Abstract
Previously, we reported a critical period (around postnatal day (P) 12–13 in the rat) in respiratory network development, when distinct neurochemical, metabolic, and physiological changes occur. Since serotonin 2A (5-HT2A) receptors play an important role in respiratory modulation, we hypothesized that they may undergo developmental adjustments during the critical period. Semi-quantitative immunohistochemical analyses were conducted in labeled neurons in a number of brain stem nuclei with or without known respiratory functions from P2 to P21 in rats. Our data indicate that the expressions of 5-HT2A receptors in neurons of the pre-Bötzinger complex, the nucleus ambiguus, and the hypoglossal nucleus were maintained within a relatively narrow range between P2 and P21, with a dip at P3–4 and a significant reduction only at P12. This change was not observed in the non-respiratory cuneate nucleus. These results suggest that reduced expressions of 5-HT2A receptors at P12 contributes to neurochemical imbalance within brain stem respiratory nuclei at that time, and may be involved in decreased hypoxic ventilatory response at this critical period of development.
Keywords: development, hypoglossal nucleus, nucleus ambiguus, Pre-Bötzinger complex, respiratory nuclei
INTRODUCTION
5-Hydroxytryptamine (5-HT, serotonin), an indoleaminergic neurotransmitter, plays an important role in various physiological functions, such as cardiorespiratory, thermoregulatory, motor and sensory activities, as well as cognitive, affective, aggressive, sexual, and arousal behaviors (20, 31, 51). It also affects smooth muscle fibers, causing a major effect on the vascular bed and the digestive tract (51). 5-HT's influences are mediated via four classes of receptors and at least 15 receptor subtypes, such as 5-HT1(A–F), 5-HT2 (A, B, C), 5-HT3, and 5-HT4/6/7 (19, 51).
5-HT2A receptors are G-protein-coupled, seven-transmembrane receptors that are expressed widely in many brain regions (2). In addition to its suprapontine distribution (35, 37), 5-HT2A receptors are also found in several pontomedullary regions (9), including the pre-Bötzinger complex (PBC, postulated as an essential part of the respiratory network (42)), the nucleus ambiguus (Amb), and the hypoglossal nucleus (XII). These regions are all, to varying degrees, involved in respiratory functions. Apart from mediating 5-HT effects on motor, viscerosensory, cardiovascular, sexual, and sleep functions (51), 5-HT2A receptors reportedly also play an important role in respiratory regulations, including respiratory rhythmogenesis (36), respiratory long-term facilitation (1, 4, 12), and gasping (46).
Previously, we found that a transient neurochemical imbalance exists at and around postnatal day (P) 12 in several rat brain stem nuclei related to respiratory control, including the PBC, Amb, and XII (27, 29, 49). The level of cytochrome oxidase, a metabolic indicator of neuronal activity (48), was also significantly reduced at P12 (25, 27, 28, 49). Around that time, significant changes in normoxic ventilation and hypoxic ventilatory response (HVR) were also evident, and the HVR was at its lowest (26). These findings strongly suggest that the end of the second postnatal week is a critical period of development for brainstem respiratory nuclei in the rat.
Since 5-HT2A receptors play an important role in respiratory modulation, we hypothesize that they may also undergo transient changes during the critical period of postnatal development. The current study was undertaken to test this hypothesis in several brain stem respiratory nuclei of the rat. The cuneate nucleus (CN) was chosen as a negative control, since this nucleus is known for its relay function in somatosensory transduction but is not generally regarded as having any respiratory function.
MATERIALS AND METHODS
Tissue preparation
A total of 120 Sprague-Dawley rats from 16 litters were used. All experiments and animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health), and all protocols were approved by the Medical College of Wisconsin Animal Care and Use Committee.
At postnatal day (P) 2, 3, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 and 21, rats were deeply anesthetized with 4% chloral hydrate (1 ml/100 g IP; Fisher Scientific, Fair Lawn, NJ) and perfused through the aorta with 4% paraformaldehyde in 0.1 M sodium phosphate buffered saline (PBS), pH 7.4, with 4% sucrose. Eight rats from eight different litters were used at each time point for most of the postnatal days, whereas six rats from six different litters were used for each of P8, 9, 15, and 16 samples. After perfusion, brain stems were removed and immersion fixed in the same fixative for 3 h at 4°C. They were then cryoprotected in increasing concentrations of sucrose (10, 20, and 30%) in 0.1 M PBS at 4°C, frozen on dry ice, and stored at −80°C until use.
