n=92) |
||||||
| Demographics | |||||||
| Age | 63.4 ± 10.5 | 63.8 ± 10.2 | 61.6 ± 11.8 | 0.021* | 60.0 ± 12.7 | 62.7 ± 11.2 | 0.170 |
| White race | 88.6% | 89.3% | 84.9% | 0.118 | 86.7% | 83.7% | 0.617 |
| Male | 78.6% | 80.1% | 70.4% | 0.008* | 66.7% | 72.8% | 0.416 |
| Past Medical History | |||||||
| Hypertension | 43.0% | 42.6% | 44.7% | 0.630 | 41.7% | 46.7% | 0.539 |
| Diabetes mellitus | 25.4% | 25.6% | 24.3% | 0.743 | 18.3% | 28.3% | 0.163 |
| Angina | 26.0% | 26.3% | 24.3% | 0.608 | 21.7% | 26.1% | 0.535 |
| Prior myocardial infarction | 68.4% | 68.8% | 65.8% | 0.457 | 60.0% | 69.6% | 0.224 |
| Physical Examination | |||||||
| Heart rate | 76.5 ± 12.6 | 76.4 ± 12.6 | 77.4 ± 12.9 | 0.382 | 77.9 ± 12.7 | 77.0 ± 13.1 | 0.657 |
| Systolic blood pressure (mmHg) | 126.6 ± 20.2 | 126.9 ± 20.2 | 124.8 ± 19.7 | 0.247 | 123.0 ± 20.1 | 126.0 ± 19.5 | 0.360 |
| Pulmonary crackles | 71.0% | 70.9% | 71.7% | 0.838 | 71.7% | 71.7% | 0.992 |
| S3 gallop | 49.4% | 49.4% | 49.3% | 0.990 | 41.7% | 54.3% | 0.126 |
| Edema | 50.2% | 48.9% | 57.2% | 0.059 | 61.7% | 54.3% | 0.373 |
| Jugular venous distention | 52.2% | 51.0% | 59.2% | 0.061 | 60.0% | 58.7% | 0.873 |
| Medications (Baseline) | |||||||
| Prior digoxin | 49.9% | 49.8% | 50.7% | 0.838 | 45.0% | 54.3% | 0.260 |
| ACE inhibitor | 96.2% | 96.1% | 96.7% | 0.732 | 96.7% | 96.7% | 0.980 |
| Potassium sparing diuretic | 11.5% | 11.1% | 13.8% | 0.337 | 18.3% | 10.9% | 0.192 |
| Other diuretic | 75.7% | 75.6% | 76.3% | 0.851 | 76.7% | 76.1% | 0.935 |
| Nitrates | 36.2% | 35.5% | 40.1% | 0.276 | 40.0% | 40.2% | 0.979 |
| Hydralazine | 1.0% | 1.0% | 1.3% | 0.693 | 0.0% | 2.2% | 0.250 |
| Laboratory Value (baseline) | |||||||
| eGFR (mL/min/1.73m2) | 70.0 ± 21.7 | 71.4 ± 22.3 | 62.1 ± 15.3 | <0.001* | 63.7 ± 15.9 | 61.1 ± 15.0 | 0.297 |
| Serum creatinine (mg/dL) | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.3 | 0.001* | 1.3 ± 0.3 | 1.4 ± 0.3 | 0.339 |
