Abstract
Cocaine dependence is associated with increased stress and drug cue-induced craving and physiological arousal but decreased prefrontal activity to emotional and cognitive challenge. As these changes are associated with relapse risk, we investigated the effects of α2 receptor agonist guanfacine on these processes. Twenty-nine early abstinent treatment-seeking cocaine dependent individuals were randomly assigned to either daily placebo or guanfacine (up to 3 mg) for four weeks. In a laboratory experiment, all patients were exposed to three 10-min guided imagery conditions (stress/stress, drug cue/drug cue, stress/drug cue), one per day, consecutively in a random, counterbalanced order. Subjective craving, anxiety and arousal as well as cardiovascular output were assessed repeatedly. Brain response to stress, drug cue and relaxing imagery was also assessed during a functional magnetic resonance (fMRI) imaging session. In the current study, guanfacine was found to be safe and well-tolerated. Lower basal heart rate and blood pressure was observed in the guanfacine versus placebo group. Guanfacine lowered stress and cue-induced nicotine craving and cue-induced cocaine craving, anxiety and arousal. The guanfacine group also showed increased medial and lateral prefrontal activity following stress and drug cue exposure compared with placebo. Data suggest further exploration of guanfacine is warranted in terms of its potential for reducing stress-induced and cue-induced drug craving and arousal.
Keywords: Cocaine abuse, drug craving, drug cue, fMRI, guanfacine, prefrontal activation, stress
Introduction
Cocaine dependence remains a serious global public health problem and despite the availability of efficacious behavioral treatments for cocaine dependence, no FDA approved medications currently exist, and cocaine relapse rates remain high (Alterman et al.,1996; Kang et al., 1991). When considering pharmacotherapy development for cocaine dependence, it is important to note that a majority of cocaine dependent individuals are also regular smokers (Patkar et al., 2006; Wiseman and McMillan, 1998). Moreover, cigarette smoking is more prevalent in cocaine abusers than alcohol and marijuana (Budney et al., 1993; Sees and Clark, 1991) and may serve to increase craving (Epstein et al., 2010; Reid et al., 1998) and relapse risk (Dackis and O’Brien, 2001; McKay et al., 1999). As a result, efficient relapse prevention strategies could benefit from investigating the effects of novel medications on craving for both concomitant cocaine and nicotine dependence.
Chronic cocaine dependence is associated with allostatic changes in stress and reward pathways that are known to increase drug seeking in laboratory animals (Bossert et al., 2005; Koob, 2009 for review; Shalev et al., 2002). A number of human studies have also shown increases in basal and phasic cardiovascular responses (e.g. heart rate, blood pressure) as well as dysregulated sympathetic and hypothalamic pituitary adrenal (HPA) activity, and sensitized negative mood to be associated with the stress-induced and cue-induced craving state (Back et al., 2005; Fox et al., 2008, 2009; Sinha et al., 1999, 2000, 2003). In support of preclinical research, these stress system alterations in humans, including stress and cue-induced drug craving, have been found to predict relapse outcomes in the laboratory as well as in clinical and real world settings (Paliwal et al., 2008; Shiffman, 2010; Sinha et al., 2006). As such, medications with the potential to target these dysregulated stress arousal and drug craving processes may be efficacious in reducing relapse vulnerability in cocaine dependent individuals.
Consistent with animal and human biophysiological research, neuroimaging studies of cocaine dependent individuals have also highlighted disturbances in corticostriatal-limbic regions involved in stress and reward processing. For example, one of our previous studies indicated that cocaine abusers show decreased medial pre-frontal and anterior cingulate activity during exposure to stress-related imagery (Sinha et al., 2005). Hypo-responsive activity in these brain systems during stress is clinically important for several reasons. First, these regions are integral to cognitive and emotional control (Garavan and Hester, 2007; Li et al., 2009b; Roberts et al., 1998), known to underlie impulsivity and a range of goal-oriented and organizational behaviors (Potenza et al., 2003; Witkiewitz and Marlatt, 2005) associated with addictive behaviors (Goldstein et al., 2009; Sinha and Li, 2007). Second, goal-oriented behaviors often break down under stressful or emotionally demanding circumstances, possibly due to the conflict of different regulatory goals (Baumeister et al., 1994; Fox et al., 2007; Kuhl and Koole, 2004). Empirical evidence for the role of corticostriatal activity in terms of addiction maintenance is further exemplified by the fact that increased activation of the ventromedial prefrontal cortex during a cognitive control-related Stroop task performance has been shown to correlate positively with longer durations of abstinence in cocaine dependent individuals (Brewer et al., 2008).
In view of this prior research, systematically assessing the efficacy of a medication which is potentially able to both reduce stress system arousal and strengthen pre-frontal mechanisms underlying control-related behaviors may be imperative for improving treatment outcome. Guanfacine decreases norepinephrine (NE) centrally by mimicking NE and selectively stimulating α2A-adrenoceptors in the prefrontal cortex (Arnsten and Goldman-Rakic, 1985; Arnsten and Plizska, 2011; Cai et al., 1993). Stimulation of these receptors causes a decrease the presynaptic output of sympathetic neurotransmitters, effectively down-regulating sympatho-mimetic outflow from the vasomotor center of the brain to the heart and blood vessels (Scahill, 2009). This, in turn, results in a decrease in peripheral vascular resistance and blood pressure.
Preclinical studies show that α2-adrenegic agonists like guan-facine reduce stress-related drug seeking in laboratory animals with experience of cocaine (Erb et al., 1998, 2000; Highfield et al., 2001) alcohol (Lê et al., 2005) and nicotine (Zislis et al., 2007). Guanfacine has also been shown to improve inhibitory control-related processes in non-human primates and humans with attention deficit hyperactivity disorder (ADHD) and tic disorders (Arnsten and Li, 2005; Chappell et al., 1995; Hunt et al., 1995; Jäkälä et al., 1999; Ma et al., 2005; Scahill et al., 2001; Swartz et al., 2008; Taylor and Russo, 2001). Similarly, we have previously shown that another α2-adrenergic agonist, lofexidine, decreases stress-induced and cue-induced opiate craving and associated arousal in opioid dependent individuals treated with naltrexone (Sinha et al., 2007).
We aimed to conduct a pilot study assessing the effects of guanfacine versus placebo in cocaine dependent individuals. Our objectives were to assess whether guanfacine decreases stress-induced and cue-induced anxiety, arousal, negative emotion and drug craving along with changes in cardiovascular measures in a laboratory setting; also whether guanfacine increases prefrontal brain activation during stress and cue exposure using functional magnetic resonance imaging (fMRI). As all of the cocaine abusers in the current study smoke both crack cocaine and cigarettes some additional overlap in terms of cue response is expected. In support of this, behavioral routines commonly associated with cigarette smoking have also been shown to serve as cues that trigger cocaine craving in crack abusers (Sees and Clark, 1993). As such, we also assessed nicotine craving within the laboratory. We hypothesize that guanfacine-treated patients will show decreased peripheral autonomic function alongside reduced stress and cue-related cocaine and nicotine craving and negative emotion. We also hypothesize that guanfacine will increase prefrontal activation during stress and drug cue exposure relative to placebo in the fMRI.
Methods and materials
Participants
Twenty-nine treatment-seeking cocaine dependent individuals who responded to local advertisements placed in newspapers, online and in community centers around the New Haven area participated in the current study. Current cocaine dependence was determined by using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM IV – First et al., 1995) as well as positive urine toxicology screens collected upon entry into inpatient treatment. Exclusion criteria included current (past month) DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) dependence for any drug other than cocaine, alcohol or nicotine. Participants meeting any current (past month) DSM-IV criteria for psychiatric disorders or using prescribed medications for any psychiatric or health issues were also excluded. In addition, all individuals underwent stringent medical assessments including electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic and thyroid function. For the laboratory component, 17 were randomized to guanfacine and 12 to placebo. Of these 29 participants, 15 (six guanfacine, nine placebo) were eligible and volunteered for the fMRI session. Separate written and verbal consent was obtained from all participants for the laboratory and fMRI component of the study and the procedures were approved by the Human Investigation Committee (HIC) of the Yale University School of Medicine.
Design
All cocaine dependent patients were randomly assigned to guanfacine (up to 3 mg daily) or placebo in a randomized, double blind manner.
In the laboratory component of the study, participants were exposed to three 10-minute personalized guided imagery conditions (stress/stress, drug cue/drug cue, combined stress/drug-cue) across three consecutive days, one imagery condition per day, in a randomized and counterbalanced order. Medication group (placebo versus guanfacine) was the between factor and imagery condition (stress/stress, drug cue/drug cue, combined stress/drug cue) and time-points (repeated assessments over time in the laboratory component) were the within factors.
In the fMRI component of the study, participants were exposed to brief (2-min) stress, drug cue and neutral/relaxing guided imagery conditions (two of each) in randomized blocks during one single scanning session. Medication group (placebo versus guanfacine) was the between factor and imagery condition (stress, drug cue, neutral/relaxing) was the within factor.