Immunohistochemistry
Coronal sections of frozen brain stems were cut at 12-μm thickness with a cryostat. Serial sections were mounted on gelatin-coated slides. Sections from 3–4 rats at different ages were mounted on the same slides so that they might be processed together. Ages were typically grouped as follows: P2-10-21, P3-4-5-17, P7-8-9, P11-12-13, and P14-15-16. All sections from all animals were processed under identical conditions (i.e., time, temperature, and concentration of reagents). They were blocked overnight at 4°C with 5% nonfat dry milk-5% normal rabbit serum-1% Triton X-100 in 0.1 M PBS (pH 7.4). Sections were then incubated at 4°C for 36 h in the primary antibodies (IgG derived from goat) against 5-HT2A receptors (Santa Cruz Biotechnology, Santa Cruz, CA), diluted at 1:100 in the same solution as used for blocking. Sections were incubated in the secondary antibodies: rabbit anti-goat IgG-HRP (Chemicon, Temecula, CA) at 1:100 dilution in the modified blocking solution (without Triton X-100) for 4 h at room temperature. Immunoreactivity was detected with 0.05% DAB-0.004% H2O2 in PBS (pH 7.4) for 5 min, and the reaction was stopped with cold PBS (pH 7.4). Sections were then washed with cold 0.1 M PBS (pH 7.4) three times, dehydrated, and coverslipped. Control sections were processed without the primary antibodies.
For estimates of the percentage of immunoreactive neurons in a specific nucleus, alternate sections were processed with Nissl, which stained all neuronal cell bodies.
Quantitative densitometry
The expression of 5-HT2A receptors in the cytoplasm of neurons in various nuclei studied was semi-quantitatively analyzed by optical densitometric measurements of reaction product of immunohistochemistry, performed with a Zeiss Zonax MPM 03 photometer, a ×25 objective, and a 2-μm-diameter measuring spot. White (tungsten) light was used for illumination, and all lighting conditions were held constant for all of the measurements. Since light intensity can directly affect optical densitometric values, a stepped density filter (Edmund Industrial Optics, Barrington, NJ), with 10-step increments of 0.1 from 0.1 to 1, was used to precisely adjust the intensity of the light source to a standard value identical for all samples. The boundary of each brain stem nucleus studied was determined with the aid of the Paxinos and Watson's The Rat Brain Atlas (New York: Academic, 1986), and many of them, including the PBC that was identifiable with the neurokinin-1 receptor (NK1R) labeling, were described in our previous studies (27, 28, 29). The optical densitometric value of each labeled neuron in the various brain stem nuclei studied was an average reading of two to four spots in the cytoplasm of its cell body. Only those neurons whose nuclei are clearly visible (i.e., sectioned through the middle of the cell body) were measured. To avoid measuring the same neuron more than once, values were taken from cells in sections at least 70 μm apart, since the largest neurons had a maximal diameter of 25–30 μm, with a maximal nuclear diameter of only 10 μm. The relative thinness and crisscrossing of the processes in the neuropil rendered them impossible to be measured accurately with a 2-μm-diameter measuring spot. Thus, the processes and neuropil in general were described only qualitatively with respect to the intensities of their labeling. About 100 neurons in each brain stem nucleus were examined for each rat, and a total of 800 neurons at each age for each nucleus were measured (for P8, 9, 15, and 16, a total of 600 neurons were measured for each nucleus at each time point). For statistical analysis, each sample's optical density value for each nucleus of each rat was the average of ~100 neurons. Thus, the sample number of each time point in each nucleus is eight or six in Figure 5. Mean optical density values, standard deviations, and standard errors of the means in each nucleus at each age were then obtained. Statistical comparisons were made among the age groups by using one-way ANOVA (to control for the type I comparison-wise error rate) and, when significant differences were found, comparisons were made between successive age groups (e.g., P2 vs. P3, P3 vs. P4, and P5 vs. P7) by using Tukey's Studentized range test (a post hoc multiple comparisons, to control for the type I experiment-wise error rate). Significance was set at P < 0.01 for one-way ANOVA and P < 0.05 for Tukey's test. The Bonferroni multiple comparison's test was also done and it showed comparable results as the Tukey's test.
Fig 5.