| Laboratory Value (one year) | |||||||
| eGFR (mL/min/1.73m2) | 68.4 ± 21.2 | 65.6 ± 19.9 | 83.3 ± 21.8 | <0.001* | 85.2 ± 22.4 | 82.0 ± 21.4 | 0.384 |
| Serum creatinine (mg/dL) | 1.3 ± 0.4 | 1.4 ± 0.5 | 1.0 ± 0.2 | <0.001* | 1.0 ± 0.3 | 1.1 ± 0.2 | 0.429 |
| Serum digoxin (ng/ml) † | 0.8 ± 0.6 | 0.9± 0.6 | 0.8 ± 0.5 | 0.078 | 0.0 ± 0.0 | 0.8 ± 0.5 | <0.001* |
| Serum digoxin level ≤0.08 ng/ml † | 54.7% | 52.2% | 66.7% | 0.014* | N/A | 66.7% | N/A |
| Change in eGFR (mL/min/1.73m2) | 1.6 ± 17.5 | −5.8 ± 15.2 | 21.2 ± 10.2 | <0.001* | 21.5 ± 10.7 | 21.0 ± 9.9 | 0.771 |
| Change in eGFR (%) | 0.6 ± 21.9 | −7.0 ± 15.9 | 34.5 ± 15.4 | <0.001* | 34.2 ± 15.4 | 34.7 ± 15.5 | 0.850 |
| Functional Status / Imaging | |||||||
| Ejection fraction (%) | 31.6 ± 12.2 | 31.5 ± 11.9 | 32.3 ± 13.7 | 0.481 | 32.5 ± 14.3 | 32.2 ± 13.4 | 0.887 |
| Preserved ejection fraction (>45%) | 11.7% | 11.0% | 15.8% | 0.091 | 20.0% | 13.0% | 0.250 |
| Cardio-thoracic ratio | 0.51 ± 0.07 | 0.52 ± 0.07 | 0.53 ± 0.07 | 0.082 | 0.53 ± 0.06 | 0.53 ± 0.07 | 0.806 |
| New York Heart Association class | 2.1 ± 0.7 | 2.1 ± 0.7 | 2.2 ± 0.7 | 0.090 | 2.3 ± 0.7 | 2.2 ± 0.7 | 0.157 |
ACE: Angiotensin converting enzyme inhibitor. Improvement in renal function (IRF) defined as ≥ 20% improvement in estimated glomerular filtration rate (eGFR) from randomization to one year.
Significant p value
Analysis restricted to patients randomized to digoxin.
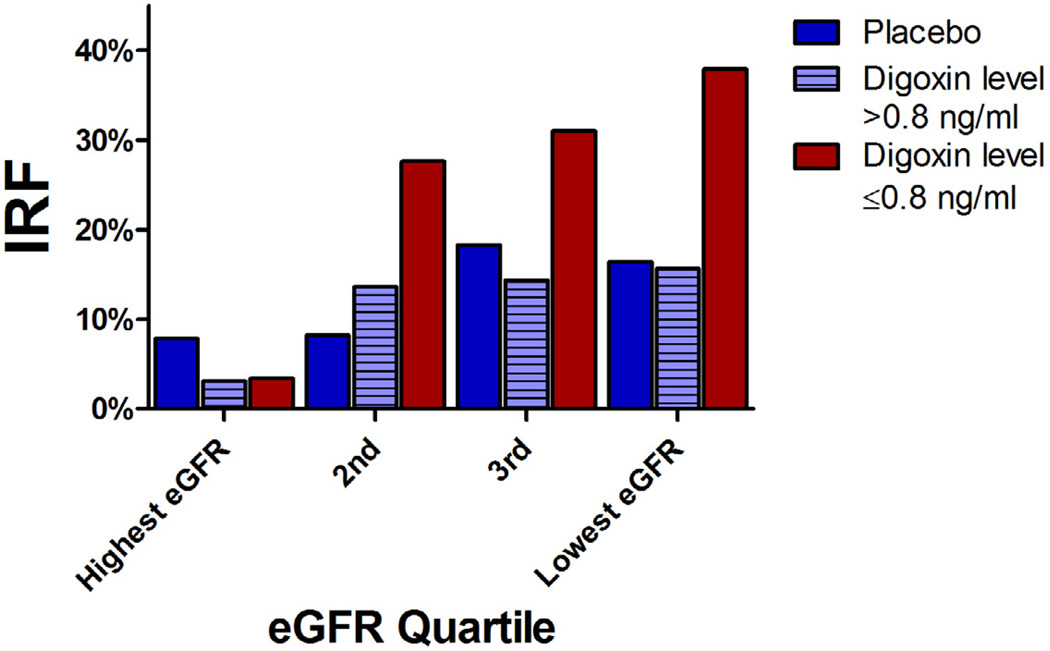
Figure 1. Incidence of improvement in renal function from baseline to one year across quartiles of baseline.
eGFR. eGFR: Estimated glomerular filtration rate. IRF: Improved renal function; defined as a ≥20% improvement in estimated glomerular filtration rate from randomization to one year. Lowest eGFR quartile < 57.9 ml/min/1.73 m2, 3rd quartile 57.9 ml/min/1.73 m2 to 70.0 ml/min/1.73 m2, 2nd quartile 70.0 ml/min/1.73 m2 to 83.7 ml/min/1.73 m2, highest quartile > 83.7 ml/min/1.73 m2.