Guanfacine dosing schedule
Guanfacine pills (marketed by Watson Pharmaceuticals) were purchased through the pharmacy located at the Connecticut Mental Health Center (CMHC) and the research pharmacist ensured that both active and placebo capsules used for medication administration appeared identical. All placebo pills contained lactose. Participants were randomly assigned to either guanfacine (2 or 3 mg) or placebo. As guanfacine has not been previously used in addicted populations, we started the study with placebo versus 2 mg dosing in a double blind manner. Guanfacine has typically been administered in a dose range of 2.0–4.0 mg/daily for the treatment of attention deficit hyperactivity disorder (ADHD) and autism in children with few side effects, and greater efficacy observed in the higher 3–4 mg dose range (Biederman et al., 2008; Chappell et al., 1995; Handen et al., 2008; Scahill et al., 2001). However, as many of these studies had used the slow-release preparation, it was not clear how the higher doses would be tolerated using the generic preparation. Therefore, we started the study using only the 2 mg dose for the active group. After the first 14 participants were studied and minimal complaints of side effects were reported, and particularly minor blood pressure changes (assessed in a blinded manner across participants), we increased the guanfacine dosing to 3 mg for subsequent participants, who were randomized to the active condition. Five days after inpatient admission all participants were initiated on a 14-day dose escalation schedule, similar to that used in previous human studies in children with ADHD (Biederman et al., 2008; Chappell et al., 1995; Handen et al., 2008; Scahill et al., 2001). All laboratory sessions were conducted in week 4, approximately 25 days after admission. The fMRI sessions were then conducted on the following day. After the laboratory and fMRI sessions, participants underwent a standard four-day taper (Strang et al., 1999). Randomization procedures were conducted by the CMHC research pharmacist, experienced in Urn randomization procedures (Stout et al., 1994).
Safety
Under the current dosing schedule guanfacine was shown to be well tolerated, with mild levels of reported side effects. Analysis of side effects data indicated that 4/17 (24%) of the guanfacine group and 1/12 (8%) of the placebo group reported mild tiredness and fatigue. Mild to moderate headaches were reported by 3/17 (18%) of the guanfacine group and severe headaches by 1/12 (8%) of the placebo group. In addition, 1/17 (6%) of the guanfacine group reported a mild sensation of numbness and 1/17 (6%) reported a mild feeling of ‘heart racing’. In the placebo group 1/12 (8%) also reported severe body aches, upset stomach and fever. All symptoms dissipated over time without further intervention and Fisher’s exact test analysis indicated no significant group differences regarding the frequency of patients reporting each of these symptoms.
General procedures
All laboratory procedures (see Figure 1a) were conducted at the Clinical Neuroscience Research Unit (CNRU) of the CMHC. Cocaine dependent patients were admitted to the CNRU for four weeks of inpatient treatment and study participation. The CNRU is a locked inpatient treatment research facility with no access to alcohol or drugs and very limited access to visitors. Drug testing was conducted regularly to ensure drug abstinence. As participants were treatment seeking, they participated in four weeks of group drug counseling treatment for cocaine addiction using the standard drug counseling manual as a guide (Mercer et al., 1994) during their inpatient stay. During the first week of inpatient stay, participants were administered structured baseline assessments measuring psychiatric and substance use history. They started on study medication on day 5, and in the second week, scripts for the guided imagery induction for the laboratory and the fMRI session were developed as described in previous studies (Sinha, 2009). The three laboratory sessions were conducted in week 4 and the fMRI session was conducted on the day following the laboratory sessions. The clinician responsible for devising the imagery scripts was also accountable for randomizing and counterbalancing presentation order as well as recording the scripts. The order of scripts was not provided to the research staff conducting both the laboratory and fMRI sessions thereby keeping them blind to the order and content of all scripts. Participants also remained blind until imagery presentation.
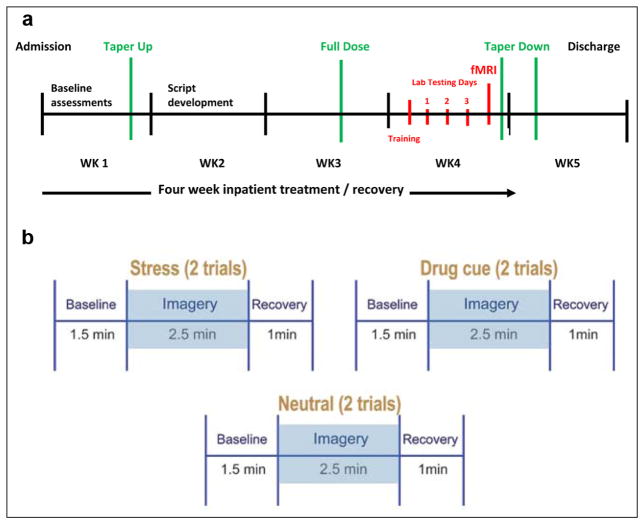
Figure 1.
(a) Time-line of study procedures. Lab testing days (1 to 3): one imagery condition (stress, cue, combined stress/cue) presented per day in a randomized and counterbalanced order across participants. (b) fMRI imagery presentation: comprising six five-minute trials. Six imagery trials per condition (stress – two, cue – two, neutral – two) presented across one testing session in a quasi-randomized order.
Imagery script development procedures
Imagery script development procedures are based on methods developed by Lang and his colleagues (Lang et al., 1980, 1983; Miller et al., 1987), and further adapted in our previous studies (Sinha et al. 1992, 1999, 2000; Sinha and Parsons, 1996). For the laboratory component of this study, participants provided two stress scenarios for the stress/stress condition, two drug cue scenarios for the drug cue/drug cue condition and a stress and drug cue scenario for the combined stress/drug cue condition. We included a combined stress and drug cue exposure condition, in addition to stress alone and drug cue alone, as both cues often occur in combination in the real-world setting. For the fMRI component of the study, all participants provided an additional two stress, two drug cue and two neutral/relaxing scenarios. Briefly, the stress scripts were based on participants’ descriptions of recent personal stressful events that were experienced as ‘most stressful’. ‘Most stressful’ was determined by having the participants rate their perceived stress on a 10-point Likert scale where 1 = not at all stressful and 10 = the most stress they felt recently in their life and only situations rated as 8 or above were accepted as appropriate for script development. Examples of stress scripts included breakup with a significant other and marital conflict situations. The drug-related cues scripts were developed by having participants identify recent situations that included drug-related stimuli and resulted in subsequent cocaine use (e.g. being at a bar, watching others smoke crack and drink alcohol). The stress and drug cue combined script elicited situations where a stressful scenario was followed by a drug-related event. Examples of such combined stress/drug cue situations are a stress event such as marital conflict scenario followed by a situation involving people, places or things relating to and leading to drug use. The neutral scripts for the fMRI component of the study comprised a relaxing script developed from the participants’ description of a personal event such as being at the beach, or a fall afternoon reading at the park, without the inclusion of drug-related stimuli.
Once the situations were elicited using Scene Construction Questionnaires, three 10-minute scripts were developed for the three laboratory sessions. Each 10-minute script consisted of two five-minute scenarios: 1) stress (5 min)/stress (5 min), 2) drug cue (5 min)/drug cue (5 min), 3) stress (5 min)/drug cue (5 min). In addition, six two-minute scripts were also developed for the fMRI session: 1) two stress scripts (2 min in length), 2) two drug cue scripts (2 min in length), 3) two neutral/relaxing scripts (2 min in length). All scripts incorporated a standardized style and format including specific stimulus and response information as described in our previous studies (see Bergquist et al., 2010 and Sinha, 2009 for detailed description of procedures). Each script was then audio-taped and presented in the laboratory and the fMRI sessions. In the laboratory sessions the three scripts (stress, cue, combined stress/cue) were presented one imagery per day, across consecutive days in a randomized and counterbalanced order. In the fMRI session, the six scripts (stress – two, cue – two, neutral – two) were presented in a quasi-randomized manner across one testing session. It was ensured that trials of the same imagery conditions were not presented consecutively.
In addition to the script development, on the day prior to the laboratory sessions, participants were brought into the testing room in order to acclimatize them to specific aspects of the study procedures including the subjective rating forms and training in relaxation and imagery procedures, as previously described by Sinha et al. (2003) and Sinha (2009).
Procedures for laboratory sessions (conducted across three consecutive days)
On each testing day, participants abstained from breakfast in order to account for dietary influences, and were brought into the testing room at 07.45. All participants were allowed an initial smoke break at 07.30 in order to reduce craving. After settling the subject in a sitting position on a hospital bed, a blood pressure cuff was placed on their preferred arm to monitor blood pressure and a pulse sensor was placed on the subject’s forefinger to obtain a measure of pulse. Self-reports of craving, anxiety and mood were completed immediately after set-up. This was followed by a 45-minute adaptation period during which the participants were instructed to practice relaxation. At 09.00 participants were provided with headphones and given the following instructions for the imagery procedure:
Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation.