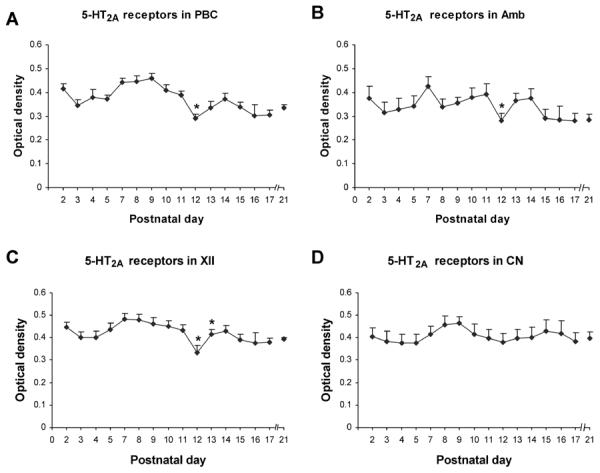
Optical densitometric measurements of immunoreactive product for serotonin 2A receptors (5-HT2AR) in the cytoplasm of neurons in the rat pre-Bötzinger complex (PBC) (A), nucleus ambiguus (Amb) (B), hypoglossal nucleus (XII) (C), and cuneate nucleus (CN) (D) from P2 to P21. The data were presented as mean and standard error of the mean. ANOVA showed significant differences among ages in the PBC, Amb, and XII (P < 0.01), but not in the CN. Tukey's Studentized range test and Bonferroni multiple comparison's test were performed on the PBC, Amb, and XII with similar results. Apart from statistically insignificant fluctuations, a significant reduction in the expression of 5-HT2AR in the PBC, Amb, and XII occurred at P12 (* P < 0.05). In the XII, this reduction was followed by a striking increase at P13 (* P < 0.05). The expression of 5-HT2AR in the CN was relatively stable from P2 to P21.
The part of the nucleus ambiguus chosen for the present study (and our previous studies) was the semicompact formation and rostral loose formation innervating upper airway muscles and representing pharyngolaryngomotor functions (3). For the remaining nuclei, measurements were made from the central main portion of each nucleus.
RESULTS
In general, 5-HT2A receptor(R)-immunoreactive (-ir) product was clearly visible in subpopulations of neurons in each of the brain stem nuclei studied. The size of 5-HT2AR-ir neurons increased with age and was relatively stable after P10–11 (Figs. 1–4). The neuropil of all brain stem nuclei examined exhibited relatively stable expression of 5-HT2AR during the first three postnatal weeks, with minor fluctuations and some depressions at P3–4, 12–13, and 17, as well as some elevations at P14–15. Control sections showed no specific labeling above background (data not shown). Quantitative analysis with ANOVA indicated significant differences in the intensities of immunolabeling among ages in neurons of the PBC, Amb, and XII (P < 0.01), but not in the cuneate nucleus CN. Tukey and Bonferroni tests for adjacent age comparisons were then done for the first three nuclei.
Fig 1.
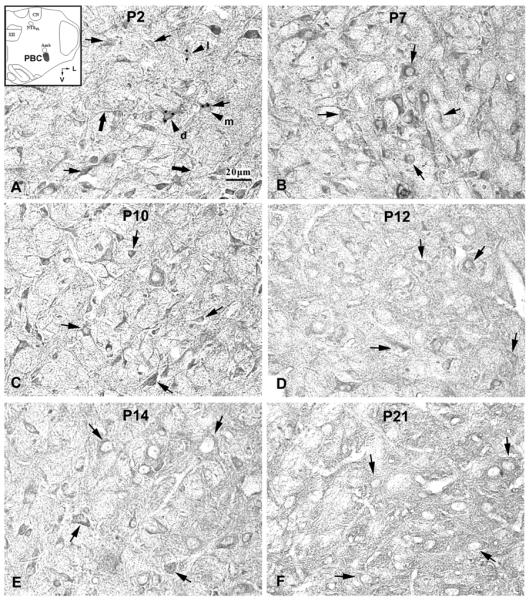
Serotonin 2A receptor (5-HT2AR) immunoreactive (-ir) neurons in the rat pre-Bötzinger complex (PBC) at P2 (A), P7 (B), P10 (C), P12 (D), P14 (E), and P21 (F). The inset in A indicates the location of PBC in a diagrammatic cross-section of the medulla (V = ventral; L = lateral). The expression of 5-HT2A receptors in the cytoplasm of PBC neurons was relatively high at P2, P7, and P10, but significantly reduced at P12, followed by increases at P14 and P21. The black arrows indicate 5-HT2A-ir neurons distributed along the boundary of the PBC. The black arrowheads in A show 5-HT2A-ir neurons with dark (d), moderate (m), or light (l) labeling in the PBC. Their optical density values (taken at 2 or more cytoplasmic sites designated by black dots) were 0.517, 0.438, and 0.332, respectively. The thick black arrows in A indicate 5-HT2A-ir processes. Scale bar: 20 μm for all.
Fig 4.