Digoxin Dose, Level and IRF
There was no significant difference in the dose of study drug, both immediately after randomization (p=0.39) or at 12 months (p=0.53), between those with and without IRF. The same lack of association remained when the analysis was restricted to patients randomized to digoxin (dose immediately after randomization, p=0.33; dose at 12 months, p=0.60). In the overall sample, there was a weak inverse relationship between digoxin level at 12 months and IRF (0.78 ± 0.47 ng/ml in IRF vs. 0.89 ± 0.54 ng/ml in non-IRF, p=0.046) that did not persist after controlling for 12 month eGFR (p=0.75). However, there was a significant interaction between baseline eGFR, digoxin level, and IRF (p interaction=0.011) such that, amongst patients with baseline RD (eGFR <60 ml/min/1.73 m2), the one year digoxin level was substantially lower in the IRF group (0.7 ± 0.4 ng/ml vs. 1.1 ± 0.5 ng/ml in those without IRF, p<0.001; OR for IRF=0.86 per 0.1 ng/ml increase, p<0.001). Importantly, this association was unchanged by adjustment for 12 month eGFR (adjusted OR=0.84 per 0.1 ng/ml increase, p=0.008). The strong relationship between study drug assignment, digoxin level, and the incidence of IRF is depicted in Figure 1.
Associations with Death or Hospitalization
Over a median follow-up of 1,151 days, a total of 277 (28.3%) patients were hospitalized and 269 (27.4%) died of any cause. The composite endpoint of death or hospitalization occurred in 418 (42.7%) patients. Similar to the overall trial results, in the subset of patients included in the current analysis, randomization to digoxin did not influence the rate of death (HR=0.96, 95% CI 0.75–1.2, p=0.71). The point estimate for reduction in hospitalization was consistent with that reported in the overall trial (HR=0.85), but did not meet statistical significance in the subset of patients with 1 year creatinine levels available (HR=0.89, 95% CI 0.70–1.1, p=0.31).(11) Similar to the previous analysis of the overall population, there was no interaction between baseline eGFR and the rate of death or hospitalization with study drug assignment in this subset (p interaction=0.89).(16) Amongst patients that did not experience IRF, randomization to digoxin was not associated with a reduction in death or hospitalization, nor the component endpoints (Table 2). However, in the group with IRF, randomization to digoxin was associated with a substantial reduction in death or hospitalization (Table 2). Similar results were obtained after substitution of MDRD eGFR with CKD-EPI eGFR for death or hospitalization (p interaction=0.02), the components of death (p interaction=0.06), and hospitalization (p interaction=0.05).These associations tended to strengthen after controlling for baseline or 12 month eGFR (Table 2). Adjustment for baseline characteristics associated with mortality (age, race, ejection fraction, heart rate, systolic blood pressure, New York Heart Association class, diabetes mellitus, baseline use of digoxin, hydralazine, nitrates, diuretics, or angiotensin converting enzyme inhibitors, physical examination findings, cardio thoracic ratio, and baseline eGFR) minimally altered these associations (Table 2). The reduction in the component endpoint of death did not meet statistical significance (Table 2) and only with adjustment for baseline eGFR was the reduction in the rate of hospitalization significant in patients with IRF (Table 2). Nonetheless, the point estimates were similar amongst all endpoints and the interaction terms remained significant for both death and hospitalization suggesting that the results noted with the composite endpoint were unlikely to have been driven by the reduction in hospitalization alone (Table 2). Although limited by power, these results did not appear to differ significantly by baseline eGFR as the interaction remained significant in those with a baseline eGFR above (adjusted p interaction = 0.096) or below (adjusted p interaction = 0.078) the median (3 way p interaction = 0.48). Notably, in patients with a baseline eGFR below the median (68.9 ml/min/1.73m2), the risk of death or hospitalization associated with randomization to digoxin remained significantly reduced in patients with IRF (adjusted HR=0.49, 95% CI 0.27–0.91, p=0.025) whereas the group without IRF had no benefit attributable to digoxin (adjusted HR=1.0, 95% CI 0.78–1.4, p=0.78).
Table 2.