During imagery presentation, heart rate was monitored continuously, and blood pressure every two minutes using a continuous monitoring Dinamap (GE Medical Systems, Tampa, FL, USA). Craving, anxiety, arousal and negative emotion ratings, as well as heart rate and blood pressure, were collected prior to, immediately following imagery and then periodically every 15 minutes after the imagery period up to 60 minutes (+15, +30, +45, +60). At the end of each session, relaxation instructions were provided and the session was terminated at 10.30. All laboratory procedures were identical across the three consecutive days with the exception of the imagery condition presented (i.e. stress, cue, combined stress/cue).
Laboratory assessments
Subjective measures
Drug craving scales
Two separate 10-point visual analog scales (VASs) were used to assess desire for cocaine and a cigarette: Please rate the intensity of your desire to use cocaine/nicotine at the moment, in which 1 = ‘not at all’ and 10 = ‘extremely high’.
Anxiety
This was also measured using a similar 10-point VAS anchored as above.
Differential Emotion Scale
The Differential Emotion Scale (DES) (Izard, 1972) comprises 30 specific emotional adjectives (or items) and participants are required to rate on a five-point scale the extent to which each word describes the way s/he feels at the current time. The subscales of anger and sadness were collapsed to form a general Negative Emotions scale including items such as downhearted, upset, distressed, annoyed, irritated and mad. The arousal subscale was also analyzed and comprised items such as tense, nervous, jittery, aroused, excited and anxious.
Cardiovascular measures
Critikon Dinamap 120 Patient Monitor (GE Medical Systems, Tampa, FL, USA) was used to assess blood pressure and pulse rate in a continuous manner prior to, immediately following imagery and then periodically every 15 minutes after the imagery period up to 60 minutes (+15, +30, +45, +60).
Statistical analyses for laboratory component
A series of Linear Mixed Effect (LME) models (Laird and Ware, 1982) were implemented to analyze the data for each dependent variable, both at baseline and following imagery exposure, using SPSS software (version 17). Within factors of condition 3 (stress/stress drug cue/drug cue, combined stress/drug-cue), time-point 6 (varying levels) and the between factors of medication group 2 (guanfacine/placebo) were the fixed effects. Participants represented the random effect. In order to account for baseline variability across each testing day, change from baseline was used for all measures in order to assess response to the imagery exposure. A series of T-tests and chi-square analyses were used in order to compare the treatment and placebo medication groups on demographic and drug use variables. As gender has been shown to moderate both the subjective and bio-physiological aspects of stress response (Fox et al., 2006, 2009) we co-varied for gender in all analyses.
The Sidak test for multiple comparisons was used to analyze simple effects. A series of T-tests and chi-square analyses were used in order to compare the treatment and placebo medication groups on demographic and drug use variables.
Procedures for fMRI component
fMRI acquisition
MRI images were collected using a 3-T Siemens Trio MRI system equipped with a single-channel standard quadrature head coil, using T2*-sensitive gradient-recalled single shot echo planar pulse sequence. Anatomical images were obtained with spin echo imaging in the axial plane parallel to the AC-PC line with TR = 300 ms, echo time (TE) = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle (FA) = 60°, field of view = 220 mm × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm with no gap. Subsequently functional images were acquired with a single-shot gradient echo planar imaging sequence; 32 axial slices parallel to the AC-PC line covering the whole brain were obtained with TR = 2000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, FA = 85°, field of view = 220 mm × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4 mm and no gap, 190 measurements. Then, a high resolution 3D Magnetization Prepared Rapid Gradient Echo sequence was utilized to acquire sagittal images for multi-subject registration (TR = 2530 ms; TE =3.34 ms; bandwidth = 180 Hz/pixel; FA = 7°; slice thickness = 1 mm; field of view = 256 mm × 256 mm; matrix = 256 × 256).
fMRI stimulus presentation
Scripts for the stress, drug cue and neutral/relaxing imagery were presented as separate trials in a single fMRI scanning session. Six 5-min trials were acquired, with each trial comprising a 1.5-min quiet baseline period followed by a 2.5-min imagery period (2 min of read-imagery) and a 1-min quiet recovery period as in our previous studies (Jastreboff et al., 2010; Li et al., 2005; Seo et al., 2010; Sinha et al., 2005). During the baseline, participants were instructed to stay still without engaging in any mental activity. During the recovery period, participants were asked to stop imagining and lie still in the scanner. Each script was presented only once and scripts from the same condition were not presented consecutively. Between fMRI blocks, all participants participated in progressive relaxation for 2 min in order to normalize any residual anxiety or craving from previous trials (see Figure 1b).
fMRI analysis
All imaging data were converted from Digital Imaging and Communication in Medicine (DICOM) format to analyze format using X MedCon (Nolfe, 2003). During the conversion process, the first 10 images at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between radio frequency pulsing and relaxation, leaving 180 measurements for analysis. fMRI data were preprocessed with slice time correction using a custom routine in MATLAB (The Math-Works, 2000, Natick, MA) and then motion corrected using Statistical Parametric Mapping (SPM 5) (Wellcome Department of Cognitive Neurology, 2005). Images were motion corrected for three translational and three rotational directions (Friston et al., 1996) and any trials with linear motion in excess of 1.5 mm or rotation greater than 2° were removed. Following preprocessing, General Linear Model (GLM) was used to analyze individual subject data on each voxel in the entire brain volume with a regressor (time during imagery, 2.5 min) for each block per condition relative to the baseline (1.5 min) using Bioimagesuite (www.bioimagesuite.org) (Duncan et al., 2004). Each block was modeled using a boxcar function convolved with a canonical hemodynamic response function. Drift correction was included in the GLM model in the commonly used manner, in which drift regressors were used to remove the mean time course, linear trend, quadratic trend and cubic trend for each trial. The recovery period (1 min) was excluded from the data analysis due to potential carryover effects from the imagery period. Functional images for each condition were spatially smoothed with a 6 mm Gaussian kernel and the output maps were normalized beta-maps in the acquired space (3.44 mm × 3.44 mm × 4 mm).
To adjust for individual anatomical differences, three registrations were applied sequentially using the Yale BioImage Suite software package (Duncan et al., 2004). First, a linear registration between the individual subject raw functional image and that subject’s 2D anatomical image was performed. Second, the 2D anatomical image was linearly registered to the individual’s 3D anatomical image (1 mm × 1 mm × 1 mm resolution). Finally, a non-linear registration was performed between the individual 3D anatomical image and a reference 3D image, which is the Colin27 Brain (Holmes et al., 1998) in Montreal Neurological Institute (MNI) space (Evans et al., 1993). Then the output maps from the individual-subject level were converted to Analysis of Functional Neuro Images (AFNI) format and submitted to a group-level analysis.
Statistical analyses for fMRI component
For group analysis, a voxel-wise LME model was implemented for the whole brain with the 3dLME program within AFNI software (Cox, 1996), including two factors, group and condition (neutral, drug cue, stress). Two tailed t test was used to further investigate main effects and interactions. Then, the Family Wise Error rate (FWE) correction for multiple comparisons was utilized using Monte Carlo simulations (Xiong et al., 1995) conducted in AlphaSim in AFNI (Cox, 1996) and a threshold of p < 0.05 (FWE corrected, two-tailed) was set for all analyses.
Results
Participants
There were no significant differences between the placebo and guanfacine medication groups with regards to gender, race, age, years spent in education, recent prior cocaine, alcohol and nicotine use as well as other clinical characteristics (see Table 1). There were also no statistical differences in the baseline nicotine craving, cocaine craving, anxiety and arousal measures among the guanfacine and placebo groups in the laboratory sessions and also no differences in craving and anxiety ratings at baseline in those who participated in the fMRI session.
Table 1.