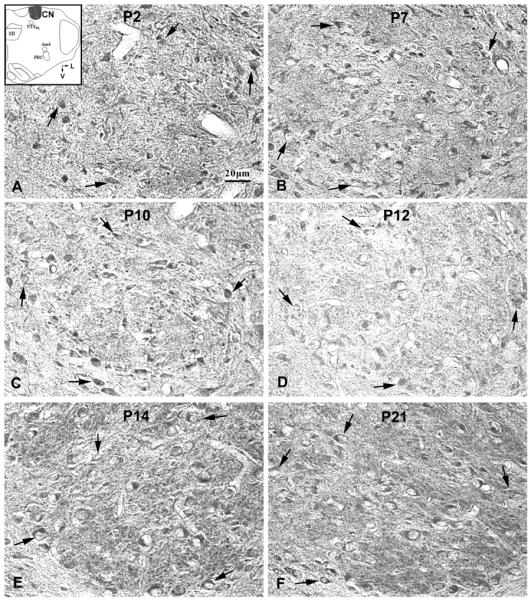
Serotonin 2A receptor (5-HT2AR) immunoreactive (-ir) neurons in the rat cuneate nucleus (CN) at P2 (A), P7 (B), P10 (C), P12 (D), P14 (E), and P21 (F). The inset in A indicates the location of CN in a diagrammatic cross-section of the medulla (V = ventral; L = lateral). The expression of 5-HT2AR in the cytoplasm of labeled neurons was relatively stable with slight but statistically insignificant fluctuations from P2 to P21. The black arrows indicate 5-HT2A-ir neurons distributed within the boundary of the CN.
5-HT 2AR-ir neurons in the pre-Bötzinger complex (PBC)
5-HT2AR-ir was observed in ~50% of neurons in the PBC. Labeled neurons were distributed evenly within the PBC, and immunoreactivity was present in the cytoplasm, proximal processes, and the rest of the neuropil (Fig. 1). Labeled neurons were multipolar, fusiform, or oval in shape, and mainly small or medium in size (Fig. 1). The size of small neurons ranged from 6.5 – 8.5 μm in diameter at P2 to 8 – 10.5 μm at P21, whereas medium-sized neurons ranged from 10 – 11.5 μm at P2 to 11 – 16 μm at P21. The expression of 5-HT2AR in the cytoplasm of neurons exhibited a relative plateau from P2 to P11, with a slight but statistically insignificant dip at P3 to P5 (Fig. 5A). However, a significant decrease in 5-HT2AR-ir (by ~25% from P11) occurred at P12 (P < 0.05), at which time the level was the lowest during the first three postnatal weeks, followed by smaller fluctuations until P21 (Figs. 1, 5A). P12 was the only time point in the three weeks that showed statistical significance with the Tukey's test.
5-HT2AR -ir neurons in the upper airway representation of the nucleus ambiguus (Amb)
5-HT2AR-ir was observed in ~80~90% of neurons in the Amb. Labeled neurons were medium to small in size and mainly multipolar in shape (Fig. 2). The size of small neurons ranged from 7.5 – 9.5 μm in diameter at P2 to 9 – 12.5 μm at P21, whereas medium-sized neurons ranged from 10 – 12.5 μm at P2 to 13.5 – 16 μm at P21. Occasionally large neurons were labeled (16.5 – 22 μm in diameter at P11 – 21). The expression of 5-HT2AR in the cytoplasm of neurons was relatively stable from P2 to P11, with a small but statistically insignificant peak at P7 (Fig. 5B). However, a significant drop in 5-HT2AR-ir (by ~28% from P11) occurred at P12 (P < 0.05), followed by a statistically insignificant rise at P13–14, and a relative plateau thereafter until P21 (Figs. 2, 5B). Again, P12 was the only time point in the 3 wk that showed statistical significance with the Tukey's test.
Fig 2.
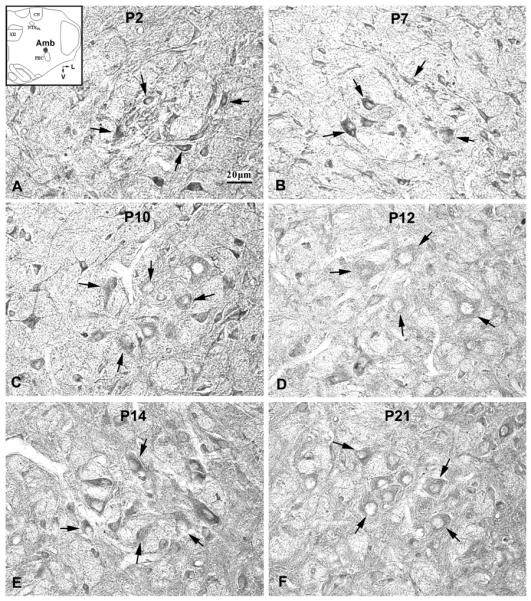
Serotonin 2A receptor (5-HT2AR) immunoreactive (-ir) neurons in the rat nucleus ambiguus (Amb) at P2 (A), P7 (B), P10 (C), P12 (D), P14 (E), and P21 (F). The inset in A indicates the location of Amb in a diagrammatic cross-section of the medulla (V = ventral; L = lateral). The expression of 5-HT2AR in the cytoplasm of neurons was relatively high at P2, P7, and P10, followed by a significant decrease at P12, then a rise at P14, followed by a reduction until P21. The black arrows indicate 5-HT2A-ir neurons distributed along the boundary of the Amb.
