Abstract
A novel therapy for Epstein-Barr virus (EBV)-positive tumors involves the intentional induction of the lytic form of EBV infection combined with ganciclovir (GCV) treatment. Virally encoded kinases (thymidine kinase and BGLF4) which are expressed only during the lytic form of infection convert GCV (a nucleoside analogue) into its active, cytotoxic form. However, tightly latent EBV infection in B cells has made it difficult to identify drugs that can be used clinically to induce lytic viral infection in B-cell lymphomas. Here we demonstrate that gemcitabine and doxorubicin (but not 5-azacytidine, cis-platinum, or 5-fluorouracil) induce lytic EBV infection in EBV-transformed B cells in vitro and in vivo. Gemcitabine and doxorubicin both activated transcription from the promoters of the two viral immediate-early genes, BZLF1 and BRLF1, in EBV-negative B cells. This effect required the EGR-1 motif in the BRLF1 promoter and the CRE (ZII) and MEF-2D (ZI) binding sites in the BZLF1 promoter. GCV enhanced cell killing by gemcitabine or doxorubicin in lymphoblastoid cells transformed with wild-type EBV, but not in lymphoblastoid cells transformed by a mutant virus (with a deletion in the BZLF1 immediate-early gene) that is unable to enter the lytic form of infection. Most importantly, the combination of gemcitabine or doxorubicin and GCV was significantly more effective for the inhibition of EBV-driven lymphoproliferative disease in SCID mice than chemotherapy alone. In contrast, the combination of zidovudine and gemcitabine was no more effective than gemcitabine alone. These results suggest that the addition of GCV to either gemcitabine- or doxorubicin-containing chemotherapy regimens may enhance the therapeutic efficacy of these drugs for EBV-driven lymphoproliferative disease in patients.
Epstein-Barr virus (EBV) is a human herpesvirus with tropism for B cells and epithelial cells that establishes a life-long persistent infection in more than 90% of the world's population. The primary infection with the virus is usually asymptomatic but can result in the self-limiting disease infectious mononucleosis. Long-term carriage of the virus is also implicated in the development of certain malignancies. The EBV genome is frequently present in African Burkitt's lymphomas, Hodgkin's disease, and lymphoproliferative disease in immunosuppressed patients as well as nasopharyngeal carcinomas and some gastric carcinomas (31, 43). In addition, EBV is possibly present in a subset of breast cancers (7), liver cancers (47), lung cancers, colon cancers, and prostate cancers (25). The presence of the EBV genome in certain malignancies could serve as a potential target for novel antitumor therapies (4, 20, 21, 27, 30, 40, 52).
One potential therapeutic strategy that takes advantage of the EBV genome in tumor cells involves induction of the lytic form of EBV infection in tumor cells, followed by administration of the nucleoside analogue ganciclovir (GCV). Like all herpesviruses, EBV manifests two distinct phases in its life cycle: latency and lytic replication. During latency, EBV expresses a limited number of viral genes, which are involved in tasks such as stimulating cell proliferation, inhibiting apoptosis, blocking viral lytic replication, and assuring accurate and equal partitioning of the episomal viral genome to daughter cells (31, 43). However, during the lytic replication phase of the EBV life cycle, many more viral genes are expressed which encode proteins involved in viral DNA replication and viral particle synthesis. In addition, during the lytic form of infection, two virally encoded kinases, the EBV thymidine kinase (EBV-TK) and the BGLF4 gene product, which phosphorylate the prodrug GCV and convert it into its active cytotoxic form (11, 33, 37, 41, 53), are expressed. Phosphorylated GCV inhibits not only the virally encoded DNA polymerase but also the cellular DNA polymerase, leading to premature termination of the nascent DNA and cell death (14, 23, 34). In addition, phosphorylated GCV can be transferred to adjacent cells, thus inducing “bystander” killing (23). Lytic EBV infection also confers sensitivity to the cytotoxic effects of zidovudine (AZT), possibly by inducing AZT phosphorylation (10, 28, 41, 52). However, GCV and AZT are not generally effective for treating EBV-positive tumors because most tumor cells are infected with the latent form of EBV and therefore do not express the kinases which activate these drugs.
Thus, it is necessary to first convert the latent form of EBV infection in tumor cells into the lytic form in order for GCV (or AZT) treatment to be successful (11, 41, 53). The switch from latent to lytic infection in host cells requires activation of the two EBV immediate-early (IE) genes, BZLF1 and BRLF1, which are not expressed during the latent form of infection. BZLF1 and BRLF1 both encode transcriptional activators, and together these proteins induce transcription of the entire lytic viral gene program (31, 43). Previous studies showed that certain chemotherapy agents (fluorouracil [5-FU] and cis-platinum) efficiently induce a lytic infection in EBV-positive epithelial cell tumors in vivo and in vitro and that the addition of GCV greatly enhances the ability of both 5-FU and cis-platinum to inhibit NPC tumor growth in nude mice (20). However, EBV infection is primarily latent in B cells (in contrast to epithelial cells, in which the virus infection is often lytic), and consequently it has been more difficult to identify agents that can efficiently induce a lytic EBV infection in B-cell tumors. Although γ-irradiation induces lytic EBV infection and expression of the viral TK in EBV-positive lymphoblastoid cell lines (LCLs) both in vitro and in vivo (45, 52) and might be useful for treating localized EBV-positive lymphomas combined with GCV, this method cannot be used to treat widely disseminated EBV-positive B-cell lymphomas.
For this paper, we have examined the ability of various chemotherapy or demethylating agents to induce a lytic EBV infection in B cells and have studied the mechanism for the lytic induction effect. We demonstrate that doxorubicin and gemcitabine (but not cis-platinum, 5-FU, or 5-azacytidine) induce lytic EBV infection in LCLs both in vitro and in vivo. The induction of lytic EBV infection by these agents requires several differential signal transduction pathways, including cellular stress mitogen-activated protein kinase (MAPK) p38, phosphatidylinositol 3-kinase (PI3 kinase), and MEK, but not cellular apoptosis per se. In addition, we show that the induction of lytic infection by both doxorubicin and gemcitabine is mediated through activation of the two viral IE genes (BZLF1 and BRLF1) and that this activation requires specific transcription factor binding motifs (cyclic AMP-responsive element [CRE], MEF2D, and EGR-1) in the promoters of these genes. Furthermore, we demonstrate that chemotherapy and GCV synergistically kill EBV-positive LCLs transformed by wild-type EBV, but GCV does not enhance the killing effect of chemotherapy in LCLs that cannot enter the lytic form of viral infection due to a deletion in the viral BZLF1 gene. Finally, we demonstrate that treatment of EBV-positive lymphoblastoid tumors in SCID mice with chemotherapy and GCV together is much more effective than either chemotherapy or GCV alone. However, the combination of AZT and gemcitabine was no more effective than gemcitabine alone. Our data suggest that GCV may enhance the therapeutic effect of certain chemotherapy agents for treating EBV-associated lymphoproliferative disease.