Participant demographic and clinical characteristics: means (standard deviations)
| n = 29 | Laboratory study
|
fMRI study
|
||
|---|---|---|---|---|
| Placebo n = 12 | Guanfacine n = 17 | Placebo n = 9 | Guanfacine n = 6 | |
| Gender – male | 7 (58%) | 12 (71%) | 5 (55.6%) | 3 (50.0%) |
| Race | ||||
| African American | 9 (75%) | 11 (64.7%) | 6 (66.7%) | 5 (83.3%) |
| Caucasian | 0 | 4 (23.5%) | 0 | 1 (16.7%) |
| Hispanic | 3 (25%) | 2 (11.8%) | 3 (33.33%) | 0 |
| Age | 38.6 ± 9.6 | 40.9 ± 6.7 | 35.78 ± 9.19 | 42.83 ± 3.6 |
| Years in education | 12.5 ± 0.9 | 11.6 ± 1.3 | 12.44 ± 1.59 | 11.83 ± 0.75 |
| Smoking status – no. regular smokers | 12 (100%) | 15 (88%) | 9 (100%) | 6 (100%) |
| Years of cocaine use | 11.16 ± 8.05 | 14.18 ± 7.13 | 9.89 ± 8.70 | 14.0 ± 6.42 |
| No. of days cocaine used in past month | 18.17 ± 11.42 | 19.94 ± 10.6 | 17.78 ± 12.51 | 23.0 ± 9.19 |
| No. of grams per month | 48.23 ± 80.18 | 35.0 ± 38.79 | 58.74 ± 91.14 | 31.73 ± 28.67 |
| No. meeting criteria for alcohol dependence | 5 (41.6%) | 7 (41.2%) | 3 (33%) | 3 (50%) |
| Years of alcohol use | 15.42 ± 9.87 | 16.94 ± 11.07 | 11.44 ± 7.25 | 16.67 ± 11.98 |
| No. of days used in past month | 14.25 ± 11.77 | 13.82 ± 11.09 | 15.22 ± 13.41 | 15.0 ± 12.36 |
| No. of drinks per month | 217.71 ± 311.70 | 316.8 ± 409.42 | 257.38 ± 361.09 | 132.0 ± 141.72 |
| Lifetime depression | 3 (27%) | 2 (11.8%) | 3 (37.5%) | 1 (16.67%) |
| Lifetime anxiety (incl. PTSD) | 4 (33.3%) | 4 (23.5%) | 4 (44.44%) | 3 (50%) |
| Lifetime anxiety (without PTSD) | 2 (16.7%) | 0 | 2 (22.22%) | 0 |
Note: All group comparisons are insignificant at p < 0.05 level.
Results for laboratory sessions
Medication group effects at baseline
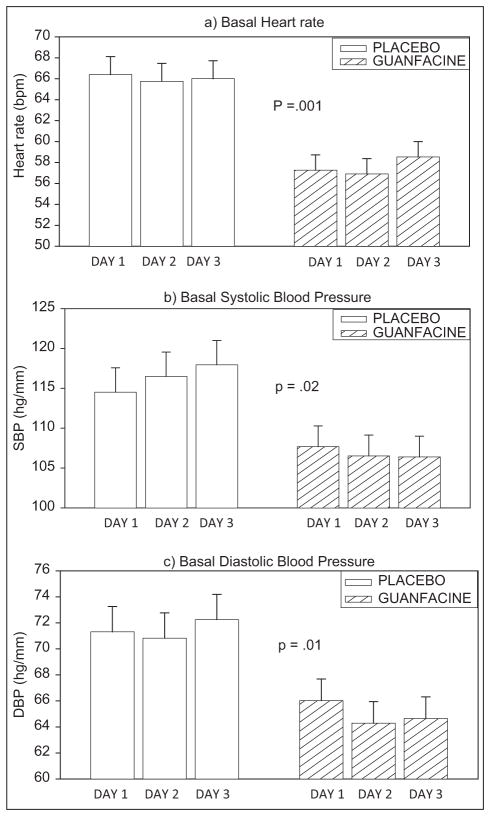
The guanfacine group demonstrated lower levels of basal heart rate [F (1, 23) = 16.2; p = 0.001], basal SBP [F (1, 23) = 6.2; p = 0.02] and basal DBP [F (1, 23) = 7.4; p = 0.01] across all three testing days compared with the placebo group (see Figure 2a–c). No other baseline differences were observed.
Figure 2.
Mean and standard error (SE) for basal heart rate and systolic and diastolic blood pressure across the three laboratory testing days in placebo versus guanfacine treated cocaine dependent individuals.
Medication effects in response to imagery
Cocaine craving
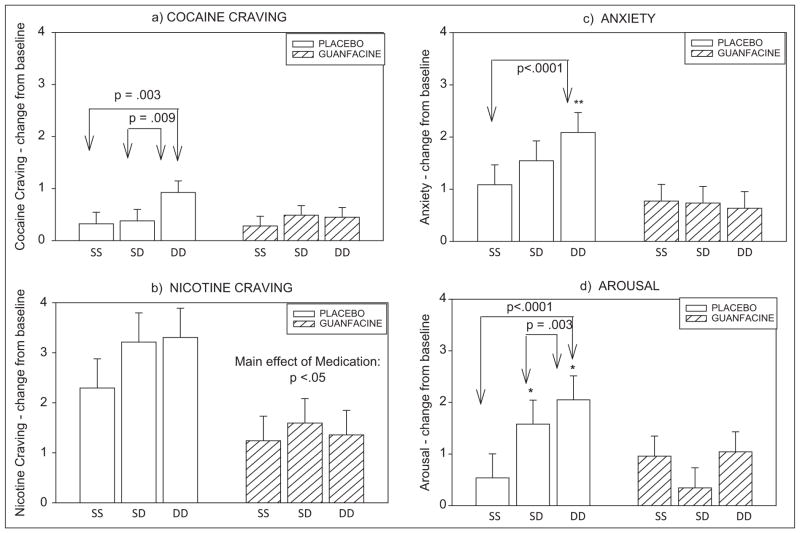
A main effect of condition was observed [F (2, 458) = 5.4; p = 0.005] where all participants reported significantly greater craving ratings in the cue/cue condition compared with the stress/stress condition (p = 0.004). A significant medication group X condition interaction [F (2, 458) = 3.2; p = 0.04] further indicated that the typical increases in cue-related craving compared with stress-related craving (Chaplin et al., 2008; Fox et al., 2007, 2008, 2009; Sinha et al., 2003, 2007, 2009) were observed in the placebo, but not the guanfacine group. Craving ratings during cue exposure were higher than those during stress (p = 0.003) and combined stress/cue (p = 0.009) exposure in the placebo group only (Figure 3a).
Figure 3.
Change from baseline for subjective measures in response to imagery in placebo versus guanfacine treated cocaine dependent individuals. (As no significant time-point interactions were observed, bars show data averaged across time-points; means and standard errors shown). (a) Cocaine craving; (b) nicotine craving; (c) anxiety; (d) arousal.
SS: stress/stress condition, SD: stress/drug cue condition, DD: drug cue/drug cue condition. Group differences: *p ≤ 0.05, **p < 0.01
Nicotine craving
A main effect of condition was observed [F (2, 458) = 6.2; p = 0.002] indicating that higher nicotine craving was reported following cue (p = 0.01) and combined stress/cue (p = 0.004) compared with stress imagery. A significant main effect of medication group [F (1, 26) = 4.4; p < 0.05] also indicated that higher nicotine craving was reported in the placebo compared with the guanfacine group following exposure to all three imagery conditions (Figure 3b).
Anxiety
A main effect of condition was observed [F (2, 458) = 3.6; p < 0.03], showing higher ratings following exposure to cue compared with stress (p = 0.02) across all participants. A significant medication group X condition interaction [F (2, 458) = 6.2; p = 0.002] was observed, resulting from significantly higher anxiety in response to cue relative to stress imagery (p < 0.0001) in the placebo group. This elevation in cue-related anxiety was not observed in the guanfacine-treated group. Furthermore, guanfacine-treated patients reported significantly lower anxiety following exposure to cue-related imagery compared with the placebo group (p = 0.006; Figure 3c).
Arousal
A main effect of condition was observed [F (2, 458) = 8.0; p < 0.0001], showing higher ratings following exposure to cue compared with stress (p < 0.0001) and combined stress and cue (p < 0.02) in all participants. A significant medication group X condition interaction [F (2, 458) = 9.5; p < 0.0001] was also observed, indicating significantly lower ratings of arousal following cue (p = 0.05) and combined stress/cue imagery (p < 0.05) in the guanfacine group compared with the placebo group. In addition, significantly higher ratings of arousal were observed following exposure to cue compared with the stress (p < 0.0001) and combined stress/cue imagery (p = 0.003) in the placebo group only. This increase in cue-related arousal was not reported in the guanfacine group (Figure 3d).
Negative emotion
A condition effect of imagery approached statistical significance [F (2, 458) = 2.8; p = 0.06], indicating that all participants demonstrated a trend for reporting higher negative emotion following stress and combine stress/cue compared with the cue imagery condition. No group effects of medication or significant medication X condition or time-point interactions were observed.
Heart rate and blood pressure
No significant main effects or interactions were observed for cardiovascular measures following exposure to the imagery conditions.
Exploratory analyses
Guanfacine dose
Current findings indicated that chronic guanfacine dosing (combined across 2 or 3 mg) was efficacious in reducing drug cue-related cocaine craving, but had no effect on these processes following stress exposure. We therefore conducted an exploratory analysis to ascertain whether these findings were potentially related to dose, and assess whether the higher guanfacine dose (3 mg daily) might be required to reduce craving and arousal following exposure to stress. Using mixed analysis of variance (ANOVA), we found significant main effects of dose (placebo, guanfacine – 2 mg and 3 mg) for cocaine craving (p = 0.05), and nicotine craving (p = 0.004) across all time-points. For both of these drug craving measures, lower levels of craving were observed in the guanfacine 3 mg group compared with either the placebo and/or the guanfacine 2 mg group.