5-HT2AR-ir neurons in the hypoglossal nucleus (XII)
5-HT2AR-ir was found in ~60~70% of neurons in the XII. Labeled neurons were mainly medium and large in size and multipolar or fusiform in shape (Fig. 3). The size of medium neurons ranged from 10.5 – 14 μm in diameter at P2 to 13 – 16 μm at P21, whereas large neurons ranged from 16.5 – 18 μm at P2 to 16.5 – 22 μm at P21. The expression of 5-HT2AR in the cytoplasm of labeled neurons was quite stable from P2 to P11, with a slight but statistically insignificant dip at P3 to P5 (Fig. 5C). However, a significant reduction (by ~23% from P11) was noted at P12 (P < 0.05), when the value reached its lowest during the first 3 wk of life, followed by a significant increase at P13 (P < 0.05) to the P11 level, and a plateau thereafter (Figs. 3, 5C). P12 and P13 were the only time points in the 3 wk that showed statistical significance with the Tukey's test.
Fig 3.
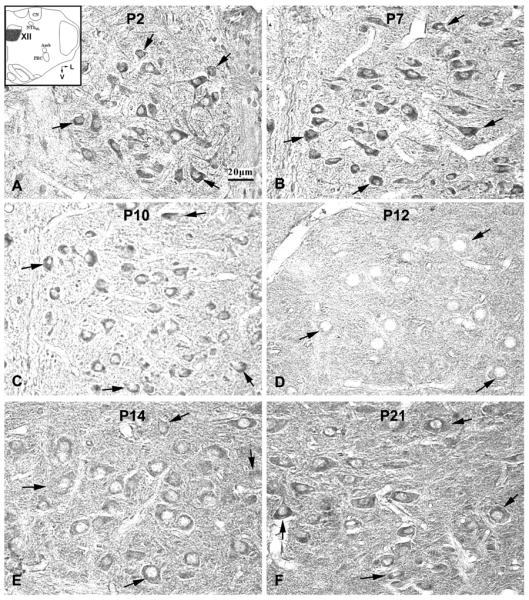
Serotonin 2A receptor (5-HT2AR) immunoreactive (-ir) neurons in the rat hypoglossal nucleus (XII) at P2 (A), P7 (B), P10 (C), P12 (D), P14 (E), and P21 (F). The inset in A indicates the location of XII in a diagrammatic cross-section of the medulla (V = ventral; L = lateral). The expression of 5-HT2AR in the cytoplasm of labeled neurons was relatively high at P2, P7, and P10, but strikingly decreased at P12, followed by a significant increase at P13 and P14, then a plateau until P21. The black arrows indicate 5-HT2A-ir neurons distributed within the boundary of the XII.
5-HT2AR-ir neurons in the cuneate nucleus (CN)
5-HT2AR-ir was evident in ~35~50% of neurons in the CN. Labeled neurons were small or medium in size and multipolar in shape (Fig. 4). The size of small neurons ranged from 5 – 8.5 μm in diameter at P2 to 7 – 10.5 μm at P21, whereas medium-sized neurons ranged from 10 – 12 μm at P2 to 11 – 14 μm at P21.The expression of 5-HT2AR in the cytoplasm of labeled neurons was relatively stable from P2 to P21, but with minor fluctuations that were not statistically significant (Figs. 4, 5D). No prominent dip in 5-HT2AR-ir was noted in neuronal cell bodies at or around P12.
DISCUSSION
Our current data indicate that the expressions of 5-HT2A receptors in neurons of the PBC, the Amb, and the XII were maintained within a narrow range between P2 and P21, with a dip at P3–4 and a significant reduction only at P12. This change was not observed in the CN.