MATERIALS AND METHODS
Cell lines.
DG75 cells are an EBV-negative BL cell line. LCL-1 and LCL-2 were obtained by transforming human B cells with the B95-8 strain of EBV. LCL-3 (wild-type) and an LCL-BZLF1 knockout (LCL-Z-KO) were established from B cells from one individual infected with the wild-type B95-8 strain of the EBV genome containing an inserted green fluorescent protein (GFP) gene or a B95-8 virus (containing the GFP gene) with a deletion in the BZLF1 gene. The wild-type and BZLF1 knockout GFP-positive viruses were constructed by use of BAC technology as previously described (19). Raji is an EBV-positive Burkitt's lymphoma cell line. BL30 is an EBV-negative Burkitt's lymphoma cell line. BL30-EBV was established from BL-30 cells infected with the wild-type B95-8 strain of the EBV genome containing an inserted GFP gene. The culture medium used was RPMI medium with 10% fetal bovine serum.
EBV promoter plasmids.
Plasmid RpCAT contains the BRLF1 IE promoter (Rp) sequences (from −962 to +5 relative to the mRNA start site) linked to the heterologous reporter gene, chloramphenicol acetyltransferase (CAT), in a pBS phagemid vector (Stratagene) (54). RpCAT (ΔZif) contains Rp sequences (from −962 to +5) with two site-directed mutations which delete the upstream (positions 125 to 131) and downstream (positions 42 to 44) Zif268 (EGR-1) binding sites (55). Plasmid ZpCAT contains the BZLF1 IE promoter (Zp) (from −221 to +12 relative to the mRNA start site) linked to CAT (22). Plasmid ZpCAT (ΔZIA/B) contains Zp sequences with two of the ZI motifs, ZIA and ZIB (previously shown to bind to MEF2D as well as Sp1/3) (46), mutated as previously described (22). ZpCAT (ΔZII) contains Zp sequences with a site-directed mutation in the ZII (CRE) site (22).
Lytic induction assays.
LCLs were treated with either 5-azacytidine (1 or 2.5 μM) (Sigma Chemical Co.), 5-FU (5 μg/ml) (Pharmacia Upjohn Co.), cis-platinum (1 μg/ml) (American Pharmaceutical Partners, Inc.), doxorubicin (0.2 μM) (Sigma Chemical Co.), or gemcitabine (1 μg/ml) (Eli Lilly and Company) for 3 to 5 days. Western blot analysis was performed as previously described (3), using anti-BMRF1 (1:100) (Capricon), anti-BZLF1 (1:100) (Argene), anti-BRLF1 (1:100) (Argene), and β-actin (1:5,000) (Sigma Chemical Co.) antibodies and an ECL detection kit (Amersham Pharmacia). Anti-LMP1 (1:100) (DAKO) and anti-EBNA2 (1:100) (DAKO) antibodies were used to detect the expression of LMP1 and EBNA2. Cell killing was determined by trypan blue exclusion before harvesting for Western blot analysis.
Immunofluorescence.
LCL-1 cells treated with no drug or gemcitabine (1 μg/ml) for 3 days were fixed in cold 50% acetone-50% methanol for 10 min at −20°C. Cells were then stained with anti-BMRF1 antibody (1:500) (Argene) or an isotype control antibody for 60 min at room temperature. BMRF1 staining was visualized with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G antibody (1:100) (Sigma) by fluorescence microscopy. DAPI (4′,6′-diamidino-2-phenylindole) staining was also performed to visualize cell nuclei.
Examination of signal transduction pathways.
LCLs were pretreated for 1 h with either no agent, PI3 kinase inhibitor LY294002 (15 μM), p38 MAPK inhibitor SB202190 (20 μM), MEK inhibitor PD98059 (50 μM), or caspase pan-inhibitor Z-VAD-fmk (50 μM) (all from Calbiochem). Cells were then treated for 72 h with or without gemcitabine (1 μg/ml) or doxorubicin (0.2 μM) in the presence or absence of the inhibitors above and were harvested for immunoblot analysis of BMRF1, BZLF1, and BRLF1 expression.
Reporter gene assays.
Plasmid DNA was purified by use of a Qiagen kit as described by the manufacturer. DNA was transfected into EBV-negative DG75 cells by electroporation at 1,500 V with a Zapper electroporation unit (Medical Electronics Shop, University of Wisconsin) using 5 μg of DNA. Transfected cells were then treated with or without doxorubicin (0.2 μM) or gemcitabine (1 μg/ml) 12 h later. Cell extracts were prepared at 72 h postchemotherapy and incubated at 37°C with [14C]chloramphenicol in the presence of acetyl coenzyme A, as described previously (24). The percent acetylation of chloramphenicol was quantified by thin-layer chromatography followed by PhosphorImager screening (Molecular Dynamics).
In vitro cell killing studies with chemotherapy and GCV.
LCL-3 and LCL-Z-KO cells were treated with no drug; doxorubicin (0.05 μM), gemcitabine (1 μg/ml), or GCV (10 μg/ml) (Warner-Lambert Co.) alone; or doxorubicin or gemcitabine combined with GCV. Cell killing was determined by trypan blue exclusion at 8 days posttreatment.
In vivo tumor studies.
All animal experiments were conducted in accordance with the guidelines of the Animal Care Committee. LCL-1 cells (5 × 107) were implanted subcutaneously into the flanks of 6-week-old SCID mice. For determination of whether chemotherapy can induce lytic EBV infection in vivo, mice with tumors were treated with no drug or one dose of gemcitabine (60 mg/kg of body weight administered intraperitoneally [i.p.]), doxorubicin (10 mg/kg of body weight administered i.p.), or 5-azacytidine (50 mg/kg of body weight). Seventy-two hours later, mice were euthanized and tumors were removed surgically. Tumors were processed as previously described (52), and protein extracts from tumors were subjected to Western blot analysis as described above.
For tumor treatment studies, mice were treated (at 8 days postinjection) with no drug (eight tumors), one dose of gemcitabine (60 mg/kg of body weight administered i.p.) (eight tumors) or doxil (2 mg/kg of body weight administered intravenously) (Ben Venue Laboratories, Inc.) alone, GCV (100 mg/kg of body weight administered i.p. twice a day for 5 days) alone, or the combination of GCV and one dose of gemcitabine (eight tumors) or GCV and one dose of doxorubicin (eight tumors). The mice were examined and tumor measurements were obtained three times per week after drug treatment was initiated. In a separate study, mice received either AZT alone (50 mg/kg/day administered i.p. for 5 days) (Catalytica Pharmaceuticals, Inc.), one dose of gemcitabine alone (60 mg/kg of body weight administered i.p.), or gemcitabine followed by 5 days of AZT. Mice were euthanized when the tumor size exceeded 1 cm. Statistical analysis was performed by using the t test.