Alcohol dependence
As a sub-sample of the current cocaine dependent participants also met criteria for current alcohol dependence (42% placebo and 41% guanfacine) secondary analyses was conducted using alcohol dependence as a fixed factor (dependent versus non-dependent) in all LME models. No significant medication group X alcohol dependence interactions were observed for any of the dependent variables, and all medication effects remained unchanged.
Results for fMRI component
BOLD activation in guanfacine and placebo groups
The results from voxel-based analysis using AFNI revealed significant group differences in brain activation during both drug cue and the stress conditions (p < 0.05 (two-tailed), whole-brain FWE corrected; see Table 2 and Figure 4).
Table 2.
Brain regions showing significant activation in the guanfacine group relative to placebo during drug cue and stress imagery conditions (p < 0.05, two-tailed, FWE corrected)
| Lat. regions of activation | BA | Coordinates
|
Volume (mm3) | t | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Drug cue | Dorsolateral/ventromedial PFC | ||||||
| L middle/inferior frontal gyrus | 9, 44, 45, 46 | −23 | 54 | 9 | 9388 | 2.35 | |
| medial/orbitofrontal gyrus | 10, 11 | ||||||
| Lateral PFC/premotor cortex | |||||||
| L middle/inferior frontal gyrus | 6, 44, 45 | −41 | −2 | 27 | 7737 | 2.37 | |
| pre-/post-central gyrus | 3, 6 | ||||||
| Limbic-temporal regions | |||||||
| L amygdala, hypothalamus superior/middle TG | 21, 28, 34, 38 | −36 | 4 | −28 | 5703 | 2.52 | |
| R amygdala, hippocampus | 20, 21, 22, 28 | 37 | −3 | −21 | 18,807 | 2.41 | |
| superior/middle/inferior TG | 38 | ||||||
| Cerebellum/inferior occipital gyrus B | 18 | −28 | 73 | −36 | 13,546 | 2.34 | |
| Stress | Ventrolateral PFC/insula | ||||||
| L inferior frontal gyrus | 44, 45, 47 | −43 | −4 | 8 | 11,817 | 2.4 | |
| insula, putamen, superior TG | 13, 22, 41 | ||||||
| R inferior frontal gyrus | 44, 45, 47 | 48 | 6 | 4 | 9696 | 2.36 | |
| insula, putamen, superior TG | 13, 22, 38 | ||||||
| precentral gyrus | 6 | ||||||
| Premotor cortex | |||||||
| L superior/middle FG, precentral gyrus | 6 | −25 | −10 | 52 | 11,092 | 2.61 | |
| Medial PFC/cingulate gyrus | |||||||
| B superior medial frontal gyrus | 6, 8, 9 | 11 | −7 | 45 | 30,079 | 2.42 | |
| anterior/middle cingulated gyrus | 23, 24, 31, 32 | ||||||
Significant activation at p < 0.05 (two-tailed, whole-brain FWE corrected). No significant differences between groups were observed in neutral/relaxing trials.
Lat.: laterality, B: bilaterality, L: left, R: right, BA: Brodmann’s area, PFC: prefrontal cortex, TG: temporal gyrus. MNI coordinates were used.
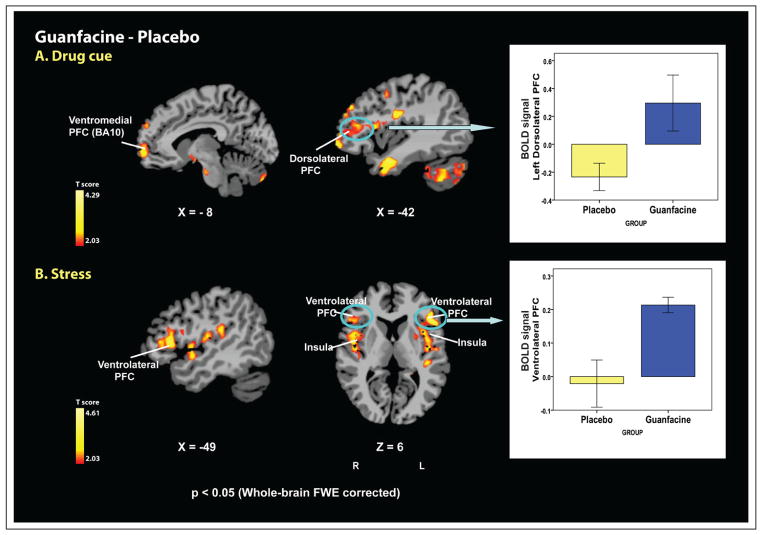
Figure 4.
Whole brain voxel-based fMRI images showing differential activations between guanfacine versus placebo groups during drug cue and stress exposure (p < 0.05 [two-tailed], whole-brain FWE corrected). (A) Drug cue: during exposure to drug cue, the guanfacine group displayed greater activity in the left dorsolateral PFC, ventromedial PFC, lateral PFC, premotor cortex, temporal lobe, inferior occipital lobe and cerebellum compared with the placebo group. The bar graph highlights the significant group difference in brain activation in the left dorsolateral PFC, a region typically associated with working memory (means and standard error shown). (B) Stress: during exposure to stress, the guanfacine group showed increased activity in the ventrolateral PFC, dorsomedial PFC, anterior/middle cingulate gyrus, insula, putamen, superior temporal lobe and premotor cortex compared with the placebo group. The bar graph highlights the significant group difference in brain activation in the ventrolateral PFC, also associated with working memory (means and standard error shown). For the neutral condition, no differences between the two groups were found with corrections for multiple comparisons at p < 0.05. Coordinates are given in MNI space.
During the drug cue condition, the guanfacine group showed significantly greater activation in five regional clusters relative to the placebo group: the left dorsolateral prefrontal cortex (DLPFC)/ventromedial prefrontal cortex (involving the left DLPFC, ventro-medial prefrontal and orbitofrontal cortex [OFC]); a cluster including the left lateral prefrontal cortex and premotor cortex; bilateral limbic-temporal clusters (amygdala, hippocampus, hypothalamus, superior/middle/inferior temporal lobe); a cerebellar-inferior occipital cluster. During stress exposure, the guanfacine group displayed greater activation in four regional clusters compared with the placebo group; bilateral ventrolateral prefrontal cortex (VLPFC)/insula clusters (VLPFC, insula, putamen, superior temporal gyrus, right precentral gyrus); a left premotor cortex cluster (BA 6); a medial prefrontal cortex/cingulate cluster (dorso-medial prefrontal cortex, anterior/middle cingulate gyrus). For the neutral condition, no differences between two groups were found with corrections for multiple comparisons at p < 0.05. No brain regions were more active in the placebo group than in the guanfacine group at p < 0.05 with correction for multiple comparisons.
Discussion
In this initial laboratory study we investigated the effects of chronic guanfacine dosing versus placebo on basal and stress-induced and drug cue-induced craving, anxiety and negative emotion as well as subjective and physiological arousal in early abstinent cocaine dependent smokers. Guanfacine treatment relative to placebo decreased basal heart rate and blood pressure, showing lower autonomic reactivity. Guanfacine also significantly decreased stress- and cue-induced nicotine craving, as well as cue-related cocaine craving, anxiety and arousal. In a second fMRI study using a sub-sample from the laboratory study, we examined the functional neural systems associated with response to stress and cue in guanfacine versus placebo groups. Findings showed that guanfacine treatment increased activation in specific regulatory regions of the medial and lateral prefrontal cortex in response to stress and drug cue exposure, as well as increasing insula activation during stress imagery exposure compared with placebo. Both studies combined demonstrate a preliminary, but unique, profile of guanfacine’s ability to selectively reduce craving, anxiety and arousal in peripheral and central pathways involved in subjective, physiological and self-control processes.
Although guanfacine did not impact phasic heart rate and blood pressure response, its efficacy in lowering autonomic tone may itself represent a key mechanism underlying the changes in subjective response to stress and cue. For example, up-regulated heart rate and blood pressure are often characteristic of the non-specific physiological adaptations observed in chronic cocaine dependence, especially during early abstinence (Fox and Sinha, 2009; Kampman et al., 2001, 2006). Importantly, in addition to increasing oxygen demand and chances of muscular and endothelial damage (Sáez et al., 2011; Schindler, 1996), increased autonomic arousal and cardiovascular output is also associated with risk-related behaviors, including persistent cocaine and alcohol craving (Fox et al., 2008; Robbins et al., 1997; Sinha et al., 2003, 2009). It is also postulated that high tonic autonomic arousal per se, may act as a cue or trigger for cocaine craving and increased anxiety during early abstinence (Kampman et al., 2006). As such, the guanfacine-related decrease in peripheral physiological arousal observed in this study may contribute to decreasing subjective anxiety, arousal and craving response to provocation.