Activation of 5-HT2A receptors is critical for the generation of respiratory rhythm in vitro (36). In vivo, it appears that intraperitoneal application of a 5-HT2A/2C receptor agonist depresses respiratory frequency and tidal volume in the newborn (P0–P3) but increases the frequency (but still suppresses tidal volume) in the adult (6). It is not known whether the effect is via the 2A or 2C receptor, or both, or the exact site of action, or when the switch occurs. Additionally, endogenous 5-HT2A receptor activity may also play an important role in the recovery of network rhythmicity and eupneic activity after hypoxic exposure (46). Several studies have indicated that 5-HT2A receptor activity was important for long-term facilitation of phrenic activity after intermittent hypoxia (1, 12). Alterations of 5-HT2A receptors may also be involved in respiratory disorders, such as sudden infant death syndrome (SIDS), in which serotonin receptor (including 5-HT2A receptor) binding in several brain stem nuclei (such as the arcuate nucleus, raphé obscurus, inferior olive, and intermediate reticular zone) were significantly decreased (22, 34). The immunoreactive expression of 5-HT2A receptors in SIDS was also reduced in the dorsal motor nucleus of the vagus, the solitary tract nucleus, and the ventrolateral medulla, where PBC and Amb are located (33).
The PBC was presumed to contribute to respiratory rhythmogenesis (38, 41, 42). The activation of 5-HT2A receptors in the PBC is reportedly required for the generation of the respiratory rhythm in vitro (36). Activation of 5-HT2A can increase the frequency of respiratory activity, whereas the blockade of 5-HT2A receptors decreases the frequency, amplitude, and regularity of populational respiratory activity (36). Cadmium-insensitive (CI) pacemakers in the PBC presumably rely on the persistent sodium current, which requires endogenously activated 5-HT2A receptors for maintaining fictive respiratory activity in the brainstem slice by modulating sodium conductance via a protein kinase C (PKC) pathway (36). Blockade of endogenous 5-HT2A receptor activity can eliminate CI-pacemaker activity and fictive gasping, suggesting that 5-HT2A receptor activity and CI-pacemaker activity are required for fictive gasping rhythm generation in vitro (46). Activation of 5-HT2A receptors in the PBC is reportedly critical for gasping (46), a presumed major mechanism for autoresuscitation from hypoxic respiratory arrest (13). Failure of such autoresuscitation may underlie sudden infant death syndrome (SIDS) (43, 46). On the other hand, other studies showed that the blockage of 5-HT2A receptors did not eliminate fictive eupnea and gasping activity (45), although it did impair them when applied during the hypoxic challenge (44). Whether decreased 5-HT2A receptors' expression in the PBC at P12 in the rat would attenuate the gasping mechanism requires further in vivo investigation.
The XII motoneurons innervate the tongue musculature and play an important role in maintaining upper airway patency during respiration (16). A loss of the XII motoneuronal activity may result in obstructive apnea (39). Serotonergic neurons in the caudal medullary raphé innervate the XII motoneurons (30) and exert excitatory effects on them (5, 10, 24). This is reportedly mediated by 5-HT2A receptors, which are the predominant serotonin receptor subtype in the XII motoneurons (10, 50). The activity of brainstem serotonin neurons is highest in wakefulness, reduced in non- rapid-eye-movement (NREM) sleep and minimal in REM sleep (15, 23). The genioglossus (GG) muscles of the tongue are critical in obstructive sleep apnea (OSA) in humans (16, 18). Once GG activity is suppressed in REM sleep, it is difficult to reactivate the muscle, even in the presence of high respiratory drive (17). Thus during REM sleep, the XII motoneurons' activity is decreased by the withdrawal of serotonin (when medullary raphé neurons are silent), which renders the upper airway particularly vulnerable to collapse (15), and obstructive sleep apnea may occur. In some SIDS cases, episodic airway obstruction was found during sleep before death (14, 22). Our data suggest that during the critical period (around P12–13 in rats), the upper airway may be even more vulnerable to collapse due to the reduction in 5-HT2A receptors' expression in the XII as well as in the Amb (see below).
The Amb is involved in controlling the upper airway via the innervation of pharyngolaryngeal and laryngeal muscles (3, 21). Serotonin has excitatory effects on the laryngeal motoneurons, and excitation can be abolished by methysergide, a blocker of multiple 5-HT receptors (11). 5-HT2 receptor agonist can reversibly and transiently excite central fictive inspiratory activity, but multiple applications of such an agonist leads to long-lasting inhibition of fictive inspiration-related GABAergic neurotransmission to cardioinhibitory vagal neurons in the Amb (8); however, it is not clear whether the action is mediated via 2A or 2C, or both. An attenuation in the expression of 5-HT2A receptors in the Amb during the critical period, as we observed in the present study, may contribute to the loss of muscle tone in maintaining upper airway patency during sleep. It may also lead to atypical regulation of inspiratory activity as well as cardiovascular activity.