Risk of death or hospitalization associated with randomization to digoxin stratified by improvement in renal function status.
| No IRF | Yes IRF | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | p interaction | |
| Death or Hospitalization | |||||
| (n events = 418) | |||||
| Unadjusted | 0.99 (0.80–1.2) | 0.910 | 0.60 (0.37–0.98) | 0.042* | 0.066* |
| Adjusted for baseline eGFR | 0.99 (0.80–1.2) | 0.909 | 0.56 (0.34–0.92) | 0.023* | 0.043* |
| Adjusted for 12 month eGFR | 1.0 (0.80–1.2) | 0.995 | 0.59 (0.33–0.92) | 0.028* | 0.029* |
| Adjusted for baseline characteristics | 0.96 (0.77–1.2) | 0.665 | 0.49 (0.29–0.81) | 0.006* | 0.026* |
| Death (n events = 269) | |||||
| Unadjusted | 1.0 (0.81–1.4) | 0.742 | 0.59 (0.32–1.1) | 0.100 | 0.095* |
| Adjusted for baseline eGFR | 1.0 (0.81–1.4) | 0.752 | 0.58 (0.31–1.1) | 0.090 | 0.078* |
| Adjusted for 12 month eGFR | 1.1 (0.82–1.4) | 0.636 | 0.58 (0.31–1.1) | 0.089 | 0.058* |
| Adjusted for baseline characteristics | 1.0 (0.80–1.3) | 0.793 | 0.60 (0.32–1.1) | 0.120 | 0.064* |
| Hospitalization (n events = 277) | |||||
| Unadjusted | 0.96 (0.74–1.2) | 0.725 | 0.58 (0.31–1.1) | 0.082 | 0.149 |
| Adjusted for baseline eGFR | 0.96 (0.74–1.2) | 0.721 | 0.51 (0.28–0.96) | 0.035* | 0.106 |
| Adjusted for 12 month eGFR | 0.97 (0.75–1.2) | 0.802 | 0.50 (0.27–0.94) | 0.030* | 0.078* |
| Adjusted for baseline characteristics | 0.92 (0.71–1.2) | 0.523 | 0.44 (0.23–0.84) | 0.013* | 0.053* |
Improvement in renal function (IRF) defined as a ≥20% increase in estimated globular filtration rate (eGFR) from randomization to one year. Baseline characteristics adjusted for: age, race, ejection fraction, heart rate, systolic blood pressure, NHYA class, diabetes mellitus, baseline use of digoxin, hydralazine, nitrates, diuretics, or angiotensin converting enzyme inhibitors, physical examination findings, cardio-thoracic ratio, and baseline eGFR.
Significant p value.
Death or Hospitalization, IRF and Digoxin Level
Given the previously reported interaction between serum digoxin levels and the effectiveness of digoxin in reducing adverse events, a subanalysis of only patients with a serum digoxin level ≤0.8 ng/ml at one year (a threshold previously demonstrated to confer the greatest benefit) was undertaken.(14) Similar to the findings in the overall sample, in patients with a digoxin level ≤0.8 ng/ml and no IRF; the rate of hospitalization, death, or the combined endpoint was not better with digoxin compared to placebo (Table 3). However, amongst patients with IRF and a digoxin level ≤0.8 ng/ml at one year, the rate of both the combined endpoint and all-cause mortality were substantially reduced (Table 3 and Figure 2). Unlike mortality, restricting the analysis to patients with a digoxin level ≤0.8 at one year minimally improved the point estimates for the reduction in hospitalization (Table 3).
Table 3.
Risk of death or hospitalization associated with randomization to digoxin stratified by improvement in renal function status, in the subgroup of patients with one year serum digoxin levels ≤0.8 ng/ml.
