Abstract
In the present study, an experiment was conducted to show that A. awamori modifies lipid metabolism in mammals. A total number of 24 rats at 6 weeks of age were divided into 2 groups (10% and 30% fat dietary groups), and each group was further divided into control and experimental groups (6 rats per group). Rats in the experimental groups were given diets containing 0.05% A. awamori. The diets were administered for 3 weeks to evaluate the effects of A. awamori on growth, plasma lipid profile, and the expressions of genes related to lipid metabolism in the liver. After the rats were fed A. awamori, body weight gain was increased, while food intake was decreased; therefore, food efficiency was increased in both A. awamori groups. Plasma triglycerides, LDL cholesterol, and glucose levels were decreased, but plasma HDL cholesterol levels were increased. Furthermore, saturated fatty acids were decreased while; unsaturated fatty acids were increased in the liver. The liver mRNA levels of FAS, ACC, delta-6-desaturase, and HMG-CoA reductase were increased, while the mRNA level of LDL receptor was decreased. From these data, it is proposed that A. awamori could be used as an effective probiotic to prevent lifestyle-related diseases in humans.
1. Introduction
It has been reported that Aspergillus provides beneficial effects on a host's health by affecting the host's intestinal microflora. Their beneficial effects on human health, including the alleviation of lactose intolerance, immunomodulation, hypocholesterolemic effects, and a reduction in the risk of gastrointestinal disease have been demonstrated previously [1–3]. Aspergillus awamori (A. awamori), a variant of Aspergillus niger, is a fungus that has long been used for food processing in Japan. The products that are processed by A. niger are given GRAS (Generally Recognised as Safe) status from the FDA [4]. A. awamori is also known to produce enzymes that enhance carbohydrates and proteins digestion [5]. Furthermore, we have reported that unsaturated fatty acid levels are increased, while saturated fatty acid levels are decreased in skeletal muscle after A. awamori feeding [6]. Polyunsaturated fatty acids reduce the risk of cardiovascular diseases by reducing blood lipids levels and platelet reactivity and aggregation, indicating that A. awamori feeding may be effective in reducing the risk of lifestyle-related diseases in humans. Here, we report that A. awamori modifies the plasma lipids profile and changes the liver fatty acids composition in rats with no harmful effects.
2. Materials and Methods
2.1. Animals and Diets
The animal experiment was conducted in accordance with the guidelines of Kagoshima University.
Twenty-four male Sprague-Dawley rats at 5 weeks of age were obtained from SLC (Shizuoka, Japan) and individually housed in stainless-steel wire-mesh cages in a temperature-controlled room at 24°C with 55% relative humidity and a 12 h light: dark cycle. The rats were allowed free access to food and water. During a 7 d prefeeding period, the rats were fed a control diet. The rats were divided into 2 groups (10% and 30% fat dietary groups), and each group was further divided into control and experimental groups (6 rats per group). Rats in the experimental groups were given diets containing 0.05% A. awamori (Table 1). The diets were administered for 3 weeks to evaluate the effects of A. awamori on growth, digestibility of energy and crude protein, abdominal fat content, organs weights, plasma lipid profile, liver fatty acids profile and the expressions of genes related to lipid metabolism in the liver. At the end of the experimental period, all rats were killed by decapitation and their organs were dissected out and used for analysis.
Table 1.
Compositions of experimental diets.
| % | ||||
|---|---|---|---|---|
| Ingredients | 10% Butter diet | 30% Butter diet | ||
| Control | Experiment | Control | Experiment | |
| Starch | 50.00 | 49.95 | 30.00 | 29.95 |
| Sucrose | 10.00 | 10.00 | 10.00 | 10.00 |
| Casein | 20.00 | 20.00 | 20.00 | 20.00 |
| Cellulose | 5.00 | 5.00 | 5.00 | 5.00 |
| Butter | 10 | 10 | 30 | 30 |
| Mineral mix1 | 3.50 | 3.50 | 3.50 | 3.50 |
| Vitamin mix2 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 |
| Methionine | 0.30 | 0.30 | 0.30 | 0.30 |
| Aspergillus awamori | — | 0.05 | — | 0.05 |
1Mineral supplied per kilogram of feed: 154 mg of Mn, 121 mg of Zn, 176 mg of Fe, 33 mg of Cu, 1.1 mg of I, and 0.7 mg of Se.