To our knowledge, there are no comparable detailed studies to date on the expression of 5-HT2A receptors in brain stem nuclei involved in the control of respiration. Roth et al. (40) reported that 5-HT2A (called 5-HT2 in previous studies) receptor binding sites in the whole brain increased 8-fold from embryonic day 17 (E17) to postnatal P13, whereas the brain mRNA levels increased 13-fold between E17 and P5, and were reduced by 50% after P25-27. By examining the 5-HT2A receptor-induced phosphatidyl inositide breakdown in the rat cortex, Claustre et al. (7) demonstrated that these receptors appeared to be functional in young immature (P8) rat cortex, and the stimulating effect of 5-HT was six times greater in immature than in adult rats. These two sets of data (7, 40) were obtained from whole brain or brain regions (such as whole medulla-pons or cortex). Thus, they did not distinguish 5-HT2A receptor expression within distinct nuclei or neuronal populations. Volgin et al. (47) examined only five time points (P5, 8, 12, 15, and 19), with one animal per time point, for the expression of 5-HT2A receptor immunoreactivity in the hypoglossal nucleus. They reported a steady increase in the intensity of labeling from P5 to P15 before tapering off to adult levels by P19. However, they did not examine P11 and P13, so the dip that we observed in the present study at P12 probably escaped their detection. Moreover, their immuno-density values were taken from the entire XII nucleus rather than from individual neurons, and probably included non-labeled structures such as nuclei of neurons and glia, bundles of myelinated and unmyelinated axons, and blood vessels in the neuropil. Interestingly, their mRNA data are more comparable to our present data on XII nucleus, with low values at P3 and P5, essentially a plateau between P7 and P33, and a dip at P14 (to 80% of the plateau level). However, due to the limited number of animals used (1–2 per time point), the change was not statistically significant at P14.
The significant reduction in the expression of 5-HT2A receptors at P12 in the PBC, Amb, and XII may be related to concurrent and significant increase in GABAB receptor expression in the same nuclei reported previously (27, 29), since GABAB receptor activation is postulated to inhibit 5-HT release (32). Thus reduction in 5-HT2A receptor expression contributes further to transient neurochemical imbalance at P12 (27, 28, 29, 49).
The suppressed expression of 5-HT2A receptors in the PBC at P12 may reduce the firing frequency, amplitude, and regularity of cadmium-insensitive pacemaker neurons that exhibit persistent sodium current in the PBC, since these neurons rely on 5-HT2A receptors for their normal functioning (36). Reduced 5-HT2A receptor expression in the XII and Amb may lead to attenuated airway patency (10, 11) at P12. When challenged with a respiratory stressor, such as hypoxia, reduced 5-HT2A receptor expression in various brain stem nuclei may contribute to attenuated hypoxic ventilatory response observed during the critical period of respiratory network development (at the end of the second postnatal week) in the rat (26). It will also increase the risk of reduced respiratory long-term facilitation (1, 4, 12) and ability to gasp (46) at the same time.
Acknowledgments
GRANTS This study was supported by National Institute of Child Health and Human Development grant HD-048954 and a grant from the Children's Hospital and Health System Foundation, Milwaukee, WI.