RESULTS
Gemcitabine and doxorubicin induce lytic EBV infection in EBV-immortalized lymphoblastoid cells in vitro.
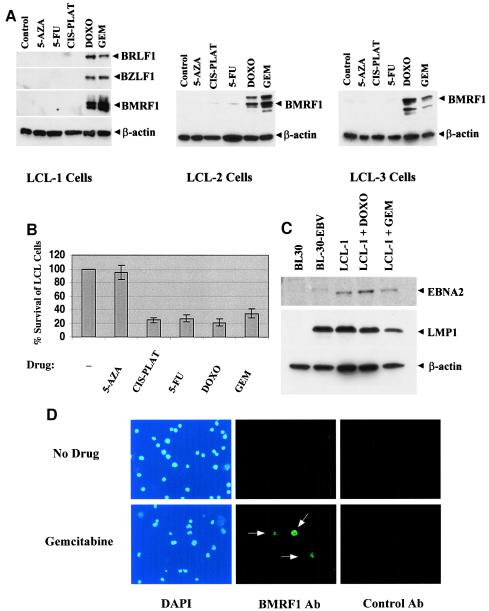
EBV-transformed B cells (lymphoblastoid cells) are usually tightly latent. However, it is known that demethylation of the viral genome induces lytic EBV genome expression in a subset of Burkitt's lymphoma cell lines (6), and it was previously shown that 5-FU and cis-platinum induce lytic EBV infection in epithelial cell lines (20). We therefore examined the ability of various chemotherapy agents (5-FU, cis-platinum, gemcitabine, and doxorubicin) and a demethylating agent (5-azacytidine) to induce lytic EBV infection in three different LCLs. Cells were treated for 5 days with the various agents and then the expression level of several different lytic EBV proteins was quantitated by immunoblot analysis. As shown in Fig. 1A, both gemcitabine and doxorubicin induced the expression of lytic EBV proteins in each of three independently derived LCLs. However, 5-azacytidine, 5-FU, and cis-platinum did not significantly activate lytic EBV infection in any of the three LCLs. Interestingly, each of the chemotherapy drugs produced a similar level of toxicity in the LCL-1 line (Fig. 1B), suggesting that cell killing per se is not sufficient to induce the lytic form of EBV infection. LMP1 and EBNA2 expression was not significantly affected by either doxorubicin or gemcitabine treatment of LCL-1 cells (Fig. 1C), in contrast to the recently reported effects of the cytotoxic drug cidofovir (1).
FIG. 1.
Gemcitabine and doxorubicin induce lytic EBV infection in B cells in vitro. (A) EBV-positive lymphoblastoid cells derived from three different hosts (LCL-1, LCL-2, and LCL-3 cells) were treated with 5-azacytidine (5-AZA; 2.5 μM), 5-FU (5 μg/ml), cis-platinum (CIS-PLAT; 1 μg/ml), doxorubicin (DOXO; 0.2 μM), or gemcitabine (GEM; 1 μg/ml). Cells were harvested 5 days later and analyzed for expression of the EBVIE proteins BZLF1 and BRLF1 or the viral early antigen BMRF1 by immunoblot analysis. The same blots were also probed with anti-β-actinantibody. (B) LCL-1 cells were treated with 5-azacytidine, 5-FU, cis-platinum, doxorubicin, or gemcitabine, and the percent cells surviving (relative to untreated cells) was determined by trypan blue exclusion at 5 days posttreatment. (C) LMP1 and EBNA2 expression was assayed by Western blotting with an EBV-negative (BL30) or EBV-positive (BL30-EBV) Burkitt's lymphoma cell line or with untreated versus doxorubicin- or gemcitabine-treated LCL-1 cells. (D) Expression of the lytic viral antigen BMRF1 was examined by immunofluorescence in untreated LCL-1 cells or LCL-1 cells treated with gemcitabine for 3 days.
To investigate the proportion of cells that switched to the lytic type of EBV infection upon treatment with gemcitabine, we used immunofluorescence to detect expression of the viral early antigen BMRF1. As shown in Fig. 1D, in the absence of chemotherapy, very few LCL-1 cells expressed BMRF1. After 3 days of gemcitabine treatment, approximately 10% of the LCL-1 cells expressed BMRF1; this number may be an underestimate due to poor antibody staining of chemotherapy-treated cells.
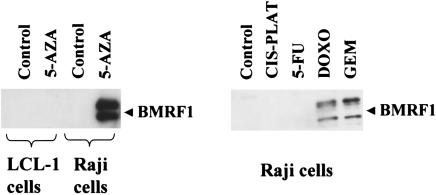
In contrast to its lack of effect in lymphoblastoid cells, 5-azacytidine efficiently induced lytic EBV gene expression in the Raji Burkitt cell line (Fig. 2). Similar to the effects observed in the LCLs, the induction of lytic EBV infection in Raji cells was induced much more efficiently with gemcitabine and doxorubicin than with 5-FU and cis-platinum. These results indicate that agents which induce lytic EBV infections in epithelial cells, or Burkitt lines, are not necessarily able to induce lytic EBV infections in LCLs. Of the various agents tested, gemcitabine and doxorubicin were clearly the most effective drugs for inducing lytic EBV infections in lymphoblastoid cells in vitro.
FIG. 2.
Gemcitabine, doxorubicin, and 5-azacitidine induce lytic EBV infection in Raji cells. EBV-positive Raji Burkitt's lymphoma cells, or LCL-1 cells, were treated with 5-azacytidine (5-AZA; 1 μM), 5-FU (5 μg/ml), cis-platinum (CIS-PLAT; 1 μg/ml), doxorubicin (DOXO; 0.2 μM), or gemcitabine (GEM; 1 μg/ml) and analyzed 5 days later for expression of the viral early antigen BMRF1 by immunoblot analysis.
Inhibitors of PI3 kinase, p38 MAPK, and MEK block the induction of lytic EBV infection by gemcitabine and doxorubicin.