| No IRF | Yes IRF | p interaction |
|||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
| Death or Hospitalization | |||||
| (n events = 305) | |||||
| Unadjusted | 0.86 (0.66–1.1) | 0.277 | 0.46 (0.25–0.82) | 0.010* | 0.054* |
| Adjusted for baseline eGFR | 0.95 (0.72–1.2) | 0.676 | 0.41 (0.24–0.80) | 0.007* | 0.023* |
| Adjusted for 12 month eGFR | 0.97 (0.74–1.3) | 0.827 | 0.42 (0.23–0.77) | 0.005* | 0.014* |
| Adjusted for baseline Characteristics | 0.92 (0.70–1.2) | 0.524 | 0.30 (0.16–0.58) | <0.001* | 0.018* |
| Death (n events = 190) | |||||
| Unadjusted | 0.84 (0.60–1.2) | 0.316 | 0.37 (0.16–0.84) | 0.017* | 0.066* |
| Adjusted for baseline eGFR | 0.91 (0.65–1.3) | 0.576 | 0.36 (0.16–0.82) | 0.015* | 0.042* |
| Adjusted for 12 month eGFR | 0.96 (0.68–1.3) | 0.807 | 0.36 (0.16–0.81) | 0.014* | 0.029* |
| Adjusted for baseline characteristics | 0.93 (0.66–1.3) | 0.662 | 0.35 (0.15–0.79) | 0.012* | 0.023* |
| Hospitalization (n events = 208) | |||||
| Unadjusted | 0.86 (0.62–1.2) | 0.354 | 0.58 (0.29–1.1) | 0.112 | 0.301 |
| Adjusted for baseline eGFR | 0.95 (0.69–1.3) | 0.755 | 0.54 (0.28–1.1) | 0.079 | 0.164 |
| Adjusted for 12 month eGFR | 0.98 (0.71–1.4) | 0.891 | 0.52 (0.26–1.0) | 0.060 | 0.120 |
| Adjusted for baseline characteristics | 0.88 (0.64–1.2) | 0.456 | 0.38 (0.17–0.82) | 0.014* | 0.145 |
Improvement in renal function (IRF) defined as a ≥20% increase in estimated globular filtration rate (eGFR) from randomization to one year. Patients in the placebo group were assumed to have a serum digoxin level less than 0.8 ng/ml. Baseline characteristics adjusted for: age, race, ejection fraction, heart rate, systolic blood pressure, NHYA class, diabetes mellitus, baseline use of digoxin, hydralazine, nitrates, diuretics, or angiotensin converting enzyme inhibitors, physical examination findings, cardio-thoracic ratio, and baseline eGFR.
Significant p value.
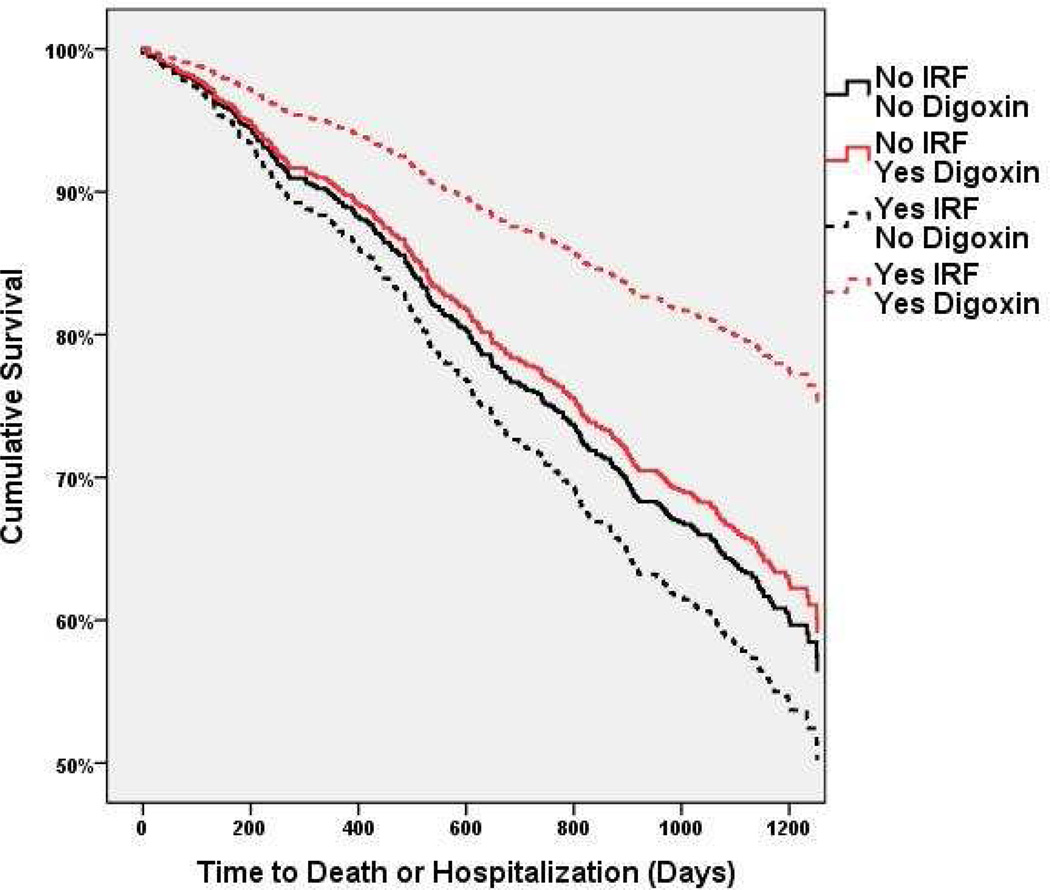
Figure 2. Adjusted survival curves grouped by randomization to digoxin or placebo and subsequent improved renal function status, in patients with a serum digoxin level ≤0.8 ng/ml.