2Vitamin supplied per kilogram of feed: 3784 mcg of vitamin A, 0.066 mcg of vitamin D, 110.11 mcg of vitamin E, 12 mg of vitamin B12, 1.37 mg of retinol, 0.13 mg of cholecalciferol, 6.50 mg of riboflavin, 2.60 mg of thiamine hydrochloride, 1.30 mg of pyridoxamine hydrochloride, 0.03 mg of cyanocobalamin, 10.40 mg of D-pantothenic acid, 26.00 mg of nicotinic acid, 1.05 mg of vitamin K3, 0.52 mg of pteroylglutamic acid, 0.78 mg of choline chloride, 0.07 mg of biotin, and 2.54 g of sucrose.
A. awamori was prepared at Bio genkoji Research Institute (Kagoshima, Japan). A. awamori was made as described by Yamamoto et al. [7]. Briefly, 6 tons of wheat bran and 1 ton of SDBP were mixed in a well-ventilated container with blender and were heated (100°C) and sterilized by steam. After cooling down the sterilized materials to 35°C, 6 kg of A. awamori spores and 70 kg of sterilized used frying oil were added. During the first 5 days of the prefermentation A. awamori grew fully. Thereafter, 1 ton of sterilized SDBP and 70 kg of used frying oil were added daily up to 70 days. During the fermentation, the ventilation fans were used to keep the temperature around 40°C which is the optimal temperature for A. awamori. In addition, the blender of the container kept working to homogenize the contents. After 70 days of the fermentation, the fermented product was cooled down by continuous ventilation. During the fermentation process, many types of enzymes are produced by the A. awamori. One gram of fermented product contained 2.56 U of glucoamylase, 0.05 U of a-glucosidase, 2.09 U of a-amylase, and 2345 U of acidic protease. The fungus was mixed into the diets. The numbers of A. awamori spores given was approximately 25 × 104/g feed.
2.2. Biochemical Analysis
2.2.1. Blood and Liver
Blood samples were collected into heparinised test tubes, quickly centrifuged at 5,900 ×g for 10 minutes at 4°C to separate the plasma, and stored at −30°C for analysis. Total cholesterol, triglycerides, HDL, LDL, GOT, and glucose levels in the plasma were measured by an automated Fuji DRY-CHEM 3500 (Fuji Medical Systems, Tokyo, Japan) according to the manufacturer's instructions. The concentration of plasma thiobarbituric acid-reactive substance (TBARS) was measured using the method of Ohkawa et al. [8]. The plasma 3-methylhistidine concentration was measured by HPLC according to the method of Hayashi et al. [9].
2.2.2. Digestibility of Energy and Crude Protein
The dietary and faecal contents of crude protein and gross energy were measured by a macrocorder machine (J-Science Lab Co., Ltd, Kyoto, Japan) and bomb calorie meter (Yoshida, Tokyo, Japan), respectively. The following calculations were made: protein digestibility = (total crude protein intake − total faecal crude protein)/total crude protein intake × 100 and energy digestibility = (total energy intake − total faecal energy)/total energy intake × 100.
2.2.3. RNA Isolation and Real-Time PCR
Total RNA was extracted from a piece of liver (approximately 100 mg) using an RNeasy Fibrous Tissue Mini Kit (Qiagen, Tokyo, Japan) according to the manufacturer's protocol. The RNA concentration and purity were determined spectrophotometrically using A 260 and A 280 values in a photometer (BioPhotometer, Eppendorf, Hamburg, Germany). The ratio of A 260/A 280 for all samples was between 1.8 and 2.0. cDNA was synthesised at 400 ng RNA per 10 μL of reaction solution with the PrimeScript RT reagent Kit (Perfect Real Time, Takara, Shiga, Japan) by the program temperature control system PC320 (Astec, Fukuoka, Japan), which was set at reverse transcription at 37°C for 15 min, inactivation of reverse transcriptase at 85°C for 5 s, and cooling at 4°C for 5 min. Real-time PCR primers were prepared according to the method of Nakashima [10]. Gene expression was measured by real-time PCR using the 7300 Real Time PCR system (Applied Biosystems, Foster City, CA USA) with SYBR Premix Ex Taq (Perfect Real-Time, TaKaRa). The thermal cycle was as follows: 1 cycle at 95°C for 10 s, and 60 cycles at 95°C for 5 s, 60°C for 31 s. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal standard and was not significantly different among the experimental groups. Gene expression results are shown as % of the control value.