While guanfacine demonstrated efficacy in decreasing nicotine craving across stress, cue and combined stress/cue imagery conditions, its effects on cocaine craving were more selective to drug cue-induced craving. This is largely inconsistent with pre-clinical data and suggests some remaining vulnerability for relapse risk in the use of guanfacine for cocaine relapse prevention. It is important to note, however, that preclinical models of stress and cue-related craving have developed via the examination of various aspects of self-administration schedules within stress-related and cue presentation paradigms. These have included assessing breaking points within progressive ratio schedules, duration of extinction responding, conditioned reinforcement through repeated pairings of drug and a neutral stimulus, and second-order schedule paradigms (Markou et al., 1993 for review). While the efficacy of guanfacine has not been directly assessed within these paradigms, studies using the stress-reinstatement model have shown evidence for the role of guanfacine in reducing the incentive–motivational properties of cocaine, nicotine and alcohol following stress (Cleck and Blendy, 2008; Erb et al., 2000; Highfield et al., 2001; Lê et al., 2005; Shaham et al., 2000; Stewart, 2003; Zislis et al., 2007).
Another potential explanation for the selective cue-related efficacy in reducing cocaine craving may be related to dose. It may be that 2 mg guanfacine daily is successfully able to reduce drug cue-related cocaine craving; however, a higher dose of 3 mg daily may be required to decrease stress-induced cocaine craving. As this was an initial preliminary study assessing guanfacine’s effects on drug craving and stress arousal, there was little previous research regarding the optimal guanfacine dosing levels for drug craving-related outcomes. Indeed, exploratory analyses in the present study provided an initial positive signal for a higher, 3 mg, dose of guanfacine, relative to the 2 mg and the placebo dose, to be beneficial in decreasing stress-induced cocaine craving and stress-induced nicotine craving. This initial dose-related exploratory analysis suggests the need to conduct a dose-related study to systematically examine the effects of guanfacine 2 mg daily and 3 mg daily relative to placebo on stress and drug cue-related craving and stress arousal and this is currently under study.
In the present study, guanfacine also demonstrated greater efficacy in reducing cue-related, rather than stress-related, anxiety and arousal, and these findings may again be consistent with the significantly higher levels of provoked cocaine and nicotine craving observed following the cue condition (Sinha et al., 2003; Fox et al., 2007, 2008). Notably, in our prior research using analogous imagery paradigms, we have shown that stress and cue exposure elicit dissociable craving-related emotional states. While cue-related craving reflects a greater anticipatory deprivation state characterized by anxiety, fear and jitteriness, stress is more associated with higher levels of negative emotion including sadness and anger (Fox et al., 2007). In addition to this, extensive pre-clinical research has shown that the specific arousal state can affect the pharmacodynamics of many psychoactive agents, possibly due to the potential for synergistic effects on CRF-NE feed forward pathways, common to both substance abuse as well as acute and chronic anxiety (Baker et al., 2005; Bremner et al., 1997; Bruijnzeel and Gold, 2005; Heim et al., 2000 for review). Adrenergic medications such as guanfacine may therefore show greater efficacy during these increased anxiety and arousal states, which, in the current study, is more likely to be associated with the cue condition. It is notable for example that an increased trend in negative emotion in this study was demonstrated in both groups following stress and combined stress and cue compared with the cue condition and guanfacine had no effect on provoked negative emotion (sadness and anger) following either stress or cue.
Findings from the current study may therefore suggest that the cue-related dissociations observed in guanfacine’s efficacy may be associated with selective underlying emotional arousal states and offer an added potential explanation for the more robust effects of guanfacine on cue-related anxiety, arousal, and cocaine craving compared with stress-related response. The possibility that different components of emotional distress may underpin the stress-related and cue-related craving state may further help clarify the potential need for a higher guanfacine dose to reduce arousal and craving following exposure to the stress imagery condition. For example, several laboratory studies in humans have demonstrated discrete emotion-specific blood pressure responses within major categories of emotions (Montoya et al., 2005; Sinha et al, 1992). In one particular study conducted by Montoya et al. (2005), while anger was associated with increased cardiac output, fear was associated with decreased cardiac output and increased total peripheral resistance in a group of healthy subjects. As the stress- and cue-related craving state has been shown to be dissociable in terms of subjective and physiological aspects of emotions such as anger and fear (Fox et al., 2007; Montoya et al., 2005; Sinha et al., 1992), varying doses of adrenergic medications such as guanfacine may be required to decrease arousal.
The ability of guanfacine to reduce this cue-related anxiety, arousal and craving is clinically important. From a broad perspective, the early tension-reduction hypothesis of addiction proposed that substance use represented a means to reduce stress and anxiety (Cappel and Herman, 1972; Conger, 1951, 1956). In addition, extensive preclinical studies have modeled the sensitization of withdrawal-induced anxiety, via assessment of locomotor activation and social interaction, and its association with increased drug seeking and relapse (Bossert et al., 2005; Shalev et al., 2002). Moreover, in human research, recent studies have shown that subjective, bio-physiological and behavioral/bodily indicators of arousal and anxiety are both robustly associated with the negative reinforcing effects of cocaine and alcohol (Bergquist et al., 2010; Breese et al., 2011; Chaplin et al., 2010; Fox and Sinha, 2009).
Findings from this study also indicated a more robust effect of guanfacine in terms of reducing nicotine, compared with cocaine, craving. Such variation in efficacy between craving for different drugs may have been influenced by differences in consumption expectancies in the current research design. While participants were all treatment seeking for cocaine dependence and kept on a locked inpatient facility with no access to cocaine or other illicit drugs, they were allowed four regular smoke breaks per day including prior to and following the laboratory study, in order to curb nicotine withdrawal-related symptoms. As early research studies have indicated that consumption expectancies exert an influence on cue-related craving (Berg et al., 1981; Kaplan et al., 1984; Marlatt et al., 1973), future research is warranted to investigate in greater depth how both dose and consumption expectancies contribute to medication effects on stress and cue-related craving.
Another possible explanation for differences in guanfacine’s efficacy in reducing cocaine craving compared with nicotine craving may be related to pharmacodynamic variations between the two drugs in terms of dopamine (DA) release within the nucleus accumbens (NAc). While cocaine inhibits DA uptake in the NAc, creating high levels of extracellular DA (Weiss et al., 1992), nicotine use leads to an enhanced fraction of DA release via presynaptic terminals (Sulzer, 2011 for review). While preclinical evidence has shown that α2A agonists, including guanfacine, do demonstrate efficacy in terms of counteracting the hyperdopaminergic state of certain drugs (Jentsch et al., 2008), it is possible that the long-term effects of nicotine and cocaine abuse on dopamine pathways may vary and could potentially contribute to the differential effects of guanfacine on nicotine versus cocaine craving.
Data from the imaging component of this study expands upon the subjective and autonomic laboratory data in showing that the therapeutic effects of guanfacine may also extend to the mediation of central catecholaminergic function in cocaine dependent individuals. While the laboratory findings highlight the potential of guanfacine to reduce peripheral autonomic arousal as well as drug cue-related subjective arousal, craving and anxiety; the imaging data confirms the ability of guanfacine to increase activation in prefrontal regions associated with distractibility and alertness (Sara, 2009) as well as the cognitive and emotional regulatory mechanisms underpinning craving and treatment outcome (Aron et al., 2007; Kober et al., 2010; Li et al., 2009a; Sinha et al., 2005). This is consistent with research indicating that guanfacine may improve prefrontal network connectivity at the level of ion channels by stimulating α2 adrenergic receptors in the prefrontal cortex (Arnsten and Pliszka, 2011 for review; Wang et al., 2007). Furthermore, the two studies combined highlight both a peripheral and central adrenergic mechanism for strengthening autonomic, emotional and cognitive/control-related function common to provoked anxiety, arousal and craving in cocaine dependent individuals.
Specifically, cocaine dependent individuals who were administered guanfacine demonstrated increased activation in medial and lateral prefrontal regions during the stress (ventrolateral PFC, dorsomedial PFC, anterior/middle cingulate gyrus) and the drug cue (left dorsolateral PFC, ventromedial PFC, lateral PFC) related imagery conditions relative to those administered placebo. Human imaging studies have shown activation in these dorsolateral pre-frontal regions to be associated with strategic working memory processes and regulatory control (Baker et al., 1996; Lazeron et al., 2000; Owen et al., 1996; Rowe et al., 2001; Yochim et al., 2008) known to underlie goal-oriented behaviors sensitive to stress (Baumeister et al., 1994; Kuhl and Koole, 2004) such as planning, organizational skills, self-monitoring, decision making (Blume and Marlatt, 2009) and the inability to resist environmental cues (de Wit and Richards, 2004). As such, the ability to optimize dorsolateral and ventromedial function in the face of stress and/or environmental challenge may also highlight an important pharmacotherapeutic mechanism associated with guanfacine. Although not directly assessed in the current study, some support for this is provided by the fact that several studies have documented guanfacine-induced increased activation within these pre-frontal regions alongside improved executive function (Avery et al., 2000; Jäkälä et al., 1999). Moreover, cognitive control techniques have been a helpful therapeutic strategy for reducing both negative affective responses and craving (Blume and Marlatt, 2009; Kober et al., 2010; Ochsner and Gross, 2005; O’Connell et al., 2007).