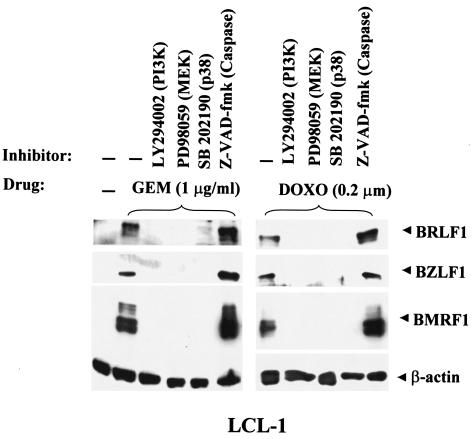
Since previous reports suggested that the PI3 kinase, p38 MAPK, and MAPK/ERK pathways are required for the activation of lytic EBV infection by certain agents (2, 9, 16, 18, 20), we examined the effects of specific inhibitors of PI3 kinase, p38 MAPK, and MEK on the induction of lytic EBV infection by gemcitabine and doxorubicin. LCL-1 cells were pretreated for 1 h with PI3 kinase, p38 MAPK, or MEK inhibitors before the addition of gemcitabine or doxorubicin, and 3 days later the expression of various EBV lytic proteins was analyzed by immunoblotting. As shown in Fig. 3, LY294002 (PI3 kinase inhibitor), SB202190 (p38 MAPK inhibitor), and PD98059 (MEK inhibitor) each prevented the induction of lytic EBV gene expression by both gemcitabine and doxorubicin. These findings suggest that the PI3 kinase, p38 MAPK, and MEK pathways may be involved in the induction of lytic EBV infection by gemcitabine and doxorubicin. However, the induction of lytic EBV infection was not blocked by treatment with a pan-caspase inhibitor (Z-VAD-fmk). Thus, apoptosis per se does not appear to be required. Similar results were obtained previously with regard to the signal transduction pathways required for cis-platinum and 5-FU induction of lytic EBV infection in epithelial cell lines (20).
FIG. 3.
Induction of lytic EBV infection by gemcitabine and doxorubicin requires activation of PI3 kinase, p38 kinase, and MEK pathways. LCL-1 cells were pretreated for 1 h with or without the indicated inhibitors and then were treated with either gemcitabine or doxorubicin. The cells were harvested 72 h later and analyzed for the EBV IE and early proteins by immunoblot analysis.
Gemcitabine and doxorubicin activate the two EBV IE promoters (Rp and Zp) in EBV-negative DG75 cells.
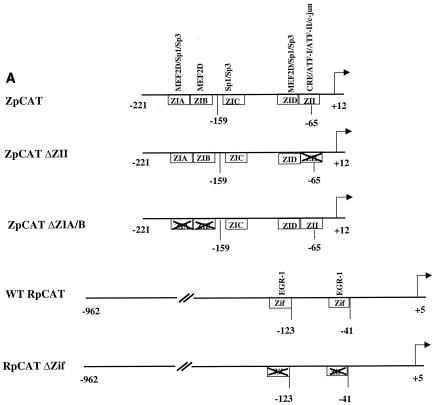
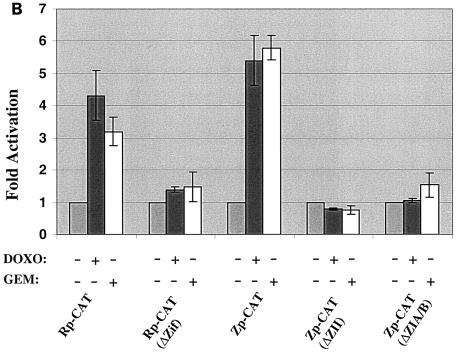
The immunoblot analysis shown in Fig. 1A indicated that gemcitabine and doxorubicin both induce expression of the two EBV IE proteins, BZLF1 and BRLF1. For determination of whether these agents directly activate transcription of the BZLF1 (Zp) and BRLF1 (Rp) promoters, EBV-negative B cells (DG75) were transfected with reporter gene constructs containing the wild-type Rp (RpCAT) or Zp (ZpCAT) promoters linked to the CAT gene. In addition, constructs containing site-directed mutations of transcription factor binding sites in each promoter that are known to be important for the induction of Zp and Rp by other stimuli were also examined for the ability to be activated by chemotherapy. Treatment of DG75 cells with either gemcitabine or doxorubicin significantly increased the CAT activity derived from either the RpCAT or ZpCAT construct (Fig. 4B), while having no effect on the promoterless control construct (data not shown). Thus, both chemoagents enhance the activity of the BZLF1 and BRLF1 promoters in EBV-negative cells. To further define the promoter elements required for chemotherapy induction of the BRLF1 promoter, we examined whether two Zif268 (EGR-1) binding sites which have previously been shown to be required for phorbol ester activation of the promoter (55) are also required for chemotherapy activation by using an RpCAT construct missing the two binding sites. As shown in Fig. 4B, neither gemcitabine nor doxorubicin significantly activated the BRLF1 promoter in the absence of the binding sites, indicating that these sites are important for chemotherapy activation. We also examined the importance of two ZI sites (which bind to MEF2D and Sp1/3) (8, 35, 46), as well as the ZII (CRE) site (which binds to CREB, ATF-1, ATF-2, and c-Jun) (2, 51), in the BZLF1 promoter for chemotherapy induction, since these motifs have been previously shown to be required for the induction of BZLF1 transcription by phorbol esters as well as by calcium ionophores (46). Neither the ZpCAT (ΔZIA/B) construct, which is missing the ZIA and ZIB binding sites, nor the ZpCAT (ΔZII) construct, which is missing the ZII (CRE) site, could be activated by either chemotherapy agent (Fig. 4). These results indicate that chemotherapy activates the BRLF1 and BZLF1 promoters through the same transcription factor binding sites that are required for other methods of lytic induction.
FIG. 4.
Doxorubicin and gemcitabine activate the two EBV IE promoters (Rp and Zp) in EBV-negative DG75 cells. (A) Constructs containing the wild-type or mutant BZLF1 (Zp-CAT) or BRLF1 (Rp-CAT) promoters linked to the CAT gene were constructed as described in Materials and Methods. Transcription factors known to bind to the promoters are indicated. (B) EBV-negative DG75 cells were transfected with 5 μg of each promoter construct, as indicated, and then were treated with no drug, doxorubicin, or gemcitabine for 72 h. CAT assays were performed as described previously (24). The average fold activation by each drug is shown.
The ability of GCV to enhance chemotherapy killing of EBV-transformed B cells requires lytic EBV infection.