2.2.4. Fatty Acids Analysis
Lipids were extracted from the liver with a mixture of chloroform and methanol (2 : 1) in a separatory funnel. The funnel was shaken carefully for 15 min and left to stand for 4 h to separate the organic layer. The organic layer was collected, passed through a glass funnel containing anhydrous sodium sulphate, and evaporated to near dryness using a vacuum evaporator. The extracted fat (100 mg) was placed into a 10 mL volumetric flask, and 2.5 mL of 0.5 N methanolic NaOH was added. The mixture was then heated in a steam bath for approximately 5 min. Four millilitres of BF3/MeOH was then added to the flask and the mixture was boiled for 2 min in a water bath. After cooling, a saturated NaCl solution was added to the mixture to reach a total volume of 8 mL. The mixture was then transferred to a separation funnel and extracted with 6 mL petroleum ether. The ether phase was then evaporated in a water bath at 60°C. The obtained methyl ester of the fatty acid fraction was dissolved in 1 mL of hexane and used for the fatty acid analysis. Fatty acids were separated by GC-MS (Thermo Fisher, MA, USA) on a capillary column (30 m × 0.25 mm i.d. DB-1 coated with a 0.25 μm film of dimethyl polysiloxane) (J&W Scientific, CA, USA) using 1 μL of sample. The temperature of the column was 150°C at the time of injection, then was programmed to increase 5°C min−1 to 250°C and was maintained at that temperature for 5 min. Injection was performed with a split ratio of 10 : 1. The flow rate was 1.0 mL min−1, helium was used as the carrier gas and the injector temperature was 250°C. The MS detection conditions were as follows: interface temperature, 230°C; ionisation mode, EI+; electron energy, 70 eV; full scan acquisition mode; and mass range, 33–450 amu. Fatty acids were identified using authentic standards and online NIST-library spectra.
2.3. Statistical Analysis
The differences among treatment groups and control groups were analysed by the General Liner model using SPSS v. 17.0 (Statistical Packages for the Social Sciences, released August 23, 2008). The significant differences among the treatments were compared by Duncan's new multiple-range test. P ≤ 0.05 was set as the limit of significance.
3. Results
The effects of feeding A. awamori on growth and digestibility in rats are summarised in Table 2. After A. awamori feeding, body weight gain was increased, while food intake was decreased; therefore food efficiency was increased in both A. awamori groups. The digestibility of protein and energy was improved by feeding A. awamori.
Table 2.
Effect of Aspergillus awamori feeding on growth and digestibility in rats.
| 10% Butter | 30% Butter | |||
|---|---|---|---|---|
| Control | Experiment | Control | Experiment | |
| Initial body weight (g) | 175 ± 3.40 | 175 ± 3.51 | 175 ± 4.11 | 175 ± 3.85 |
| Body weight gain (g/21 days) | 140 ± 3c | 161 ± 3b | 164 ± 6b | 189 ± 5a |
| Food intake (g/21 days) | 426 ± 10a | 385 ± 12b | 375 ± 11b | 341 ± 8c |
| Food efficiency (%) | 33 ± 1.4c | 42 ± 1.3b | 44 ± 1.5a | 55 ± 1.3a |
| Protein digestibility (%) | 74 ± 3c | 80 ± 5b | 82 ± 5ab | 85 ± 4a |
| Energy digestibility (%) | 75 ± 4c | 83 ± 4ab | 80 ± 3b | 86 ± 4a |
Values are expressed as means ± standard error. Data were analysed by two-way analysis of variance and Duncan's multiple range test. Means with different superscripts significantly differ from each other. a-b-cMeans with different superscripts differ from each other (P< 0.05).
The effect of feeding A. awamori on organ weights is summarised in Table 3. The weights of abdominal fat, heart, and kidney were decreased after A. awamori feeding, while spleen weight was not affected. Gastrocnemius muscle and liver weights were increased.
Table 3.
Effect of Aspergillus awamori feeding on organ relative weights.
| 10% Butter | 30% Butter | |||
|---|---|---|---|---|
| Control | Experiment | Control | Experiment | |
| Abdominal fat (g/100 g BW) | 3.4 ± 0.3a | 2.1 ± 0.3c | 3.8 ± 0.4a | 2.8 ± 0.1b |
| Gastrocnemius muscle (g/100 g BW) | 1.95 ± 0.05c | 2.38 ± 0.09b | 2.26 ± 0.13b | 2.64 ± 0.06a |
| Liver (g/100 g BW) | 7.7 ± 0.4ab | 9.0 ± 0.4a | 7.4 ± 0.4b | 8.0 ± 0.2ab |
| Heart (g/100 g BW) | 0.73 ± 0.03a | 0.61 ± 0.01b | 0.64 ± 0.03b | 0.52 ± 0.02c |
| Kidney (g/100 g BW) | 1.50 ± 0.07a | 1.30 ± 0.04b | 1.30 ± 0.07b | 1.10 ± 0.04b |
| Spleen (g/100 g BW) | 0.35 ± 0.01 | 0.39 ± 0.02 | 0.33 ± 0.01 | 0.34 ± 0.03 |
Values are expressed as means ± standard error. Data were analyzed by two-way analysis of variance and Duncan's multiple range test. Means with different superscripts significantly differ from each other. a-b-cMeans with different superscripts differ from each other (P< 0.05).