In terms of study limitations, most notably, any interpretation of findings is limited by the small subject sample and must be viewed in terms of preliminary data. Also, as no previous clinical study had tested guanfacine’s effects in the laboratory or on clinical outcomes in drug abuse, we administered up to 3 mg daily doses without systematically testing for a dose effect of 2 versus 3 mg. In addition, although guanfacine reduced cue-related cocaine craving in the laboratory sessions, this was only comparative with other imagery conditions that also evoked a craving response. While this is theoretically supported by a large number of studies using the same imagery paradigm and which typically show craving to be significantly higher following exposure to cue compared with stress (Chaplin et al., 2008; Fox et al., 2007, 2008, 2009; Sinha et al., 2003, 2007, 2009) future research may be encouraged to consider the use of a non-arousing comparison condition.
Additionally, with regard to our fMRI study, it is important to consider the possibility that functional brain activation may have been indirectly affected by the non-specific anti-hypertensive effects of guanfacine (Kalisch et al., 2001; Tuor et al., 2002), producing a potential confound in terms of response to the stress and cue-related stimuli. While this remains a possibility, there were no differences in brain response between placebo and guanfacine treated groups in the neutral/relaxing control condition, which would be expected with any non-specific anti-hypertensive effect of guanfacine. Rather, brain activation in the current study was observed in discrete, rather than widespread, regions of the pre-frontal cortex that are consistent with those previously observed in rats administered non-sedative doses of guanfacine (Easton et al., 2006) as well as those affected by ADHD (Arnsten, 2011).
Despite these limitations, this preliminary study is the first to assess guanfacine’s effects on stress- and drug cue-related drug craving as well as peripheral and neural stress arousal responses. Findings suggest that guanfacine may reduce stress- and cue-induced nicotine craving as well as cue-induced cocaine craving, anxiety and arousal in cocaine dependent individuals possibly by decreasing peripheral arousal and increasing BOLD activation within regulatory prefrontal regions. Exploratory data also suggest that a higher dose of 3 mg daily may be required to decrease stress-induced subjective cocaine craving and arousal, possibly due to dissociations in the emotional processes underlying stress and cue exposure. These data suggest that further dose-related studies of guanfacine in decreasing stress, drug craving and relapse risk in cocaine dependence are warranted.
Acknowledgments
We would like to thank the staff at The Clinical Neuroscience Research Unit, The Substance Abuse Center at The Connecticut Mental Health Center, The Yale Center for Clinical Research and the Yale Magnetic Resonance Research Center for their assistance in completing this study.
Funding
This work was supported by the National Institute of Health (NIH) and the NIH Common Fund Grants R01-DA027130 (to RS), UL1-DE019586 (to RS) and PL1-DA024859 (to RS), and the Connecticut Department of Mental Health and Addiction Services.
Footnotes
Reprints and permission: sagepub.co.uk/journalsPermissions.nav
Conflict of interest
The authors declare that they have no competing financial interests pertaining to the aims and results of this study.
References
- Alterman AI, Snider EC, Cacciola JS, May DJ, Parikh G, Maany I, Rosenbaum PR. A quasi-experimental comparison of the effectiveness of 6- versus 12-hour per week outpatient treatments for cocaine dependence. J Nerv Ment Dis. 1996;184(1):54–6. doi: 10.1097/00005053-199601000-00010. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. The use of 2A adrenergic agonists for the treatment of attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2011;10:1595–1605. doi: 10.1586/ern.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Catecholamines and cognitive decline in aged nonhuman primates. Ann N Y Acad Sci. 1985;444:218–234. doi: 10.1111/j.1749-6632.1985.tb37592.x. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–13384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR. Catecholamine influences on pre-frontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011 Feb 2; doi: 10.1016/j.pbb.2011.01.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;3:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, et al. Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 2005;162:992–994. doi: 10.1176/appi.ajp.162.5.992. [DOI] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RSJ, et al. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF, Tice DM. Losing Control: How and Why People Fail at Self-regulation. San Diego, CA: Academic Press; 1994. [Google Scholar]
- Berg JC, Laberg A, Skutle A, Ohman A. Instructed versus pharmacological effects of alcohol in alcoholics and social drinkers. Behav Res Ther. 1981;19:55–66. doi: 10.1016/0005-7967(81)90112-1. [DOI] [PubMed] [Google Scholar]
- Bergquist KL, Fox HC, Sinha R. Self-reports of interoceptive responses during stress and drug cue related experiences in cocaine and alcohol dependent individuals. Exper Clin Psychopharmacol. 2010;18:229–237. doi: 10.1037/a0019451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. SPD503 Study Group. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2008;121:e73–e84. doi: 10.1542/peds.2006-3695. [DOI] [PubMed] [Google Scholar]
- Blume AW, Marlatt GA. The role of executive cognitive functions in changing substance use: what we know and what we need to know. Ann Behav Med. 2009;37:117–25. doi: 10.1007/s12160-009-9093-8. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Cai JX, Ma YY, Xu L, Hu XT. Reserpine impairs spatial working memory performance in monkeys: reversal by the alpha 2-adren-ergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- Cappel H, Herman CP. Alcohol and tension reduction: a review. Q J Stud Alcohol. 1972;33:33–64. [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Freiburger MB, Mayes LC, Sinha R. Prenatal cocaine exposure, gender, and adolescent stress response: a prospective longitudinal study. Neurotoxicol Teratol. 2010;32:595–604. doi: 10.1016/j.ntt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PB, Riddle MA, Scahill L, Lynch KA, Schultz R, Arnsten A, et al. Guanfacine treatment of comorbid attention-deficit hyperactivity disorder and Tourette’s syndrome: preliminary clinical experience. J Am Acad Child Adolesc Psychiatry. 1995;34:1140–1146. doi: 10.1097/00004583-199509000-00010. [DOI] [PubMed] [Google Scholar]
- Cleck JN, Blendy JA. Making a bad thing worse: adverse effects of stress on drug addiction. J Clin Invest. 2008;118:454–461. doi: 10.1172/JCI33946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JJ. The effects of alcohol on conflict behavior in the albino rat. Q J Stud Alcohol. 1951;12:1–29. [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem and challenge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brains reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH. Geometric strategies for neuroanatomic analysis from MRI. Neuroimage. 2004;23(Suppl 1):S34–S45. doi: 10.1016/j.neuroimage.2004.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton N, Shah YB, Marshall FH, Fone KC, Marsden CA. Guanfacine produces differential effects in frontal cortex compared with striatum: assessed by phMRI BOLD contrast. Psychopharmacology (Berl) 2006;189(3):369–385. doi: 10.1007/s00213-006-0558-1. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: craving and use during daily life. Addict Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;21:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Evans A, Collins D, Mills S, Brown E, Kelly R, Peters T. 3D statistical neuroanatomical models from 305 MRI volumes. In: Proceedings of the IEEE. Nuclear Science Symposium and Medical Imaging Conference; 1993. pp. 1813–1817. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Washington DC: American Psychiatric Press Inc; 1995. Patient Edition. [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress system changes: implications for treatment in substance-abusing women. Harv Rev Psychiatry. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Garcia M, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug-cue in cocaine dependent individuals. Psychopharmacology. 2006;185:348–357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Berquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong K-I, Siedlarz KM, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz KM, Bergquist KL, Anderson GM, Kreek MJ, et al. Gender dissociations in autonomic and HPA responses to stress and cues in alcohol dependent patients with cocaine abuse. Alcohol Alcohol. 2009;44:575–585. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Goldstein RA, DesLauriers C, Burda AM. Cocaine: history, social implications, and toxicity – a review. Dis Mon. 2009;55:6–38. doi: 10.1016/j.disamonth.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Handen BL, Sahl R, Hardan AY. Guanfacine in children with autism and/or intellectual disabilities. J Dev Behav Pediatr. 2008;29:303–308. doi: 10.1097/DBP.0b013e3181739b9d. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypo-cortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Highfield D, Yap J, Grimm JW, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced but not drug cues-induced reinstatement of a heroin–cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Hunt RD, Arnsten AFT, Asbell MD. An open trial of guanfacine in the treatment of attention deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Izard C. Patterns of emotions: A new analysis of anxiety and depression. New York: Academic Press; 1972. [Google Scholar]