REFERENCES
- 1.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg KA, Harvey JA, Spampinato U, Clarke WP. Physiological relevance of constitutive activity of 5-HT2A and 5-HT2C receptors. Trends Pharmacol Sci. 2005;26:625–630. doi: 10.1016/j.tips.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- 4.Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol. 2002;87:2964–2971. doi: 10.1152/jn.2002.87.6.2964. [DOI] [PubMed] [Google Scholar]
- 5.Brandes IF, Zuperku EJ, Stucke AG, Jakovcevic D, Hopp FA, Stuth EA. Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs. J Neurophysiol. 2006;95:3449–3459. doi: 10.1152/jn.00823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cayenot F, Gros F, Larnicol N. Postnatal changes in the respiratory response of the conscious rat to serotonin 2A/2C receptor activation are reflected in the developmental pattern of fos expression in the brain stem. Brain Res. 2002;942:51–57. doi: 10.1016/s0006-8993(02)02690-2. [DOI] [PubMed] [Google Scholar]
- 7.Claustre Y, Rouquier L, Scatton B. Pharmacological characterization of serotonin-stimulated phosphoinositide turnover in brain regions of the immature rat. J Pharmacol Exp Ther. 1988;244:1051–1056. [PubMed] [Google Scholar]
- 8.Dergacheva O, Griffioen KJ, Wang X, Kamendi H, Gorini C, Mendelowitz D. 5-HT(2) receptor subtypes mediate different long-term changes in GABAergic activity to parasympathetic cardiac vagal neurons in the nucleus ambiguus. Neuroscience. 2007;149:696–705. doi: 10.1016/j.neuroscience.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–345. [PubMed] [Google Scholar]
- 10.Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- 11.Fenik V, Kubin L, Okabe S, Pack AI, Davies RO. Differential sensitivity of laryngeal and pharyngeal motoneurons to iontophoretic application of serotonin. Neurosci. 1997;81:873–885. doi: 10.1016/s0306-4522(97)00215-7. [DOI] [PubMed] [Google Scholar]
- 12.Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- 13.Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest. 1975;56:1371–1377. doi: 10.1172/JCI108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horne RS, Ferens D, Watts AM, Vitkovic J, Lacey B, Andrew S, Cranage SM, Chau B, Adamson TM. The prone sleeping position impairs arousability in term infants. J Pediatr. 2001;138:811–816. doi: 10.1067/mpd.2001.114475. [DOI] [PubMed] [Google Scholar]
- 15.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 16.Horner RL. Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol. 2007;85:155–165. doi: 10.1139/y06-089. [DOI] [PubMed] [Google Scholar]
- 17.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO(2) in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 18.Horner RL, Shea SA, McIvor J, Guz A. Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea. Q J Med. 1989;72:719–735. [PubMed] [Google Scholar]
- 19.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International union of pharmacology classification of receptors of 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 20.Jacobs BL, Fornal CA. Serotonin and behavior. A general hypothesis. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven Press; New York: 1995. pp. 461–469. [Google Scholar]
- 21.Jordan D. Central nervous pathways and control of the airways. Respir Physiol. 2001;125:67–81. doi: 10.1016/s0034-5687(00)00205-x. [DOI] [PubMed] [Google Scholar]
- 22.Kinney HC. Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol. 2005;8:507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- 23.Kubin L, Davies RO. Mechanisms of airway hypotonia. In: Pack AI, editor. Sleep apnea: pathogenesis, diagnosis and treatment. Dekker; New York: 2002. pp. 99–154. [Google Scholar]
- 24.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Kim J, Cinotte J, Homolka P, Wong-Riley MTT. Carotid body denervation effect on cytochrome oxidase activity in pre-Bötzinger complex of developing rats. J Appl Physiol. 2003;94:1115–1121. doi: 10.1152/japplphysiol.00765.2002. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Lowry TF, Wong-Riley MTT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Wong-Riley MTT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Bötzinger complex. J Appl Physiol. 2002;92:923–934. doi: 10.1152/japplphysiol.00977.2001. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Wong-Riley MTT. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J Appl Physiol. 2003;95:2285–2291. doi: 10.1152/japplphysiol.00638.2003. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Wong-Riley MTT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- 30.Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- 31.Mason P, Leung CG. Physiological functions of pontomedullary raphe and medial reticular neurons. Prog Brain Res. 1996;107:269–282. doi: 10.1016/s0079-6123(08)61870-1. [DOI] [PubMed] [Google Scholar]
- 32.Metz A, Goodwin GM, Green AR. The administration of baclofen to mice increases 5-HT2-mediated head-twitch behaviour and 5-HT2 receptor number in frontal cortex. Neuropharmacology. 1985;24:357–360. doi: 10.1016/0028-3908(85)90145-5. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- 34.Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- 35.Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- 36.Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 38.Rekling JC, Feldman JL. Pre-Bötzinger complex and pacemaker neurons; hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- 39.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 40.Roth BL, Hamblin MW, Ciaranello RD. Developmental regulation of 5-HT2 and 5-HT1c mRNA and receptor levels. Brain Res Dev Brain Res. 1991;58:51–58. doi: 10.1016/0165-3806(91)90236-c. [DOI] [PubMed] [Google Scholar]
- 41.Smith JC, Butera RJ, Koshiya N, Negro CD, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir Physiol. 2000;122:131–147. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- 42.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol. 2003;36:113–122. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- 44.St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of {alpha}1-adrenergic receptors and serotonin 5-HT2 receptors. J Appl Physiol. 2008;104:665–673. doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- 45.Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–227. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- 46.Tryba AK, Pena F, Ramirez JM. Gasping acti-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2 A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- 48.Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- 49.Wong-Riley MTT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neurosci. 2002;113:145–154. doi: 10.1016/s0306-4522(02)00137-9. [DOI] [PubMed] [Google Scholar]
- 51.Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacol Rev. 1992;44:401–458. [PubMed] [Google Scholar]