The effect of feeding A. awamori on plasma GOT, TBARS, and 3-methylhistidine levels is summarised in Table 4. Plasma GOT was decreased by feeding 30% butter with 0.05% A. awamori. Plasma TBARS was decreased significantly by A. awamori feeding despite the high levels of dietary fat. Plasma 3-methylhistidine was decreased by A. awamori feeding.
Table 4.
Effect of Aspergillus awamori feeding on plasma GOT, TBARS, and 3-methylhistidine.
| 10% Butter | 30% Butter | |||
|---|---|---|---|---|
| Control | Experiment | Control | Experiment | |
| Plasma GOT (I/U) | 151 ± 5a | 148 ± 8a | 152 ± 6a | 136 ± 5b |
| Plasma TBARS (nmol MDA/mL of plasma) | 3.55 ± 0.1b | 2.42 ± 0.09c | 5.62 ± 0.14a | 3.74 ± 0.16b |
| Plasma 3-methylhistidine (µmol/mL) | 17 ± 4a | 12 ± 4c | 14 ± 4b | 10 ± 4c |
Values are expressed as means ± standard error. Data were analyzed by two-way analysis of variance and Duncan's multiple range test. Means with different superscripts significantly differ from each other. a-b-cMeans with different superscripts differ from each other (P< 0.05).
Table 5 shows the effect of feeding A. awamori on the liver fatty acids profile. The levels of saturated fatty acids (palmitic acid and stearic acid) were significantly decreased while the levels of unsaturated fatty acids (oleic acid, arachidonic acid, linoleic acid, and linolenic acid) were all significantly increased by A. awamori feeding.
Table 5.
Effect of Aspergillus awamori feeding on liver fatty acid profile.
| Butter 10% | Butter 30% | |||
|---|---|---|---|---|
| Control | Experiment | Control | Experiment | |
| Palmitic acid (mg/100 mg fat) | 0.9 ± 0.03c | 0.8 ± 0.03c | 1.8 ± 0.04a | 1.4 ± 0.02b |
| Stearic acid (mg/100 mg fat) | 0.43 ± 0.01b | 0.25 ± 0.02c | 0.99 ± 0.04a | 0.68 ± 0.03b |
| Oleic acid (mg/100 mg fat) | 0.7 ± 0.05c | 1.2 ± 0.11b | 1.9 ± 0.12ab | 2.3 ± 0.16a |
| Arachidonic acid (mg/100 mg fat) | 0.89 ± 0.04c | 1.32 ± 0.16b | 1.44 ± 0.15ab | 1.84 ± 0.12a |
| Linoleic acid (mg/100 mg fat) | 1.6 ± 0.14c | 2.8 ± 0.16b | 2.9 ± 0.25b | 4.1 ± 0.22a |
| Linolenic acid (mg/100 mg fat) | 0.083 ± 0.004d | 0.176 ± 0.006c | 0.214 ± 0.005b | 0.293 ± 0.002a |
Values are expressed as means ± standard error. Data were analyzed by two-way analysis of variance and Duncan's multiple range test. Means with different superscripts significantly differ from each other. a-b-cMeans with different superscripts differ from each other (P< 0.05).
Figure 1 shows the effect of feeding A. awamori on plasma characteristics. Plasma cholesterol, triglyceride, LDL, and glucose levels were all decreased, while plasma HDL levels were increased by A. awamori feeding.
Figure 1.