Improved renal function (IRF) defined as a ≥20% improvement in estimated glomerular filtration rate (eGFR) from randomization to one year. Covariates adjusted for: age, race, ejection fraction, heart rate, systolic blood pressure, NHYA class, diabetes, baseline use of digoxin, hydralazine, nitrates, diuretics, or angiotensin converting enzyme inhibitors, physical examination findings, cardio-thoracic ratio, and baseline eGFR. Overall between group differences p=0.028. Comparisons of the Yes IRF/Yes Digoxin (n=58) to the No IRF/No Digoxin group (n=409, p=0.007), No IRF/Yes Digoxin (n=213, p=0.026), and Yes IRF/No Digoxin group (n=60, p=0.004) were all statistically significant.
IRF and Death or Hospitalization
There was no association between IRF and death or hospitalization in the overall cohort, both before (HR=0.98, 95% CI 0.75–1.3, p=0.85) and after adjustment for eGFR at 12 months (to focus on the change in renal function rather than the absolute eGFR at 12 months; HR=1.2, 95% CI 0.93–1.6, p=0.16). Amongst patients randomized to digoxin, the lack of association between IRF and death or hospitalization persisted after adjustment for 12 month eGFR (HR=0.96, 95% CI 0.65–1.4, p=0.83). Despite similar baseline characteristics, in patients randomized to placebo, IRF at 1 year was strongly associated with increased subsequent death or hospitalization (adjusted for 12 month eGFR; HR=1.7, 95% CI 1.2–2.6, p=0.008, p interaction=0.029). There were also significant associations between IRF and individual components of death (HR=1.8, 95% CI 1.1–2.9, p=0.028, p interaction=0.058) and hospitalization (HR=1.8, 95% CI 1.1–3.0, p=0.023, p interaction=0.078) in patients in the placebo group. Adjustment for baseline characteristics associated with mortality did not alter the differential association between IRF and death or hospitalization between patients randomized to digoxin or placebo (p interaction=0.022).
Discussion
The principal observations of this proof of concept study are: 1) randomization to digoxin appears to be associated with a significantly greater incidence of IRF at one year and 2) digoxin was associated with the greatest reduction in death or hospitalization in the subset of patients that had an improvement in renal function. Amongst patients with stable renal function from baseline to one year, randomization to digoxin or placebo was associated with nearly identical rates of death, hospitalization, or the combination. However, patients assigned to digoxin that had IRF at one year (potentially identifying patients that have both baseline cardiorenal dysfunction and physiology responsive to cardiac glycosides), the rate of death or hospitalization was significantly reduced. Importantly, when this analysis was restricted to patients with a serum digoxin level ≤0.8 ng/ml, the association between randomization to digoxin and the reduction in death or hospitalization in patients with IRF strengthened, a finding largely driven by a further reduction in mortality. Although significant limitations inherent to these data exist, these results provide preliminary evidence to suggest that cardiac glycosides may provide benefit with respect to longterm renal and clinical outcomes in selected patients with cardio-renal dysfunction.
With the possible exception of discontinuation of angiotensin converting enzyme inhibitor therapy (which was rare in this population and similar between groups), there are no commonly used therapeutic maneuvers in HF that can induce IRF independent of a true improvement in the function of the kidney. As such, the finding of IRF strongly suggests that the patient had some degree of baseline cardio-renal dysfunction. Moreover, since the primary defect in most chronic intrinsic kidney disease is an irreversible loss of nephrons, the finding of IRF also helps to distinguish patients with reversible RD from those with chronic intrinsic kidney disease. Given the absence of methodology with which HF- induced RD can be identified, IRF may represent an approach to facilitate identification of patients that will ultimately have improved long term outcomes with cardio-renal therapeutics.