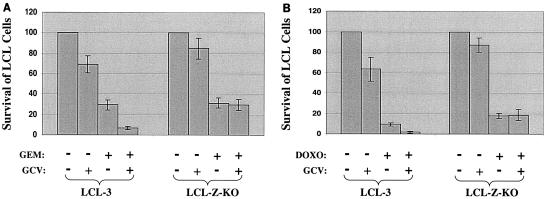
Although it is clear that lytically infected cells express virally encoded kinases which can phosphorylate GCV, it has been somewhat controversial whether EBV-dependent killing by GCV actually requires the lytic form of infection (28). To further examine this issue, we immortalized B cells from the same host with either wild-type (B95-8) virus (LCL-3 line) or a virus with a deletion in the BZLF1 gene that can latently infect cells but is unable to cause the lytic type of infection. These viruses were constructed as previously described (19). The wild-type LCL-3 line and BZLF1 knockout (Z-KO) line were treated with GCV alone, gemcitabine (Fig. 5A) or doxorubicin (Fig. 5B) alone, or the combination of GCV and chemotherapy. The number of surviving cells was determined by trypan blue staining at 8 days posttreatment. As shown in Fig. 5, GCV alone was somewhat more toxic in the wild-type LCL-3 cells than in the BZLF1 knockout cells, presumably reflecting the fact that this particular wild-type LCL contained some cells with the lytic form of viral infection even in the absence of inducing agents (data not shown). The cytotoxic effect of gemcitabine alone was similar in the wild-type and BZLF1 knockout lines, whereas doxorubicin was somewhat more toxic in the wild-type line. Most importantly, however, while the addition of GCV clearly enhanced the killing effect of both gemcitabine and doxorubicin in the wild-type LCLs, it had no effect on chemotherapy killing in the cells that were immortalized with the BZLF1 knockout virus. These results clearly indicate that the ability of GCV to enhance chemotherapy-mediated killing of EBV-positive tumor cells requires the lytic form of viral infection.
FIG. 5.
Synergistic killing between GCV and chemotherapy requires lytic EBV infection. EBV-positive LCL cells derived from the wild-type virus (LCL-3) or a mutant virus with a deletion in the BZLF1 gene (LCL-Z-KO) were treated with GCV alone, gemcitabine alone (A), gemcitabine plus GCV (A), doxorubicin alone (B), or doxorubicin plus GCV (B). Cell viability (relative to untreated cells) was determined 8 days later by trypan blue exclusion.
Gemcitabine and doxorubicin induce lytic EBV infection in lymphomas in vivo.
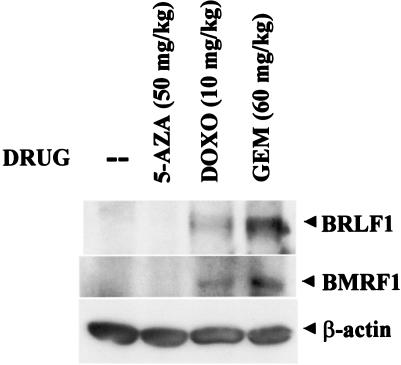
We next examined the ability of doxorubicin, gemcitabine, or 5-azacytidine to induce lytic EBV infection in lymphomas in vivo. LCL-1 cells (5 × 107) were injected subcutaneously into the flanks of SCID mice. After the development of tumors, mice were treated with a single dose of either 5-azacytidine (i.p), gemcitabine (i.p.), or doxorubicin (i.p.) and then euthanized 72 h later. Tumors were analyzed by immunoblotting for expression of the EBV IE protein BRLF1 or the early lytic protein BMRF1. As shown in Fig. 6, the demethylating agent 5-azacytidine could not induce lytic EBV infection in lymphomas derived from lymphoblastoid cells. However, expression of the EBV IE protein BRLF1 and the early protein BMRF1 was clearly induced by a single dose of gemcitabine and doxorubicin. These results indicate that gemcitabine and doxorubicin can both induce lytic EBV infection in lymphomas at clinically relevant doses.
FIG. 6.
Doxorubicin and gemcitabine induce lytic EBV infection in lymphomas in vivo. Lymphomas were made by injecting mice subcutaneously in the flanks with LCL-1 cells. Mice with tumors were treated with no drug or a single i.p. dose of 5-azacytidine, doxorubicin, or gemcitabine. Tumors were harvested 72 h after treatment and analyzed for expression of the EBV IE protein BRLF1 and the early viral protein BMRF1 by immunoblot analysis.
GCV enhances the therapeutic effect of gemcitabine and doxorubicin in EBV-positive lymphoproliferative disease in SCID mice.
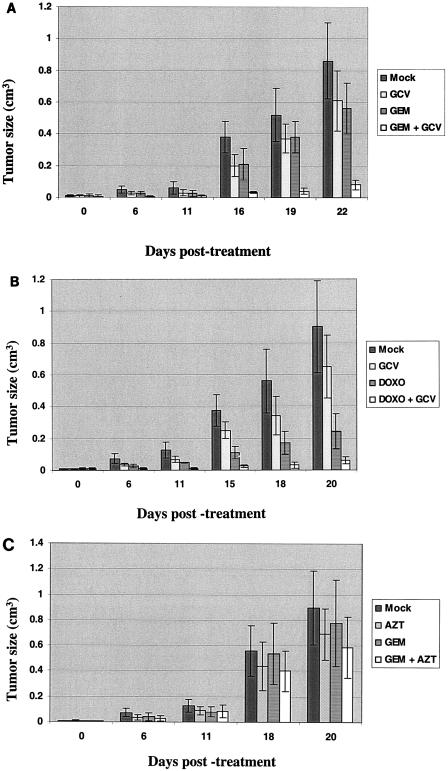
To determine whether GCV can enhance the efficacy of chemotherapy for EBV-driven lymphoproliferative disease in vivo, we injected 5 × 107 LCL-1 cells subcutaneously into the flanks of SCID mice and treated the mice 8 days after cell injection with either no drug, GCV alone for 5 days, one dose of gemcitabine alone, or one dose of gemcitabine followed by a 5-day GCV treatment (Fig. 7A). In a separate experiment, the effect of a single dose of doxorubicin (2 mg/kg administered intravenously), with or without subsequent GCV treatment, was also assessed (Fig. 7B). As shown in Fig. 7, GCV alone produced some inhibition of EBV-driven lymphoproliferation, presumably due to the small percentage of lymphoblastoid cells containing the lytic form of EBV infection. Treatment with gemcitabine alone had only a small antitumor effect, whereas doxorubicin alone, as expected, significantly inhibited growth. Most importantly, the lymphoproliferative tumors in mice treated with gemcitabine or doxorubicin plus GCV were significantly smaller than the tumors in mice treated with gemcitabine (P = 0.009) or doxorubicin (P = 0.039) alone. In contrast, we did not find that the combination of gemcitabine and AZT was significantly more effective than either drug alone (Fig. 7C). Thus, although GCV and AZT have both been reported to preferentially kill lytically infected EBV-positive cells in vitro (41, 52), GCV was considerably more effective than AZT for the treatment of EBV-associated lymphoproliferative disease (in combination with chemotherapy) in this mouse model.
FIG. 7.
GCV enhances the therapeutic effect of gemcitabine and doxorubicin on EBV-driven lymphoproliferative disease in mice. EBV-positive lymphoblastoid cells (5 × 107 LCL-1 cells) were implanted subcutaneously into the flanks of SCID mice. Tumors (eight tumors in each group) were treated 8 days after injection with either no drug, GCV alone, gemcitabine alone or gemcitabine followed by 5 days of GCV treatment (A), doxorubicin with or without GCV treatment (B), or AZT alone, gemcitabine alone, or gemcitabine and AZT (C). Tumor volumes at different time points (means ± standard errors) are shown.