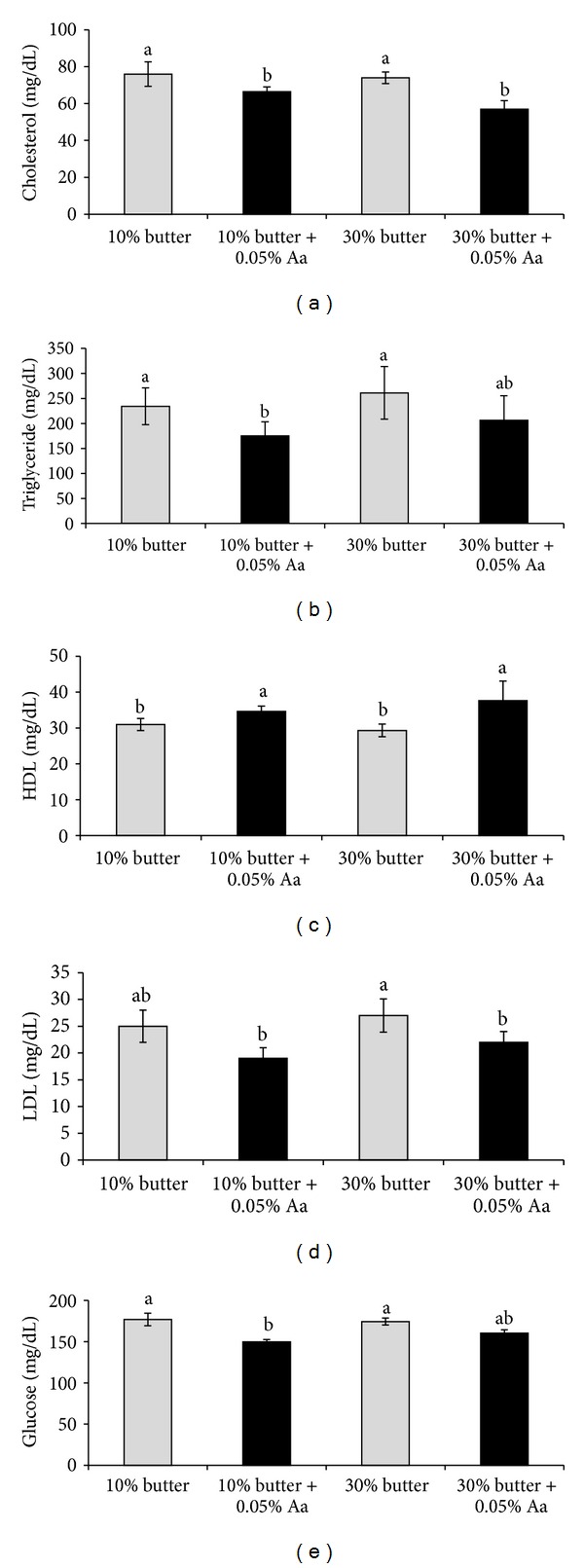
Effect of Aspergillus awamori feeding on the plasma levels of cholesterol (a), triglycerides (b), HDL (c), LDL (d), and glucose (e). Values are expressed as the means ± standard error. Data were analysed by two-way analysis of variance and Duncan's new multiple range tests. a-bMeans with different superscripts differ from each other (P < 0.05).
Figure 2 shows the effect of feeding A. awamori on the liver mRNA levels of fatty acid synthesis (FAS) (a), acetyl CoA carboxylase (ACC) (b), delta-6desaturase (c), LDL receptor (d), and HMG-CoA reductase (e). The mRNA levels of FAS, ACC, delta-6desaturase, and HMG-CoA reductase were all increased, while the mRNA level of the LDL receptor was decreased after A. awamori feeding.
Figure 2.
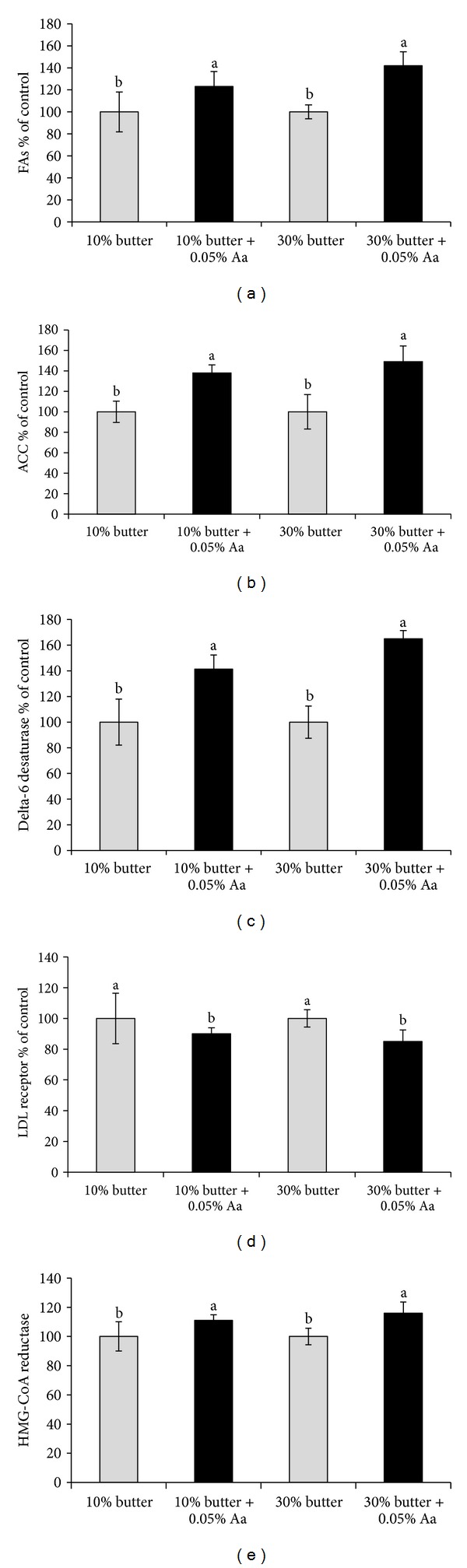
Effect of Aspergillus awamori feeding on the liver mRNA levels of FAS (a), ACC (b), delta-6 desaturase (c), LDL receptor (d), and HMG-CoA reductase (e). Values are expressed as % of the control values (means ± S.D); a-bMeans with different superscripts differ from each other (P < 0.05).