The current findings also support the concept that the factors driving changes in renal function in HF populations may be more important than the actual changes in GFR. Illustrating this, amongst patients that developed IRF in the current study, the magnitude of improvement in eGFR at one year was nearly identical between the digoxin and placebo groups. However, there was significantly worsened survival in patients with IRF in the placebo group but no impact on mortality in the digoxin group. Consistent with these findings, we have recently reported that IRF is fairly common in HF, often transient, and associated with a significantly worsened survival.(3,5) Given that it is highly unlikely that an improvement in GFR itself would be causal of poor outcomes, it is probable that IRF identifies a “sicker” cohort of patients with limited cardio-renal reserve. Since these patients are “sicker” at baseline, it is understandable how this group could have greater rates of adverse clinical outcomes, but also derive particular clinical benefit from an agent that can provide a sustained improvement in overall hemodynamic and neurohormonal status. Furthermore, it is reasonable to assume that a “cardio-renal” intervention with the potential to improve clinical outcomes must have a net benefit to the overall cardio-renal axis, which digoxin may provide. A therapeutic intervention that improves renal function but worsens the overall heart failure (i.e., stopping ACE-I or diuretic therapy) would be expected have an overall net negative impact on clinical outcomes.
Although limited by the small number of observations, the association between a lower serum digoxin level at one year and both an increased incidence of IRF and greater net clinical benefit of digoxin in patients that develop IRF, has potential mechanistic implications. It is known that digoxin toxicity is linearly related to serum levels, but the positive neurohormonal and hemodynamic effects are not.(9,17) Notably, the positive neurohormonal effects of digoxin occur at doses where there is minimal effect on contractility or hemodynamics.(18) However, as the dose is further increased, hemodynamic improvements occur without significant additional neurohormonal benefit.(19) As the dose is further increased, there actually appears to be an increase in neurohormonal activity.(9) Since digoxin is renally cleared, an argument could be made that the association between IRF and lower one year digoxin levels in our study is the result of improved clearance of the drug. However, this association was unchanged after adjustment for the 12 month eGFR, indicating that improved digoxin clearance is not entirely driving this association. These pharmacodynamic effects of digoxin, in conjunction with the finding that lower serum levels were associated with both a greater incidence of IRF and freedom from death or hospitalization, suggests that the neurohormonal effects could be the predominant mechanism driving the cardio-renal benefits of digoxin in these patients.
Regardless of the mechanism, the fact remains that essentially all of the clinical benefit from digoxin observed in this population was found in the subset of patients that experienced IRF. A possible explanation for this finding is that patients responding to digoxin with IRF had enough baseline cardio-renal dysfunction and pathophysiology responsive to cardiac glycosides to offset the toxicity of this agent. The neutral effect of digoxin in patients without IRF may indicate that these patients did not have a baseline substrate for which the risk benefit profile of digoxin was favorable. Unfortunately, tailoring therapy based on monitoring for subsequent IRF at one year is cumbersome, requires temporary exposure of non-responders to the risks of therapy, and was in no way tested in this study (i.e., discontinuation of digoxin in patients without IRF may worsen outcomes). In light of the above and the limitations inherent to this data, the most direct application of this study is proof of concept that a high yield target group exists for digoxin and possibly other cardio-renal therapeutics.