DISCUSSION
EBV, a gammaherpesvirus with potent B-cell-transforming activity, is linked to Burkitt's lymphoma, Hodgkin's lymphoma, and lymphoproliferative diseases in immunosuppressed patients (31, 43). The persistent expression of certain EBV-encoded gene products is likely required for the continued growth of many EBV-associated lymphomas. Although antiviral therapy is effective for treating lytic EBV infection (for example, oral hairy leukoplakia lesions), it has not been clearly shown that antiviral agents have any therapeutic effect for treatment of EBV-associated lymphoproliferative diseases, which primarily contain the latent form of infection (13). However, in combination with strategies for inducing the lytic form of EBV infection in tumor cells, the cytotoxic effects of certain antiviral drugs, such as GCV, could potentially be used to kill EBV-positive tumor cells. In this study, we have examined the ability of various chemotherapy drugs, as well as 5-azacytidine, to induce lytic EBV infection in EBV-positive lymphoblastoid cells and have defined the mechanisms for the lytic-inducing effects. Gemcitabine and doxorubicin were both shown to induce lytic EBV infection in lymphoblastoid cells through activation of the promoters driving the two viral IE genes, BZLF1 and BRLF1. Furthermore, the addition of GCV to either gemcitabine or doxorubicin greatly enhanced the ability of these drugs to inhibit the growth of EBV-driven lymphoproliferative disease in SCID mice. In contrast, AZT did not enhance the antitumor effect of gemcitabine in this mouse model of an EBV-positive lymphoma.
The switch from latent to lytic EBV infection in host cells is mediated by the transcriptional effects of the two EBV IE proteins, BZLF1 and BRLF1, and overexpression of either one of these proteins in latently infected cells is sufficient to induce the lytic form of EBV infection (12, 15, 42, 44, 49, 56). In latently infected B cells, the BZLF1 (Zp) and BRLF1 (Rp) promoters are inactive. However, ligation of the B-cell receptor (48), phorbol ester treatment (58), calcium ionophores (17), transforming growth factor β1 (18), demethylating agents (41), and agents which induce histone acetylation (38, 39, 52) are known to activate expression of the BZLF1 and BRLF1 IE promoters in at least a portion of EBV-positive B-cell lines. In the case of the BZLF1 IE promoter (Zp), the two promoter elements which appear to be essential for stimulation by most, if not all, of these inducing factors are termed the ZI and ZII motifs (46). Several of the ZI motifs are bound by the MEF2D cellular transcription factor, as well as by Sp1/Sp3 (8, 35), and MEF2D has been shown to be an important regulator of EBV infection in host cells (26, 46). The ZII motif is a CRE site which is bound by CREB, ATF-1, c-Jun, and ATF-2 (2, 51). The results presented here indicate that the ability of both gemcitabine and doxorubicin to activate BZLF1 transcription in EBV-negative cells requires both the ZI and ZII binding motifs of the BZLF1 promoter. Gemcitabine and doxorubicin also activated the BRLF1 IE promoter in EBV-negative cells. This effect was shown to require the presence of two EGR-1 (Zif268) binding sites in the promoter which were previously shown to be important for phorbol ester-induced activation of the promoter (55).
Our data also indicate that the ability of gemcitabine and doxorubicin to induce lytic EBV gene expression requires several different signal transduction pathways, including the PI3K, p38 MAPK, and MEK pathways. These same pathways were previously shown to be required for lytic viral induction following ligation of the B-cell receptor (2, 9, 16) as well as for lytic induction induced by transforming growth factor β1 (18). The requirement for the p38 MAPK stress pathway may reflect the ability of this kinase to activate both the c-Jun and ATF-2 transcription factors (36, 50), which bind to the CRE site in the BZLF1 promoter. In addition, it has been reported that at least some members of the MEF2 family are activated by p38 kinase phosphorylation (57). The treatment of cells with chemotherapy agents has been shown to activate p38 kinase (5, 32), and thus it is likely that activation of this pathway is at least partially responsible for lytic EBV induction following chemotherapy. In addition, doxorubicin has been reported to induce activation of the cellular IE protein EGR-1, through both p38 kinase-dependent (5) and MEK-dependent (32) mechanisms, and our results indicate that the ability of both doxorubicin and gemcitabine to activate the BRLF1 IE promoters requires EGR-1 binding motifs. Thus, chemotherapy is likely to induce BZLF1 and BRLF1 transcription by activating several different signal transduction pathways which have downstream activating effects on the cellular transcription factors binding to EGR-1, CRE, and MEF2D binding motifs. However, apoptosis per se does not appear to be required, as a pan-caspase inhibitor did not prevent lytic induction, nor is cell killing alone sufficient, since cis-platinum and 5-FU were as toxic as gemcitabine and doxorubicin in LCL-1 cells. The fact that gemcitabine (a pyrimidine analogue) and doxorubicin (which intercalates into DNA) have very different killing mechanisms suggests that the exact mechanism of cell killing does not predict the ability of different agents to induce lytic EBV infection.
The data presented here also demonstrate that EBV-dependent GCV toxicity requires the lytic form of viral infection. This is not particularly surprising, since previous reports (40, 52) have suggested that lytically infected cells are more susceptible to GCV cytotoxicity and since it is known that virally encoded kinases expressed only during the lytic form of EBV infection (EBV TK and BGLF4) can confer GCV susceptibility (39, 41, 53). Nevertheless, the data presented here, for which lymphoblastoid cells from the same host were immortalized with either a wild-type or lytic-defective (with a deletion in BZLF1) virus, very clearly demonstrate that the ability of GCV to enhance the cytotoxicity of chemotherapy requires the lytic form of EBV infection. Thus, it is extremely unlikely that sufficient GCV phosphorylation could be achieved to be clinically useful in tumors containing completely latent EBV infections. Nevertheless, certain tumors may contain enough cells with the lytic form of EBV infection for GCV by itself to have some clinical benefit (45). Although we and others have previously shown that lytic EBV infection (or the EBV TK alone) confers sensitivity to the cytotoxic effects of AZT in vitro (41, 52), we were unable to demonstrate this effect here with our mouse model of EBV-positive lymphomas. Nevertheless, it remains possible that AZT at a dose higher than that used in this particular study might have had some benefit.