4. Discussion
The major aim of the present study was to demonstrate that feeding A. awamori reduces the lipid levels in the abdomen and plasma and modifies the plasma lipid profile in rats with no harmful effects. Body weight gain was significantly increased (P < 0.05) when the rats were fed A. awamori. The increased weight gain and feed efficiency due to A. awamori may be partially due to the increase in the digestibility of energy and protein. Enzymes such as cellulase and xylanase which are required for the digestion of soluble nonstarch polysaccharides (NSPs) are produced by A. awamori. Enzymes contained in the fungus stimulate digestion and improve growth. Amsal found that A. awamori stimulates the digestion of raw starches [11].
An increase in abdominal fat is associated with a higher risk of heart disease, hypertension, insulin resistance, and diabetes [12]. Iwashita et al. reported that a high-fat diet (HFD) is one of the factors that lead to obesity, and the long term-intake of HFD evokes a significant increase in abdominal fat in mammals [13]. However, our findings show that abdominal fat was decreased by A. awamori feeding indicating that A. awamori feeding might contribute to minimising the risk of heart disease in humans.
Muscle weight was increased after A. awamori feeding. This finding might be correlated with the observed changes in lipid metabolism. Yamamoto et al. reported that when diets containing 0.05% or 1% of A. awamori were administered, the breast muscle weight tended to increase in broiler chickens [14]. The growth-promoting effect of A. awamori can be explained by its effect on plasma 3-methylhistidine concentrations, as reported by Kamizono et al. and Saleh et al. [15, 16]. We used the plasma 3-methylhistidine concentration to track changes in muscle protein degradation, as reported by Nagasawa et al. [17]. The plasma 3-methylhisitidine level was decreased by A. awamori feeding, indicating a decreased rate of skeletal muscle protein degradation. The present results are consistent with previous results showing that A. awamori feeding decreases the plasma level of 3-methylhisitidine in broiler chickens [18]. The plasma 3-methylhisitidine level in this study was significantly decreased by A. awamori feeding, indicating a decreased rate of skeletal muscle protein degradation (Table 4).
The concentrations of polyunsaturated fatty acids in the liver were increased by A. awamori feeding (Table 5). This finding is important because polyunsaturated fatty acids play important roles in reducing the incidence of lifestyle-related diseases such as coronary artery disease, hypertension, and diabetes, as well as certain inflammatory diseases such as arthritis and dermatitis, in humans [19]. Several studies have shown that oleic and linoleic acids are the most common unsaturated fatty acids that are produced by Aspergillus and linoleic acid is a major constituent of fungal lipids [20–23]. Aspergillus produces desaturase, which converts saturated fatty acids to unsaturated fatty acids [22]. Srianta reported that Aspergillus terreus produces linolenic acid, but the production of other polyunsaturated fatty acids has not been studied [24]. It is probable that the increases in oleic, linoleic, and linolenic acids in the muscle are a result of the intestinal activities of A. awamori. In the present study, liver unsaturated fatty acid levels were increased, while saturated fatty acid levels were decreased after A. awamori feeding.
Plasma cholesterol, triglycerides, LDL cholesterol, and glucose levels were decreased, while plasma HDL levels were increased after A. awamori feeding (Figure 1). Kim et al. found that A. oryzae at a concentration of 0.1% in the diet significantly lowered serum cholesterol and triglyceride levels in chickens [25]. Saleh et al. [16] and Hajjaj et al. [26] found that the mechanism underlying the cholesterol-lowering effect of Aspergillus could be related to an inhibitor of 3-hydroxyl-3-methylglutaryl-coenzyme (HMG-CoA) reductase. It is well known that an HMG-CoA reductase inhibitor (statin) was extracted from a fungus [27]. Statin is recognised as safe and is widely used to treat patients with hypercholesterolemia [28]. An HMG-CoA reductase inhibitor that is present in A. awamori might be responsible for the decrease in carcass fat deposition. In addition, Aspergillus might affect fat deposition by influencing the activities of hormone-sensitive lipase and malate dehydrogenase enzyme in adipose tissues [29, 30].