- Jäkälä P, Riekkinen M, Sirviö J, Koivisto E, Kejonen K, Vanhanen M, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Jastreboff AM, Potenza MN, Lacadie C, Hong KA, Sherwin RS, Sinha R. Body mass index, metabolic factors, and striatal activation during stressful and neutral–relaxing states: an FMRI study. Neuropsychopharmacology. 2010;36:627–637. doi: 10.1038/npp.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Sanchez D, Elsworth JD, Roth RH. Clonidine and guanfacine attenuate phencyclidine-induced dopamine overflow in rat prefrontal cortex: mediating influence of the alpha-2A adrenoceptor subtype. Brain Res. 2008;1246:41–46. doi: 10.1016/j.brainres.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Elbel GK, Gössl C, Czisch M, Auer DP. Blood pressure changes induced by arterial blood withdrawal influence bold signal in anesthetized rats at 7 Tesla: implications for pharmacologic mri. Neuroimage. 2001;14:891–898. doi: 10.1006/nimg.2001.0890. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, et al. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Dackis C, Lynch KG, Pettinati H, Tirado C, Gariti P, et al. A double-blind, placebo-controlled trial of amantadine, pro-pranolol, and their combination for the treatment of cocaine dependence in patients with severe cocaine withdrawal symptoms. Drug Alcohol Depend. 2006;85:129–137. doi: 10.1016/j.drugalcdep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kang SY, Kleinman PH, Woody GE, Millman RB, Todd TC, Kemp J, et al. Outcomes for cocaine abusers after once-a-week psychosocial therapy. Am J Psychiatry. 1991;148:630–635. doi: 10.1176/ajp.148.5.630. [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Meyer RE, Virgilio LM. Physiological reactivity to alcohol cues and the awareness of an alcohol effect in a double-blind placebo design. Br J Addict. 1984;79:439–442. doi: 10.1111/j.1360-0443.1984.tb03893.x. [DOI] [PubMed] [Google Scholar]
- Kober H, Kross EF, Mischel W, Hart CL, Ochsner KN. Regulation of craving by cognitive strategies in cigarette smokers. Drug Alcohol Depend. 2010;106(1):52–55. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl J, Koole SL. Workings of the will. In: Greenberg J, Koole SL, Pyszczynski T, editors. Handbook of Experimental Existential Psychology. New York: Guildfors Press; 2004. pp. 411–430. [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: conceptual structure and pattern of somato-visceral response. Psychophysiology. 1980;17:179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. J Abnorm Psychol. 1983;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Lazeron RH, Rombouts SA, Machielsen WC, Scheltens P, Witter MP, Uylings HB, et al. Visualizing brain activation during planning: the Tower of London test adapted for MR imaging. Am J Neuroradiol. 2000;21:1407–1414. [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Li CS, Kemp K, Milivojevic V, Sinha R. Neuroimaging study of sex differences in the neuropathology of cocaine abuse. Gend Med. 2005;2:174–182. doi: 10.1016/s1550-8579(05)80046-4. [DOI] [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol Clin Exp Res. 2009a;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Zhang S, Duann JR, Yan P, Sinha R, Mazure CM. Gender differences in cognitive control: an extended investigation of the stop signal task. Brain Imaging Behav. 2009b;3:262–276. doi: 10.1007/s11682-009-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CL, Arnsten AF, Li BM. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Biol Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Demming B, Reid JB. Loss of control drinking in alcoholics: an experimental analogue. J Abnorm Psychol. 1973;81:233–241. doi: 10.1037/h0034532. [DOI] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Rutherford MJ, Cacciola JS, McLellan AT. The relationship of alcohol use to cocaine relapse in cocaine dependent patients in an aftercare study. J Stud Alcohol. 1999;60:176–180. doi: 10.15288/jsa.1999.60.176. [DOI] [PubMed] [Google Scholar]
- Mercer D, Carpenter G, Daley D. Unpublished measure. Center for Psychotherapy Research, Department of Psychiatry, University of Pennsylvania Medical School; 1994. The adherence and competence scale for group addiction counseling for TCACS. [Google Scholar]
- Miller GA, Levin DN, Kozak MJ, Cook EW, McLean A, Jr, Lang PJ. Individual differences in imagery and the psychophysiology of emotion. Cognition Emotion. 1987;1:367–390. [Google Scholar]
- Montoya P, Campos JJ, Schandry R. See red? Turn pale? Unveiling emotions through cardiovascular and hemodynamic changes. Span J Psychol. 2005;8:79–85. doi: 10.1017/s1138741600004984. [DOI] [PubMed] [Google Scholar]
- Nolfe E. XMedCon – An open-source medical image conversion toolkit. Eur J Nucl Med. 2003;30(Supp 2):S246. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Hosein VL, Schwartz JE, Leibowitz RQ. How does coping help people resist lapses during smoking cessation? Health Psychol. 2007;26:77–84. doi: 10.1037/0278-6133.26.1.77. [DOI] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW. Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain. 1996;119:1597–1615. doi: 10.1093/brain/119.5.1597. [DOI] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Mannelli P, Peindl K, Murray HW, Meier B, Leone FT. Changes in tobacco smoking following treatment for cocaine dependence. Am J Drug Alcohol Abuse. 2006;32:135–148. doi: 10.1080/00952990500479209. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, et al. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, Weiskrantz L. The Prefrontal Cortex Executive And Cognitive Functions. Oxford: Oxford University Press; 1998. [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Owen AM, Johnsrude IS, Passingham RE. Imaging the mental components of a planning task. Neuropsychologia. 2001;39:315–327. doi: 10.1016/s0028-3932(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Sáez CG, Olivares P, Pallavicini J, Panes O, Moreno N, Massardo T, et al. Increased number of circulating endothelial cells and plasma markers of endothelial damage in chronic cocaine users. Thromb Res. 2011;128:e18–e23. doi: 10.1016/j.thromres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Scahill L. Alpha-2 adrenergic agonists in children with inattention, hyperactivity and impulsiveness. CNS Drugs. 2009;23(Suppl 1):43–49. doi: 10.2165/00023210-200923000-00006. [DOI] [PubMed] [Google Scholar]
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Schindler CW. Cocaine and cardiovascular toxicity. Addict Biol. 1996;1:31–47. doi: 10.1080/1355621961000124676. [DOI] [PubMed] [Google Scholar]
- Sees KL, Clark HW. Substance abusers want to stop smoking! Alcohol. Clin Experiment Res. 1991;15:152. [Google Scholar]
- Sees KL, Clark HW. When to begin smoking cessation in substance abusers. J Subst Abuse Treat. 1993;10:189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp. 2010;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Smoking-cessation treatment utilization: the need for a consumer perspective. Am J Prev Med. 2010;38(Suppl 3):S382–S384. doi: 10.1016/j.amepre.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sinha R, Parsons OA. Multivariate response patterning of fear and anger. Cognition Emotion. 1996;10:173–198. [Google Scholar]
- Sinha R, Lovallo WR, Parsons OA. Cardiovascular differentiation of emotions. Psychosom Med. 1992;54:422–435. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary–ACDrenal axis and sympatho- ACDreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, et al. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic–pituitary–adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology (Berl) 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist KL, Bhagwagar Z, Siedlatz K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am J Addict. 2003;12:1–17. [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Strang J, Bearn J, Gossop M. Lofexidine for opiate detoxification: review of recent randomized and open controlled trials. Am J Addict. 1999;8:337–348. doi: 10.1080/105504999305749. [DOI] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz BE, McDonald CR, Patel A, Torgersen D. The effects of guanfacine on working memory performance in patients with localization-related epilepsy and healthy controls. Clin Neuropharmacol. 2008;31:251–260. doi: 10.1097/WNF.0b013e3181633461. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Russo J. Comparing guanfacine and dextroamphet-amine for the treatment of adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- Tuor UI, McKenzie E, Tomanek B. Functional magnetic resonance imaging of tonic pain and vasopressor effects in rats. Magn Reson Imaging. 2002;20:707–712. doi: 10.1016/s0730-725x(02)00599-4. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Weiss F, Paulus MP, Lorang MT, Koob GF. Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: effects of acute and repeated administration. J Neurosci. 1992;12:4372–4380. doi: 10.1523/JNEUROSCI.12-11-04372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Department of Imaging Neuroscience. Statistical Parametric Mapping Software. London, UK: 2005. [accessed 1 December 2005]. Available at: http://www.fil.ion.ucl.ac.uk/spm/ [Google Scholar]
- Wiseman EJ, McMillan DE. Relationship of cessation of cocaine use to cigarette smoking in cocaine-dependent outpatients. Am J Drug Alcohol Abuse. 1998;24:617–625. doi: 10.3109/00952999809019611. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Emphasis on interpersonal factors in a dynamic model of relapse. Am Psychol. 2005;60:341–342. doi: 10.1037/0003-066X.60.4.341. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao J-H, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. [Google Scholar]
- Yochim BP, Baldo JV, Kane KD, Delis DC. D-KEFS Tower Test performance in patients with lateral prefrontal cortex lesions: the importance of error monitoring. J Clin Exp Neuropsychol. 2008;24:1–6. doi: 10.1080/13803390802448669. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]