Although we have concentrated here on activating lytic gene transcription from the endogenous EBV genome in tumor cells, another approach for inducing lytic EBV infection in tumors would be to introduce the BZLF1 or BRLF1 genes under the control of a strong heterologous promoter by gene delivery methods. It was recently shown that direct delivery of the two EBV IE genes into nasopharyngeal carcinomas by using adenovirus vectors induces lytic EBV gene expression in the tumors and inhibits tumor growth (20). However, adenovirus delivery of the two EBV IE genes to B-cell lymphomas would be limited by the fact that B cells express very little (if any) of the major receptor (CAR) for adenovirus, although this hurdle could potentially be overcome by the use of bispecific antibody techniques (29). Nevertheless, drug-based strategies for inducing lytic EBV infection in widely disseminated EBV-induced lymphoproliferative disease are more likely to be successful clinically than gene delivery strategies. Although we and others have previously shown that gamma irradiation can also induce lytic EBV infection in B-cell lymphomas (45, 52), the combination of gamma irradiation and GCV can be used only for localized malignancies.
Another implication of the studies presented here is that the ability of various chemotherapy drugs to induce lytic EBV induction is clearly cell type dependent. While cis-platinum and 5-FU were previously shown to be highly successful for inducing lytic EBV infection in epithelial tumors, these drugs did not effectively induce lytic EBV infection in EBV-immortalized lymphoblastoid cells. Likewise, although the demethylating agent 5-azacytidine induces lytic EBV infection in certain Burkitt's lymphoma cell lines (6) and treatment with 5-azacytidine has been suggested as a potential way to induce lytic EBV infection in patient tumors (41), we did not find this approach to be successful for inducing lytic EBV infection in EBV-positive lymphoblastoid cells. Previous reports have shown that sodium butyrate (which induces histone acetylation) effectively induces the lytic form of EBV infection in at least some Burkitt's lymphoma cells (39, 52), and the combination of butyrate and GCV to treat EBV lymphomas in patients is currently being studied (40). The data presented in this report indicate that the addition of GCV to either gemcitabine- or doxorubicin-containing regimens may be useful for enhancing the efficacy of these agents for treating EBV-induced lymphoproliferative disease in immunosuppressed patients, since these tumors have a similar pattern of EBV latent gene expression (type III) as the lymphoblastoid cells used to make tumors in SCID mice for this study. Whether EBV-positive lymphomas containing other types of EBV latency, such as type I Burkitt's lymphomas, would also respond favorably to the combination of GCV and gemcitabine or doxorubicin needs to be further studied.
Acknowledgments
This work was supported by NIH grants R01 CA 66519, P01-CA19014, and R01-CA58853.
We thank Natalie Edmund for help with the animal experiments, Erik Flemington for the ZpCAT plasmids, and Robert Orlowski for the gift of doxil.
REFERENCES
- 1.Abdu, B., S. Sabri. D. Zelenika, E. Deutsch, V. Frascogna, J. Klijanienko, W. Vainchenker, I. Joab, and J. Bourhis. 2003. Antiviral agent cidofovir decreases Epstein-Barr virus (EBV) oncoproteins and enhances the radiosensitivity in EBV-related malignancies. Oncogene 22:2260-2271. [DOI] [PubMed] [Google Scholar]
- 2.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z (S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. [DOI] [PubMed] [Google Scholar]
- 4.Ambinder, R., K. Robertson, S. Moore, and J. Yang. 1996. Epstein-Barr virus as a therapeutic target in Hodgkin's disease and nasopharyngeal carcinoma. Semin. Cancer Biol. 7:217-227. [DOI] [PubMed] [Google Scholar]
- 5.Arai, M., A. Yoguchi, T. Takizawa, T. Yokoyama, T. Kanda, M. Kurabayashi, and R. Nagai. 2000. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca(2+)-ATPase gene transcription. Circ. Res. 86:8-14. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Sasson, S. A., and G. Klein. 1981. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int. J. Cancer 28:131-135. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet, M., J. M. Guinebretiere, E. Kremmer, V. Grunewald, E. Benhamou, G. Contesso, and I. Joab. 1999. Detection of Epstein-Barr virus in invasive breast cancers. J. Natl. Cancer Inst. 91:1376-1381. [DOI] [PubMed] [Google Scholar]
- 8.Borras, A. M., J. L. Strominger, and S. H. Speck. 1996. Characterization of the ZI domains in the Epstein-Barr virus BZLF1 gene promoter: role in phorbol ester induction. J. Virol. 70:3894-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant, H., and P. J. Farrell. 2002. Signal transduction and transcription factor modification during reactivation of Epstein-Barr virus from latency. J. Virol. 76:10290-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cazaux, C., M. Tiraby, L. Loubiere, L. Haren, D. Klatzmann, and G. Tiraby. 1998. Phosphorylation and cytotoxicity of therapeutic nucleoside analogues: a comparison of alpha and gamma herpesvirus thymidine kinase suicide genes. Cancer Gene Ther. 5:83-91. [PubMed] [Google Scholar]
- 11.Chen, M. R., S. J. Chang, H. Huang, and J. Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV) encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an early EBV promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen, J. I. 2000. Epstein-Barr virus infection. N. Engl. J. Med. 343:481-492. [DOI] [PubMed] [Google Scholar]
- 14.Conners, T. A. 1995. The choice of prodrugs for gene directed enzyme prodrug therapy of cancer. Gene Ther. 2:702-709. [PubMed] [Google Scholar]
- 15.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr virus after gene transfer with a small cloned fragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darr, C. D., A. Mauser, and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J. Virol. 75:6135-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faggioni, A., C. Zompetta, S. Grimaldi, G. Barile, L. Frati, and J. Lazdins. 1986. Calcium modulation activates Epstein-Barr virus genome in latently infected cells. Science 232:1554-1556. [DOI] [PubMed] [Google Scholar]
- 18.Fahmi, H., C. Cochet, Z. Hmama, P. Opolon, and I. Joab. 2000. Transforming growth factor beta 1 stimulates expression of the Epstein-Barr virus BZLF1 immediate-early gene product ZEBRA by an indirect mechanism which requires the MAPK kinase pathway. J. Virol. 74:5810-5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng, W.-H., B. Israel, N. Raab-Traub, P. Busson, and S. C. Kenney. 2002. Chemotherapy induces lytic Epstein-Barr virus (EBV) replication and confers ganciclovir susceptibility to EBV-positive tumors. Cancer Res. 62:1920-1926. [PubMed] [Google Scholar]