Acetyl CoA carboxylase and FAS are thought to play major roles in fatty acid synthesis, and delta 6-fatty acid desaturase (D6DES) plays a key role in the synthesis of polyunsaturated fatty acids (PUFAs) [31]. On the other hand, LDL receptor, which is found in the liver, is essential for producing low-density lipoprotein. In the present experiment, the levels of mRNAs related to fatty acid synthesis, including acetyl CoA carboxylase and delta 6-fatty acid desaturase, were all increased after A. awamori feeding (Figure 2). However, liver LDL receptor mRNA levels were decreased after A. awamori feeding. The present results are consistent with our previous results indicating that A. awamori feeding increases the mRNA levels of FAS, ACC, and delta-6 desaturase in chickens [6]. Sakuradani et al. found that A. oryzae produces delta-6 desaturase, which plays an important role in converting saturated fatty acids to unsaturated fatty acids [32].
5. Conclusions
We conclude that A. awamori modifies the plasma lipid profile and changes the liver fatty acid composition in rats with no harmful effects. Thus, A. awamori feeding might be effective in reducing the risk of lifestyle-related diseases in humans.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. All authors contributed equally in this work.
References
- 1.Isolauri E, Salminen S, Ouwehand AC. Probiotics. Best Practice and Research. 2004;18(2):299–313. doi: 10.1016/j.bpg.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Ljungh A, Wadström T. Lactic acid bacteria as probiotics. Current Issues in Intestinal Microbiology. 2006;7(2):73–89. [PubMed] [Google Scholar]
- 3.Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Current Issues in Molecular Biology. 2008;10(1):37–54. [PubMed] [Google Scholar]
- 4.Bigelis R, Lasure L. Fungal enzymes and primary metabolites used in food processing. In: Beuchat LR, editor. Food and Beverage Mycology. 2nd edition. Westport, Conn, USA: Avi; 1987. pp. 473–516. [Google Scholar]
- 5.Gracia MI, Araníbar MJ, Lázaro R, Medel P, Mateos GG. α-amylase supplementation of broiler diets based on corn. Poultry Science. 2003;82(3):436–442. doi: 10.1093/ps/82.3.436. [DOI] [PubMed] [Google Scholar]
- 6.Saleh AA, Eid Y, Ebaid T, Ohtsuka A, Hayashi K. The modification of the muscle fatty acid profile by dietary supplementation with Aspergillus awamori in broiler chickens. British Journal of Nutrition. 2012;108:1596–1602. doi: 10.1017/S0007114511007069. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, Saleh F, Hayashi K. A fermentation method to dry and convert Shochu distillery by-product to a source of protein and enzymes. Journal of Poultry Science. 2004;(41):275–280. [Google Scholar]
- 8.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi K, Maeda Y, Toyomizu M, Tomita Y. High-performance liquid chromatographic method for the analysis of N tau-methylhistidine in food, chicken excreta, and rat urine. Journal of Nutritional Science and Vitaminology. 1987;33(2):151–156. doi: 10.3177/jnsv.33.151. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima K, Yamazaki M, Abe H. Effects of serum deprivation on expression of proteolytic-related genes in chick myotube cultures. Bioscience, Biotechnology and Biochemistry. 2005;69(3):623–627. doi: 10.1271/bbb.69.623. [DOI] [PubMed] [Google Scholar]
- 11.Amsal A, Takigami M, Ito H. Increased digestibility of raw starches by mutant strains of Aspergillus awamori . Food Science and Technology Research. 1999;5(2):153–155. [Google Scholar]
- 12.Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 13.Iwashita S, Tanida M, Terui N, et al. Direct measurement of renal sympathetic nervous activity in high-fat diet-related hypertensive rats. Life Sciences. 2002;71(5):537–546. doi: 10.1016/s0024-3205(02)01707-1. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Saleh F, Tahir M, Ohtsuka A, Hayashi K. The effect of Koji-feed (fermented distillery by-product) on the growth performance and nutrient metabolizability in broiler. Journal of Poultry Science. 2007;44(3):291–296. [Google Scholar]