Limitations
This study was a post-hoc, post-randomization, sub-analysis of a clinical trial with significant missing data, and thus significant potential for confounding exists. As a result, this analysis should be interpreted as hypothesis generating only. The DIG trial was not designed to investigate changes in renal function and treating physicians were not blinded to measures of renal function obtained for clinical use. As a result, treatment strategies may have been modified in response to this data. Patients with severe renal insufficiency (creatinine >3.0 mg/dL) were excluded from the DIG trial, limiting generalization to this group of patients. Although randomization to digoxin was associated with a greater survival advantage in patients that developed IRF, this finding was the result of a post-randomization subgroup analysis. As a result, causality cannot be assumed and it is possible that the associations presented in these studies arose from post-randomization differences in the groups unrelated to study drug assignment. Furthermore, since all patients had to (by definition) be alive at one year post randomization to have IRF assessed, significant differences in the patients alive at one year compared to those initially randomized at baseline likely exist. Additionally, the lack of ability to determine who is likely to experience IRF with digoxin at baseline is a significant limitation given that it is impractical to wait a year to evaluate for renal response to determine if the patient is to derive benefit. As a result, it is of particular importance that the findings with respect to clinical outcomes be regarded as hypothesis-generating only. It is impossible to discern what percentage of the digoxin/IRF group had IRF as a direct result of randomization to digoxin. As a result, there are an unknown percentage of subjects in the digoxin group that likely had spontaneous IRF unrelated to digoxin (much like the placebo group), possibly reducing the effect size. Furthermore, given that patients with IRF on average had lower eGFR at baseline than the remainder of the cohort, regression to the mean likely was responsible for some of the signal for IRF. Although the DIG trial took place prior to the routine use of therapies such as beta blockers, aldosterone antagonists, and implantable cardioverter defibrillators (limiting generalizability to contemporary HF populations); it is exceedingly unlikely that a large randomized trial of digoxin in unselected HF patients will ever be repeated and thus the DIG trial may represent the highest quality data source ultimately available for this clinical question.
Conclusion
In this subset of the DIG trial, randomization to digoxin was associated with a significantly greater incidence of improvement in kidney function, and the reduction in death or hospitalization attributable to digoxin was primarily derived from the group of patients demonstrating this favorable renal response. These hypothesis generating results suggest that treatment of patients with baseline cardio-renal dysfunction and physiology responsive to treatment may ultimately provide benefit with respect to long term renal and clinical outcomes. These positive proof of concept findings suggest that future research is warranted to evaluate the role for cardiac glycosides in the treatment of cardio-renal dysfunction and to develop methodology to prospectively identify patients likely to derive benefit from this therapy.
Supplementary Material
Acknowledgements
Funding Sources: NIH Grant 5T32HL007843-15 and 1K23HL114868-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationships to industry:None
References
- 1.McAlister FA, Ezekowitz JA, Tarantini L, et al. Renal Dysfunction in Heart Failure Patients with Preserved versus Reduced Ejection Fraction: Impact of the New CKD-EPI Formula. Circulation Heart failure. 2012 doi: 10.1161/CIRCHEARTFAILURE.111.966242. [DOI] [PubMed] [Google Scholar]
- 2.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol. 2010;106:1763–1769. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heywood JT, Fonarow GC, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail. 2011;17:993–1000. doi: 10.1016/j.cardfail.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 7.Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Konstam MA, Burnett JC, et al. Short-term Clinical Effects of Tolvaptan, an Oral Vasopressin Antagonist, in Patients Hospitalized for Heart Failure: The EVEREST Clinical Status Trials. JAMA: The Journal of the American Medical Association. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 9.Gheorghiade M, Harinstein ME, Filippatos GS. Digoxin for the treatment of chronic and acute heart failure syndromes. Acute Card Care. 2009;11:83–87. doi: 10.1080/17482940902883246. [DOI] [PubMed] [Google Scholar]
- 10.Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 11.The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 15.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak MG, Smith GL, Rathore SS, Massie BM, Krumholz HM. Renal function, digoxin therapy, and heart failure outcomes: evidence from the digoxin intervention group trial. J Am Soc Nephrol. 2004;15:2195–2203. doi: 10.1097/01.ASN.0000135121.81744.75. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113:2556–2564. doi: 10.1161/CIRCULATIONAHA.105.560110. [DOI] [PubMed] [Google Scholar]
- 18.Newton GE, Tong JH, Schofield AM, Baines AD, Floras JS, Parker JD. Digoxin reduces cardiac sympathetic activity in severe congestive heart failure. J Am Coll Cardiol. 1996;28:155–161. doi: 10.1016/0735-1097(96)00120-9. [DOI] [PubMed] [Google Scholar]
- 19.Gheorghiade M, Hall VB, Jacobsen G, Alam M, Rosman H, Goldstein S. Effects of increasing maintenance dose of digoxin on left ventricular function and neurohormones in patients with chronic heart failure treated with diuretics and angiotensin-converting enzyme inhibitors. Circulation. 1995;92:1801–1807. doi: 10.1161/01.cir.92.7.1801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.