- 21.Feng, W.-H., E. Westphal, A. Mauser, N. Raab-Traub, M. L. Gulley, P. Busson, and S. C. Kenney. 2002. Use of adenovirus vectors expressing Epstein-Barr virus (EBV) immediate-early protein BZLF1 or BRLF1 to treat EBV-positive tumors. J. Virol. 76:10951-10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flemington, E., and S. H. Speck. 1990. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman, S. M., C. N. Abboud, K. A. Whartenby, C. H. Packman, D. S. Koeplin, F. L. Moolten, and G. N. Abraham. 1993. The “bystander” effect: tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 53:5274-5283. [PubMed] [Google Scholar]
- 24.Gorman, C. M., L. F. Moffat, and B. H. Howard. 1982. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinstein, S., M. V. Preciado, P. Gattuso, P. A. Chabay, W. H. Warren, E. D. Matteo, and V. E. Gould. 2002. Demonstration of Epstein-Barr virus in carcinomas of various sites. Cancer Res. 62:4876-4878. [PubMed] [Google Scholar]
- 26.Gruffat, H., E. Manet, and A. Sergeant. 2000. MEF2-mediated recruitment of class II HDAC at the EBV immediate early gene BZLF1 links latency and chromatin remodeling. EMBO Rep. 3:141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guitierrez, M. I., J. G. Judde, I. Magrath, and K. G. Bhatia. 1996. Switching viral latency to viral lysis: a novel therapeutic approach for Epstein-Barr virus-associated neoplasia. Cancer Res. 56:969-972. [PubMed] [Google Scholar]
- 28.Gustafson, E. A., A. C. Chillemi, D. R. Sage, and J. D. Fingeroth. 1998. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob. Agents Chemother. 42:2923-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Israel, B. I., R. J. Pickles, D. M. Segal, R. D. Gerard, and S. C. Kenney. 2001. Enhancement of adenovirus vector entry into CD70-positive B-cell lines by using a bispecific CD70-adenovirus fiber antibody. J. Virol. 75:5215-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenney, S., J.-Q. Ge, E. M. Westphal, and J. Olsen. 1998. Gene therapy strategies for treating Epstein-Barr virus-associated lymphomas: comparison of two different Epstein-Barr virus-based vectors. Hum. Gene Ther. 9:1131-1141. [DOI] [PubMed] [Google Scholar]
- 31.Kieff, E. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 32.Lim, C. P., N. Jain, and X. Cao. 1998. Stress-induced immediate-early gene, egr-1, involves activation of p38/JNK1. Oncogene 16:2915-2926. [DOI] [PubMed] [Google Scholar]
- 33.Lin, J. C., D. Nelson, C. Lambe, and E. Cho. 1986. Metabolic activation of 9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)guanine in human lymphoblastoid cell lines infected with Epstein-Barr Virus. J. Virol. 60:569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, J. C., C. Smith, and J. Pagano. 1984. Prolonged inhibitory effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine against replication of Epstein-Barr virus. J. Virol. 50:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, S., P. Liu, A. Borras, T. Chatila, and S. H. Speck. 1997. Cyclosporin A-sensitive induction of the Epstein-Barr virus lytic switch is mediated via a novel pathway involving a MEF2 family member. EMBO J. 16:143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livingstone, C., G. Patel, and N. Jones. 1995. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 14:1785-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loubiere, L., M. Tiraby, C. Cazaux, E. Brisson, M. Grisoni, J. Zhao-Emonet, G. Tiraby, and D. Klatzmann. 1999. The equine herpes virus 4 thymidine kinase is a better suicide gene than the human herpes virus 1 thymidine kinase. Gene Ther. 6:1638-1642. [DOI] [PubMed] [Google Scholar]
- 38.Luka, J., B. Kallin, and G. Klein. 1979. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology 94:228-231. [DOI] [PubMed] [Google Scholar]
- 39.Mentzer, S. J., J. Fingeroth, J. J. Reilly, S. P. Perrine, and D. V. Faller. 1998. Arginine butyrate-induced susceptibility to ganciclovir in an Epstein-Barr virus-associated lymphoma. Blood Cells Mol. Dis. 24:114-123. [DOI] [PubMed] [Google Scholar]
- 40.Mentzer, S. J., S. P. Perrine, and D. Faller. 2001. Epstein-Barr virus post-transplant lymphoproliferative disease and virus-specific therapy: pharmacological reactivation of viral target genes with arginine butyrate. Transplant Infect. Dis. 3:177-185. [DOI] [PubMed] [Google Scholar]
- 41.Moore, S. M., J. S. Cannon, Y. Tanhehco, F. Hamzeh, and R. Ambinder. 2001. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob. Agents Chemother. 45:2082-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein disrupts latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 44.Rooney, C., N. Taylor, J. Countryman, H. Jenson, J. Kolman, and G. Miller. 1988. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc. Natl. Acad. Sci. USA 85:9801-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roychowdhury, S., R. Peng, R. A. Baiocchi, D. Bhatt, S. Vourganti, J. Grecula, N. Gupta, C. F. Eisenbeis, G. J. Nuovo, W. Yang, P. Schmalbrock, A. Ferketich, M. Moeschberger, P. Porcu, R. F. Barth, and M. A. Caligiuri. 2003. Experimental treatment of Epstein-Barr virus-associated primary central nervous system lymphoma. Cancer Res. 63:965-971. [PubMed] [Google Scholar]
- 46.Speck, S. H., T. Chatila, and E. Flemington. 1997. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol. 5:399-405. [DOI] [PubMed] [Google Scholar]
- 47.Sugawara, Y., M. Makuuchi, and K. Takada. 2000. Detection of Epstein-Barr virus DNA in hepatocellular carcinoma tissues from hepatitis C-positive patients. Scand. J. Gastroenterol, 35:981-984. [DOI] [PubMed] [Google Scholar]
- 48.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27-32. [DOI] [PubMed] [Google Scholar]
- 49.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. Transactivation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dam, H., D. Wilhelm, I. Herr, A. Steffen, P. Herrlich, and P. Angel. 1995. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 14:1798-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, Y. C., J. M. Huang, and E. A. Montalvo. 1997. Characterization of proteins binding to the ZII element in the Epstein-Barr virus BZLF1 promoter: transactivation by ATF1. Virology 227:323-330. [DOI] [PubMed] [Google Scholar]
- 52.Westphal, E. M., W. Blachstock, W.-H. Feng, B. Israel, and S. C. Kenney. 2000. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 60:5781-5788. [PubMed] [Google Scholar]
- 53.Westphal, E. M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. C. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 54.Zalani, S., E. A. Holley-Guthrie, D. E. Gutsch, and S. C. Kenney. 1992. The Epstein-Barr virus immediate-early promoter BRLF1 can be activated by the cellular Sp1 transcription factor. J. Virol. 66:7282-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1995. The Zif268 cellular transcription factor activates expression of the Epstein-Barr virus immediate-early BRLF1 promoter. J. Virol. 69:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao, M., L. New, V. V. Kravchenko, Y. Kato, H. Gram, F. di Padova, E. N. Olson, R. J. Ulevitch, and J. Han. 1999. Regulation of the MEF2 family of transcription factors by p38. Mol. Cell. Biol. 19:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.zur Hausen, H., F. J. O'Neill, U. K. Freese, and E. Hecker. 1978. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature 272:373-375. [DOI] [PubMed] [Google Scholar]