- 15.Kamizono T, Nakashima K, Ohtsuka A, Hayashi K. Effects of feeding hexane-extracts of a shochu distillery by-product on skeletal muscle protein degradation in broiler chicken. Bioscience, Biotechnology and Biochemistry. 2010;74(1):92–95. doi: 10.1271/bbb.90587. [DOI] [PubMed] [Google Scholar]
- 16.Saleh AA, Eid YZ, Ebeid TA, Kamizono T, Ohtsuka A, Hayashi K. Effects of feeding Aspergillus awamori and Aspergillus niger on growth performance and meat quality in broiler chickens. Journal of Poultry Science. 2011;48(3):201–206. [Google Scholar]
- 17.Nagasawa T, Hirano J, Yoshizawa F, Nishizawa N. Myofibrillar protein catabolism is rapidly suppressed following protein feeding. Bioscience, Biotechnology and Biochemistry. 1998;62(10):1932–1937. doi: 10.1271/bbb.62.1932. [DOI] [PubMed] [Google Scholar]
- 18.Saleh AA, Eid YZ, Ebeid TA, Ohtsuka A, Yamamoto M, Hayashi K. Feeding Aspergillus awamori reduces skeletal muscle protein breakdown and stimulates growth in broilers. Animal Science Journal. 2012;83(8):594–598. doi: 10.1111/j.1740-0929.2011.00999.x. [DOI] [PubMed] [Google Scholar]
- 19.Simopoulos AP. Human requirement for N-3 polyunsaturated fatty acids. Poultry Science. 2000;79(7):961–970. doi: 10.1093/ps/79.7.961. [DOI] [PubMed] [Google Scholar]
- 20.Mazur P, Nakanishi K, El-Zayat AAE, Champe SP. Structure and synthesis of sporogenic psi factors from Aspergillus nidulans . Journal of the Chemical Society. 1991;20:1486–1487. [Google Scholar]
- 21.Calvo AM, Gardner HW, Keller NP. Genetic Connection between fatty acid metabolism and sporulation in Aspergillus nidulans . Journal of Biological Chemistry. 2001;276(28):25766–25774. doi: 10.1074/jbc.M100732200. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RA, Calvo AM, Chang P-K, Keller NP. Characterization of the Aspergillus parasiticus Δ12desaturase gene: a role for lipid metabolism in the Aspergillus-seed interaction. Microbiology. 2004;150(9):2881–2888. doi: 10.1099/mic.0.27207-0. [DOI] [PubMed] [Google Scholar]
- 23.Tsitsigiannis DI, Zarnowski R, Keller NP. The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulanss . Journal of Biological Chemistry. 2004;279(12):11344–11353. doi: 10.1074/jbc.M310840200. [DOI] [PubMed] [Google Scholar]
- 24.Srianta I, Nugerahani I, Kusumawat N. Production of polyunsaturated fatty acids with Rhizomucor miehei by submerged fermentation. Journal of Agricultural & Food Industrial Organization. 2010;3(02):293–300. [Google Scholar]
- 25.Kim SH, Park SY, Yu DJ, Lee SL, Ryu KS, Lee DG. Effects of feeding Aspergillus oryzae ferments on performance, intestinal microbiota, blood serum components and enviromental factors in broiler. Korean Journal of Poultry Science. 2003;30:151–159. [Google Scholar]
- 26.Hajjaj H, Duboc P, Fay LB, Zbinden I, Macé K, Niederberger P. Aspergillus oryzae produces compounds inhibiting cholesterol biosynthesis downstream of dihydrolanosterol. FEMS Microbiology Letters. 2005;242(1):155–159. doi: 10.1016/j.femsle.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Endo A. Compactin (ML-236B) and related compounds as potential cholesterol-lowering agents that inhibit HMG-CoA reductase. Journal of Medicinal Chemistry. 1985;28(4):401–405. doi: 10.1021/jm00382a001. [DOI] [PubMed] [Google Scholar]
- 28.Serruys PWJC, De Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. Journal of the American Medical Association. 2002;287(24):3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 29.Mersmann HJ. Lipoprotein and hormone-sensitive lipases in porcine adipose tissue. Journal of Animal Science. 1998;76(5):1396–1404. doi: 10.2527/1998.7651396x. [DOI] [PubMed] [Google Scholar]
- 30.Shen T, Wang JY, Zhao BD. Biochemistry. Beijing, China: Higher Education Press; 1991. [Google Scholar]
- 31.García-Maroto F, Garrido-Cárdénas JA, Rodríguez-Ruiz J, et al. Cloning and molecular characterization of the Δ6-desaturase from two Echium plant species: Production of GLA by heterologous expression in yeast and tobacco. Lipids. 2002;37(4):417–426. doi: 10.1007/s1145-002-0910-6. [DOI] [PubMed] [Google Scholar]
- 32.Sakuradani E, Kobayashi M, Shimizu S. Δ6-Fatty acid desaturase from an arachidonic acid-producing Mortierella fungus: gene cloning and its heterologous expression in a fungus, Aspergillus . Gene. 1999;238(2):445–453. doi: 10.1016/s0378-1119(99)00359-5. [DOI] [PubMed] [Google Scholar]


